Abstract
Chloride was recently recognized to play an important role in the pathophysiology of heart failure (HF). Chloride manipulation, including the use of acetazolamide, may be a requisite therapeutic target in HF treatment. An 87-year-old male patient with advanced HF and hypertrophic cardiomyopathy was admitted to the hospital due to hypochloremia (94 mEq/L) and hyponatremia (134 mEq/L) under diuretic treatment with azosemide, spironolactone, and tolvaptan. On admission, HF-related signs of overhydration were lacking, but B-type natriuretic peptide was moderately elevated. The etiology of the hypochloremia/natremia state was depletion of both electrolytes based on serum analysis and spot urinary concentrations. Immediately after admission, acetazolamide (500 mg/d) was prescribed to correct the hypochloremia in parallel with cessation of the preceding administration of azosemide and spironolactone, and tapering off of the tolvaptan over 7 days. Under treatment, both serum chloride and sodium concentrations recovered to normal (108 mEq/L and 148 mEq/L, respectively), and the serum potassium concentration decreased from 3.9 mEq/L to 2.4 mEq/L. Urinary concentrations of sodium and potassium increased from 18 mEq/L to 31 mEq/L and from 19 mEq/L to 51.5 mEq/L respectively, in concordance with the changes in serum concentrations, but the chloride concentration decreased from 18 mEq/L to 12 mEq/L, opposite the changes in the serum concentration.
<Learning objective: The present case confirms that the classic drug acetazolamide, although rarely used now for HF treatment, is a potent “chloride-regaining” or “chloride-retaining diuretic” with concomitant activity to reduce serum potassium. Additionally, this case study highlights the importance of monitoring both serum and urinary electrolyte concentrations to determine the electrolyte disturbance and efficacy of diuretic treatment through analytic evaluation of changes in the serum electrolytes and their tubular reabsorption in the kidney.>
Keywords: Heart failure, Acetazolamide, Chloride, Diuretics, Electrolyte disturbance
Introduction
Chloride was recently identified as having an important role in heart failure (HF) pathophysiology. Grodin et al. reported that upon admission for worsening HF, the serum chloride concentration, but not the serum sodium concentration, is an independent prognostic marker of acutely decompensated HF [1]. I recently proposed a unifying hypothesis of the “chloride theory” for HF pathophysiology during worsening HF and its therapeutic resolution [2], [3]. According to the “chloride theory” [2], chloride manipulation, including the use of acetazolamide [4], [5], [6], may be an essential therapeutic target in HF treatment.
Case report
An 87-year-old man with advanced HF patient (New York Heart Association functional class III) and hypertrophic cardiomyopathy was admitted to the hospital due to complaints of general fatigue and loss of appetite. Physical examination on admission revealed no HF-related physical signs of neck vein distension, peripheral edema, extra-cardiac sounds, or bilateral pulmonary rales, but he presented with weight loss of 2 kg over 3 months, hypotension (92/72 mmHg), irregular pulse (98 bpm), and peripheral coldness. A 12-lead electrocardiogram revealed atrial fibrillation with an irregular heart rate of 106 beats/min and a complete right bundle branch pattern. A chest X-ray revealed moderate cardiomegaly (cardiothoracic ratio, 63%), but no signs of pulmonary congestion or pleural effusion. Ultrasonography showed diastolic dysfunction with reduced left ventricular ejection fraction (40%), non-dilated diastolic volume (74 cc), marked asymmetric left ventricular hypertrophy (interventricular septum, 35 mm; posterior wall, 20 mm), and a moderately enlarged left atrium. Ultrasound revealed no pleural effusion, but a moderately expanded inferior vena cava with minimal respiratory change. Blood examination revealed a modestly increased serum B-type natriuretic peptide concentration (720 pg/ml), reduced renal function (estimated glomerular filtration rate of 31 mL/min), and a moderate degree of hyponatremia (134 mEq/L) and hypochloremia (94 mEq/L) under diuretic treatment with a combination of azosemide (90 mg/d), spironolactone (12.5 mg/d), and tolvaptan (22.5 mg/d).
