ABSTRACT
In South Korea, the National Immunization Program offers a 23-valent pneumococcal polysaccharide vaccine (PPSV23) for the elderly; however, the 13-valent pneumococcal conjugate vaccine (PCV13) is not included, and vaccination is not offered to younger, at-risk populations. This study offers a comparative analysis of PCV13 and PPSV23 in Korea's adults, stratified by age and risk group. A Markov model with a lifetime horizon was developed from the healthcare perspective. Data sources included the Health Insurance Review & Assessment Service, Korea Centre for Disease Control & Prevention and Korean medical institutions. An expert panel tested data validity. The CAPiTA trial and Cochrane meta-analysis were used to obtain vaccine effectiveness data. Regardless of co-morbidity, when the sequential PCV13-PPSV23 strategy was compared to that using PPSV23-only, in elderly populations, the incremental cost-effectiveness ratio (ICER) was 3,300 USD per quality-adjusted life years (QALY). For the risk group aged ≥65 years, the ICER of the addition of PCV13 over the existing PPSV23-only strategy was 3,404 USD/QALY. However, on replacing PPSV23 with PCV13, for all elderly populations, an ICER of 1,421 USD/QALY resulted; for the risk group aged ≥65 years, the ICER was 1,736 USD/QALY. For the 18–64 year-old risk group, the sequential PCV13-PPSV23 strategy yielded an ICER of 3,629 USD/QALY over the PPSV23-only strategy, and 6,643 USD/QALY compared to no vaccination. Thus, the PCV13→PPSV23 combination strategy for elderly populations was found to be a cost-effective alternative to the current National Immunization Program regardless of co-morbidity. This finding was the same as that for younger, at-risk populations.
KEYWORDS: Streptococcus pneumoniae, pneumococcal vaccination, cost-effectiveness analysis, PCV13, PPSV23, Korea
Introduction
Streptococcus pneumoniae causes a broad spectrum of diseases, including mild respiratory diseases such as otitis media, sinusitis, and non-bacteremic pneumococcal pneumonia (NBPP) and serious invasive pneumococcal disease (IPD) such as meningitis and bacteremia.1,2 Among these, the major clinical syndromes of pneumococcal disease are NBPP and IPD.2 Annually, the number of NBPP and IPD cases reported in the United States is approximately 500,000 and 8,700, respectively.3-5 Pneumococcal pneumonia in adults needs special attention because it accounts for more than 90% cases of pneumococcal diseases and most of these cases are of non-bacteremic pneumonia.6 In Korean adults, the annual incidence rates of community-acquired pneumonia (CAP) range from 538 to 707 cases per 100,000 persons, which increases with age.7 Among CAP cases, S. pneumoniae accounts for 21.1- 43.6%,8-18 and 5.9% of the cases result in death.10 The average per-capita medical fee was reported at 1,887 USD.10 Although not common, the burden of IPD is also substantial; the case fatality rate was 30.9%, and the per-capita medical fee for Korean adults was 7,452 USD.19 Both age and comorbidities are known to be important risk factors for pneumococcal disease20,21 and the mortality rate increases with age.19 In elderly patients, the mortality associated with bacteremia and meningitis due to S. pneumoniae was reported to be up to 60% and 80%, respectively.4 In addition to the substantial burden of pneumococcal disease, an increasing resistance to various antibiotics has made treatment more difficult22-24; therefore, awareness on the importance of prevention is on the rise.
Vaccination is the most powerful preventative measure against IPD, and several countries such as the United States, Australia and the United Kingdom include pneumococcal vaccination in their national immunization programs.25-27 In South Korea, the National Immunization Program has been offering the 23-valent pneumococcal polysaccharide vaccine (PPSV23) to elderly populations since May 2013; however, the 13-valent pneumococcal conjugate vaccine (PCV13) is not included in the program.28
PPSV23 includes polysaccharide capsule antigens of 23 S. pneumoniae serotypes.29 It has long been in use, and is advantageous in that there is an abundance of clinical evidence pertaining to its preventative effect against invasive pneumococcal disease.30,31 However, in general, polysaccharide vaccines induce immunoreaction without the involvement of T cells, and, therefore, neither can long-term immunity be sustained, nor can it be enhanced through additional vaccination.32-34 Moreover, the efficacy of PPSV23 in preventing NBPP, the majority of pneumococcal disease, is known to be poor and inconsistent.
The chemical conjugation of pneumococcal polysaccharide to a carrier protein creates a T-cell dependent vaccine (pneumococcal conjugate vaccine) that stimulates better antibody response, mucosal immunity, and immunological memory in children and adults.35 PCV13 prevents IPD; it is also an effective preventative agent against NBPP and acute otitis media.30 A herd immunity effect, which points to a reduced occurrence of IPD in adults who have not received pneumococcal vaccine after pediatric PCV13 use, has also been reported.36
Cost-effectiveness analyses between PPSV23 and PCV13 have been conducted in many countries, but not in Korea.37-41 In Korea, research on the pneumococcal disease index, effectiveness of vaccines, and total disease burden among infants as well as adults and the elderly, is limited. There is also a need for cost-effectiveness analysis data based on such research. Thus, this paper aims to compare the effects of PCV13 in Korea's adult population, particularly in the elderly, with those of PPSV23, a vaccine currently included in the country's National Immunization Program.
Results
Primary analysis
Regardless of co-morbidity, the addition of PCV13 to the current vaccination strategy in elderly adults aged ≥65 years, compared to the use of PPSV23 alone, yielded an incremental cost-effectiveness ratio (ICER) of 3,300 USD/ quality-adjusted life years (QALY) (Table 1). For the risk group, among the elderly population, the addition of PCV13, compared to the use of the existing PPSV23, yielded an ICER of 3,404 USD/QALY (Table 1).
Table 1.
