Abstract
Chemical investigation of the fungus Penicillium sp. SCSIO Ind16F01 derived from deep-sea sediment sample afforded a new xanthone, 3,8-dihydroxy-2-methyl-9-oxoxanthene-4-carboxylic acid methyl ester (1) and a new chromone, coniochaetone J (2), together with three known xanthones, 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (3), 7,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (4), 1,6,8-trihydroxy-3-(hydroxymethyl)anthraquinone (5), three known chromones, coniochaetone B (6), citrinolactones B (7), epiremisporine B (8), and four reported rare class of N-methyl quinolone lactams: quinolactacins B (9), C1 (10), and C2 (11), and quinolonimide (12). The structures of new compounds were determined by analysis of the NMR and MS spectroscopic data. Those isolated compounds were evaluated for their antiviral (EV71 and H3N2) and cytotoxic activities.
Keywords: marine-derived fungus, Penicillium sp. SCSIO Ind16F01, xanthone, chromone, N-methyl quinolone lactams
1. Introduction
Marine microorganisms, especially the marine fungi, tend to produce structurally unique and biologically active natural products which have been documented in recent years [1,2,3,4]. Recently, microorganisms from deep-sea habitats, including the hydrothermal vents, have become an interesting and newly emerging source of novel bioactive compounds, which play an important role in drug discovery [5].
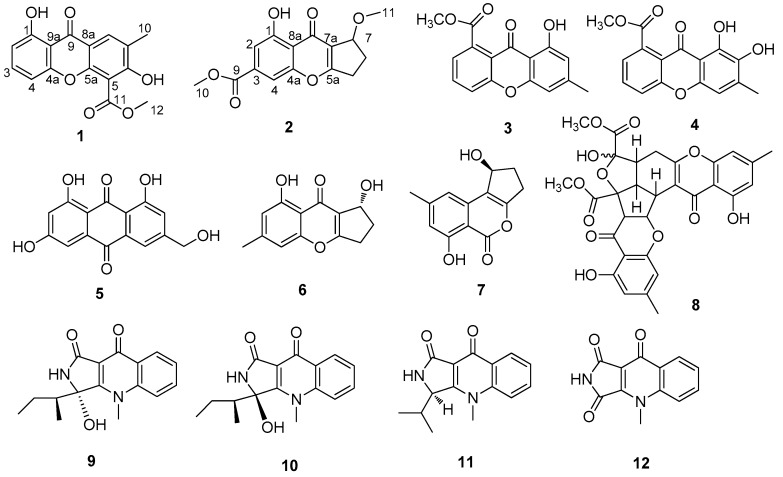
As a part of our progressive program to explore the antiviral potential of marine fungi, the secondary metabolites of the strain SCSIO Ind16F01 were examined. Two new compounds: 3,8-dihydroxy-2-methyl-9-oxoxanthene-4-carboxylic acid methyl ester (1) and a new chromone, coniochaetone J (2), together with ten known compounds: 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (3), 7,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (4), 1,6,8-trihydroxy-3-(hydroxymethyl)anthraquinone (5), coniochaetone B (6), citrinolactones B (7), epiremisporine B (8), quinolactacins C1 (9), C2 (10), and B (11), and quinolonimide (12) (Figure 1) were isolated from the ethyl acetate crude extracts of the rice medium. Their structures were established on the basis of extensive spectroscopic techniques. The isolated compounds were evaluated for their anti-H3N2, anti-enterovirus 71 (EV71) and cytotoxic activities, respectively. We present herein the fermentation, isolation, structural elucidation, bioactive assay of compounds 1–12.
Figure 1.
Structures of metabolites 1–12.