To identify the background mechanisms of the hypochloremia and hyponatremia in this patient, longitudinal changes in serum and spot urinary electrolytes [7] before and after the present hospital admission were examined in parallel with evaluation of changes in other laboratory tests (Table 1). Approximately four months prior to the present hospital admission, he was admitted to another hospital (March, 2017) and underwent evaluation of dietary effects on the changes in serum and spot urinary solutes under a high- (12 g/d) or low-salt (6 g/d) diet. That evaluation clearly revealed high and low urinary excretion of both sodium and chloride in accordance with the amount of dietary salt. The etiology of hypochloremia and hyponatremia at the present admission (July, 2017) was determined to be the depletion of both sodium and chloride electrolytes from the body based on the evaluation of serum and spot urinary concentrations of the solutes, i.e. excretion of both chloride and sodium was extremely reduced compared with the previous examination. Acetazolamide (500 mg/d) was prescribed on day 1 of the present admission to correct the hypochloremia, and replace the previously prescribed diuretics. In parallel, the preceding administration of azosemide (90 mg/d) and spironolactone (12.5 mg/d) was discontinued immediately, and tolvaptan was tapered off over 7 days from 22.5 mg/d before admission to 15 mg/d, 7.5 mg/d, and ultimately cessation. Under such treatment, both the serum chloride and sodium concentrations recovered to normal values (108 mEq/L and 148 mEq/L, respectively), and the serum potassium concentration decreased from 3.9 mEq/L to 2.4 mEq/L on day 8 of hospitalization. Urinary concentrations of sodium and potassium were increased (sodium, from 18 mEq/L to 31 mEq/L; potassium, from 19 mEq/L to 51.5 mEq/L) in accordance with the changes in the serum concentrations, but the urinary chloride concentration was decreased (from 18 mEq/L to 12 mEq/L) opposite the changes in the serum concentration. Thereafter, the hypokalemia was treated by increasing the dose of oral spironolactone (37.5 mg/d) and administering a potassium supplement (Slow-K, 1200 mg/d). As shown in Table 1, the serum potassium concentration returned to the normal range (4.5 mEq/L) on day 14 of hospitalization. At this time, the appetite loss and hypotension improved, worsening of physical HF-related signs did not appear, and the serum creatinine concentration improved compared to the admission evaluation (from 1.67 mg/dL to 1.47 mg/dL).
Table 1.
Changes in serum and urinary electrolytes before and after administration of acetazolamide.
| Admission to another hospital (March/2017) |
Admission to the present hospital (July/2017) |
|||||
|---|---|---|---|---|---|---|
| Before acetazolamide therapy — day 1 | After acetazolamide therapy |
|||||
| High-salt diet (12 g/d) | Low-salt diet (6 g/d) | Day 4 | Day 8 | Day 14 | ||
| (March 15) | (March 21) | (July 4) | (July 8) | (July 12) | (July 18) | |
| A. Heart failure-related examination | ||||||
| Body weight (kg) | 50 | – | 48.1 | – | – | – |
| Blood pressure (mmHg) | 115/82 | – | 92/71 | 85/66 | 94/67 | 104/77 |
| Heart rate (bpm) | 91 | – | 98 | 95 | 83 | 80 |
| Sings of body fluid retention | No | – | No | No | No | No |
| B-type natriuretic peptide (pg/mL) | 711 | 720 | – | – | – | |
| B. Peripheral blood examination | ||||||
| Hemoglobin (g/dL) | 14 | 14.6 | 14.4 | 14.5 | 14.8 | 13.5 |
| Hematocrit (%) | 42.2 | 42.4 | 42.1 | 42.8 | 44.8 | 39.7 |
| MCV (fL) | 100 | 98.6 | 102 | 105 | 107 | 106 |
| Serum electrolytes | ||||||
