Abstract
In our continuing study of biologically active natural products from the fruit of Alpinia galanga (Zingiberaceae), we newly isolated three new labdane-type diterpenes, termed galangalditerpenes A–C (1–3), along with four known sesquiterpenes (4–7) and two diterpenes (8 and 9). The stereostructures of 1–3 were elucidated on the basis of their spectroscopic properties. The melanogenesis inhibitory activities in theophylline-stimulated murine B16 melanoma 4A5 cells of these isolates, including the new diterpenes (1–3, IC50 = 4.4, 8.6, and 4.6 μM, respectively), were found to be more than 6–87-fold higher than that of arbutin (174 μM), a commercially available positive control.
Keywords: Alpinia galanga, melanogenesis inhibitor, galangaldeterpene, labdane-type diterpene
1. Introduction
Labdanes, belonging to the bicyclic diterpenoid group, have been found as secondary metabolites in the tissues of fungi, insects, and marine organisms, and in essential oils, resins, and tissues of higher plants. Among plant materials, the gymnosperms, as well as the Asteraceae, Lamiaceae, and Zingiberaceae families, are the most important sources of labdane-type diterpenoids [1,2]. Labdanes have been reported to have a broad spectrum of biological activities, including antimicrobial, antiviral, cytotoxic, radical scavenging, anti-hypertensive, hepatoprotective, and anti-inflammatory activities [1,2]. The Zingiberaceae plant Alpinia galanga Swartz, which is known as Greater Galangal in English and Kulanjan in Hindi, is widely cultivated in China, India, and Southeast Asian countries such as Thailand, Indonesia, and the Philippines [3]. The fruit of this plant has been used for the treatment of stomachache, dyspepsia, emesis, diarrhea, asthma, osteoarthritis, and rheumatoid arthritis in several traditional medicine systems such as Ayurveda and Siddha [3,4,5,6,7,8,9,10]. In the course of our studies on the chemical constituents of A. galanga, we have isolated several phenylpropanoid, neolignan, and sesquineolignan constituents from 80% aqueous acetone extracts of the rhizome [11,12] and the fruit parts of A. galanga [13]. We have also reported that several phenylpropanoids showed gastroprotective [11], antiallergic [14], anti-inflammatory [12,15,16], and melanogenesis inhibitory activities [13]. Further separation of the constituents in the extract from the fruit part allowed us to isolate three new labdane-type diterpenes, galangalditerpenes A (1), B (2), and C (3), along with four known sesquiterpenes (4–7) and two known diterpenes (8 and 9). Here, we describe the isolation and structural elucidation of 1–3 as well as their inhibitory effects on theophylline-stimulated melanogenesis in mouse murine B16 melanoma 4A5 cells.
2. Results and Discussion
2.1. Isolation
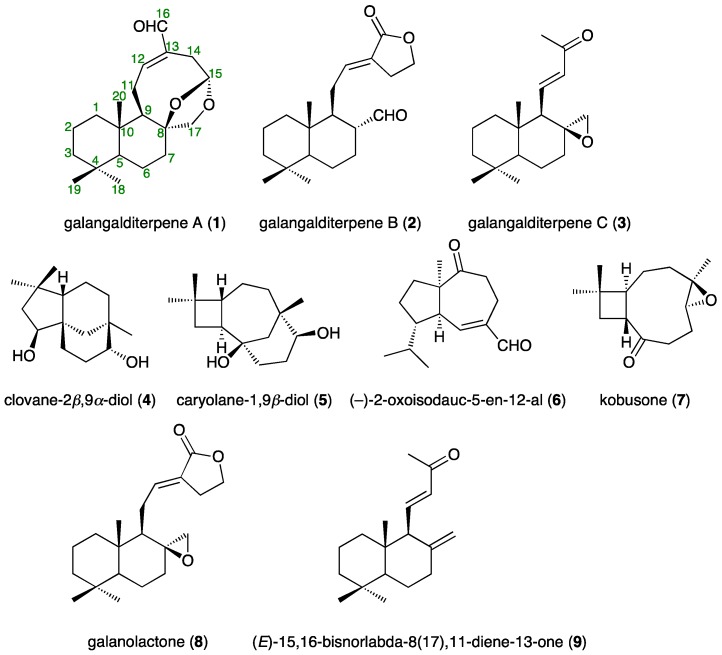
In the present study, we isolated galangalditerpenes A (1, 0.00180%), B (2, 0.00121%), and C (3, 0.00475%), clovane-2β,9α-diol (4, 0.01341%) [17], caryolane-1,9β-diol (5, 0.00916%) [17], (−)-2-oxoisodauc-5-en-12-al (6, 0.00098%) [18], kobusone (7, 0.00066%) [17], galanolactone (8, 0.00148%) [7], and (E)-15,16-bisnorlabda-8(17),11-diene-13-one (9, 0.00136%) [19], from an 80% aqueous acetone extract of the fruit of A. galanga [13] using normal-phase silica gel and reversed-phase ODS CC, and finally preparative HPLC (Figure 1).
Figure 1.
Structures of isolates (1–9) from the fruit of A. galanga.
