Abstract
Four novel compounds—two phenylpropionamides, one piperidine, and one phenolic derivatives—were isolated and identified from the fruit of a medicinal plant, Ailanthus altissima (Mill.) Swingle (Simaroubaceae), together with one known phenylpropionamide, 13 known phenols, and 10 flavonoids. The structures of the new compounds were elucidated as 2-hydroxy-N-[(2-O-β-d-glucopyranosyl)phenyl]propionamide (1), 2-hydroxy-N-[(2-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)phenyl]propionamide (2), 2β-carboxyl-piperidine-4β-acetic acid methyl ester (4), and 4-hydroxyphenyl-1-O-[6-(hydrogen-3-hydroxy-3-methylpentanedioate)]-β-d-glucopyranoside (5) based on spectroscopic analysis. All the isolated compounds were evaluated for their inhibitory activity against Tobacco mosaic virus (TMV) using the leaf-disc method. Among the compounds isolated, arbutin (6), β-d-glucopyranosyl-(1→6)-arbutin (7), 4-methoxyphenylacetic acid (10), and corilagin (18) showed moderate inhibition against TMV with IC50 values of 0.49, 0.51, 0.27, and 0.45 mM, respectively.
Keywords: Ailanthus altissima, Simaroubaceae, phenylpropionamide, piperidine, phenols, flavonoid, Tobacco mosaic virus (TMV)
1. Introduction
Ailanthus altissima (Mill.) Swingle (Simaroubaceae), a deciduous tree (6–20 m in height), is native to Mainland China and now naturalized in many temperate regions of the world [1,2]. The stem and root bark have been used as traditional Chinese medicines for the treatment of colds, bleeding, and gastric diseases [3,4]. Phytochemical studies, especially on the stem and root bark of A. altissima, have led to the characterization of quassinoids [5,6], alkaloids [7,8], triterpenoids [9,10], coumarins [9], lignans [11], as well as sterols, lipids, and other phenolic derivatives [12]. However, little is known concerning the constituents of the fruit of A. altissima, which was also used as traditional Chinese medicine for bleeding and antibacterial. By far, previous phytochemical studies have demonstrated the identification of only four quassinoid glycosides [13], and several stigmasterols [14,15] from the fruit. We report in this paper the isolation and structure elucidation of four novel compounds—two phenylpropionamides (1 and 2), one piperidine (4) and one phenolic (5) derivatives—as well as 24 known constituents—one known phenylpropionamide (3), 13 phenols (6–18), and 10 flavonoids (19–28). All compounds were investigated for their anti-Tobacco mosaic virus (TMV) activity.
2. Results
2.1. Extraction, Isolation, and Sructure Elucidation
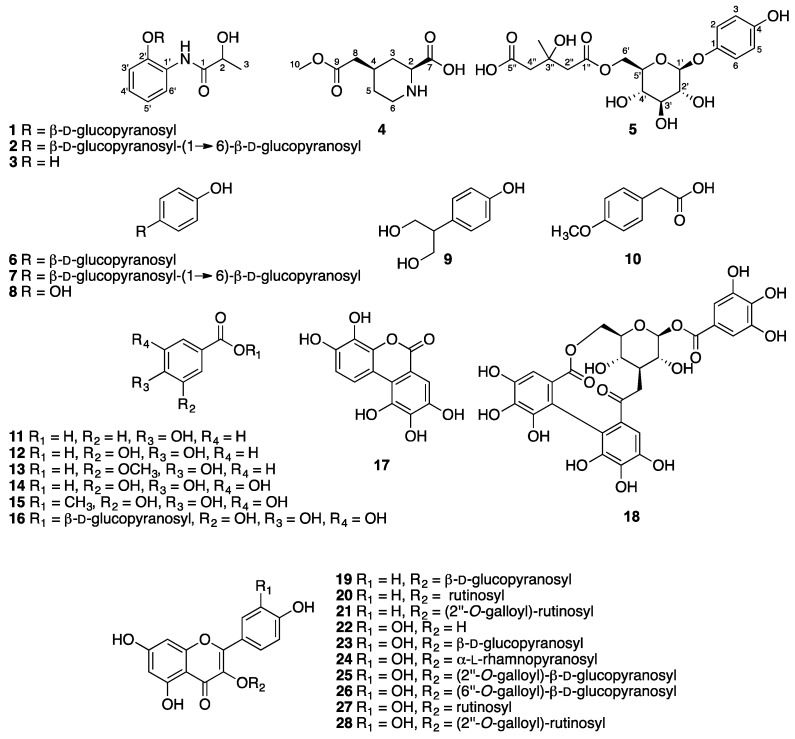
Chromatographic purification of the n-BuOH-soluble fraction from MeOH extract of the dried A. altissima fruit afforded 28 compounds, including four new ones (1, 2, 4, and 5, Figure 1).
