Abstract
Device failure from unexpected battery depletion is uncommon but can be life-threatening. Lithium cluster formation at the cathode is a novel mechanism of sudden implantable cardioverter-defibrillator (ICD) battery depletion that was first reported in 2014. We report a rare case of a 78-year-old woman with an ICD battery failure due to lithium cluster formation. Although she had never received ICD therapy, the battery voltage had dropped from 2.9 V to 2.54 V (end of life) unexpectedly for only 2 days. The prevalence of this rare phenomenon was reported to be 0.004% in 2014. However, it had gone up to 0.21% in October 2016. Both device manufacturers and clinicians should be aware of this phenomenon, and remote monitoring systems and vibratory patient notifier alerts should be considered for early detection and early treatment.
<Learning objective: Lithium cluster formation is a novel mechanism of premature ICD battery depletion that was first reported in 2014. Although the prevalence of this rare phenomenon was first reported as 0.004% in 2014, according to the data of St. Jude Medical, it had gone up to 0.21% in October 2016. We should be aware of this rare but important phenomenon and use remote monitoring systems and vibratory patient notifier alerts for early detection and treatment.>
Keywords: Battery depletion, Lithium cluster, Short circuit, Implantable cardioverter-defibrillator, St. Jude Medical
Introduction
Battery or capacitor malfunctions are the most common causes of implantable cardioverter-defibrillator (ICD) generator malfunction [1]. Lithium cluster formation at the cathode is a novel mechanism of premature ICD battery depletion, that was first reported by Pokorney et al. recently [2]. Although the authors showed that the prevalence of this phenomenon was 0.004%, it might actually be higher. Herein, we report a rare case of unexpected early battery depletion caused by lithium cluster formation in an ICD.
Case report
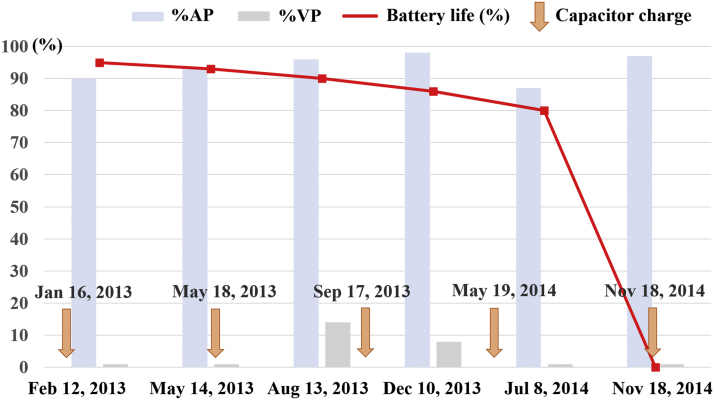
A 78-year-old woman without structural heart disease, but with a history of hypertension, paroxysmal atrial fibrillation, and third-degree atrioventricular block underwent the implantation of an ICD with a Medtronic Secura DR generator (Medtronic, Minneapolis, MN, USA) for secondary prevention (sustained ventricular tachycardia) in 2010. Due to normal battery depletion, the first generator was changed to a St. Jude Fortify ST DR CD2235-40 generator (St. Jude Medical, St. Paul, MN, USA) in January 2013. After the first generator exchange, no shock or anti-tachycardia pacing therapy was delivered, and parameters such as the lead and shock impedances and the sensing and pacing thresholds were stable (threshold: atrial 0.75 V, ventricular 1.0 V; amplitude: atrial 2.3 mV, ventricular 6.7 mV; impedance: atrial 410 Ω, ventricular 460 Ω). Although the residual battery longevity was 80% in July 2014, the patient’s device was noted to be at its end of life on November 18, 2014 (Fig. 1). She was immediately referred to our hospital to undergo another generator exchange. Her device was replaced with a St. Jude Fortify Assura DR CD2359-40C (St. Jude Medical), and we returned her explanted device to St. Jude Medical for analysis. The manufacturer’s analysis demonstrated that the battery voltage had dropped from 2.9 V to 2.54 V (end of life) unexpectedly between November 17 and 18, 2014. The device’s microelectronics were normal, and there were no abnormalities in the circuitry even when testing it in conditions of temperature fluctuations and high humidity. The battery was sent back to its manufacturer for a detailed examination. Upon inspection of the battery, a lithium cluster was observed on the cathode (on the top side of the battery) and was found to touch the anode (Fig. 2). This cluster caused a short circuit between the cathode and the anode, leading to a high current drain and rapid battery failure.
Fig. 1.
Time course of residual battery longevity. Although the residual battery longevity was 80% in July 2014, the device was found to have reached its end of life on November 18, 2014. Capacitor charges were conducted five times for regular maintenance of the capacitor.