2.2. Structures of Galangalditerpenes A–C (1–3)
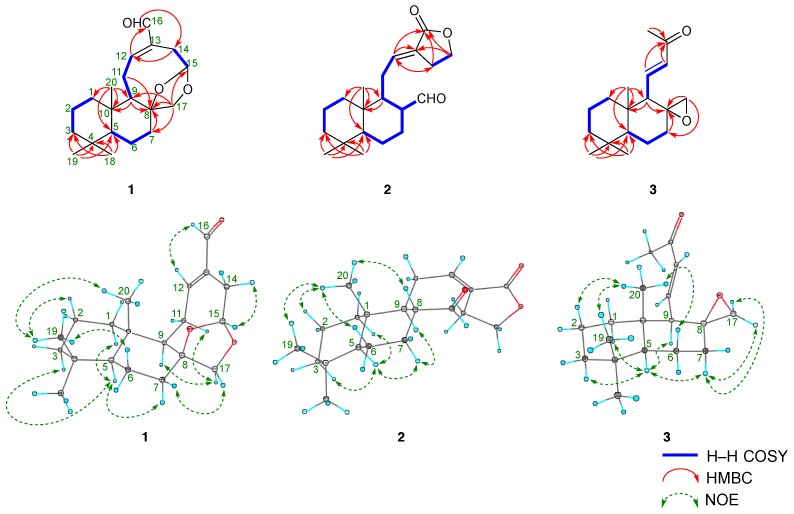
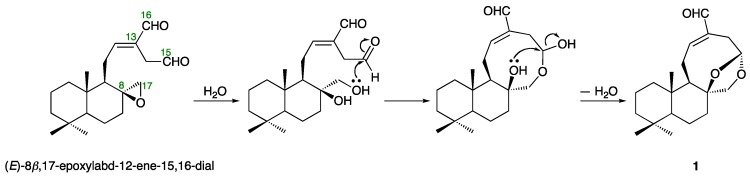
Galangalditerpene A (1) was obtained as a white powder with negative optical rotation ([α] −13.8 in CHCl3). The positive-ion EIMS spectrum of 1 showed a molecular ion peak at m/z 318 (M+), and the molecular formula was determined as C20H30O3 by high-resolution MS. The UV spectrum exhibited maximum absorption at 230 nm, while the IR spectrum showed absorption bands at 1686 cm−1 ascribable to the conjugated aldehyde function. The 1H- and 13C-NMR spectra of 1 (CDCl3, Table 1), which were assigned with the aid of distortion-less enhancement by polarization transfer (DEPT), 1H–1H correlation spectroscopy (COSY), heteronuclear multiple-quantum correlation (HMQC), and heteronuclear multiple bond correlation (HMBC) experiments (Figure 2 and Figure S1), showed signals assignable to three methyls (δ 0.80, 0.81, and 0.89 (3H each, all s, H3-18, 20, and 19)), six methylenes and a methylene bearing an oxygen function (δ (1.04 (1H, ddd, J = 3.2, 12.8, 12.8 Hz), 1.87 (1H, ddd, J = 2.4, 3.2, 12.8 Hz), H2-1), (1.15 (1H, dddd, J = 2.4, 3.2, 12.8, 13.6 Hz), 1.74 (1H, dddd, J = 2.4, 4.4, 4.4, 13.6 Hz), H2-6), (1.19 (1H, ddd, J = 4.0, 13.6, 13.6 Hz), 1.41 (ddd, J = 2.4, 2.4, 13.6 Hz), H2-3), (1.51 (1H, ddddd, J = 2.4, 3.2, 12.8, 13.6, 14.4 Hz), 1.60 (1H, m), H2-2), (1.64 (1H, m), 1.84 (1H, ddd, J = 2.4, 3.2, 12.8 Hz), H2-7), (2.27 (1H, ddd, J = 2.4, 3.2, 16.0 Hz), 3.13 (1H, dd, J = 5.6, 16.0 Hz), H2-14), (2.40 (1H, br dd, J = ca. 9, 13 Hz), 2.57 (1H, ddd, J = 8.8, 10.4, 12.8 Hz), H2-11), 3.62 and 4.35 (1H each, both d, J = 9.6 Hz, H2-17)), two methines and an acetal methine (δ 0.96 (1H, dd, J = 2.4, 12.8 Hz, H-5), 1.64 (1H, m, H-9), 5.44 (1H, dd, J = 3.2, 5.6 Hz, H-15)), a tri-substituted olefin (δ 6.87 (1H, ddd, J = 2.4, 8.8, 8.8 Hz, H-12)), and an aldehyde function (δ 9.35 (1H, s, H-16)). As shown in Figure 2 and Figure S1, the 1H–1H COSY experiment on 1 indicated the presence of partial structures, shown by bold lines. In the HMBC experiment on 1, long-range correlations were observed between the following proton and carbon pairs: H-7 and C-8; H-9 and C-8, 10; H-12 and C-16; H-14 and C-12; H-16 and C-14; H2-17 and C-7–9, 15; H3-18 and C-3–5, 19; H3-19 and C-3–5, 18; H3-20 and C-1, 5, 9, 10. Thus, the linkage positions of the quaternary carbons (C-4, 8, 10, and 13) were clarified unambiguously, and the planar structure of 1 was elucidated. Next, the stereostructure of 1 was investigated using a nuclear Overhauser effect spectroscopy (NOESY) experiment, in which NOE correlations were observed between the following proton pairs: H-1α (δ 1.04) and H-5; H-2β (δ 1.51) and H3-19; H-3α (δ 1.19) and H-5; H-5 and H-7α (δ 1.64); H-6α (δ 1.15) and H3-19; H-7β (δ 1.84) and H-17β (δ 4.35); H-9 and H-17α (δ 3.62); H-12 and H-16; H-14α (δ 2.27) and H-15; H3-19 and H3-20, as shown in Figure 2. A hypothetical pathway from the corresponding diterpene (E)-8β,17-epoxylabd-15,16-dial, which was isolated from the seeds of A. galanga [7], to 1 is proposed in Figure 3. On the basis of the above-mentioned evidence, the stereostructure of 1 was determined to be as shown. To the best of our knowledge, this is the first report of the isolation of a labdane-type diterpene having a 5-formyl-8,10-dioxabicyclo[5.2.1]dec-4-en structure from natural sources.
Table 1.
NMR data (800 MHz, CDCl3) for galangalditerpenes A–C (1–3).
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.04 (ddd, 3.2, 12.8, 12.8, Hα), 1.87 (ddd, 2.4, 3.2, 12.8, Hβ) | 39.4 | 1.02 (ddd, 4.0, 12.8, 12.8, Hα), 1.76 (m, Hβ) |
39.0 | 0.96 (ddd, 3.6, 12.8, 12.8, Hα), 1.42 (m, Hβ) | 40.5 |
| 2 | 1.60 (m, Hα), 1.51 (ddddd, 2.4, 3.2, 12.8, 13.6, 14.4, Hβ) | 18.6 | 1.76 (ddddd, 2.4, 3.2, 4.0, 4.0, 14.4, Hα), 1.50 (ddddd, 3.6, 4.0, 12.8, 13.4, 14.4, Hβ) | 18.5 | 1.51 (m, Hα), 1.41 (m, Hβ) | 18.3 |
| 3 | 1.19 (ddd, 4.0, 13.6, 13.6, Hα), 1.41 (ddd, 2.4, 2.4, 13.6, Hβ) | 41.6 | 1.20 (ddd, 4.0, 13.4, 13.4, Hα), 1.76 (ddd, 2.4, 3.6, 13.4, Hβ) |
41.8 | 1.17 (ddd, 3.2, 13.6, 13.6, Hα), 1.43 (m, Hβ) | 41.9 |
| 4 | 33.4 | 33.3 | 33.5 | |||
| 5 | 0.96 (dd, 2.4, 12.8) | 55.5 | 0.96 (dd, 2.4, 12.4) | 54.5 | 1.00 (dd, 2.4, 12.0) | 54.2 |