Results of the primary and secondary analyses.
| Population | Vaccine strategy | Comparator | ICER (USD/QALY) |
|---|---|---|---|
| ≥65 years, total | PCV13 →PPSV23 | PPSV23 | 3,300 |
| ≥65 years, risk group | PCV13 →PPSV23 | PPSV23 | 3,404 |
| ≥65 years, total | PCV13 | PPSV23 | 1,421 |
| ≥65 years, risk group | PCV13 | PPSV23 | 1,736 |
| 18–64 years, risk group | PCV13 →PPSV23 | PPSV23 | 3,629 |
| PCV13 →PPSV23 | No vaccination | 6,643 |
PCV13: 13-valent pneumococcal conjugate vaccine, PPSV23: 23-valent pneumococcal polysaccharide vaccine, ICER: incremental cost-effectiveness ratio, QALY: quality-adjusted life years.
Secondary analysis
When PCV13 was used as a replacement for PPSV23 (PCV13 vs. PPSV23), the ICER in the elderly population aged ≥65 years was 1,421 USD/QALY, and that of adults aged ≥65 years, in the risk group, was 1,736 USD/QALY (Table 1).
When the vaccination group was changed to include individuals from the risk group, aged 18–64 years, the use of both PCV13 and PPSV23 resulted in an ICER of 3,629 USD/QALY compared to the cases of PPSV23 use, and 6,643 USD/QALY compared to the cases of no vaccination (Table 1).
Sensitivity analysis
The results of one-way deterministic sensitivity analysis identified the vaccine effectiveness of PCV13 against NBPP, and disease incidence of NBPP as the two most sensitive parameters (Fig. 1(a)). Despite the ±25% variation in the base parameter inputs, all the ICERs were within the cost-effective range. In addition, the results of the probabilistic sensitivity analysis (Fig. 1(b)) showed that all 1,000 random simulations were cost-effective.
Figure 1a.
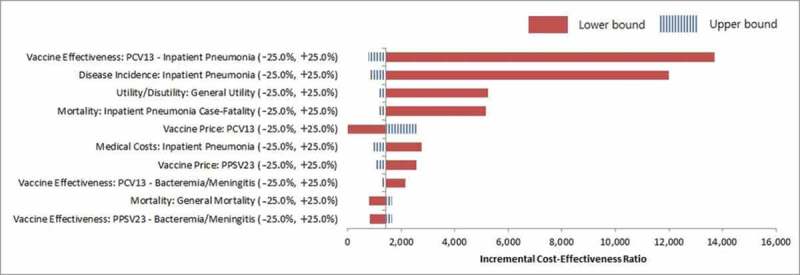
One-way deterministic sensitivity analysis tornado diagram.
Figure 1b.
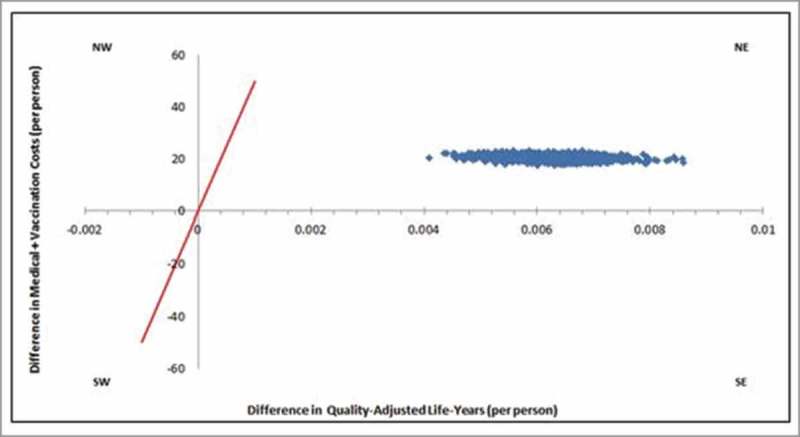
Probabilisc sensivity analysis ICER scaerpolt, of elderly adults aged > 65 years old.
The results of additional sensitivity analysis are described in Tables 2 and 3. When the vaccine effectiveness of PCV13 against NBPP was lowered in all those aged ≥65 years, the result yielded an ICER of 16,283 USD/QALY, compared to the result of 1,746 USD/QALY obtained upon enhancement. In the case of the risk group aged ≥65 years, the lower bound ICER was 25,103 USD/QALY compared to the upper bound ICER of 1,647 USD/QALY. Regarding the disease incidence of NBPP, a reduction in the proportion of all-cause pneumonia due to S.pneumoniae resulted in an ICER of 14,217 USD/QALY, while its increase resulted in an ICER of 1,993 USD/QALY, among all adults aged ≥65 years. Similarly, the risk group aged ≥65 years showed an ICER of 23,708 USD/QALY upon reduction, in contrast to the 2,001 USD/QALY observed on its increase. With some variations in the serotypes, reflected in the estimation of the indirect effect, the result yielded an ICER of 3,237–3,937 USD/QALY among the total population aged ≥65 years (Table 2), and 3,476–3,961 USD/QALY among the risk groups (Table 3).
Table 2.
Results of the sensitivity analysis for the total population aged ≥65 years.
| Parameters |
Range valuesa |
ICER (USD/QALY) |
|
|---|---|---|---|
| Base case | 3,300 | ||
| PCV13 vaccine effectiveness against NBPP | Lower | 16,283 | |
| Upper | 1,746 | ||
| Disease incidence of NBPP | Lower | 14,217 | |
| Upper | 1,993 | ||
| Discount rate | Lower | 2,023 | |
| Upper | 4,363 | ||
| Serotype reflected in indirect effect estimation | |||
| 3,6A,7F,19A (except serotypes 1,5) | 3,539 | ||
| 1,5,6A,7F,19A (except serotype 3) | 3,237 | ||
| 6A,7F,19A (except serotypes 1,3,5) | 3,937 | ||
PCV13: 13-valent pneumococcal conjugate vaccine, NBPP: non-bacteremic pneumococcal pneumonia, ICER: incremental cost-effectiveness ratio, QALY: quality-adjusted life years.
See Supplementary Table 3.
Table 3.