2. Results
Compound 1 was isolated as white amorphous solid. Based on the HRESIMS ion peak at m/z 299.0569 [M − H]− (calcd. for 299.0561), the molecular formula was established as C16H12O6 indicating eleven degrees of unsaturations (Figure S5). The 1H NMR spectrum of 1 showed characteristic signals for three vicinal protons as an AMX spin system at δH 6.80 (1H, dd, J = 0.7, 8.5 HZ, H-4), 7.05 (1H, dd, J = 0.7, 8.5 HZ, H-2), 7.72 (1H, t, J = 8.5 HZ, H-3), and one olefinic proton at δH 7.57 (1H, d, J = 1.0 HZ, H-8) (Figure S1). Additionally, one intramolecularly hydrogen-bonded hydroxyl group at δH 12.32, a methoxy singlet at δH 3.87 and an aromatic methyl singlet at δH 2.38 were observed. The 13C NMR data of 1, with the aid of DEPT and HSQC experiments (Table 1, Figures S2 and S3), showed resonances for two methyl groups (one methoxy), twelve aromatic carbons with four protonated, one carboxylic carbon (δC 167.0), and one α,β-unsaturated ketone carbon (δC 180.5). These data showed great similarities to those of 2,8-dihydroxy-3-methyl-9-oxoxanthene-1-carboxylic acid methyl ester [6] with the difference in ring C. The HMBC correlations of H-8 to C-6, C-5a, C-8a, and C-9, CH3-12 to C-11 and C-5, CH3-10 to C-6, C-7 and C-8 revealed that a phenolic OH was attached to C-6 and a carboxymethoxy moiety was attached to C-5 (Figure 2 and Figure S4). The positioning of the phenolic OH at C-6 was further supported by the base peak observed at m/z 268 [M+ − MeOH] (Figure S8), as previously illustrated for 2,8-dihydroxy-3-methyl-9-oxoxanthene-1-carboxylic acid methyl ester [6]. Thus, compound 1 was suggested to be 1,6-dihydroxy-7-methyl-9-oxoxanthene-5-carboxylic acid methyl ester.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR and HMBC data for 1 (DMSO-d6) and 2 (CDCl3).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC Type | δH (J in Hz) | δC Type | |
| 1 | 160.7 qC | 161.4 qC | ||
| 2 | 7.05 dd (0.7, 8.5) | 110.0 CH | 7.41 s | 112.6 CH |
| 3 | 7.72 t (8.5) | 137.3 CH | 135.7 qC | |
| 4 | 6.80 dd (0.7, 8.5) | 107.2 CH | 7.54 s | 108.4 CH |
| 4a | 155.5 qC | 156.9 qC | ||
| 5 | 118.7 qC | 3.20 m | 30.2 CH2 | |
| 2.82 m | ||||
| 5a | 149.5 qC | 175.1 qC | ||
| 6 | 148.9 qC | 2.32 m | 27.6 CH2 | |
| 2.17 m | - | |||
| 7 | 138.7 qC | 4.94 d (6.5) | 79.3 CH | |
| 7a | 120.7 qC | |||
| 8 | 7.57 d (1.0) | 120.1 CH | 181.0 qC | |
| 8a | 115.2 qC | 113.5 qC | ||
| 9 | 180.5 qC | 165.4 qC | ||
| 9a | 107.9 qC | |||
| 10 | 2.38 s | 17.4 CH3 | 3.94 s | 52.7 10-OMe |
| 11 | 167.0 qC | 3.49 s | 57.5 11-OMe | |
| 12 | 3.87 s | 52.3 11-OMe | ||
| 1-OH | 12.32 s | 12.64 s | ||
Figure 2.
Key HMBC (half arrows) correlations of compounds 1 and 2.
Compound 2 was isolated as white amorphous solid. Its molecular formula was assigned as C28H36N3O5 based on the HRESIMS at m/z: 313.0687 [M + Na]+ (calcd. for C15H14NaO6 313.0683), accounting for nine degrees of unsaturation (Figure S13). The 1H NMR spectrum of 2 showed two olefinic proton at δH 7.41 (1H, s, H-2) and 7.54 (1H, s, H-4) one oxygenated methine at δH 4.94 (1H, d, J = 6.5 Hz, H-7), and two oxygenated methyl at δH 3.94 (3H, s, H-11) and 3.49 (3H, s, H-10) (Figure S9). Its 13C NMR (DEPT) spectrum include signals for two methyl groups, two methines, one oxygenated methylene, eight aromatic/olefinic carbons with two protonated, one carboxylic carbon (δC 165.4), and one α, β-unsaturated ketone carbon (δC 181.0). The 1H and 13C NMR spectra of 2 are similar to those of coniochaetone B, with the exception that the methyl at C-3 of the latter were replaced by a carboxymethoxy moiety and the hydroxy at C-7 was replaced by a methoxy group (Table 1, Figures S10 and S11). The HMBC correlations of OH-1 to C-1, C-2, and C-8a, H-2 to C-3, C-1, C-8a, and C-9, H-4 to C-4a, C-2, C-8a and C-9, CH3-10 to C-9, and CH3-11 to C-7 revealed the positioning of the phenolic OH at C-1, a carboxymethoxy moiety at C-3 and a methoxy at C-7 (Figure S12). The configuration of similars at C-7 were determined on the basis of optical rotation in the literature, which the 7S absolute configuration showed negative optical rotation and 7R absolute configuration showed positive sign [7,8]. However, compound 2 didn’t show optical rotation value or cotton effect in the CD spectrum. Thus, compound 2 was a racemate.