| 6 | 1.74 (dddd, 2.4, 4.4, 4.4, 13.6, Hα), 1.15 (dddd, 2.4, 3.2, 12.8, 13.6, Hβ) | 20.5 | 1.74 (m, Hα), 1.35 (dddd, 4.0, 12.0, 12.4, 12.4, Hβ) | 20.0 | 1.71 (br ddd, ca. 2, 6, 14, Hα), 1.66 (dddd, 4.0, 12.0, 13.6, 14.4, Hβ) | 19.9 |
| 7 | 1.64 (m, Hα), 1.84 (ddd, 2.4, 3.2, 12.8, Hβ) | 40.1 | 1.40 (dddd, 4.0, 12.0, 12.4, 12.4, Hα), 1.74 (m, Hβ) | 26.8 | 1.97 (ddd, 5.6, 13.6, 14.4, Hα), 1.42 (m, Hβ) | 18.3 |
| 8 | 84.7 | 2.28 (dddd, 3.2, 5.6, 12.0, 14.4) | 53.8 | 58.2 | ||
| 9 | 1.64 (m) | 57.4 | 1.54 (ddd, 3.2, 6.4, 14.4) | 50.3 | 1.42 (br d, ca. 10) | 56.9 |
| 10 | 39.5 | 37.5 | 39.5 | |||
| 11 | 2.40 (br dd, ca. 9, 13, Hα), 2.57 (ddd, 8.8, 10.4, 12.8, Hβ) | 24.7 | 1.81 (ddd, 6.4, 9.6, 12.8), 2.40 (ddd, 3.2, 6.4, 12.8) |
29.9 | 6.49 (dd, 10.4, 16.8) | 144.4 |
| 12 | 6.87 (ddd, 2.4, 8.8, 8.8) | 157.6 | 6.62 (dd, 6.4, 9.6) | 139.6 | 6.01 (d, 16.8) | 135.8 |
| 13 | 140.4 | 126.2 | 198.4 | |||
| 14 | 2.27 (ddd, 2.4, 3.2, 16.0, Hα), 3.13 (dd, 5.6, 16.0, Hβ) | 29.6 | 2.70 (m), 2.74 (m) | 25.3 | ||
| 15 | 5.44 (dd, 3.2, 5.6) | 100.4 | 4.34 (ddd, 6.4, 7.2, 14.4), 4.37 (ddd, 6.4, 7.6, 14.4) |
65.4 | ||
| 16 | 9.35 (s) | 194.1 | 171.1 | 2.23 (3H, s) | 26.4 | |
| 17 | 3.62 (d, 9.6, Hα), 4.35 (d, 9.6, Hβ) | 70.4 | 9.28 (d, 5.6) | 203.8 | 2.31 (d, 4.8, Hα), 2.38 (d, 4.8, Hβ) | 48.8 |
| 18 | 0.80 (3H, s) | 21.5 | 0.84 (3H, s) | 21.7 | 0.90 (3H, s) | 21.9 |
| 19 | 0.89 (3H, s) | 33.5 | 0.89 (3H, s) | 33.4 | 0.91 (3H, s) | 33.5 |
| 20 | 0.81 (3H, s) | 14.9 | 0.87 (3H, s) | 14.2 | 1.09 (3H, s) | 15.6 |
Figure 2.
1H–1H COSY, HMBC, and NOESY correlations of 1–3.
Figure 3.
Hypothetical pathway to galangalditerpene A (1).
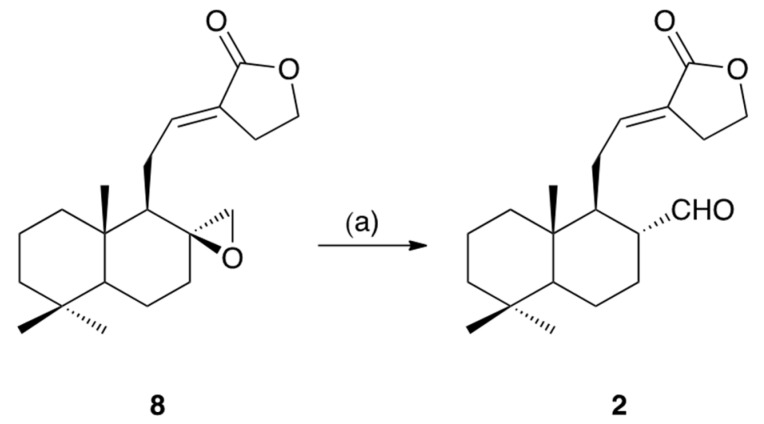
Galangalditerpene B (2) was obtained as a colorless oil with negative optical rotation ([α] −2.8 in CHCl3). In the positive-ion ESIMS profile, a sodium adduct ion peak was observed at m/z 341 [M + Na]+, and high-resolution ESIMS analysis revealed the molecular formula to be C20H30O3. The UV spectrum exhibited absorption maximum at 230 nm, while the IR spectrum showed absorption bands at 1728, 1712, and 1647 cm−1 ascribable to α,β-unsaturated lactone and aldehyde functions. The 1H- and 13C-NMR spectroscopic properties of 2 were generally superimposable onto those of galanolactone (8), but a difference was due to the 17α-formyl group in 2. As shown in Table 1 (in CDCl3), these were three methyls (δ 0.84, 0.87, and 0.89 (3H each, all s, H3-18, 20, and 19)), seven methylenes and a methylene bearing an oxygen function (δ (1.02 (1H, ddd, J = 4.0, 12.8, 12.8 Hz), 1.76 (1H, m), H2-1), (1.20 (1H, ddd, J = 4.0, 13.4, 13.4 Hz), 1.76 (1H, ddd, J = 2.4, 3.6, 13.4 Hz), H2-3), (1.35 (1H, dddd, J = 4.0, 12.0, 12.4, 12.4 Hz), 1.74 (1H, m), H2-6), (1.40 (1H, dddd, J = 4.0, 12.0, 12.4, 12.4 Hz), 1.74 (1H, m), H2-7), (1.50 (1H, ddddd, J = 3.6, 4.0, 12.8, 13.4, 14.4 Hz), 1.76 (1H, ddddd, J = 2.4, 3.2, 4.0, 4.0, 14.4 Hz), H2-2), (1.81 (1H, ddd, J = 6.4, 9.6, 12.8 Hz), 2.40 (1H, ddd, J = 3.2, 6.4, 12.8 Hz), H2-11), 2.70 and 2.74 (1H each, both m, H2-14), (4.34 (1H, ddd, J = 6.4, 7.2, 14.4 Hz), 4.37 (1H, ddd, J = 6.4, 7.6, 14.4 Hz), H2-15)), three methines (δ 0.96 (1H, dd, J = 2.4, 12.4 Hz, H-5), 1.54 (1H, ddd, J = 3.2, 6.4, 14.4 Hz, H-9), 2.28 (1H, dddd, J = 3.2, 5.6, 12.0, 14.4 Hz, H-8)), a tri-substituted olefin (δ 6.62 (1H, dd, J = 6.4, 9.6 Hz, H-12)), and an aldehyde function (δ 9.28 (1H, d, J = 5.6 Hz, H-17)). As shown in Figure 2 and Figure S1, the connectivity of the quaternary carbons in 2 was determined using 1H–1H COSY and HMBC experiments. NOE correlations in the NOESY experiment were observed between the following proton pairs, as shown in Figure 2: H-1α (δ 1.02) and H-5; H-2β (δ 1.50) and H3-20; H-3α (δ 1.20) and H-5; H-5 and H-7α (δ 1.40); H-6β (δ 1.35) and H3-20; H-7α (δ 1.40) and H-9; H-8 and H3-20; H3-19 and H3-20, and on this basis, the relative stereostructure of 2 was determined. To elucidate the absolute stereostructure, we carried out the conversion of 8 into 2. As shown in Scheme 1, the corresponding epoxide 8, the absolute configuration of which has been reported [20], was rearranged in the presence of boron trifluoride diethyl ether complex (BF3·Et2O) to afford 2 [21]. Consequently, the absolute stereostructure of 2 was determined to be 5S, 8R, 9S, and 10R.