Results of the sensitivity analysis for the risk group aged ≥65 years.
| Parameters |
Range valuesa |
ICER (USD/QALY) |
|
|---|---|---|---|
| Base case | 3,404 | ||
| PCV13 vaccine effectiveness against NBPP | Lower | 25,103 | |
| Upper | 1,647 | ||
| Disease incidence of NBPP | Lower | 23,708 | |
| Upper | 2,001 | ||
| Discount rate | Lower | 2,254 | |
| Upper | 4,732 | ||
| Serotype reflected in indirect effect estimation | |||
| 3,6A,7F,19A (except serotypes 1,5) | 3,476 | ||
| 1,5,6A,7F,19A (except serotype 3) | 3,961 | ||
| 6A,7F,19A (except serotypes 1,3,5) | 3,677 | ||
PCV13: 13-valent pneumococcal conjugate vaccine, NBPP: non-bacteremic pneumococcal pneumonia, ICER: incremental cost-effectiveness ratio, QALY: quality-adjusted life years.
See Supplementary Table 3
Discussion
Due to increase in the incidence of pneumonia and the associated burden in Korea, especially among the elderly, pneumococcal vaccination has been administered free of charge as a public health policy since 2013, and the vaccination rate had increased to 59.2% as of December 2015.28 In recent times, PCV13, a new pneumococcal vaccine formulation, has been used as an optional vaccine in Korean adults, since its approval in 2012. There is a need for the adequacy and necessity of the current vaccination policy to be assessed using cost-effectiveness analysis.
This study aimed to offer a comparative analysis of PCV13 use versus PPSV23 use in Korea's adults, in various age- and risk-specific populations with either sequential or replacement vaccination strategy. Currently in Korea, the cost-effectiveness analysis of medical treatment considers 1 GDP per capita (27,633 USD) per QALY,42,43 as a commonly-cited threshold value. This means that an alternative ICER below this threshold could be interpreted as cost-effective, in relation to a comparator. Therefore, in both the primary analyses, the strategy using PCV13 followed by PPSV23, for adults aged ≥65 years, targeted at either the total population or risk group, can be concluded as a cost-effective alternative compared to the existing PPSV23-only strategy (ICER of 3,300 USD/QALY and 3,404 USD/QALY, for the total population and risk group, respectively). Similarly, the results of the secondary analysis, using PCV13 as a replacement for PPSV23, for the same population aged ≥65 years, indicated that this strategy is also cost-effective (ICER of 1,421 USD/QALY and 1,736 USD/QALY for the total population and risk group, respectively). A similar result was observed when, in the risk group aged <65-years, the use of PCV13 was compared to that of both PPSV23 and no vaccination (ICER of 3,629 USD/QALY vs. PPSV23, and 6,643 USD/QALY vs. no vaccination). Furthermore, after conducting sensitivity analysis, with regards to the identified parameters – vaccine effectiveness of PCV13 against NBPP and disease incidence of NBPP – the primary cases revealed that all the ICER values could be considered cost-effective.
PPSV23, which has long been in use, has an advantage, in terms of the presence of the clinical evidence of its preventative effects against 23 serotypes of invasive pneumococcal infection disease.30,31 In South Korea, the National Immunization Program has been offering the PPSV23 to elderly adults, since May 2013. However, the efficacy of PPSV23 in preventing NBPP is known to be poor and inconsistent, especially in elderly adults and adults with chronic disease, based on the results of several meta-analyses.3,31 However, PCV13 (Prevnar13; Pfizer), licensed in 2009, has expanded its coverage to include serotypes 3, 6A, and 19A compared to PCV10.29 In October 2013, Korea's Ministry of Food and Drug Safety approved PCV13 administration in adults aged ≥50 years, and the age was expanded to ≥18 years, in 2015. Given the demographic characteristics of elderly adults and the prevalence of pneumococcal disease, the prevention of the large burden of disease associated with NBPP should be a major objective, from a healthcare perspective.3-5,44,45 Meanwhile, owing to the unmet medical needs in effectively preventing NBPP, the CAPiTA trial was conducted to confirm the efficacy of PCV13 in preventing the first episode of confirmed vaccine-serotype pneumococcal pneumonia in adults aged ≥65 years.46
Based on these reasons and backgrounds, the United States Advisory Committee on Immunization Practice (ACIP) stepped up its recommendation in the 2014 revision of the pneumococcal vaccination recommendation to advise that all individuals aged ≥65 years be administered PCV13 and PPV23, sequentially.47 This recommendation took into consideration the insufficient herd immunity level among adults aged ≥65 years, as a result of infant vaccination, the preventative effect of PCV13 against pneumococcal disease, and cost-effectiveness analysis. Moreover, the current recommendation of the Korea Society of Infectious Disease (KSID), in terms of pneumococcal vaccination for adults, advises the use of either PCV13 or PPSV23 for low- risk adults aged ≥65 years; however, for moderate and high-risk patients aged ≥65 years, due to the high risk of pneumococcal diseases caused by various serotypes, it is recommended that PCV13 is used first, followed by the addition of PPSV23 after 6- 12 months (with a minimum interval of 8 weeks). Meanwhile, PPSV23 has been offered to those aged ≥65 years as part of the National Immunization Program; however, there is a lack of cost-effectiveness analysis data on PCV13 among elderly adults aged ≥65 years in Korea. Thus, this research sought to provide economic evidence data which may support minimizing the gap between the current recommendation and the National Immunization Program, in the formulation of a better program in the future.
This research is limited in terms of reflecting the real status of PCV13 use in Koreans. The study drew the effect and utility values of the vaccine from foreign clinical literature and used estimates, although they were based on expert consultation when there were insufficient data such as detailed values, for each risk group. However, to preserve the credibility of the data, as well as to reflect the reality of the local situation, we adopted the efficacy data of PCV13 from a verified large-scale, double-blind, randomized, placebo-controlled research (CAPiTA).46 In addition, epidemiological and cost data on pneumococcal diseases were taken from existing local sources,7,8,10,48-50 as completely as possible. Lastly, the risk group ratio reflected in this study (Supplementary Table 1) is considered to be lower than what is usually estimated by expert groups in reality.
Based on the ICER results in this research, it is suggested that PCV13, either in combination with or as a replacement to PPSV23, is a cost-effective alternative in Koreans aged ≥65 years. In addition, for individuals in the risk group aged 18- 64 years, PCV13 is a cost-effective alternative to PPSV23 alone or no vaccination. Our findings largely support the KSID's recommendation and indicate the need for the National Immunization Program to include PCV13 for adults aged ≥65 years, and even for those aged 18- 64 years with risk conditions. Such results, however, should be considered along with the change in the serotype distribution after its introduction in the National Immunization Program, as well as the real disease burden of pneumococcal disease. Policy decisions should be based on such considerations to determine the ideal strategy for pneumococcal vaccine administration for adults in Korea.