By comparing the 1H, 13C NMR and MS data with the literature values, the known compounds were identified as 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (3) [9], 7,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (4) [10], 1,6,8-trihydroxy-3-(hydroxymethyl)anthraquinone (5) [10], coniochaetone B (6) [8,10], citrinolactone B (7) [11], epiremisporine B (8) [7,12], quinolactacins C1 (9) [13,14,15], C2 (10) [13,14,15], and B (11) [16], and quinolonimide (12) [15]. The stereochemistry of 6, 7, 9 and 11 was confirmed by the optical rotation, and comparison with literature values. Compound 10 was obtained as a mixture of quinolactacins C1 and C2. The configurations of the compound 8 were not determined.
The isolated compounds were evaluated for their antiviral (H3N2 and EV71) and cytotoxic activities (Table S1). Among them, compounds 2 and 8 exhibited weak inhibitory activity against EV71 in vitro, with IC50 values of 81.6 and 19.8 μM. In addition, compound 8 also exhibited inhibitory activity against H3N2 with IC50 values of 24.1 μM, and cytotoxic effects on the tested cancer cell lines (K562, MCF-7, and SGC7901) with IC50 values of 16.6, 16.3, and 15.8 μM, respectively.
3. Materials and Methods
General experimental procedures. 1H-, 13C-NMR, DEPT and 2D-NMR spectra were recorded on a Bruker AC 500 NMR spectrometer with TMS as an internal standard. HR-ESI-MS data were measured on a Bruker microTOF-QII mass spectrometer. CD spectra were measured with a Chirascan circular dichroism spectrometer (Applied Photophysics, Surrey, UK). Optical rotation values were measured with a PerkineElmer 341 polarimeter. Column chromatography were performed on silica gel (200–300 mesh; Qingdao Marine Chemical Factory, Qingdao, China), YMC gel (ODS-A, 12 nm, S-50 µm) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden), respectively. The silica gel GF254 used for TLC were supplied by the Qingdao Marine Chemical Factory, Qingdao, China. All solvents used were of analytical grade (Tianjin Fuyu Chemical and Industry Factory, Tianjin, China). HPLC was carried on Hitachi L-2400 with YMC ODS column. Spots were detected on TLC under UV light or by heating after spraying with 5% H2SO4 in EtOH (v/v).
Fungal Material. The culture of Penicillium sp. SCSIO Ind16F01 was isolated from a deep-sea sediment sample collected in the Indian Ocean (10.00371667° N, 88.72803333° E) in 2013. The strain (accession No. MF945609) was identified as Penicillium sp. based on sequence analysis of the internal transcribed spacer (ITS) region. The DNA was amplified and ITS region sequence was compared with the GenBank database and shared a similarity of 100% with Penicillium citrinum. This strain was stored on MB agar slants at 4 °C and then deposited at CAS Key Laboratory of Tropical Marine Bio-resources and Ecology.
Fermentation and Extraction. Strain stored on PDA slants at 4 °C was cultured MB agar (malt extract 15 g, sea salt 10 g, agar 15 g, distilled water 1 l, pH 7.4–7.8) plates. Seed medium (malt extract 15 g, sea salt 10 g, distilled water 1 L, pH 7.4–7.8) in 50-mL Erlenmeyer flasks was inoculated with strain SCSIO Ind16F01 and incubated at 25 °C for 48 h on a rotating shaker (180 rpm). Production medium of solid rice in 1000 mL flasks (rice 200 g, NaCl 0.5 g, distilled water 200 mL) was inoculated with 10 mL seed solution. Flasks were incubated at 25 °C under static stations and daylight. After 60 days, cultures from 30 flasks were harvested for the isolation of substances.
The total rice solid culture was crushed and extracted with acetone three times. The acetone extract was evaporated under reduced pressure to afford an aqueous solution, and then the aqueous solution was extracted with EtOAc to yield 62 g of a crude gum. The H2O layer (120 g) was further partitioned n-Butyl alcohol to yield n-Butyl alcohol (55.6 g) fractions.