Scheme 1.
Reagent and condition: (a) BF3·Et2O/toluene, 0–10 °C, 30 min, 23%.
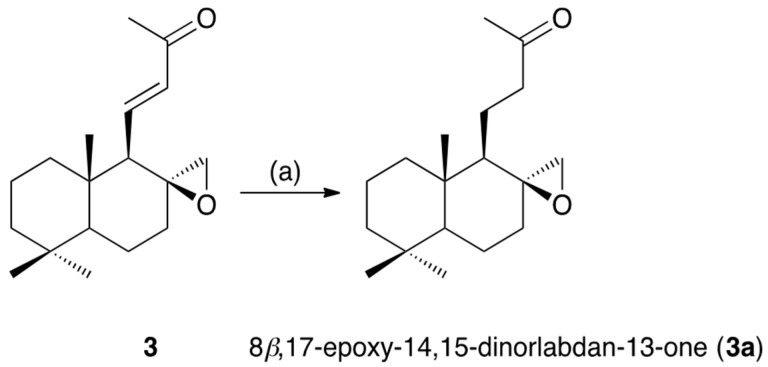
Galangalditerpene C (3) was obtained as a colorless oil with positive optical rotation ([α] +6.1 in CHCl3), and its molecular formula, C18H28O2, was determined using EIMS and high-resolution EIMS measurements. The 1H- and 13C-NMR spectra of 3 (CDCl3, Table 1) showed signals assignable to four methyls (δ 0.90, 0.91, 1.09, 2.23 (3H each, all s, H3-18, 19, 20, and 16)), five methylenes and a methylene bearing an oxygen function (δ (0.96 (1H, ddd, J = 3.6, 12.8, 12.8 Hz), 1.42 (1H, m), H2-1), (1.17 (1H, ddd, J = 3.2, 13.6, 13.6 Hz), 1.43 (1H m), H2-3), 1.41 and 1.51 (1H each, both m, H2-2), (1.42 (1H, m), 1.97 (1H, ddd, J = 5.6, 13.6, 14.4 Hz), H2-7), (1.66 (1H, dddd, J = 4.0, 12.0, 13.6, 14.4 Hz), 1.71 (1H, br ddd, J = ca. 2, 6, 14 Hz), H2-6), and 2.31 and 2.38 (1H each, both d, J = 4.8 Hz, H2-17)), two methines (δ 1.00 (1H, dd, J = 2.4, 12.0 Hz, H-5), 1.42 (1H, br d, J = ca. 10 Hz, H-9)), and a di-substituted trans-olefin (δ 6.01 (1H, d, J = 16.8 Hz, H-12), 6.49 (1H, dd, J = 10.4, 16.8 Hz, H-11)). The 1H and 13C-NMR spectroscopic properties of 3 were quite similar to those of (E)-15,16-bisnorlabda-8(17),11-diene-13-one (9), except for the signal due to the 17-position [19]. As shown in Figure 2, the 1H–1H COSY experiment indicated the presence of partial structures, shown in bold lines. In the HMBC experiment, long-range correlations were observed as shown in Figure 2. Thus, the linkage positions of the quaternary carbons in 3 were determined unambiguously. The configuration of 3 was characterized using a NOESY experiment, which showed NOE correlations between the following proton pairs, as shown in Figure 2: H-1α (δ 0.96) and H-5; H-2β (δ 1.41) and H3-20; H-3α (δ 1.17) and H-5; H-5 and H-7α (δ 1.97), H-9; H-6β (δ 1.66) and H3-20; H-7α and H-17α (δ 2.31); H-7β (δ 1.42) and H-17α; H3-19 and H3-20. Finally, the absolute stereostructure of 3 was determined as chemically related to 3a, the absolute configuration of which has been reported [22]. As shown in Scheme 2, the hydrogenation of 3 in the presence of 5% palladium-carbon (Pd/C)-ethylenediamine complex catalyst [Pd/C(en)] in tetrahydrofuran (THF) yielded 3a [23]. Consequently, the absolute stereostructure of 3 was determined to be 5S, 8S, 9R, and 10S.
Scheme 2.
Reagent and condition: (a) H2, 5% Pd/C(en)/THF, r.t., 6 h, 90%.
2.3. Effects on Theophylline-Stimulated Melanogenesis Inhibitory Activity
Melanin is a broad term for a group of natural pigments found in bacteria, fungi, plants, and animals. It is a heterogeneous, polyphenol-like biopolymer with a complex structure; the color varies from yellow to black through its development. The colors of mammalian skin and hair are determined by several factors, the most important being the degree and distribution of melanin pigmentation. The role of melanin is to protect the skin from ultra-violet (UV) damage by absorbing UV light and removing reactive oxygen species. However, excess production of melanin due to prolonged exposure to sunlight causes dermatological disorders such as melisma, freckles, post-inflammatory melanoderma, and solar lentigines. Melanin is secreted from melanocytes distributed in the basal layer of the dermis. Melanocytes are known to be stimulated by various factors, including UV radiation, α-melanocyte-stimulating hormone (α-MSH), or a phosphodiesterase inhibitor, theophylline [13,24,25,26,27,28,29]. In our previous investigation of naturally occurring compounds possessing melanogenesis inhibitory activity, we reported that several alkaloids [24,25,26,28], phenylethanoid glycosides [26], phenylpropanoids [13], neolignans [13], methoxyflavones [27], and diterpenes [29] exhibited significant positive effects against theophylline-stimulated melanogenesis in B16 melanoma 4A5 cells. As a continuation of the above study, melanogenesis inhibitors from the fruit of A. galanga were further explored. The results revealed that galangalditerpenes A (1, IC50 = 4.4 μM), B (2, 8.6 μM), and C (3, 4.6 μM), clovane-2β,9α-diol (4, 17.7 μM), caryolane-1,9β-diol (5, 9.4 μM), (−)-2-oxoisodauc-5-en-12-al (6, 2.9 μM), kobusone (7, 29.8 μM), galanolactone (8, 5.2 μM), and (E)-15,16-bisnorlabda-8(17),11-diene-13-one (9, 2.0 μM) showed potent activity without notable cytotoxic effects at effective concentrations (Table 2). The melanogenesis inhibitory activity was found to be more than 6–87-fold higher than that of arbutin (174 μM), a commercially available positive control [13,24,25,26,27,28,29].