| Sodium (mEq/L) | 142 | 138 | 134 | 138 | 148 | 147 |
| Potassium (mEq/L) | 4.1 | 4.3 | 3.9 | 3.2 | 2.4 | 4.5 |
| Chloride (mEq/L) | 104 | 105 | 94 | 102 | 108 | 109 |
| Serum blood urea nitrogen (mg/dL) | 48 | 50 | 66 | 45 | ||
| Serum creatinine (mg/dL) | 1.64 | 1.8 | 1.67 | 1.78 | 2.38 | 1.47 |
| C. Urinary examination | ||||||
| Urinary electrolytes | ||||||
| Sodium (mEq/L) | 92 | 54.3 | 18 | – | 31 | – |
| Potassium (mEq/L) | 10.8 | 17.9 | 19 | – | 51.5 | – |
| Chloride (mEq/L) | 90 | 59.1 | 18 | – | 12 | – |
| Urinary creatinine (mg/dL) | 25.8 | – | 46.7 | – | 71.2 | – |
| Urinary electrolytes corrected by urinary creatinine | ||||||
| Sodium/Cr (10mEq/g Cr) | 3.57 | – | 0.39 | – | 0.44 | – |
| Potassium/Cr (10mEq/g Cr) | 0.42 | – | 0.41 | – | 0.72 | – |
| Chloride/Cr (10mEq/g Cr) | 3.49 | – | 0.39 | – | 0.17 | – |
| D. Diuretic treatment (daily dose) | ||||||

| ||||||
Cr, Creatinine; d, day; MCV, mean corporeal red cell volume.
Thoracic and abdominal X-ray computed tomographic examination revealed no evidence of malignancy. The appetite loss and fatigue were considered to be due to advanced age, low cardiac output syndrome, and frailty.
Discussion
The findings of the present case confirm that acetazolamide, the oldest diuretic among those commercially available, is a potent agent for “chloride-regaining or retaining diuretic” with concomitant pharmacologic action to reduce serum potassium [4], [5], [6], [8]. Additionally, this case study highlights the importance of monitoring both serum and urinary concentrations of solutes [7].
Clinical significance of hypochloremia in HF
Electrolyte abnormalities, including hypochloremia, may be multifactorial and interrelated, resulting from neurohormonal activation, renal dysfunction, medications, and dietary intake [8], [9]. In the patient whose case is described here, analysis of comparative changes in electrolytes between the serum and spot urinary concentrations of electrolytes during the clinical course (Table 1) revealed that hypochloremia was associated with the preceding natriuretic diuretic use and insufficient dietary salt intake. Hypochloremia in HF patients was recently identified as an important prognostic marker, and reaffirmed [10] as the genesis of diuretic refractoriness [4], [5], [6]. Thus, the chloride dynamics are clinically important to determine and treat, if necessary, in HF patients.
Monitoring urinary electrolytes in HF pathophysiology
Monitoring the urinary electrolyte concentrations [7] greatly contributed to our understanding of the serum electrolyte disturbance in this case. Namely, longitudinal observations from concomitant spot urinary and serum concentrations of the solutes (Table 1) revealed that the electrolyte concentrations were greatly influenced by the amount of dietary salt (March, 2017). The spot urinary test on the subsequent hospital admission in July, 2017 suggested that the etiology of the patient’s hypochloremia was electrolyte depletion possibly due to the persistent use of a natriuretic diuretic, azocemide, and a mineralocorticoid receptor antagonist, spironolactone, especially with low dietary salt intake. Concomitant administration of the aquauretic diuretic tolvaptan was expected to correct the hyponatremia and hypochloremia [2], but this did not occur in the present case because hyponatremia and hypochloremia appeared even under the high dose of this agent. Thus, these agents were discontinued and tapered off after hospital admission. The preceding prescription of these agents was replaced by acetazolamide with the intention to correct hypochloremia in this patient.
