Abstract
We report a case of multiple coronary spasms leading to life-threatening ventricular arrhythmia, during left atrium (LA) ablation. Coronary artery spasm is a rare complication during radiofrequency catheter ablation of atrial fibrillation (AF). Previous reports mention that autonomic imbalance leads to coronary artery spasm and ST-segment elevation in the inferior leads, during trans-septal LA catheterization and AF ablation procedures. However, there are few reports detailing the association between ablation sites and changes in the electrocardiogram. We encountered transient ST-segment elevation and refractory ventricular arrhythmia, associated with coronary artery spasm, during radiofrequency ablation of ganglionated plexuses (superior surface of left and right atrium).
<Learning objective: Our case report shows that ablation of ganglionated plexus sites during pulmonary vein isolation, can induce ST-segment elevation in various leads, and cause multiple coronary spasms associated with refractory ventricular fibrillation. There is an inherent risk of multiple coronary spasms during catheter ablation of atrial fibrillation (AF), in patients with vasospastic angina. We recommend continuous intravenous infusion of nitroglycerin during ablation for AF to prevent coronary spasms in patients with vasospastic angina.>
Keywords: Multiple coronary spasms, Ganglionated plexus, Atrial fibrillation, Ventricular fibrillation, Autonomic imbalance
Introduction
Coronary artery spasm is a rare complication associated with radiofrequency catheter ablation of the left atrium. Previous reports mentioned that various mechanisms including direct thermal injury, progressive inflammation, or stimulation of ganglionated plexuses (GP) might lead to coronary artery spasm. However, there are few reports about the relationship between ablation of GP sites and changes on the electrocardiogram (ECG). We present here, ECG changes associated with GP stimulation, and the risk of life-threatening coronary spasm.
Case report
A 57-year-old man was admitted to our hospital for acute heart failure with atrial fibrillation (AF). However, he had no symptoms such as palpitations or chest pain. We suspected that the AF was associated with heart failure. Thus, he was admitted for pulmonary vein isolation (PVI) procedure for AF. The patient was placed under general anesthesia using propofol and dexmedetomidine. Two 8 Fr. Swartz sheaths (St Jude Medical Inc., Saint Paul, MN, USA) and AgilisNxT (St Jude Medical Inc.) were inserted into the left atrium, through a punctured trans-septal hole. The patient was administered 8000 units of heparin, and heparin was continued until a maintenance dose was reached, with minimal activated clotting time of 300 s. We flushed the sheaths carefully using saline with heparin to prevent air or thrombus. We used an irrigated tip radiofrequency (RF) ablation catheter (Navistar ThermoCoolSmartTouch; Biosense Webster, Irvine, CA, USA). Ablation was performed at a maximum temperature of 43 °C and power limit of 30 W. We used the CARTO3 system automated lesion-tagging module (Biosense Webster), based on catheter stability information and force-time integral (FTI). Our target FTI was 400 gs. RF was performed using the point-by-point technique. Ablation was initiated at the inferior wall of the left inferior pulmonary vein (IPV), and posterior ablation was performed sequentially upward, to the superior wall of the left superior pulmonary vein (SPV). Subsequently, anterior ablation was performed downward toward the left IPV. The patient’s