Methods
Vaccine strategy and study population
This paper assessed the cost-effectiveness of a combined strategy using PCV13 and PPSV23 compared to the existing PPSV23 strategy (sequential PCV13-PPSV23 vs. PPSV23-only) in Korean adults, assuming 100% vaccine coverage. The study population, considering the co-morbidity risks, was categorized into the total population (including the low risk group), and at-risk group (consisting only of moderate- and high-risk groups), and the groups were defined as follows:51
* The low-risk group comprised individuals without co-morbidity
* The moderate-risk group comprised individuals who had one or more of the following conditions or lifestyle behaviors: chronic heart disease, chronic liver disease, chronic respiratory disease, diabetes, asthma, alcohol abuse and smoking
* The high-risk group comprised individuals who had one or more of the following conditions: chronic renal disease, nephrotic syndrome, malignant tumor (hematologic malignancy, solid tumor), and immune deficiency (HIV, organ or bone-marrow transplant, low immunoglobulin, asplenia, sickle-cell disease, or immunosuppressive therapy).
The primary endpoints of this study were to analyze the cost-effectiveness of the PCV13 and PPSV23 combination strategy in: 1) age ≥65 years, total population and 2) age ≥65 years, risk group.
Secondary analysis was conducted using various vaccination strategies (replacement with PCV13; PCV13 vs. PPSV23), age, and risk groups (1) age ≥65 years, total population, PCV13 vs. PPSV23; (2) age ≥65 years, risk group, PCV13 vs. PPSV23; (3) age 18–64 years, risk group, PCV13→PPSV23 vs. PPSV23; and (4) age 18–64 years risk group, PCV13→PPSV23 vs. no vaccination.
The demographic data of the study population were obtained from the 2016 Korea Statistical Information Service,48 whereas those of the risk groups were obtained from the 2013 Health Insurance Review & Assessment Service (HIRA) big data7 (Supplementary Table 1).
Model analysis
The Markov model, from a healthcare perspective for a full lifetime, was used for data analysis (Fig. 1b). The model depicts the clinical and economic impact of pneumococcal vaccination strategies, including PCV13, in a hypothetical > 18-year-old adult population, specifically characterized based on age and risk profiles. The Prevenar13 Public Health and Economic Impact Model for Adults (the PREVENT Adults) Sequential Simulation Model and the PREVENT Adults Deterministic Cohort Model were used for sequential and replacement vaccine strategies, respectively. Both models are designed to determine the outcomes based on user-specified inputs such as study population, risks and costs of pneumococcal disease, vaccine coverage, effectiveness and costs. A base discount rate of 3% was applied to both costs and benefits. Collected base data were analyzed by an expert to generate input for the cost-effectiveness analysis model. The final outcomes of the analysis, estimated using the obtained input from the model, were expressed as ICERs, which indicates the cost change of each alternative per QALY.
Base data collection
During the base data collection, pneumococcal diseases were categorized into IPD and NBPP; the data were also categorized according to age and risk group. For IPD, data on bacteremia and meningitis were collated, and for NBPP, data collection was limited to inpatients because it was difficult to obtain accurate data on visit rates and almost all patients with pneumonia are hospitalized in Korea,9,52 and their cost burdens were considered low.
Epidemiological and cost data analyses, including incidence rates, mortality, and serotype coverage of pneumococcal diseases (IPD and NBPP), were taken from the HIRA's big data,7 Korea Centre for Disease Control & Prevention (KCDCP) reports,49 and research data from Korean medical institutions, such as the Korea University Guro Hospital.50 An expert panel was also consulted to verify the validity of the data (Table 4).
Table 4.
Model key inputs: pneumococcal disease incidence, vaccine effectiveness and direct medical costs.
| Incidence (cases /100,000 persons/year) |
PPSV23 effectiveness (%) |
PCV13 effectiveness (%) |
Direct medical costs (USD) (/case) |
Serotype coverage of PPSV23 (%)/PPV13 (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age(years)/ risk | IPD | Pneumoniaa | VT-IPD | Pneumoniaa | VT-IPD | Pneumoniaa | IPD | Pneumoniaa | IPD | NBPP | |
| 18–49 | Low | 0.9 | 85 | 82.0 | 0 | 75.0 | 5.6 | 6,849 | 1,055 | 59.5/35.1 | 49.8/35.2 |
| Mod | 5.5 | 497 | 65.6 | 0 | 63.8 | 5.6 | 5,404 | 2,234 | |||
| High | 3.2 | 287 | 20.0 | 0 | 58.5 | 3.7 | 6,990 | 2,986 | |||
| 50–64 | Low | 4.8 | 288 | 82.0 | 0 | 75.0 | 5.4 | 8,528 | 1,406 | 59.5/35.1 | 49.8/35.2 |
| Mod | 12.0 | 723 | 65.6 | 0 | 63.8 | 5.4 | 8,756 | 2,624 | |||
| High | 22.7 | 1367 | 20.0 | 0 | 58.5 | 3.5 | 7,135 | 2,871 | |||
| 65–74 | Low | 19.0 | 1299 | 82.0 | 0 | 75.0 | 5.1 | 8,557 | 1,701 | 65.5/38.1 | 54.9/38.2 |
| Mod | 21.1 | 1437 | 65.6 | 0 | 63.8 | 5.1 | 6,964 | 2,651 | |||
| High | 47.7 | 3266 | 20.0 | 0 | 58.5 | 3.3 | 8,609 | 2,800 | |||
| 75+ | Low | 58.8 | 5013 | 82.0 | 0 | 75.0 | 4.8 | 8,090 | 1,812 | 65.5/38.1 | 54.9/38.2 |
| Mod | 48.2 | 4118 | 65.6 | 0 | 63.8 | 4.8 | 5,730 | 2,390 | |||
| High | 92.4 | 7865 | 20.0 | 0 | 58.5 | 3.1 | 7,880 | 2,538 | |||
PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine, IPD: invasive pneumococcal disease, NBPP: non-bacteremic pneumococcal pneumonia, VT-IPD: vaccine-type invasive pneumococcal disease, Mod: moderate-risk.
