ABSTRACT
Cellular abundance of mitochondria is dynamically regulated. We could recently show that dysfunctional mitochondria release the phosphatase PGAM family member 5 (PGAM5) into the cytosol, where it interacts with the Wnt signaling-component AXIN1 and dephosphorylates AXIN1-bound β-catenin (CTNNB1) thereby activating Wnt/β-catenin signaling. Because Wnt/β-catenin signaling induces mitochondrial biogenesis dysfunctional mitochondria trigger their own replacement by releasing PGAM5.
KEYWORDS: β-catenin, AXIN1, PGAM5, mitochondria, Wnt signaling
Mitochondria are the cellular powerhouses where most of the energy is produced by the mitochondrial respiratory chain through aerobic oxidation. Cellular abundance of mitochondria is regulated by mitochondrial biogenesis and mitophagy.6 Furthermore, mitochondrial numbers and individual sizes of mitochondria are controlled by processes of mitochondrial fusion and fission leading to the organization of complex mitochondrial networks.6 Several signaling pathways have been reported to be involved in regulating mitochondria. Best characterized, AMP-activated protein kinase (AMPK) signaling was shown to be critical for regulating mitochondrial homeostasis.4
Wnt/β-catenin signaling constitutes an evolutionary conserved signaling pathway which controls patterning of body-axis during embryonic development, stem cell biology and tissue homeostasis.3 Since Wnt/β-catenin signaling induces cell proliferation, hyperactivity of the pathway can drive carcinogenesis in several organs, most prominently in the colon. In the absence of Wnt ligands, β-catenin (CTNNB1) is phosphorylated in a destruction complex consisting of adenomatous polyposis coli (APC), AXIN1, casein kinase 1 α (CK1α) and glycogen synthase kinase 3 (GSK3) leading to ubiquitination and proteasomal degradation of phosphorylated β-catenin.3 Binding of Wnt ligands to receptors of the frizzled family (FZD) and LDL receptor related protein 5/6 co-receptors (LRP5/6) blocks β-catenin degradation resulting in its accumulation and the transcription of β-catenin target genes, e.g. MYC and CCND1 which drive cell proliferation.3
There is evidence that Wnt/β-catenin signaling induces mitochondrial biogenesis.9,8 Thus, treatment of cells with Wnt ligands or downregulation of negative pathway regulators was shown to increase mitochondrial numbers.9 This might help to ensure that enough mitochondria are generated for daughter cells after cell division. In our recent study, we could reveal a signaling axis from mitochondria to the Wnt/β-catenin pathway which is involved in regulating mitochondrial homeostasis.1 Initially, we found that the destruction complex scaffold protein AXIN1 interacts with PGAM family member 5 (PGAM5), a serine/threonine phosphatase located in mitochondria. Moreover, PGAM5 promoted dephosphorylation of β-catenin in a complex with AXIN1, leading to its stabilization and robust activation of Wnt/β-catenin signaling. These results were obtained in vitro using PGAM5 overexpression. The question arose how endogenous PGAM5, which appears to be confined to mitochondria, may be able to cross-talk to the Wnt pathway. We capitalized on previous findings that PGAM5 gets cleaved by the mitochondrial protease presenilin associated rhomboid like (PARL) after disturbance of the mitochondrial membrane potential by treatment with the uncoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP).7 PARL cleaves PGAM5 at its N-terminal membrane anchor leading to its release from mitochondrial membranes.7 We could show by sub-cellular fractionation and immunocytochemistry that a considerable fraction of cleaved PGAM5 shows up in the cytosol after CCCP treatment and that β-catenin is concomitantly dephosphorylated (Figure 1, 1.-3.).1 Importantly, β-catenin dephosphorylation was markedly diminished in PGAM5 and PARL knockout cells showing a causal relationship between both events. Similarly, challenging mitochondria by hypoxic stress led to cytosolic release of PGAM5 and to PGAM5-dependent stabilization of β-catenin. Altogether, mitochondrial stress leading to activation of PARL and cleavage of PGAM5 results in cytosolic appearance of PGAM5 which activates Wnt/β-catenin signaling by dephosphorylating β-catenin. Given previous findings that Wnt signaling increases mitochondrial numbers we also explored whether cytosolic PGAM5 alters mitochondrial mass. Indeed stable expression of cytoplasmic PGAM5 increased the number of mitochondria by 30 to 40%.1
Figure 1.
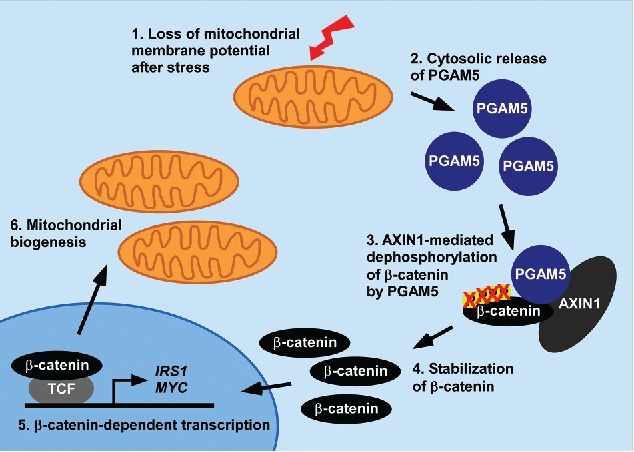
Feedback regulation of mitochondrial homeostasis via activation of Wnt/β-catenin signaling. PGAM5 released from damaged mitochondria cell-intrinsically activates Wnt/β-catenin signaling to induce mitochondrial biogenesis. IRS1: insulin receptor substrate 1. TCF: T-cell factor.