blood pressure was 89/55 mmHg and heart rate was 55 bpm while initiating ablation. However, his blood pressure decreased to 66/42 mmHg, and the heart rate suddenly dropped to 36 bpm, at the time of ablation of the atrial appendage-atrial junction (between the 19th and 24th applications). RF energy was 30 W and RF application was followed by FTI (400 gs). Cardiac tamponade was excluded on echocardiography. Within a few minutes, the patient’s blood pressure returned to baseline. Following this, RF ablation was continued and the procedure for the left PV was completed successfully. Retrospectively, we observed ST-segment elevation in leads II, III, aVF, and I, aVL with ‘mirror’ ST-segment depression, on a 12-lead ECG. The ST-segment elevation and hemodynamic changes resolved spontaneously after several minutes (Fig. 1). We continued right PVI from the inferior wall of the right IPV to the roof on the posterior side. At the time of roof ablation, the 12-lead ECG revealed occurrence of AF, and the patient’s blood pressure decreased gradually. During ablation on the anterior side of right SPV (between the 53rd and 59th applications), the 12-lead ECG revealed ST-segment elevation in aVR and recurrence of hypotension. The patient’s blood pressure dropped to 60/43 mmHg and heart rate decreased to 31 bpm. However, these changes could not be detected earlier, because only a limited number of ECG leads were displayed on the monitor. Hence, we initiated atrial pacing and continued the ablation. RF energy was 30 W and RF application did not change. Suddenly, the 12-lead ECG showed monomorphic ventricular tachycardia, which progressed to ventricular fibrillation (VF) (Fig. 2). Immediately, cardioversion was performed thrice, but it failed to control the VF. The patient then developed pulseless electrical activity. We initiated percutaneous cardiopulmonary support (PCPS) and VF was terminated using an external defibrillator. Cardiac tamponade was excluded by ultrasound sonography. The 12-lead ECG revealed ST-segment elevation in the inferior leads and in V2, V3, and V4. Therefore, we performed urgent coronary artery angiography, which demonstrated severe spasm, and reduced flow in the right and left coronary arteries (Fig. 3). Intracoronary bolus injection of nitroglycerin induced vasodilation of the coronary arteries and the ST-segment elevation in the leads improved. Subsequently, his blood pressure and general condition stabilized. We performed ablation at the residual site of the right PV, and the left atrium RF ablation was completed successfully. The patient recovered consciousness 3 days later, his hemodynamic stability was maintained, and he was taken off PCPS support. He recovered without any neurological sequelae, and was discharged. Sinus rhythm was preserved. One year later, a drug-induced coronary artery spasm test was performed. We injected acetylcholine into the right coronary artery up to a dose of 50 μg. The drug-induced coronary artery spasm test revealed total occlusion of the right coronary artery.
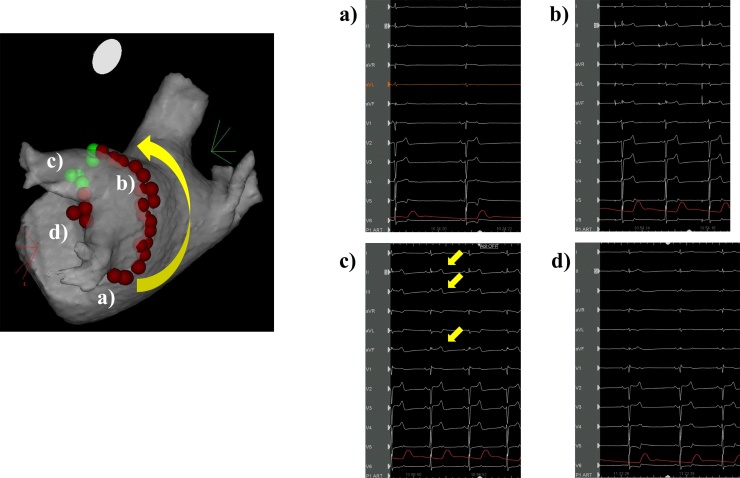
Fig. 1.
Changes in the electrocardiogram associated with radiofrequency site of the left pulmonary vein. (a) The inferior wall of the left pulmonary vein. (b) The roof of the left pulmonary vein. (c) Anterior side of the superior left pulmonary vein. Twelve-lead electrocardiogram revealed ST-segment elevation in leads II, III, aVF (arrows), and I, aVL with ‘mirror’ ST-segment depression. (d) Anterior carina of the left pulmonary vein. ST-segment elevation in the inferior leads was resolved.
