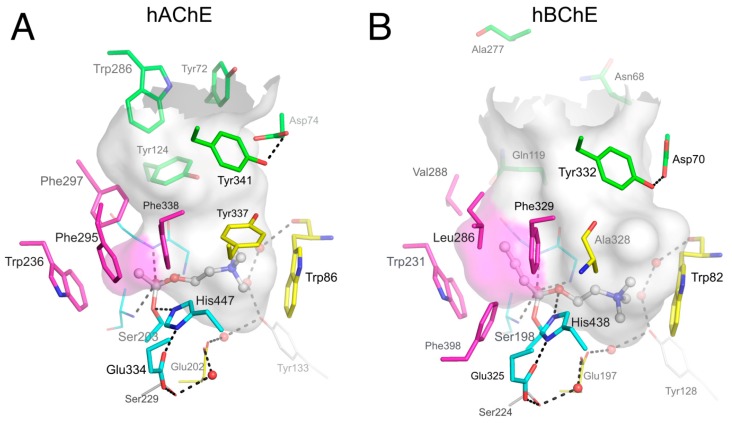
Figure 1.
Active site gorges of human acetylcholinesterase (hAChE) (A; pdb 4ey4) and human butyrylcholinesterase (hBChE) (B; pdb 1p0i). The acylation transition state of a substrate molecule of acetylcholine (ACh) or butyrylcholine (BCh) is modelled and represented in ball and stick. The gorge is depicted by its molecular surface (semi-transparent gray and magenta for the acyl-binding pocket). The main residues are represented. The catalytic triad (in sticks) and oxyanion hole residues (in lines) of the A-site are in cyan. The acyl-binding pocket of both enzymes is in magenta. The key aromatic residues of the choline-binding pocket in the A-site are in yellow. P-site residues located at the rim of the gorge are in green. Conserved structural water molecules are represented in red spheres. The dense hydrogen bond network of the A-site is represented in dashed lines.