From these studies several conclusions regarding mechanistic aspects of Wnt signaling and mitochondrial homeostasis can be drawn: (i) PGAM5 is identified as a conditional β-catenin phosphatase active after mitochondrial damage. Presumably, PGAM5 takes part in Wnt signaling only under conditions of mitochondrial challenge which might explain why Pgam5 k.o. mice show no overt “Wnt” phenotype.5 (ii) AXIN1 acts as a scaffold not only for phosphorylation of β-catenin but also for its dephosphorylation. In cells with intact mitochondria, cytosolic PGAM5 levels are low and β-catenin phosphorylation by CK1α and GSK3 scaffolded by AXIN1 prevails. In damaged mitochondria, PGAM5 gets released and AXIN1 fosters β-catenin dephosphorylation by linking it to PGAM5 (Figure 1, 3.). To our knowledge, this mechanism represents the first example of a cell intrinsic mode of Wnt pathway activation that can operate independent of Wnt ligands. (iii) Our findings suggest that mitochondrial homeostasis is regulated by a feedback loop operating from mitochondria to Wnt/β-catenin signaling. By releasing PGAM5 and activating Wnt signaling, damaged, mitophagy-prone mitochondria induce mitochondrial biogenesis thereby stimulating their own replacement (Figure 1). Interestingly, PGAM5 was described to induce mitophagy.2 Therefore, activation of PGAM5 triggers both removal of the damaged mitochondria and replenishment of the mitochondrial pool. Simultaneous regulation of mitochondrial biogenesis and mitophagy seems to be a common feature in regulation of mitochondrial homeostasis because also AMPK signaling activates both processes.4
Induction of mitochondrial biogenesis by Wnt/β-catenin signaling might have implications for colorectal carcinogenesis beyond ensuring sufficient energy production. Increased mitochondrial numbers lead to an increase in reactive oxygen species (ROS) and, thereby, an increase in DNA damage which can cause senescence through activation of DNA damage checkpoints.9 In cancer cells with impaired checkpoints, increased DNA damage will result in higher mutation rates and faster acquisition of mutations which drive tumor progression.
Funding Statement
Our work was supported by grants from the Friedrich-Alexander University of Erlangen-Nuremberg Interdisciplinary Center for Clinical Research to J.B. (D22) and D.B.B. (J58) and by a grant from the Johannes und Frieda Marohn-Stiftung to D.B.B. We apologize to colleagues whose work could not be cited due to space limitations.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Our work was supported by grants from the Friedrich-Alexander University of Erlangen-Nuremberg Interdisciplinary Center for Clinical Research to J.B. (D22) and D.B.B. (J58) and by a grant from the Johannes und Frieda Marohn-Stiftung to D.B.B. We apologize to colleagues whose work could not be cited due to space limitations.
References
- 1.Bernkopf DB, Jalal K, Bruckner M, Knaup KX, Gentzel M, Schambony A, Behrens J. Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J Cell Biol. 2018. doi: 10.1083/jcb.201708191. PMID:29438981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C et al.. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–77. doi: 10.1016/j.molcel.2014.02.034. PMID:24746696. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. PMID:22682243. [DOI] [PubMed] [Google Scholar]
- 4.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35. doi: 10.1038/nrm.2017.95. PMID:28974774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W, Karuppagounder SS, Springer DA, Allen MD, Zheng L, Chao B, Zhang Y, Dawson VL, Dawson TM, Lenardo M. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson's-like movement disorder. Nat Commun. 2014;5:4930. doi: 10.1038/ncomms5930. PMID:25222142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. 2014;56:182–8. doi: 10.1016/j.exger.2014.01.021. PMID:24486129. [DOI] [PubMed] [Google Scholar]
- 7.Sekine S, Kanamaru Y, Koike M, Nishihara A, Okada M, Kinoshita H, Kamiyama M, Maruyama J, Uchiyama Y, Ishihara N et al.. Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J Biol Chem. 2012;287:34635–645. doi: 10.1074/jbc.M112.357509. PMID:22915595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Undi RB, Gutti U, Gutti RK. LiCl regulates mitochondrial biogenesis during megakaryocyte development. J Trace Elem Med Biol. 2017;39:193–201. doi: 10.1016/j.jtemb.2016.10.003. PMID:27908414. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–18. doi: 10.1101/gad.1924910. PMID:20634317. [DOI] [PMC free article] [PubMed] [Google Scholar]