Acetazolamide, a potent “chloride-regaining” diuretic
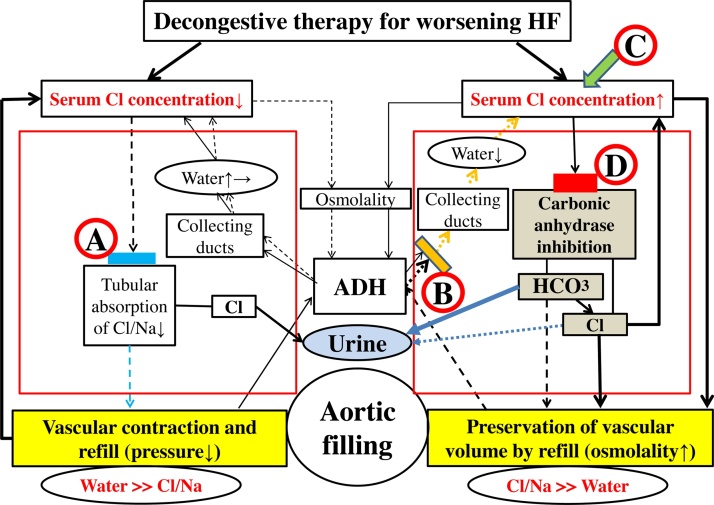
Based on my “chloride theory” for HF pathophysiology [2], manipulation of the serum chloride concentration could become an attractive therapeutic target for HF treatment as shown in Fig. 1, such as reducing the quantity and concentration of serum chloride using conventional diuretics for HF worsening with a higher concentration and retention of serum chloride (Fig. 1
 ), and preserving and enhancing the concentration of serum chloride with aquaresis using a V2-receptor antagonist (Fig. 1
), and preserving and enhancing the concentration of serum chloride with aquaresis using a V2-receptor antagonist (Fig. 1
 ) or supplemental chloride (Fig. 1
) or supplemental chloride (Fig. 1
 ) for worsening HF with a decreased serum chloride concentration. Diuretic treatment using a carbonic anhydrase inhibitor as described earlier [4] might be a suitable serum chloride-regaining therapy for refractory HF by its effects to improve hypochloremia (Fig. 1
) for worsening HF with a decreased serum chloride concentration. Diuretic treatment using a carbonic anhydrase inhibitor as described earlier [4] might be a suitable serum chloride-regaining therapy for refractory HF by its effects to improve hypochloremia (Fig. 1
 ), as predicted by the “chloride theory” [2].
), as predicted by the “chloride theory” [2].
Fig. 1.
Heart failure treatment and resolution of worsening HF based on the “chloride theory”; conventional diuretic therapy ( ), V2-receptor antagonist (
), V2-receptor antagonist ( ), chloride supplementation (
), chloride supplementation ( ), and carbonic anhydrase inhibitor (
), and carbonic anhydrase inhibitor ( ). Blue and yellow blocks represent inhibition of the absorption of chloride/sodium and water in each. Red block indicates the action of carbonic anhydrase inhibition by acetazolamide. Therapeutic effect induced by each treatment is shown by a solid or dotted line. Solid line indicates enhanced supply or excitatory effect, and dotted line indicates reduced supply or inhibitory effect. Different effect strengths are expressed by the thickness of each line. Large red square represents the kidney as an active place of the reabsorption of electrolytes and water.
). Blue and yellow blocks represent inhibition of the absorption of chloride/sodium and water in each. Red block indicates the action of carbonic anhydrase inhibition by acetazolamide. Therapeutic effect induced by each treatment is shown by a solid or dotted line. Solid line indicates enhanced supply or excitatory effect, and dotted line indicates reduced supply or inhibitory effect. Different effect strengths are expressed by the thickness of each line. Large red square represents the kidney as an active place of the reabsorption of electrolytes and water.
ADH, antidiuretic hormone; Cl, chloride; HCO3−, bicarbonate; HF, heart failure; Na, sodium.