Table 2.
Inhibitory effects of the isolates (1–9) from the fruit of A. galanga on theophylline-stimulated melanogenesis and viability in B16 4A5 cells.
| Treatment | Inhibition (%) | IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | ||
| Galangalditerpene A (1) | 0.0 ± 4.6 (100.0 ± 1.3) |
48.3 ± 4.1 ** (95.1 ± 0.9) |
56.6 ± 6.9 ** (91.4 ± 1.9) |
67.9 ± 5.9 ** (78.4 ± 2.3 #) |
86.2 ± 4.4 ** (48.6 ± 1.1 #) |
4.4 |
| Galangalditerpene B (2) | 0.0 ± 9.3 (100.0 ± 3.9) |
30.5 ± 9.1 ** (94.2 ± 6.1) |
50.9 ± 3.4 ** (107.3 ± 3.9) |
80.6 ± 2.3 ** (137.1 ± 1.6) |
85.3 ± 2.6 ** (126.5 ± 1.7) |
8.6 |
| Galangalditerpene C (3) | 0.0 ± 9.5 (100.0 ± 6.0) |
40.6 ± 2.8 ** (94.2 ± 3.7) |
67.1 ± 2.2 ** (114.9 ± 1.8) |
82.5 ± 4.4 ** (126.9 ± 4.1) |
— (13.1 ± 0.6 #) |
4.6 |
| Clovane-2β,9α-diol (4) | 0.0 ± 3.4 (100.0 ± 4.7) |
23.9 ± 3.9 ** (98.1 ± 3.0) |
37.2 ± 2.6 ** (93.8 ± 1.4) |
64.0 ± 4.7 ** (102.3 ± 3.2) |
73.6 ± 3.7 ** (76.2 ± 3.6 #) |
17.7 |
| Caryolane-1,9β-diol (5) | 0.0 ± 6.0 (100.0 ± 4.2) |
37.3 ± 3.2 ** (98.4 ± 3.3) |
53.8 ± 2.0 ** (98.2 ± 2.5) |
52.7 ± 7.3 ** (105.3 ± 1.5) |
87.9 ± 3.5 ** (96.7 ± 1.1) |
9.4 |
| (−)-2-Oxoisodauc-5-en-12-al (6) | 0.0 ± 11.8 (100.0 ± 4.2) |
45.9 ± 7.8 ** (87.7 ± 2.4) |
67.8 ± 3.3 ** (86.3 ± 2.6) |
85.1 ± 2.2 ** (90.0 ± 0.8) |
86.7 ± 4.0 ** (59.9 ± 0.3 #) |
2.9 |
| Kobusone (7) | 0.0 ± 6.6 (100.0 ± 1.8) |
−16.1 ± 3.6 (97.0 ± 2.1) |
20.5 ± 8.3 * (97.3 ± 2.2) |
51.5 ± 7.5 ** (92.1 ± 1.4) |
82.8 ± 2.7 ** (87.5 ± 0.5) |
29.8 |
| Galanolactone (8) | 0.0 ± 9.4 (100.0 ± 5.6) |
38.6 ± 4.1 ** (95.3 ± 5.2) |
64.4 ± 7.5 ** (113.6 ± 4.1) |
83.8 ± 3.0 ** (134.4 ± 4.1) |
— (39.9 ± 3.6 #) |
5.2 |
| (E)-15,16-Bisnorlabda-8(17), 11-diene-13-one (9) |
0.0 ± 5.8 (100.0 ± 2.3) |
56.8 ± 3.4 ** (91.4 ± 1.3) |
60.8 ± 2.2 ** (98.5 ± 1.6) |
71.1 ± 1.4 ** (107.3 ± 2.0) |
— (36.4 ± 1.6 #) |
2.0 |
| Arbutin [13,24,25,26,27,28,29] | 0.0 ± 1.4 (100.0 ± 2.1) |
20.4 ± 0.5 (82.4 ± 3.0) |
38.1 ± 0.9 ** (78.1 ± 1.9) |
61.5 ± 0.6 ** (79.8 ± 2.2) |
83.7 ± 0.5 ** (53.1 ± 1.8 #) |
174 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01; # cytotoxic effects were observed, and values in parentheses indicate cell viability (%) in MTT assay; commercial arubutin was purchased from Nakalai Tesque Inc., (Kyoto, Japan).
2.4. Effects on Mushroom Tyrosinase
Tyrosinase, a copper-containing enzyme widely distributed in microorganisms, animals, and plants, is a key enzyme in melanin biosynthesis and determines the color of skin and hair. It catalyzes the oxidation of both l-tyrosine and l-DOPA, following the oxidation of l-DOPA to dopaquinone and oxidative polymerization via several dopaquinone derivatives to yield melanin. Tyrosinase inhibitors are clinically used for the treatment of several dermatologic disorders associated with melanin hyperpigmentation. In addition, these inhibitors are commonly used as additives in cosmetics for skin whitening and/or depigmentation [13,24,26,27,28,29]. As shown in Table S1, none of the isolates (1–9) showed inhibitory activity at effective concentrations when either l-tyrosine or l-DOPA was used as a substrate. This suggests that tyrosinase inhibition is minimally involved in the mechanisms of action of these melanogenesis inhibitors.
3. Materials and Methods
3.1. General Experimental Procedures
The following instruments were used to obtain physical data: specific rotation, JASCO P-2200 polarimeter (JASCO Corporation, Tokyo, Japan, l = 5 cm); UV spectra, Shimadzu UV-1600 spectrometer; IR spectra, IRAffinity-1 spectrophotometer (Shimadzu Corporation, Kyoto, Japan); EIMS and HREIMS, JMS-GC-MATE mass spectrometer (JEOL Ltd., Tokyo, Japan); ESIMS and HRESIMS, Exactive Plus mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA); 1H-NMR spectra, JNM-ECA800 (800 MHz) and JNM-ECS400 (400 MHz) spectrometers (JEOL Ltd., Tokyo, Japan); 13C-NMR spectra, JNM-ECA800 (200 MHz) and JNM-ECS400 (100 MHz) spectrometers (JEOL Ltd., Tokyo, Japan) with tetramethylsilane as an internal standard; HPLC detector, SPD-10Avp UV-VIS detector (Shimadzu Co., Kyoto, Japan); HPLC column, Cosmosil 5C18-MS-II (Nacalai Tesque, Inc., Kyoto, Japan), 4.6 mm × 250 mm i.d. and 20 mm × 250 mm i.d. for analytical and preparative studies, respectively.