Isolation and Purification. The EtOAc portion was subsequently separated by Si gel column chromatography using CHCl3–MeOH gradient elution to give forty-one fractions (Fr.1–41). Fr.25 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get five subfractions (Fr.25.1–Fr.25.5). Subfraction Fr.25.1 was purified by CC on silica gel eluting with petroleum ether (PE)–ethyl acetate (EtOAc) (4:1) to get 3 (3.3 mg). Fr.33 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get six subfractions (Fr.33.1–Fr.33.6). Subfraction Fr.33.4 was purified by CC on silica gel eluting with CHCl3–MeOH (30:1) to get 2 (4.3 mg) and 4 (5.4 mg). Fr.37 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get five subfractions (Fr.37.1–Fr.37.5). Subfraction Fr.37.5 was purified by CC on silica gel eluting with CHCl3–acetone (150:1) to get 5 (4.1 mg) and 7 (9.4 mg). The total n-Butyl alcohol extract was subjected to column chromatography (CC) on ODS, eluting with a gradient MeOH–H2O (5:95–100:0) to give twenty fractions (Fr.s1–Fr.s20). Fr.s2 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get seven subfractions (Fr.s2.1–Fr.s2.7). Fr.s2.1 was purified by semipreparative RP HPLC (58% MeOH in H2O) at a flow rate of 3 mL/min to afford 6 (3.5 mg). Fr.s14 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get nine subfractions (Fr.s14.1–Fr.s14.9). Fr.s14.1 was purified by semipreparative RP HPLC (50% MeOH in H2O) at a flow rate of 3 mL/min to afford 1 (2.8 mg). Fr.s14.3 was purified by semipreparative RP HPLC (60% MeOH in H2O) at a flow rate of 3 mL/min to afford 8 (6.6 mg). Fr.s14.4 was purified by semipreparative RP HPLC (30% MeOH in H2O) at a flow rate of 3 mL/min to afford 9 (7.8 mg), and 10 (6.1 mg). Fr.s14.6 was purified by semipreparative RP HPLC (50% MeOH in H2O) at a flow rate of 3 mL/min to afford 12 (3.8 mg). Fr.s16 was separated by CC over Sephadex LH-20 (CHCl3–MeOH 1:1) to get five subfractions (Fr.s16.1–Fr.s16.5). Fr.s16.3 was purified by semipreparative RP HPLC (40% MeOH in H2O) at a flow rate of 3 mL/min to afford 11 (4.8 mg).
3,8-Dihydroxy-2-methyl-9-oxoxanthene-4-carboxylic acid methyl ester (1): White amorphous solid; UV (MeOH) λmax (log ε) 236 (0.52), 261 (0.59), 290 (0.23), 382 (0.16) nm; IR (KBr) νmax 3177, 1690, 1647, 1598, 1468, 1372, 1303, 1241, 1056, 1020, 774 cm−1; 1H and 13C NMR data: see Table 1; HRESIMS: m/z: 299.0559 [M − H]− (calcd. for C16H11O6 299.0561).
Coniochaetone J (2): White amorphous solid; [α 0 (MeOH; c 0.2); UV (MeOH) λmax (log ε) 225 (1.56), 245 (1.78), 347 (0.45) nm; IR (KBr) νmax 3094, 2949, 1721, 1647, 1618, 1448, 1246, 1080, 765 cm−1; 1H and 13C NMR data: see Table 1; HRESIMS: m/z: 313.0687 [M + Na]+ (calcd. for C15H14NaO6 313.0683), 603.1473 [2M + Na]+ (calcd. for C30H28NaO12 603.1473).
Biological Activity. Cytotoxic assay: All isolated compounds were evaluated against the three human tumor cell lines (K562, MCF-7, SGC7901) according to Bergeron et al. [17]. Cell lines, K562, MCF-7, SGC7901 were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Cells were routinely grown and maintained in mediums RPMI or DMEM with 10% FBS and with 1% penicillin/streptomycin. All cell lines were incubated in a Thermo/Forma Scientific CO2 Water Jacketed Incubator with 5% CO2 in air at 37 °C. Cell viability assay was determined by the CCK8 (DOjinDo, Kumamoto, Japan) assay. Cells were seeded at a density of 400–800 cells/ well in 384 well plates and treated with various concentration of compounds or solvent control. After 72 h incubation, CCk8 reagent was added, and absorbance was measured at 450 nm using Envision 2104 multi-label Reader (Perkin Elmer, Waltham, MA, USA). Dose response curves were plotted to determine the IC50 values using Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Antiviral assay: The antiviral activities against H3N2 were evaluated by the CPE inhibition assay in duplicate assay [18]. Oseltamivir was used as the positive control with IC50 values of 16.9 nM, respectively. Confluent MDCK cell monolayers were incubated with influenza virus at 37 °C for 1 h. After removing the virus, cells were maintained in infecting media (RPMI 1640, 4 μg/mL of trypsin) containing different concentrations of test compounds. After 48 h of incubation at 37 °C, the cells were fixed with 100 μL of 4% formaldehyde for 20 min at room temperature. After removal of the formaldehyde, the cells were stained with 0.1% crystal violet for 30 min. The plates were washed and dried, and the intensity of crystal violet staining for each well was measured in a microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. The IC50 was calculated as the compound concentration required to inhibit influenza virus yield at 48 h postinfection by 50%. Tamiflu was used as the positive control, with IC50 values of 16.9 and 18.5 nM, respectively.