%AP = percentage of atrial pacing; %VP = percentage of ventricular pacing.
Fig. 2.

Lithium cluster observed on top side of the battery. It was found on the cathode and touched the anode. The arrow shows the lithium cluster.
Discussion
Lithium clusters are composed of microscopic lithium deposits that form in the setting of high current pulses between the cathode and the anode. Lithium clusters are by-products of capacitor charges themselves, and the clustering phenomenon can theoretically happen in any high-energy device powered by a lithium battery. As the patient in our case had never received tachyarrhythmia therapy, the lithium cluster formation was caused by the regular maintenance capacitor charges. It is interesting to note that this phenomenon so far seems to be limited to the Unify/Fortify family of St. Jude Medical devices. Aggarwal et al. identified two contributing factors: (1) the shape and orientation of the battery in the Unify/Fortify devices, (2) the higher energy output of the Unify/Fortify (40 J) over the Ellipse (36 J) devices [3]. The electrical current can pass through the lithium cluster, generating a short circuit that causes overheating and damage to the battery cells, leading to rapid battery energy depletion [2]. This problem can be prevented by adding an extra layer of insulation between the anode and the cathode on the battery header to ensure that the lithium cluster does not connect the anode with the cathode. The US Food and Drug Administration (FDA) approved this change in 2015 and the latest generation of Unify/Fortify devices features this new layer of battery insulation. No case of early battery depletion caused by lithium cluster formation has arisen since that change. Pokorney et al. first reported this phenomenon in a two-patient case series in 2014. At the time, the reported prevalence of this battery failure mechanism was approximately 0.004%. Aggarwal et al. reported another four cases in 2016, and pointed out that the estimated prevalence of 0.004% was an underestimation [2], [3]. Based on October 2016 data from St. Jude Medical, 841 (0.21%) out of 398,740 Unify/Fortify devices sold globally had been returned to the manufacturer because of early battery depletion, and they presented evidence of lithium material in the form of clusters in the battery. Electrical short circuits caused by lithium clusters were confirmed in 46 of these 841 ICDs. In the remaining 795 devices, a battery short was not confirmed. However, the presence of lithium clusters was noted and no other cause for premature battery depletion was identified. The prevalence of early battery depletion caused by lithium clusters, including both confirmed and unconfirmed shorts, has since gone up to 0.21% (841 out of 398,740 devices). The FDA announced a class 1 recall of these affected devices on October 11, 2016. In some cases, full battery drainage occurred within a day to a few weeks after the device had reached an elective replacement indicator (ERI). The devices should be programmed to deliver a vibratory patient alert when the battery has reached ERI. Healthcare providers should immediately replace the devices at the time of an ERI alert. The FDA also pointed out that battery depletion may not always be reported to the manufacturer. Therefore the true number of devices affected by premature battery depletion from lithium clusters is unknown. At the time of writing, 349,852 affected devices remain actively implanted worldwide [4]. Failure is hard to predict, as battery depletion can happen within a day to a few weeks if a short circuit occurs. It might be better to replace these devices prophylactically, particularly in patients who are dependent on pacing because they suffer from bradycardia or who have a history of frequent ICD tachycardia therapies, as well as in responders to cardiac resynchronisation therapy, as these patients are at a higher risk of developing heart failure and sudden cardiac death. Remote monitoring system and a vibratory patient notifier alert should be used to facilitate early detection and early treatment.
Conclusions
Early battery depletion caused by lithium clusters is a rare complication. Both device manufacturers and clinicians should be aware of this phenomenon, and remote monitoring systems and vibratory patient notifier alerts should be considered for early detection and early treatment.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
We would like to thank St. Jude Medical for their assistance with the device analysis.
References
- 1.Maisel W.H., Moynahan M., Zuckerman B.D., Gross T.P., Tovar O.H., Tillman D.B. Pacemaker and ICD generator malfunctions: analysis of Food and Drug Administration annual reports. JAMA. 2006;295:1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 2.Pokorney S.D., Greenfield R.A., Atwater B.D., Daubert J.P., Piccini J.P. Novel mechanism of premature battery failure due to lithium cluster formation in implantable cardioverter-defibrillators. Heart Rhythm. 2014;11:2190–2195. doi: 10.1016/j.hrthm.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal A., Sarmiento J.J., Charles D.R., Parr A.R., Baman T.S. Accelerated implantable defibrillator battery depletion secondary to lithium cluster formation: a case series. Pacing Clin Electrophysiol. 2016;39:375–377. doi: 10.1111/pace.12808. [DOI] [PubMed] [Google Scholar]
- 4.FDA safety communication: premature battery depletion of St. Jude Medical ICD and CRT-D devices. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm524666.htm. [Date issued on October 11, 2016].