Refers to all-cause pneumonia.
Incidence
Since epidemiological research on IPD in Korea is limited, National Statistical Information Service data (2011- 2014) and catchment population estimates, based on data from three university hospitals (Korea University Guro Hospital, Hallym University Kangnam Sacred Heart Hospital, and Chungbuk University Hospital),50 were used to calculate the IPD incidence among Korean adults. This was done by obtaining the actual number of visitors of each university hospital and all medical institutions within the surrounding area where these university hospitals are located, for different age groups, and then applying that ratio to the population size of each age group, to calculate the catchment population of the area. Since this incidence was not calculated based on risk groups, the risk group ratios observed in the case of NBPP were applied.
The incidence rates of all-cause pneumonia were extracted from HIRA's big data,7 based on age/risk groups. Of these, the proportion of pneumococcal pneumonia incidence was assumed to be 30%, following an expert panel consultation, based on the existing research in Korea.8-18 Since bacteremic pneumococcal pneumonia is classified as IPD, and thus should be excluded, the proportion of bacteremia was set to be 7%, following an expert panel consultation, based on the existing research in Korea.8,9,12,53
Serotype coverage
A S. pneumoniae serotype report,49 which studied Korean adults in 2013–2015, was used. For NBPP, the serotype distribution was researched only among those aged ≥65 years. Since no other data that could be used for analysis existed in Korea, the NBPP serotype distribution for 18–64-year-old adults was calculated using the ratio of NBPP serotype distribution to IPD among adults aged ≥65 years (Table 4).
Utility weights
Baseline utility weights were retrieved from the Korean National Health Statistics that reported values by age groups (Supplementary Table 3).54 Due to the lack of data in Korea, the ratio between the risk groups was derived from other cost-effective analysis.55,56 Utility weights associated with IPD and NBPP were based on the values described by Smith et al.55 and the duration of each event was based on the Korean research.10,19 Because the duration of hospitalization due to NBPP was not categorized by age and risk groups,10 the value was adjusted based on the expert panel. We decided to extend the hospitalization due to NBPP to 130% (12 days) and 140% (13 days) for patients aged 65–74 years and ≥75 years, respectively, compared to the 18–64-year-old age group (9 days). In addition, we extended the hospitalization to 150% and 200% for the moderate- and high- risk group, respectively, compared to the low-risk group.
Vaccine effectiveness
PCV13 against IPD
Regardless of age, the CAPiTA research result of 75% was applied to the low-risk group46. Since data on the effectiveness in the moderate-risk group were limited, an expert panel was consulted to apply 85% effectiveness of the low-risk group. For the high-risk group, taken from the randomized clinical trial results on infants,57 the authors decided to apply 78% effectiveness of the low-risk group.
PCV13 against NBPP
Regardless of age, the CAPiTA research result of 45% was applied to the low-risk group;46 for the moderate-risk group, an expert panel consultation decided on 80% effectiveness of the low- risk group. For high-risk groups, randomized clinical trial results on infants were used to apply 65% effectiveness of the low-risk group.57 In addition, the proportion of the incidence of all-cause pneumonia due to S. pneumoniae (30%) and the serotype coverage of PCV13 in NBPP (36.9%) were taken from Korean research,49 and utilized in the study model for automatic calculation.
PPSV23 against IPD
Applying the preventive effect of PPSV23 against VT-IPD, from the Cochrane meta-analysis31 as the base value for the healthy population (low-risk group) aged 65 years, the age/time variances observed by Smith et al.32,55 were applied. For the moderate-risk group, the sub-group analysis in the Cochrane meta-analysis,31 showed that there was no significant preventive effect against IPD in patients with chronic conditions; however, owing to the heterogeneity among the studies, there was statistical insignificance. Due to the lack of statistically significant data on vaccine effectiveness in patients with chronic conditions, the authors consulted an expert panel and reduced the effect of PPSV23 for the moderate-risk group against the low-risk group by 20%. For the high-risk group, a randomized controlled trial on HIV patients in Uganda reported no vaccine effectiveness on immunocompromised patients;58 however, this was particularly conducted in patients with severe immune deficiency, and thus calls for caution during interpretation. The 2012 ACIP report, based on six homogeneous observational studies, suggested a protective effect of 49%.59 These two sets of data were used for expert consultation which concluded that the high-risk group's vaccination effect was 20% at the baseline.58,60
PPSV23 against NBPP
A study by Huss et al.,3 the Cochrane meta-analysis,31 and other studies were used for expert consultation.60,61 For the low-risk group, the direct effectiveness against NBPP was assumed to be zero. Since PPSV23 has been reported to have an insignificant effect against pneumonia even in low-risk groups, research on its effect in moderate-/high-risk groups is very limited. Thus, an expert panel estimated the vaccination effect in moderate-/high-risk groups to be 0%.
Indirect effect
To estimate the indirect effect of pediatric PCV13 vaccination on IPD, data from existing research were used62,63; the indirect effect previously observed in the case of PCV7 was assumed to be shown by the six serotypes added to PCV13 (1, 3, 5, 6A, 7F, 19A). We estimated the age-specific maximum reduction in the incidence of IPD by applying the proportion of reduction in the incidence of IPD due to the six serotypes, to the distribution of the six serotypes in Korea (Supplementary Table 2).
Indirect reductions in the proportion of IPD occurrence after the routine pediatric use of PCV13 were assumed to be comparable to those seen in the post-PCV7 era;63 and it was assumed that the maximum indirect effect would be observed in the seventh year after the routine pediatric use of PCV13. Using the year 2012 as the base year,64 and the trend observed post-PCV7, the year 2018 would be the seventh year after the routine pediatric use of PCV13. The indirect effect of PCV13 on NBPP was set at 50%, compared to the case of IPD, while PPSV23 was assumed to have no indirect preventive effect, following an expert panel consultation.