The carbonic anhydrase inhibitor acetazolamide has a unique but critical diuretic action (Fig. 1
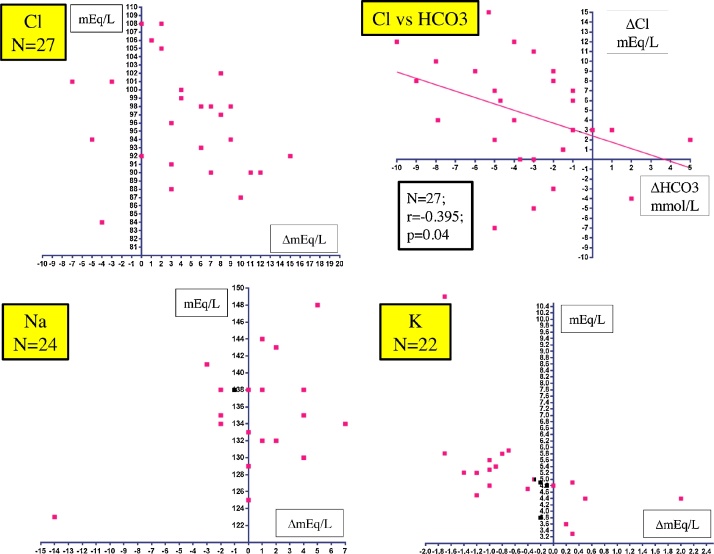
 ), a “non-reabsorbable anion-like effect”, that results in the excretion of bicarbonate (HCO3−) into the urinary tubules with interchangeable absorption of filtrated chloride into the blood, and concurrent excretion of potassium into the urine [6]. An earlier study by Relman et al. [4] examined the acute effects (3–12 days) of acetazolamide on serum solutes in 26 patients with severe HF (male, 73%; age, 56.7 ± 12.4 years) and found that the serum chloride concentration was increased in 78% of 27 evaluations, the serum sodium concentration was unchanged or increased in 73% of 24 evaluations, and the serum potassium concentration was decreased in 73% of 22 evaluations, as shown in Fig. 2. There was also a reciprocal association between the changes in the serum concentrations of chloride and HCO3−. In the present patient, concomitant monitoring of changes in the serum and urinary electrolyte concentrations (Table 1, July, 2017) clearly indicated that the gradual increase in the serum sodium concentration after admission to the hospital might be due to cessation of the loop diuretic, but the increase in the serum chloride concentration and the decrease in the serum potassium concentration were the result of active absorption of filtrated chloride and active excretion of potassium into the urinary tubules, respectively, induced by the pharmacologic actions of acetazolamide.
), a “non-reabsorbable anion-like effect”, that results in the excretion of bicarbonate (HCO3−) into the urinary tubules with interchangeable absorption of filtrated chloride into the blood, and concurrent excretion of potassium into the urine [6]. An earlier study by Relman et al. [4] examined the acute effects (3–12 days) of acetazolamide on serum solutes in 26 patients with severe HF (male, 73%; age, 56.7 ± 12.4 years) and found that the serum chloride concentration was increased in 78% of 27 evaluations, the serum sodium concentration was unchanged or increased in 73% of 24 evaluations, and the serum potassium concentration was decreased in 73% of 22 evaluations, as shown in Fig. 2. There was also a reciprocal association between the changes in the serum concentrations of chloride and HCO3−. In the present patient, concomitant monitoring of changes in the serum and urinary electrolyte concentrations (Table 1, July, 2017) clearly indicated that the gradual increase in the serum sodium concentration after admission to the hospital might be due to cessation of the loop diuretic, but the increase in the serum chloride concentration and the decrease in the serum potassium concentration were the result of active absorption of filtrated chloride and active excretion of potassium into the urinary tubules, respectively, induced by the pharmacologic actions of acetazolamide.
Fig. 2.
Scatter plot of changes in serum solutes of Na, Cl, K, and HCO3− after treatment with acetazolamide based on data obtained in the study by Relman et al. [4].
Cl, chloride; HCO3−, bicarbonate; K, potassium; Na, sodium.