The following experimental conditions were used for chromatography (CC): ordinary-phase silica gel column chromatography, silica gel 60 N (Kanto Chemical Co., Tokyo, Japan; 63–210 mesh, spherical, neutral); reverse-phase silica gel CC, Chromatorex ODS DM1020T (Fuji Silysia Chemical, Aichi, Japan; 100–200 mesh); normal-phase TLC, pre-coated TLC plates with silica gel 60F254 (Merck, Darmstadt, Germany; 0.25 mm); reversed-phase TLC, pre-coated TLC plates with silica gel RP-18 F254S (Merck, 0.25 mm); reversed-phase HPTLC, pre-coated TLC plates with silica gel RP-18 WF254S (Merck, 0.25 mm); detection was carried out by spraying 1% Ce(SO4)2–10% aqueous H2SO4 onto the plates, followed by heating.
3.2. Plant Materials
The fruit of Alpinia galanga was collected in Nakhonsithammarat Province, Thailand, in September 2011. The plant material was identified by one of the authors (S.C.), and a voucher specimen (2011.09. Raj-01) of this plant is on file in our laboratory, as described in a previous report [13].
3.3. Extraction and Isolation
Dried fruit of A. galanga (3.0 kg) were extracted four times with 80% aqueous acetone at room temperature overnight. Evaporation of the combined extracts under reduced pressure provided an aqueous acetone extract (258.7 g, 8.62%). An aliquot (230.0 g) was partitioned into an EtOAc–H2O (1:1, v/v) mixture to furnish an EtOAc-soluble fraction (131.7 g, 5.09%) and an aqueous phase. The aqueous phase was subjected to Diaion HP-20 CC (3.0 kg, H2O → MeOH) to give H2O-eluted (19.4 g, 0.75%) and MeOH-eluted (24.8 g, 0.96%) fractions, respectively. An aliquot (110.0 g) of the EtOAc-soluble fraction was subjected to normal-phase silica gel CC [3.0 kg, n-hexane–EtOAc (10:1 → 5:1 →3:1 → 1:1, v/v) → EtOAc → MeOH] to give 11 fractions (Fr. 1 (1.16 g), Fr. 2 (2.91 g), Fr. 3 (2.95 g), Fr. 4 (6.45 g), Fr. 5 (9.80 g), Fr. 6 (11.47 g), Fr. 7 (1.25 g), Fr. 8 (10.03 g), Fr. 9 (2.80 g), Fr. 10 (16.28 g), and Fr. 11 (37.70 g)), as described previously [13]. Fraction 2 (2.91 g) was subjected to reversed-phase silica gel CC (90 g, MeOH–H2O (70:30 → 80:20 → 90:10, v/v) → MeOH) to afford eight fractions (Fr. 2-1 (113.1 mg), Fr. 2-2 (=(−)-2-oxoisodauc-5-en-12-al (6, 21.2 mg, 0.00098%)), Fr. 2-3 (35.7 mg), Fr. 2-4 (165.3 mg), Fr. 2-5 (77.5 mg), Fr. 2-6 (817.5 mg), Fr. 2-7 (823.7 mg), and Fr. 2-8 (850.0 mg)). Fraction 2-4 (165.3 mg) was purified by HPLC (MeOH–H2O (80:20, v/v)) to give galangalditerpene A (1, 4.0 mg, 0.00019%). Fraction 3 (2.95 g) was subjected to reversed-phase silica gel CC (90 g, MeOH–H2O (70:30 → 80:20 → 90:10, v/v) → MeOH) to afford six fractions (Fr. 3-1 (48.0 mg), Fr. 3-2 (94.1 mg), Fr. 3-3 (375.6 mg), Fr. 3-4 (274.9 mg), Fr. 3-5 (1244.7 mg), and Fr. 3-6 (860.0 mg)]. Fraction 3-3 (375.6 mg) was purified by HPLC (MeOH–H2O (80:20, v/v)) to give (E)-15,16-bisnorlabda-8(17),11-diene-13-one (9, 29.4 mg, 0.00136%). Fraction 4 (6.45 g) was subjected to reversed-phase silica gel CC (200 g, MeOH–H2O (60:40 → 80:20, v/v) → MeOH) to afford eight fractions (Fr. 4-1 (62.4 mg), Fr. 4-2 (2.06 g), Fr. 4-3 (239.1 mg), Fr. 4-4 (53.2 mg), Fr. 4-5 (802.6 mg), Fr. 4-6 (252.8 mg), Fr. 4-7 (187.6 mg), and Fr. 4-8 (2.80 g)), as described previously [13]. Fraction 4-5 (300.0 mg) was purified by HPLC (MeOH–H2O (75:25, v/v)] to give galangalditerpene C (3, 38.4 mg, 0.00475%). Fraction 4-6 (252.8 mg) was purified by HPLC (MeOH–H2O (80:20, v/v)) to give 1 (34.9 mg, 0.00161%). Fraction 5 (9.80 g) was subjected to reversed-phase silica gel CC (300 g, MeOH–H2O (60:40 → 80:20, v/v) → MeOH) to afford nine fractions (Fr. 5-1 (63.8 mg), Fr. 5-2 (323.8 mg), Fr. 5-3 (2.57 g), Fr. 5-4 (483.9 mg), Fr. 5-5 (223.9 mg), Fr. 5-6 (2.08 g), Fr. 5-7 (827.2 mg), Fr. 5-8 (140.8 mg), and Fr. 5-9 (2.78 g)), as described previously [13]. Fraction 5-4 (483.9 mg) was purified by HPLC (MeOH–H2O (60:40, v/v)) to give kobusone (7, 14.2 mg, 0.00066%). Fraction 5-6 (502.0 mg) was purified by HPLC (MeOH–H2O (80:20, v/v)) to give galangalditerpene B (2, 6.3 mg, 0.00121%) and galanolactone (8, 0.00148%), together with galanganol D diacetate (15.2 mg, 0.00292%) and trans-p-coumaryl acetate (0.00140%), as described previously [13]. Fraction 9 (2.80 g) was subjected to reversed-phase silica gel CC (90 g, MeOH–H2O (20:80 → 30:70 → 40:60 → 50:50 → 60:40 → 70:30, v/v) → MeOH) to afford seven fractions (Fr. 9-1 (419.0 mg), Fr. 9-2 (231.0 mg), Fr. 9-3 (= caryolane-1,9β-diol (5, 198.0 mg, 0.00916%)], Fr. 9-4 (20.8 mg), Fr. 9-5 ( = clovane-2β,9α-diol (4, 289.9 mg, 0.01341%)), Fr. 9-6 (658.7 mg), and Fr. 9-7 (947.1 mg)).
Galangalditerpene A (1): A white powder; [α] −13.8 (c 0.35, CHCl3); UV (MeOH, nm (log ε)): 230 (3.42); IR (KBr) vmax cm−1: 1686, 1649, 1136, 1103; 1H- and 13C-NMR spectroscopic data, see Table 1; EIMS m/z (%): 318 (M+, 3), 279 (9), 190 (100); HREIMS m/z 318.2197 (calcd. for C20H30O3, 318.2195).