Anti-EV71 was assayed on Vero cells with the CCK8 (DOjinDo, Kumamoto, Japan) method [19]. Ribavirinwas used as the positive control with an IC50 value of 0.60 μM. Vero cells (2 × 103 cells/well) were seeded with DMEM medium (2% FBS) into a 384-wellplate. After 24 h, 1000 fold serial dilution of the compound was added in triplicate to the 348-well plate. After incubation at 37 °C for 30 min, a twofold dilution 100× the 50% tissue culture infectious dose (TCID50) of EV71 virus in DMEM supplemented with 2% FBS was added to each well. The plate was incubated at 37 °C for 72–96 h when the viral control cells showed complete CPE, the cell survival was quantified using CCK-8. The A450 of the well was measured with a microtiter platereader (Envision, PerkinElmer, Waltham, MA, USA). The 50% inhibitory concentration (IC50) of the testing compound was calculated using the GraphPad Prism software.
4. Conclusions
The chemical investigation of the deep-sea-derived fungus Penicillium sp. SCSIO Ind16F01 has led to twelve compounds, including two new metabolites. Their structures were elucidated by the detailed analysis of spectroscopic data. All compounds were evaluated for their antiviral (H3N2 and EV71) and cytotoxic effects. Among them, compounds 2 and 8 exhibited anti-EV71 activities in vitro, with IC50 values of 81.6 and 19.8 μM. In addition, compound 8 also exhibited inhibitory activity against H3N2 with IC50 values of 24.1 μM, and cytotoxic effects on the tested cancer cell lines (K562, MCF-7, and SGC7901) with IC50 values of 16.6, 16.3, and 15.8 μM, respectively. Compound 8 was a unique cyclopentachromone dimer.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (21202080, 41376162), the Guangdong Natural Science Foundation (2014A030313724, 2014A030313765, 2014A030313765), the Open Research Fund Program of Beijing Key Lab of Plant Resource Research and Development, Beijing Technology and Business University (PRRD-2017-ZD3), Pearl River S&T Nova Program of Guangzhou Scientific Research Project (201610010017), Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (20161166) and the Research Fund Program of Guangdong Key Laboratory of Marine Materia Medica (LMM2016-5).
Supplementary Materials
Supplementary materials are available online.
Author Contributions
F.-a.L. and H.T. fractionated the extract, isolated the compounds. F.-a.L. and M.C. performed the bioassays. X.Z. analyzed the data. X.L. and X.H. collected and identified Penicillium sp. SCSIO Ind16F01. B.Y. and H.T. elucidated the structures, and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–12 are available from the authors.