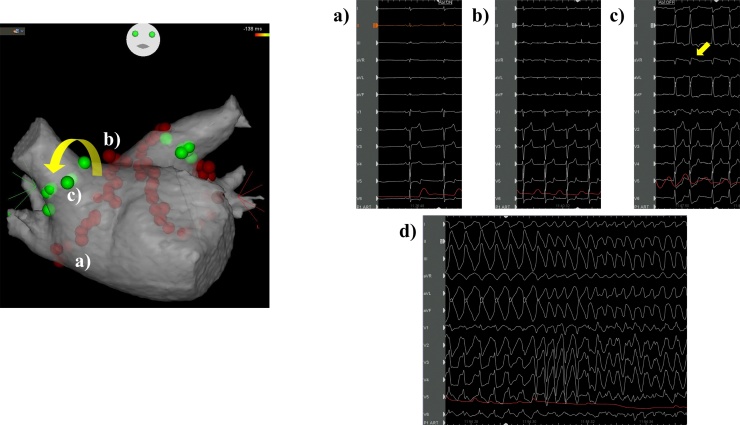
Fig. 2.
Changes in the electrocardiogram according to the radiofrequency site of the right pulmonary vein. (a) The inferior wall of the right pulmonary vein. (b) The roof of the right pulmonary vein. Heart rate increased to approximately 100 beats per minute. (c) Anterior side of the superior right pulmonary vein. Twelve-lead electrocardiogram (ECG) revealed ST-segment elevation in the aVR lead (arrow) with atrial fibrillation. (d) 12-lead ECG showed monomorphic ventricular tachycardia suddenly progressing to ventricular fibrillation after radiofrequency for the anterior carina of the right pulmonary vein.
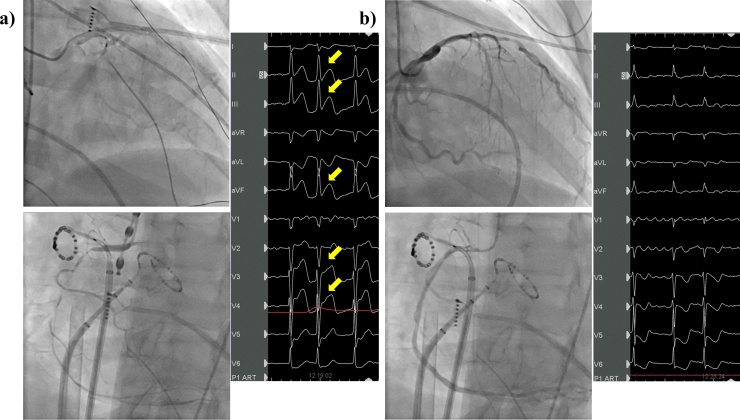
Fig. 3.
Improvement on coronary angiography and electrocardiogram (ECG). (a) Coronary angiography revealed multiple coronary spasms. Twelve-lead ECG showed ST-segment elevation in inferior leads and in V2, V3, and V4 (arrow). (b) Coronary angiography demonstrated vasodilation of the coronary artery after intracoronary bolus injection of nitroglycerin. 12-lead ECG showed improvement of ST-segment elevation in those leads.
Discussion
In this case report, we described in detail, the association between RF of GP sites of the left atrium with ST-segment elevation and multiple, near-fatal coronary spasms. Several mechanisms might explain the ST-segment elevation during RF ablation of the left atrium. Previous reports indicated the possibility of direct thermal injury or progressive inflammation from the RF energy that might damage the coronary artery [1]. However, in our case, there was enough distance between the ablation site and both coronary arteries. Thus, RF energy could not reach the coronary artery directly. Coronary embolism (air or thrombus) due to trans-septal puncture is another cause of ST-segment elevation according to previous reports [2]. In our case, we did not perform catheter exchange and maintained a heparin controlled activated clotting time of 300 s to reduce the possibility of thrombus formation. Additionally, coronary angiography did not show obstruction from bubbles or thrombus. Furthermore, infusion of nitroglycerin into the coronary vessels resolved severe stenosis of the coronary artery immediately, confirming the diagnosis of coronary spasm. It has been reported that vagal stimulation during GP ablation can cause bradycardia, low blood pressure, and transient conduction block through the atrioventricular node [3]. In our case, bradycardia and low blood pressure occurred at the time of ablation of the LSPV and RSPV. We believe these ablation sites were associated with GP. Transient ST-segment elevation occurred in our case, at the time of superior left GP ablation. Thus, we speculate that the RF energy for the GP site led to autonomic nervous tone imbalance, leading to coronary artery spasm. Coronary artery spasm has been associated with coronary endothelial dysfunction [4] and cardiac autonomic activity [5]. Autonomic cardiac GP are located deep inside the epicardial fatty tissue, as well as under the superficial cardiac muscle fascicles of the atrium and ventricle. Five major GP sites are located in the left atrium (superior left, anterior right, Marshall tract, inferior left, and inferior right GP) [6]. A previous report showed that destruction of GP during RF led to a vagal response prior to spontaneous coronary artery spasm [7]. Tada et al. speculated that stimulation of GP in the left atrium changes the balance between sympathetic and parasympathetic nerve tone and leads to coronary spasm [8]. Our case showed that ST-segment elevation in the inferior leads and hypotension occurred at the time of RF