Cost
Direct medical costs for IPD cases were estimated using data from a multicenter study in Korea;19 for NBPP, the cost of all-cause pneumonia from the HIRA data was used.7 For vaccine costs, the current distribution levels for hospitals and clinics, as of July 2016 (PPSV23 16.74 USD; PCV13 50.48 USD) were used. For vaccination costs (total 15.43 USD including diagnosis, injection, and drug management), 2015 KCDC data were used for estimation.65 Since the change in the healthcare inflation rate is little in Korea,66 we did not adjust inflation to the medical cost within 5 years. The Korean Won was converted into USD, and the exchange rate is 1 USD = 1200 KRW.
Sensitivity analysis
One-way deterministic and probabilistic sensitivity analyses were performed for all elderly adults aged ≥65 years. For the one-way sensitivity analysis, each parameter was individually varied at a ±25% level base input, while the other parameters were kept constant. For probabilistic sensitivity analysis, we performed 1,000 replications to ensure robust results, using all the input parameters from triangular distribution. Moreover, we also varied the inputs of the two most sensitive parameters, identified from the one-way sensitivity analysis results: vaccine effectiveness of PCV13 against NBPP, and disease incidence of NBPP. The range of variation in each parameter was determined following the expert panel consultation based on existing research (Supplementary Table 3).8-18,46We also included variations in the serotypes, reflected in the estimation of the indirect effect, excluding certain serotypes (3 or 1,5 or 1,3,5); the contribution of serotype 3 to disease reduction has been reported to be low,67 and serotypes 1 and 5 were considered uncommon colonizers.55 Finally, we assessed differences in cost using a 0% or 5% discount rate. Sensitivity analysis was not performed for the vaccination rate, because the result was not changed under different rates (60%, 80%, and 100%) and most conservative results were calculated under the 100% vaccination rate (data not shown). Our study only showed the results under the 100% vaccination rate. For those parameters and ranges defined, univariate sensitivity analysis was individually performed, for both those in the risk group and those in the total population, aged over 65 years.
Expert panel consultation
When appropriate data were not available, a panel of nine experts were consulted using a modified Delphi method,68 to reach a consensus. Before this process, the core panel conducted a systematic literature review to provide evidence. For the first round, experts used the Likert scale (out of nine) to give an individual, on-line response for each scenario, and for the second round, they engaged in an in-person meeting and discussion. Results were collated using descriptive statistical analysis.
Supplementary Material
Disclosure of potential conflicts of interest
In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I receive funding from a company that may be affected by the research reported in the enclosed paper. I have disclosed those interests fully to Taylor & Francis, and I have in place an approved plan for managing any potential conflicts arising from that involvement.
Funding
This research was sponsored by Pfizer.
References
- 1.Atlas RM, Wellnitz WR. Microorganisms in our world. St Louis: Mosby-Year Book; 1995. [Google Scholar]
- 2.Center for Disease Control and Prevention Pneumococcal home, about pneumococcal. [2017March21]. https://www.cdc.gov/pneumococcal/about/symptoms-complications.html.
- 3.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. Can Med Assoc J. 2009;180(1):48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson W, Wolfe S, Hamborsky J. Centers for Disease Control and Prevention Epidemiology and Prevention of Vaccine-preventable Diseases,“Pink Book,”. Washington, DC: Public Health Foundation; 2011. [Google Scholar]
- 5.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412. doi: 10.1016/j.vaccine.2011.02.088. PMID:21397721. [DOI] [PubMed] [Google Scholar]
- 6.Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28(31):4955–60. doi: 10.1016/j.vaccine.2010.05.030. PMID:20576535. [DOI] [PubMed] [Google Scholar]
- 7.Choi MJ, Song JY, Noh JY, Yoon JG, Hyun HJ, Yun JW, Kim WJ, Cheong HJ Disease Burden and Risk Factors of Hospitalized Community-Acquired Pneumonia in Korean Adults. IDWeek 2016. New Orleans, LA: LA Infectious Diseases Society of America; 2016. [Google Scholar]
- 8.Woo JH, Kang JM, Kim YS, Shin WS, Ryu JH, Choi JH, Kim YR, Cheong HJ, Uh ST, Park CS. A prospective multicenter study of community-acquired pneumonia in adults with emphasis on bacterial etiology. Korean J Infect Dis. 2001;33(1):1–7. [Google Scholar]
- 9.Chong YP, Jung K-S, Lee KH, Kim M-N, Moon SM, Park S, Hur J, Kim D-M, Jeon MH, Woo JH. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010;42(6):397–403. doi: 10.3947/ic.2010.42.6.397. [DOI] [Google Scholar]
- 10.Yoo KH, Yoo CG, Kim SK, Jung JY, Lee MG, Uh ST, Shim TS, Jeon K, Shim JJ, Lee HB. Economic burden and epidemiology of pneumonia in Korean adults aged over 50 years. J Korean Med Sci. 2013;28(6):888–95. doi: 10.3346/jkms.2013.28.6.888. PMID:23772154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung MH, Shin WS, Kim YR, Kang MW, Kim MJ, Jung HJ, Park SC, Pai H, Choi HJ, Shin HS. Etiology of community-acquired pneumonia surveyed by 7 university hospitals. Korean J Infect Dis. 1997;29(5):339–59. [Google Scholar]
- 12.Choi MJ, Song JY, Cheong HJ, Jeon JH, Kang SH, Jung EJ, Noh JY, Kim WJ. Clinical usefulness of pneumococcal urinary antigen test, stratified by disease severity and serotypes. J Infect and Chemother. 2015;21(9):672–9. doi: 10.1016/j.jiac.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu CW, Park CW, Hwang BY, Song JY, Park O, Sohn JW, Cheong HJ, Kim WJ, Kim MJ, Park SC. Clinical features and prognosisof community-acquired pneumonia in the elderly patients. Korean J Infect Dis. 2000;32(3):212–8. [Google Scholar]