References
- 1.Kobayashi J.I. Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. 2016;64:1079–1083. doi: 10.1248/cpb.c16-00281. [DOI] [PubMed] [Google Scholar]
- 2.El-Hossary E.M., Cheng C., Hamed M.M., Hamed A.N.E.-S., Ohlsen K., Hentschel U., Abdelmohsen U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017;126:631–651. doi: 10.1016/j.ejmech.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Schinke C., Martins T., Queiroz S.C.N., Melo I.S., Reyes F.G.R. Antibacterial compounds from marine bacteria, 2010–2015. J. Nat. Prod. 2017;80:1215–1228. doi: 10.1021/acs.jnatprod.6b00235. [DOI] [PubMed] [Google Scholar]
- 4.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- 5.Skropeta D., Wei L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014;31:999–1025. doi: 10.1039/C3NP70118B. [DOI] [PubMed] [Google Scholar]
- 6.Sumarah M.W., Puniani E., Blackwell B.A., Miller J.D. Characterization of polyketide metabolites from foliar endophytes of Picea glauca. J. Nat. Prod. 2008;71:1393–1398. doi: 10.1021/np800192f. [DOI] [PubMed] [Google Scholar]
- 7.Xia M.-W., Cui C.-B., Li C.-W., Wu C.-J., Peng J.-X., Li D.-H. Rare chromones from a fungal mutant of the marine-derived Penicillium purpurogenum G59. Mar. Drugs. 2015;13:5219–5236. doi: 10.3390/md13085219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H.J., Gloer J.B., Scott J.A., Malloch D. Coniochaetones A and B New antifungal benzopyranones from the coprophilous fungus Coniocheta saccardoi. Tetrahedron Lett. 1995;36:5847–5850. doi: 10.1016/0040-4039(95)01174-G. [DOI] [Google Scholar]
- 9.Macias M., Gamboa A., Ulloa M., Toscano R.A., Mata R. Phytotoxic naphthopyranone derivatives from the coprophilous fungus Guanomyces polythrix. Phytochemistry. 2001;58:751–758. doi: 10.1016/S0031-9422(01)00278-3. [DOI] [PubMed] [Google Scholar]
- 10.Trisuwan K., Rukachaisirikul V., Borwornwiriyapan K., Phongpaichit S., Sakayaroj J. Benzopyranone, benzophenone, and xanthone derivatives from the soil fungus Penicillium citrinum PSU-RSPG95. Tetrahedron Lett. 2014;55:1336–1338. doi: 10.1016/j.tetlet.2014.01.017. [DOI] [Google Scholar]
- 11.Kuramata M., Fujioka S., Shimada A., Kawano T., Kimura Y. Citrinolactones A, B and C, and sclerotinin C, plant growth regulators from Penicillium citrinum. Biosci. Biotechnol. Biochem. 2007;71:499–503. doi: 10.1271/bbb.60538. [DOI] [PubMed] [Google Scholar]
- 12.Kong F.M., Carter G.T. Remisporine B, a novel dimeric chromenone derived from spontaneous Diels-Alder reaction of remisporine A. Tetrahedron Lett. 2003;44:3119–3122. doi: 10.1016/S0040-4039(03)00518-5. [DOI] [Google Scholar]
- 13.Kakinuma N., Iwai H., Takahashi S., Hamano K., Yanagisawa T., Nagai K., Tanaka K., Suzuki K., Kirikae F., Kirikae T., et al. Quinolactacins A, B and C: Novel quinolone compounds from Penicillium sp. EPF-6 I. Taxonomy, production, isolation and biological properties. J. Antibiot. 2000;53:1247–1251. doi: 10.7164/antibiotics.53.1247. [DOI] [PubMed] [Google Scholar]
- 14.Kim W.G., Song N.K., Yoo I.D. Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J. Antibiot. 2001;54:831–835. doi: 10.7164/antibiotics.54.831. [DOI] [PubMed] [Google Scholar]
- 15.Clark B., Capon R.J., Lacey E., Tennant S., Gill J.H. Quinolactacins revisited: From lactams to imide and beyond. Org. Biomol. Chem. 2006;4:1512–1519. doi: 10.1039/b600959j. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S., Kakinuma N., Iwai H., Yanagisawa T., Nagai K., Suzuki K., Tokunaga T., Nakagawa A. Quinolactacins A, B and C: Novel quinolone compounds from Penicillium sp. EPF-6 II. Physico-chemical properties and structure elucidation. J. Antibiot. 2000;53:1252–1256. doi: 10.7164/antibiotics.53.1252. [DOI] [PubMed] [Google Scholar]
- 17.Bergeron R.J., Cavanaugh P.F., Kline S.J., Hughes R.G., Elliott G.T., Porter C.W. Antineoplastic and antiherpetic activity of spermidine catecholamide iron chelators. Biochem. Biophys. Res. Commun. 1984;121:848–854. doi: 10.1016/0006-291X(84)90755-1. [DOI] [PubMed] [Google Scholar]
- 18.Fang W., Lin X.P., Zhou X.F., Wan J.T., Lu X., Yang B., Ai W., Lin J., Zhang T.Y., Tu Z.C., et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Medchemcomm. 2014;5:701–705. doi: 10.1039/C3MD00371J. [DOI] [Google Scholar]
- 19.Ho H.Y., Cheng M.L., Weng S.F., Leu Y.L., Chiu D.T.Y. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.