Galangalditerpene B (2): A colorless oil; [α] −2.8 (c 0.29, CHCl3); UV (MeOH, nm (log ε)): 230 (3.40); IR (KBr) vmax cm−1: 1728, 1712, 1647; 1H- and 13C-NMR spectroscopic data, see Table 1; positive-ion ESIMS m/z: 341 [M + Na]+; HRESIMS m/z: 341.2104 [M + Na]+ (calcd. for C20H30O3Na, 341.2087).
Galangalditerpene C (3): A colorless oil; [α] +6.1 (c 0.47, CHCl3); UV (MeOH, nm (log ε)): 230 (3.71); IR (film) vmax cm−1: 1697, 1635, 1123; 1H- and 13C-NMR spectroscopic data, see Table 1; EIMS m/z (%): 276 (M+, 15), 258 (14), 43 (100); HREIMS m/z 276.2092 (calcd. for C18H28O2, 276.2089).
3.4. Conversion of Galanolactone (8) into Galangalditerpene B (2)
A solution of 8 (20.0mg, 0.06 mmol) in dry toluene (1.0 mL) at 0 °C was treated with BF3·Et2O (100 μL, 0.08 mmol), and the entire mixture was stirred at 0 °C for 30 min. The reaction mixture was poured into cold aqueous saturated NaHCO3 and extracted with EtOAc. The EtOAc extract was washed with brine, then dried over anhydrous Na2SO4 and filtered. Removal of the solvent under reduced pressure gave a crude product, which was purified by HPLC (CH3CN–H2O (55:45, v/v)) to furnish 2 (4.5 mg, 23%).
3.5. Hydrogenation of Galangalditerpene C (3)
A solution of 3 (5.5 mg, 0.02 mmol) in dry THF (1.0 mL) was treated with H2 in the presence of 5% Pd/C(en) (5.0 mg), and the entire mixture was stirred at room temperature for 6 h. The catalyst was removed by filtration, and the filtrate was evaporated under reduced pressure to give a crude product, which was purified by HPLC (MeOH–H2O (75:25, v/v)) to furnish 3a (5.0 mg, 90%).
3.6. Reagents for Bioassays
Dulbecco’s modified Eagle’s medium (DMEM, 4.5 g/L glucose) was purchased from Sigma-Aldrich (St. Louis, MO, USA); fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Invitrogen, Carlsbad, CA, USA); and other chemicals used in this study were purchased from Wako Pure Chemical Co., Ltd. (Osaka, Japan). The 48- and 96-well microplates (Sumilon) were purchased from Sumitomo Bakelite Co., Ltd. (Tokyo, Japan).
3.7. Cell Culture
Murine B16 melanoma 4A5 cells (RCB0557) were obtained from Riken Cell Bank (Tsukuba, Japan). The cells were grown in DMEM (glucose; 4500 mg/L) supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin (100 µg/mL) at 37 °C in 5% CO2/air. Cells were harvested by incubation in phosphate-buffered saline (PBS) containing 0.05% ethylenediaminetetraacetic acid (EDTA) and 0.02% trypsin for ca. 5 min at 37 °C and used for the subsequent bioassays.
3.8. Melanogenesis and Cell Viability
Effects on theophylline-stimulated melanogenesis and the viability of B16 melanoma 4A5 cells were determined as described previously [13,25,26,27,28,29].
3.9. Mushroom Tyrosinase
Tyrosinase activities using l-tyrosine or 3,4-dihydroxyphenyl-l-alanine (l-DOPA) as a substrate were determined as described previously [13,25,26,27,28,29].
3.10. Statistical Analysis
Values are expressed as mean ± S.E.M. One-way analysis of variance followed by Dunnett’s test was used for statistical analyses. Probability (p) values less than 0.05 were considered significant.
4. Conclusions
In conclusion, three new labdane-type diterpenes, galangalditerpenes A–C (1–3), were newly isolated from the 80% aqueous acetone extract of the fruit of A. galanga (Zingiberaceae) together with four known sesquiterpenes, clovane-2β,9α-diol (4), caryolane-1,9β-diol (5), (−)-2-oxoisodauc-5-en-12-al (6), and kobusone (7), and two diterpenes, galanolactone (8) and (E)-15,16-bisnorlabda-8(17),11-dien-13-one (9). The melanogenesis inhibitory activities in theophylline-stimulated murine B16 melanoma 4A5 cells of these isolates including the new diterpenes (1–3, IC50 = 4.4, 8.6, and 4.6 μM, respectively) were found to be more than 6–87-fold higher than that of arbutin (174 μM), a commercially used tyrosinase inhibitor. Considering the mechanisms of action, we examined the activity of the isolates against tyrosinase using either L-tyrosine or L-DOPA as a substrate. However, none of the isolates (1–9) showed inhibitory activity at the effective concentration. The detailed mechanisms of action of the melanogenesis inhibitory activity of the isolates require further examination.
Acknowledgments
This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (S1411037, T.M.), as well as JSPS KAKENHI Grant Numbers 15K08008 (T.M.), 15K08009 (K.N.), and 16K08313 (O.M.). Thanks are also due to the Kobayashi International Scholarship Foundation for financial support (T.M.).
Supplementary Materials
Supplementary materials are available online. Figure S1: NMR spectra of galangalditerpenes A–C (1–3); Table S1: Effects of the isolates (1–9) from the fruit of A. galanga on the activity of tyrosinase from mushroom.
Author Contributions
Y.M., K.N., S.C., O.M., and T.M. conceived and designed the experiments; Y.M., K.N., R.N., and Y.H. performed the experiments; Y.M., K.N., O.M., and T.M. analyzed the data; S.C., O.M., and T.M. contributed the materials; Y.M. and T.M. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds 1–9 are available from the authors.