for the superior left GP site. During ablation of the superior right GP site, multiple coronary spasms, and elevated ST-segment in aVR was observed. We hypothesize that these phenomena indicate that the RF of the GP site of the left atrium led to autonomic imbalance resulting in life-threatening coronary spasm. The reason for ST-segment elevation in the inferior leads appearing at the time of ablation of the superior left GP site remains unknown. Le et al. reviewed several situations associated with transient ST-segment elevation during procedures for AF. Their report evaluated the etiology of ST-segment elevation in inferior leads. The procedure for LA activates cardiac parasympathetic nerve selectively, distributed through the right coronary artery [9]. Kawakami et al. reported that patients with paroxysmal AF have high positive rates of drug-provoked coronary artery spasm [10]. The drug-induced coronary artery spasm test revealed coronary spasm in our patient. Thus, some patients with vasospastic angina undergoing RF of the left atrium have the risk of life-threatening arrhythmias associated with coronary artery spasm.
Conclusions
RF of GP sites during ablation for AF might provoke multiple coronary spasms, possibly due to an autonomic imbalance caused by the procedure itself. We need to confirm whether coronary spasm occurs during unexpected decrease in blood pressure and whether ST-segment elevation develops during RF ablation for AF. We recommend that continuous intravenous infusion of nitroglycerin during ablation for AF might be useful to prevent coronary spasms in patients with vasospastic angina.
Conflict of interest
None.
Acknowledgment
None.
References
- 1.Yune S., Lee W.J., Hwang J.W., Kim E., Ha J.M., Kim J.S. Acute myocardial infarction after radiofrequency catheter ablation of typical atrial flutter. J Korean Med Sci. 2014;29:292–295. doi: 10.3346/jkms.2014.29.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael K.A., Redfearn D.P., Simpson C.S., Baranchuk A. An unusual complication of a pulmonary vein isolation. J Interv Card Electrophysiol. 2009;25:203–205. doi: 10.1007/s10840-008-9357-4. [DOI] [PubMed] [Google Scholar]
- 3.Yao Y., Shi R., Wong T., Zheng L., Chen W., Yang L. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 4.Stern S., Bayes de Luna A. Coronary artery spasm. Circulation. 2009;119:2531–2534. doi: 10.1161/CIRCULATIONAHA.108.843474. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita E., Tada H., Tadokoro K., Hashimoto T., Kaseno K., Miyaji K. Left atrial catheter ablation promotes vasoconstriction of the right coronary artery. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S98–S102. doi: 10.1111/j.1540-8159.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun W., Zheng L., Qiao Y., Shi R., Hou B., Wu L. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armour J.A., Murphy D.A., Yuan B.X., Macdonald S., Hopkins D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Tada H., Naito S., Oshima S., Taniguchi K. Vasospastic angina shortly after left atrial catheter ablation for atrial fibrillation. Heart Rhythm. 2005;2:867–870. doi: 10.1016/j.hrthm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Le B.H., Black J.N., Huang S.K. Transient ST-segment elevation during transseptal catheterization for atrial fibrillation ablation. Tex Heart Inst J. 2010;37:717–721. [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami T., Ohno H., Tanaka N., Ishihara H., Kobayakawa H., Sakurai T. The relationship between paroxysmal atrial fibrillation and coronary artery spasm. Pacing Clin Electrophysiol. 2014;37:591–596. doi: 10.1111/pace.12299. [DOI] [PubMed] [Google Scholar]