- 14.Sohn JW, Park SC, Choi Y-H, Woo HJ, Cho YK, Lee JS, Sim H-S, Kim MJ. Atypical Pathogens as Etiologic Agents in Hospitalized Patient with Community-Acquired Pneumonia in Korea: A Prospective Multi-Center Study. J Korean Med Sci. 2006;21(4):602–7. doi: 10.3346/jkms.2006.21.4.602. PMID:16891800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J-H, Oh WS, Kang C-I, Chung DR, Peck KR, Ko KS, Yeom JS, Kim CK, Kim SW, Chang H-H. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents. 2008;31(2):107–14. doi: 10.1016/j.ijantimicag.2007.09.014. PMID:18162378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon EJ, Cho S-G, Shin JW, Kim JY, Park IW, Choi BW, Choi JC. The difference in clinical presentations between healthcare-associated and community-acquired pneumonia in university-affiliated hospital in Korea. Yonsei Med J. 2011;52(2):282–7. doi: 10.3349/ymj.2011.52.2.282. PMID:21319347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Chung JW, Koh Y, Lim C-M, Jung YJ, Oh YM, Shim TS, Lee SD, Kim WS, Kim D-S. The etiologies and initial antimicrobial therapy outcomes in one tertiary hospital ICU-admitted patient with severe community-acquired pneumonia. Tuberc Respir Dis. 2005;59(5):522–9. doi: 10.4046/trd.2005.59.5.522. [DOI] [Google Scholar]
- 18.Oh SY, Park SJ, Koh YM, Suh GY, Chung MP, Kim H, Choi DC, Kwon OJ, Rhee CH. Efficacy of the antibiotics chosen by ATS guideline in the treatment of Korean patients with community-acquired pneumonia admitted to a tertiary hospital. Tuberc Respir Dis. 1999;47(4):460–70. doi: 10.4046/trd.1999.47.4.460. [DOI] [Google Scholar]
- 19.Song JY, Choi JY, Lee JS, Bae I-G, Kim YK, Sohn JW, Jo YM, Choi WS, Lee J, Park KH. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013;13(1):202. doi: 10.1186/1471-2334-13-202. PMID:23641904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, George R, Miller E. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24. doi: 10.1016/j.jinf.2012.02.017. PMID:22394683. [DOI] [PubMed] [Google Scholar]
- 21.Kyaw MH, Rose CE Jr, Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG, Network ABCSPotEIP . The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192(3):377–86. doi: 10.1086/431521. PMID:15995950. [DOI] [PubMed] [Google Scholar]
- 22.Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, Ki HK, Oh WS, Suh JY, Peck KR, et al.. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004;48(6):2101–7. doi: 10.1128/AAC.48.6.2101-2107.2004. PMID:15155207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenoll A, Granizo JJ, Aguilar L, Gimenez MJ, Aragoneses-Fenoll L, Hanquet G, Casal J, Tarrago D. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47(4):1012–20. doi: 10.1128/JCM.01454-08. PMID:19225097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinert RR, van der Linden M, Seegmuller I, Al-Lahham A, Siedler A, Weissmann B, Toschke AM, von Kries R. Molecular epidemiology of penicillin-non-susceptible Streptococcus pneumoniae isolates from children with invasive pneumococcal disease in Germany. Clin Microbiol Infect. 2007;13(4):363–8. doi: 10.1111/j.1469-0691.2006.01676.x. PMID:17359319. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Vaccines and preventable disease. [2017March21]. https://www.cdc.gov/vaccines/vpd/pneumo/.
- 26.National Health Service The NHS vaccination schedule. 2016. [2017March21]. http://www.nhs.uk/Conditions/vaccinations/Pages/vaccination-schedule-age-checklist.aspx.
- 27.Australian Government Department of Health Pneumococcal disease. [2017March21]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-pneumococcal.
- 28.Korean Centers for Disease Control and Prevention Pneumococcal vaccination program for the elderly. 2016 June 8. [Accessed 2017June6]. http://cdc.go.kr/CDC/notice/CdcKrTogether0302.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0088&cid=68718.
- 29.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28(3):871–99. doi: 10.1128/CMR.00024-15. PMID:26085553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila-Corcoles A, Ochoa-Gondar O. Preventing Pneumococcal Disease in the Elderly. Drugs & Aging. 2013;30(5):263–76. doi: 10.1007/s40266-013-0060-5. [DOI] [PubMed] [Google Scholar]
- 31.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013; doi: 10.1002/14651858.CD000422.pub3.1):CD000422. doi:. PMID:23440780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–60. doi: 10.1056/NEJM199111213252101. PMID:1944423. [DOI] [PubMed] [Google Scholar]
- 33.Schenkein JG, Park S, Nahm MH. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine. 2008;26(43):5521–6. doi: 10.1016/j.vaccine.2008.07.071. PMID:18706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis. 2012;205(3):422–30. doi: 10.1093/infdis/jir750. PMID:22158565. [DOI] [PubMed] [Google Scholar]
- 35.Clutterbuck EA, Lazarus R, Yu L-M, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–16. doi: 10.1093/infdis/jis212. PMID:22457293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43. doi: 10.1016/S1473-3099(15)70044-7. PMID:25801458. [DOI] [PubMed] [Google Scholar]
- 37.S Hoshi -l, M Kondo, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PloS One. 2015;10(10):e0139140. doi: 10.1371/journal.pone.0139140. PMID:26444287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Hoek AJ, Miller E. Cost-Effectiveness of Vaccinating Immunocompetent≥ 65 Year Olds with the 13-Valent Pneumococcal Conjugate Vaccine in England. PloS One. 2016;11(2):e0149540. doi: 10.1371/journal.pone.0149540. PMID:26914907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blommaert A, Bilcke J, Willem L, Verhaegen J, Goossens H, Beutels P. The cost-effectiveness of pneumococcal vaccination in healthy adults over 50: An exploration of influential factors for Belgium. Vaccine. 2016;34(18):2106–12. doi: 10.1016/j.vaccine.2016.03.003. PMID:26988257. [DOI] [PubMed] [Google Scholar]
- 40.Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–8. doi: 10.1007/s11606-016-3651-0. PMID:26976292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangen M-JJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, van Deursen AM, van der Ende A, Grobbee DE, Sanders EA. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Resp J. 2015;46(5):1407–16. doi: 10.1183/13993003.00325-2015. PMID: 6160871.25785969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Health Insurance Review & Assessment Service Evaluation criteria for cost-effectiveness. Detailed evaluation criteria for drugs subject to negotiation including new drugs, etc., 2016 [accessed 2017June6]. http://www.hira.or.kr/dummy.do?pgmid=HIRAA020002000000&cmsurl=/cms/inform/01/1351466_27106.html#none.