Despite the unique and requisite pharmacologic actions of acetazolamide, this old but potent agent that was formerly a mainstay of diuretic treatment in the field of HF pathophysiology has long since been forgotten, although early literature indicates that this agent exerts potent diuretic activity in patients with refractory HF [4]. Subsequent several studies [5], [6] reported the importance of this agent under specific HF situations of hypochloremia and alkalosis, which frequently ensues following the use of loop diuretics and thiazide [8], [9] and may produce a refractory HF status [10]. Additionally, mineralocorticoid receptor antagonists are recommended for HF patients, but they are under-prescribed and frequently discontinued mainly when hyperkalemia develops [11]. Under such situations, however, the potassium-lowering actions of acetazolamide could provide enough ability for administration of a mineralocorticoid receptor antagonist [5], [6]. Monitoring the changes in the serum potassium concentration from the beginning of acetazolamide treatment is, of course, important after introducing this diuretic treatment because hypokalemia may appear promptly, as shown in the present study (8 days after) and the report by Relman et al. (3–12 days) [4]. The development of hypokalemia should be corrected by adding or increasing the dose of mineralocorticoid receptor antagonists and/or a potassium supplement [8], [9] to avoid serious events of malignant ventricular arrhythmias [9].
Conclusions
Acetazolamide is a potent “chloride-regaining” diuretic with concomitant activity to reduce serum potassium. The specific diuretic action of this agent greatly contributes to favorably modulate serum electrolytes in HF pathophysiology, such as correction of hypochloremia, and providing the necessary stability for the administration of mineralocorticoid receptor antagonists [5], [6]. Future studies are required to re-evaluate and determine the effects of this forgotten, but indispensable agent, on the pharmacologic, hemodynamics, neurohormonal, and prognostic aspects of HF pathophysiology.
Conflict of interest
None.
This manuscript or part of it has not been published previously. There is no relationship with industry and financial associations that might pose a conflict of interest.
Author contribution
The author has contributed to the presentation of the manuscript and also has read and agrees the contents of the manuscript.
References
- 1.Grodin J.L., Simon J., Hachamovitch R., Wu Y., Jackson G., Halkar M. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66:659–666. doi: 10.1016/j.jacc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka H. The “chloride theory”, a unifying hypothesis for renal handling and body fluid distribution in heart failure pathophysiology. Med Hypotheses. 2017;104:170–173. doi: 10.1016/j.mehy.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H. Proposal for heart failure progression based on the ‘chloride theory’: worsening heart failure with increased vs. non-increased serum chloride concentration. ESC Heart Fail. 2017 doi: 10.1002/ehf2.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relman A.S., Leaf A., Schwartz W.B. Oral administration of a potent carbonic anhydrase inhibitor (“Diamox”) N Engl J Med. 1954;250:800–804. doi: 10.1056/NEJM195405132501902. [DOI] [PubMed] [Google Scholar]
- 5.Khan M.I. Treatment of refractory congestive heart failure and normokalemic hypochloremic alkalosis with acetazolamide and spironolactone. Can Med Assoc J. 1980;123:883–887. [PMC free article] [PubMed] [Google Scholar]
- 6.Caramelo C., Albalate M., Alcázar T.R., Baldoví S., Pérez A.G., Marín M. Actuality of the use of acetazolamide as a diuretic: usefulness in refractory edema and in aldosterone-antagonist-related hyperkalemia. Nefrologia. 2008;28:234–238. [PubMed] [Google Scholar]
- 7.Verbrugge F.H., Nijst P., Dupont M., Penders J., Tang W.H.W., Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7:766–772. doi: 10.1161/CIRCHEARTFAILURE.114.001377. [DOI] [PubMed] [Google Scholar]
- 8.Grodin J.L. Pharmacologic approaches to electrolyte abnormalities in heart failure. Curr Heart Fail Rep. 2016;13:181–189. doi: 10.1007/s11897-016-0295-7. [DOI] [PubMed] [Google Scholar]
- 9.Urso C., Brucculeri S., Caimi G. Acid-base and electrolyte abnormalities in heart failure: pathophysiology and implications. Heart Fail Rev. 2015;20:493–503. doi: 10.1007/s10741-015-9482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanberg J.S., Rao V., ter Maaten J.M., Laur O., Brisco M.A., Wilson F.P. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira J.P., Rossignol P., Machu J.-L., Sharma A., Girerd N., Anker S.D. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT-CHF. Eur J Heart Fail. 2017;19:1284–1293. doi: 10.1002/ejhf.900. [DOI] [PubMed] [Google Scholar]