References
- 1.Demetzos C., Dimas K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem. 2001;25:235–292. [Google Scholar]
- 2.Chinou I. Labdanes of natural origin-biological activities (1981–2004) Curr. Med. Chem. 2005;12:1295–1317. doi: 10.2174/0929867054020990. [DOI] [PubMed] [Google Scholar]
- 3.Raviraja S.G., Monisha S. Pharmacology of an endangered medicinal plant Alpinia galanga—A review. Res. J. Pharm. Biol. Chem. Sci. 2015;6:499–511. [Google Scholar]
- 4.Matsui S., Kobayashi S., Nagahori H., Ogiso A. Constituents from seeds of Alpinia galanga Wild. And their anti-ulcer activities. Chem. Pharm. Bull. 1976;24:2377–2382. doi: 10.1248/cpb.24.2377. [DOI] [PubMed] [Google Scholar]
- 5.Morita H., Itokawa H. New diterpenes from Alpinia galanga Wild. Chem. Lett. 1986;15:1205–1208. doi: 10.1246/cl.1986.1205. [DOI] [Google Scholar]
- 6.Itokawa H., Morita H., Sumitomo T., Totsuka N., Takeya K. Antitumor principles from Alpinia galanga. Planta Med. 1987;53:32–33. doi: 10.1055/s-2006-962611. [DOI] [PubMed] [Google Scholar]
- 7.Morita H., Itokawa H. Cytotoxic and antifungal diterpenes from the seeds of Alpinia galanga. Planta Med. 1988;54:117–120. doi: 10.1055/s-2006-962365. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z.L., Zhou K.Y., Dong H., Xu L.S. Characters of nrDNA ITS region sequences of fruits of Alpinia galanga and their adulterants. Planta Med. 2001;67:381–383. doi: 10.1055/s-2001-14311. [DOI] [PubMed] [Google Scholar]
- 9.Bian M.-Q., Kang J., Wang H.-Q., Zhang Q.-J., Liu C., Chen R.-Y. Three new norsesquiterpenoids from the seeds of Alpinia galanga. J. Asian Nat. Prod. Res. 2014;16:459–464. doi: 10.1080/10286020.2014.906407. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Q.-H., Lu C.-L., Zhang X.-W., Jiang J.-G. Isolation and identification of ingredients inducing cancer cell death from the seeds of Alpinia galanga, a Chinese spice. Food Funct. 2015;6:431–443. doi: 10.1039/C4FO00709C. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda H., Pongpiriyadacha Y., Morikawa T., Ochi M., Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: Structural requirements and mode of action. Eur. J. Pharmacol. 2003;471:59–67. doi: 10.1016/S0014-2999(03)01785-0. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa T., Ando S., Matsuda H., Kataoka S., Muraoka O., Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: Structures of new 8–9′ linked neolignans and sesquineoliganns. Chem. Pharm. Bull. 2005;53:625–630. doi: 10.1248/cpb.53.625. [DOI] [PubMed] [Google Scholar]
- 13.Manse Y., Ninomiya K., Nishi R., Kamei I., Katsuyama Y., Imagawa T., Chaipech S., Muraoka O., Morikawa T. Melanogenesis inhibitory activity of a 7-O-9′-linked neolignan from Alpinia galanga fruit. Bioorg. Med. Chem. 2016;24:6215–6224. doi: 10.1016/j.bmc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H., Morikawa T., Managi H., Yoshikawa M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2003;13:3197–3202. doi: 10.1016/S0960-894X(03)00710-8. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda H., Ando S., Morikawa T., Kataoka S., Yoshikawa M. Structure-activity relationships of 1′S-1′-acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. Lett. 2005;15:1949–1953. doi: 10.1016/j.bmcl.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 16.Ando S., Matsuda H., Morikawa T., Yoshikawa M. 1′S-1′-acetoxtchavicol acetate as a new type inhibitor of interferon-β production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2005;13:3289–3294. doi: 10.1016/j.bmc.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Heymann H., Tezuka Y., Kikuchi T., Supriyatna S. Constituents of Sindora sumatrana MIQ. I. Isolation and NMR spectral analysis of sesquiterpenes from the dried pods. Chem. Pharm. Bull. 1994;42:138–146. doi: 10.1248/cpb.42.138. [DOI] [Google Scholar]
- 18.Hansson T., Wickberg B. A short enantiospecific route to isodaucane sesquiterpenes from limonene. On the absolute configuration of (+)-aphannamol I and II. J. Org. Chem. 1992;57:5370–5376. doi: 10.1021/jo00046a018. [DOI] [Google Scholar]
- 19.Itokawa H., Yoshimoto S., Morita H. Diterpenes from the rhizomes of Alpinia formosana. Phytochemistry. 1988;27:435–438. doi: 10.1016/0031-9422(88)83115-7. [DOI] [Google Scholar]
- 20.Jung M., Lee S., Yoon B. Conversion of sclareol into (+)-galanolactone and (+)-labdienedial. Tetrahedron Lett. 1997;38:2871–2874. doi: 10.1016/S0040-4039(97)00485-1. [DOI] [Google Scholar]
- 21.Winter B. Spirocyclic ethers related to Ambrox®: Synthesis and structure-odor relationships. Helv. Chim. Acta. 2004;87:1616–1627. doi: 10.1002/hlca.200490149. [DOI] [Google Scholar]
- 22.Grant P.K., Weavers R.T. Diterpene chemistry—IV transformations of 8,(17)-labdadien-13-ol. Tetrahedron. 1974;30:2385–2395. doi: 10.1016/S0040-4020(01)97108-X. [DOI] [Google Scholar]
- 23.Sajiki H., Hattori K., Hirota K. Highly chemoselective hydrogenation with retention of the epoxide function using a heterogeneous Pd/C-ethylenediamine catalyst and THF. Chem. Eur. J. 2000;6:2200–2204. doi: 10.1002/1521-3765(20000616)6:12<2200::AID-CHEM2200>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa T., Nakanishi Y., Ninomiya K., Matsuda H., Nakashima S., Miki H., Miyashita Y., Yoshikawa M., Hayakawa T., Muraoka O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2104;68:539–549. doi: 10.1007/s11418-014-0832-1. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe G., Sugano Y., Shirato M., Sonoda N., Tsutsui N., Morikawa T., Ninomiya K., Yoshikawa M., Muraoka O. Total synthesis of 4,5-didehydroguadiscine: A potent melanogenesis inhibitor from the Brazilian medicinal herb, Hornschuchia obliqua. J. Nat. Prod. 2015;78:1536–1542. doi: 10.1021/np500995z. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa T., Ninomiya K., Kuramoto H., Kamei I., Yoshikawa M., Muraoka O. Phenylethanoid and phenylpropanoid glycosides with melanogenesis inhibitory activity from the flowers of Narcissus tazetta var. chinensis. J. Nat. Med. 2016;70:89–101. doi: 10.1007/s11418-015-0941-5. [DOI] [PubMed] [Google Scholar]
- 27.Ninomiya K., Matsumoto T., Chaipech S., Miyake S., Katsuyama Y., Tsuboyama A., Pongpiriyadacha Y., Hayakawa T., Muraoka O., Morikawa T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016;70:179–189. doi: 10.1007/s11418-015-0955-z. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa T., Kitagawa N., Tanabe G., Ninomiya K., Okugawa S., Motai C., Kamei I., Yoshikawa M., Lee I.J., Muraoka O. Quantitative determination of alkaloids in lotus flower (flower buds of Nelumbo nucifera) and their melanogenesis inhibitory activity. Molecules. 2016;21:930. doi: 10.3390/molecules21070930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manse Y., Ninomiya K., Okazaki A., Okada-Nishida E., Imagawa T., Imamura-Mizushima M., Yamano Y., Kaname K., Nakamura S., Morikawa T. Melanogenesis inhibitory activity of diterpenoid and triterpenoid constituents from the aerial part of Isodon trichocarpus. Nat. Prod. Commun. 2017;12:1185–1188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.