- 43.International Monetary Fund World Economic Outlook update; 2016. [2017June6]. http://www.imf.org/external/pubs/ft/weo/2016/update/01/.
- 44.Butler JC, Schuchat A. Epidemiology of pneumococcal infections in the elderly. Drugs & Aging. 1999;15(1):11–9. doi: 10.2165/00002512-199915001-00002. [DOI] [PubMed] [Google Scholar]
- 45.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, Kurki S, Rönnberg PR, Seppä A, Soimakallio S. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Amer J Epidemiol. 1993;137(9):977–88. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 46.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi: 10.1056/NEJMoa1408544. PMID:25785969. [DOI] [PubMed] [Google Scholar]
- 47.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T, Control CfD, Prevention . Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5. PMID:25233284. [PMC free article] [PubMed] [Google Scholar]
- 48.Korea Statistical Information Service Population by age, 2016. [2016July27]. http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parentId=A#SubCont.
- 49.Kim MJ. Pneumococcal diseases and serotyping in the population over 65 years old in Korea. Korean Centers for Disease Control and Prevention. 2015. [2018 April 26]. http://www.prism.go.kr/homepage/entire/retrieveEntireDetail.do;jsessionid=087D85E7CB9309D4BED478809629F098.node02?cond_research_name=&cond_research_start_date=&cond_research_end_date=&research_id=1351000-201400151&pageIndex=288&leftMenuLevel=160. [Google Scholar]
- 50.Heo JY, Seo YB, Choi WS, Lee J, Noh JY, Jeong HW, Kim WJ, Kim MJ, Lee HY, Song JY. Incidence and mortality of community-acquired pneumonia and pneumococcal diseases among Korean adults: catchment population-based analysis. The 10th International Symposium on Pneumococci and Pneumococcal Diseases 2016; Glasgow, Scotland: ISPPD Association. [Google Scholar]
- 51.Center for Disease Control and Prevention Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Morbidity and Mortality Weekly Report. 2012;61(40):816. PMID:23051612. [PubMed] [Google Scholar]
- 52.Kim HI, Kim SW, Chang HH, Cha SI, Lee JH, Ki HK, Cheong HS, Yoo KH, Ryu SY, Kwon KT, et al.. Mortality of community-acquired pneumonia in Korea: assessed with the pneumonia severity index and the CURB-65 score. J Korean Med Sci. 2013;28(9):1276–82. doi: 10.3346/jkms.2013.28.9.1276. PMID:24015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang C-I, Song J-H, Kim SH, Chung DR, Peck KR, Thamlikitkul V, Wang H, TM-k So, Hsueh P-R, Yasin RM. Risk factors and pathogenic significance of bacteremic pneumonia in adult patients with community-acquired pneumococcal pneumonia. J Infect. 2013;66(1):34–40. doi: 10.1016/j.jinf.2012.08.011. PMID:22922634. [DOI] [PubMed] [Google Scholar]
- 54.National Health Statistics 2014. [2018February2]. https://www.seoulmentalhealth.kr/dataroom/data.jsp?cmd=view&test_num=88.
- 55.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804–12. doi: 10.1001/jama.2012.169. PMID:22357831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Y, Gauthier A, Keeping S, Carroll S. Cost-effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):913–27. doi: 10.1586/14737167.2014.950232. PMID:25189087. [DOI] [PubMed] [Google Scholar]
- 57.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–8. doi: 10.1056/NEJMoa035060. PMID:14523142. [DOI] [PubMed] [Google Scholar]
- 58.French N, Nakiyingi J, Carpenter L, Lugada E, Watera C, Moi K, Moore M, Antvelink D, Mulder D, Janoff E. 23–valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106–11. doi: 10.1016/S0140-6736(00)02377-1. PMID:10902624. [DOI] [PubMed] [Google Scholar]
- 59.Advisory Committee on Immunization Practices, Summary report, June 20–21. Atlanta: Centers for Disease control and prevention; 2012. [Google Scholar]
- 60.Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged≥ 60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58(7):909–17. doi: 10.1093/cid/ciu002. PMID:24532544. [DOI] [PubMed] [Google Scholar]
- 61.Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, Hospital I, Gomez-Bertomeu F, Raga X. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case–control study. Vaccine. 2009;27(10):1504–10. doi: 10.1016/j.vaccine.2009.01.013. PMID:19171174. [DOI] [PubMed] [Google Scholar]
- 62.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. PMID:19947881. [DOI] [PubMed] [Google Scholar]
- 63.Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O'Brien KL, Moore MR. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10(9):e1001517. doi: 10.1371/journal.pmed.1001517. PMID:24086113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013;28(9):1283–8. doi: 10.3346/jkms.2013.28.9.1283. PMID:24015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Health Insurance Review & Assessment Service Korea National Health Insurance Reimbursement Medical Expenses, 2015. [2017June6]. http://www.google.co.kr/url?url=http://opendata.hira.or.kr/co.apndFile.dir/download.do%3FfileNm%3D%25EA%25B1%25B4%25EA%25B0%2595%25EB%25B3%25B4%25ED%2597%2598%25EC%259A%2594%25EC%2596%2591%25EA%25B8%2589%25EC%2597%25AC%25EB%25B9%2584%25EC%259A%25A9.pdf&rct=j&frm=1&q=&esrc=s&sa=U&ved=0ahUKEwjsx_LPipTOAhUMymMKHS2YCTAQFggmMAM&usg=AFQjCNGPZbp-ucGDg7LjPJ6Tb_L5ZNjFHg.
- 66.Korean statistical information service (KOSIS) [assessed 2018February2]. http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=4027.
- 67.Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. doi: 10.1016/S1473-3099(14)70822-9. PMID:25042756. [DOI] [PubMed] [Google Scholar]
- 68.National Evidence-based Healthcare Collaborating Agency Handbook for clinical practice guideline developer 2015. [2017June6]. http://www.neca.re.kr/center/researcher/book_view.jsp?boardNo=CA&seq=9436&q=626f6172644e6f3d4341.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