Figure 1.
Chemical structures of 1–28.
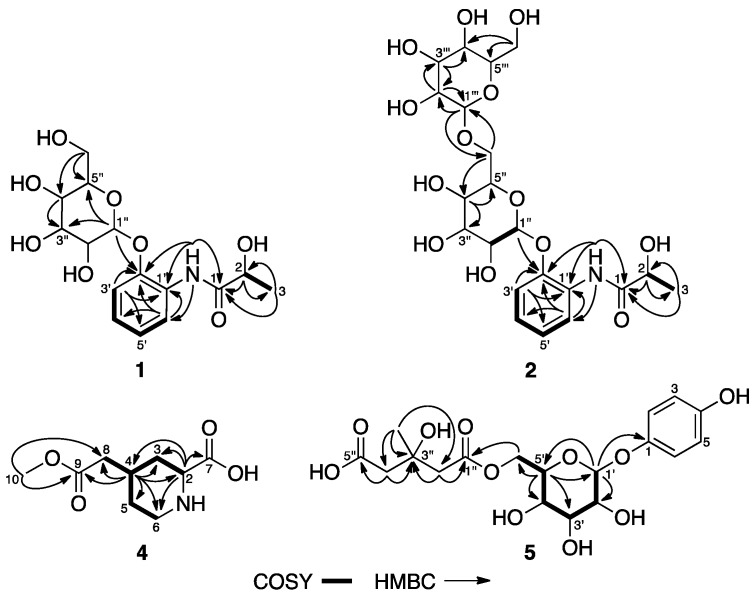
Compound 1 was isolated as a white amorphous powder. It was assigned with a molecular formula of C15H21NO8 by an HR-ESI-MS (high resolution electrospray ionization mass spectrometry) ion peak at m/z = 366.1185 [M + Na]+ (Calcd. for C15H21NO8Na, 366.1159). The IR spectrum (Supplementary Materials) exhibited absorption bands due to the presence of hydroxyl, amide, and phenyl groups (3478, 3388, 1674, 1603, 1534, and 1456 cm−1). The 1H-NMR (Table 1) and HSQC (heteronuclear single quantum coherence) spectrum of 1 indicated the presence of a 1,2-disubstituted benzene ring [δH 8.23 (1H, dd, J = 7.4, 2.3 Hz), 7.20 (1H, dd, J = 7.5, 2.0 Hz), 7.05 (1H, td, J = 7.5, 2.0 Hz), and 7.02 (1H, td, J = 7.5, 1.7 Hz)], an oxygenated methine [4.15 (1H, qd, J = 6.8, 5.1 Hz)], a methyl [δH 1.33 (3H, d, J = 6.8 Hz)], an amide proton [δH 9.42 (1H, s)], and a glucopyranosyl moiety [δH 4.85 (1H, d, J = 7.5 Hz), 3.69 (1H, ddd, J = 11.8, 5.4, 2.2 Hz), 3.49 (1H, dt, J = 11.8, 5.9 Hz), 3.27–3.32 (3H, overlap), and 3.18 (1H, td, J = 9.2, 5.4 Hz)]. Its 13C-NMR (Table 1) and DEPT (distortionless enhancement by polarization transfer) spectra showed 15 carbon resonances, including one methyl, one methylene, 10 methines, and three quaternary carbons (including one carbonyl). The HMBC (heteronuclear multiple bond correlation) correlations (Figure 2) observed from the amide proton to C-1 (δC 173.1), C-1′ (δC 128.6), C-2′ (δC 146.5), and C-6′ (δC 119.6) and from H-2 to C-1, C-2 (δC 67.8), and C-3 (δC 20.8) indicated the presence of a 2-hydroxypropionamide moiety, which was attached to C-1′ of the benzene ring via an NH linkage. The anomeric proton appearing as a doublet at δH 4.85 with a diaxial coupling constant of 7.5 Hz suggested that the glucopyranosyl moiety must be a β-anomer. Additionally, it was attached to C-2′ of the benzene ring through an oxygen, as indicated from the HMBC correlations from the anomeric proton to C-2′, which was further confirmed by the NOESY (nuclear Overhauser effect spectroscopy) correlation between H-3′/H-1‴. The acid hydrolysis of 1 afforded d-glucose, which was identified using TLC by comparison with standard sugars. Therefore, the structure of Compound 1 was established as 2-hydroxy-N-[(2-O-β-d-glucopyranosyl)phenyl]propionamide.
Table 1.
1H- (500 MHz) and 13C-NMR (125 MHz) data of 1 and 2 in DMSO-d6.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC (ppm) | δH (ppm, J in Hz) | δC (ppm) | δH (ppm, J in Hz) | |
| 1 | 173.1 | 173.2 | ||
| 2 | 67.8 | 4.15 (1H, qd, 6.8, 5.1) | 67.8 | 4.16 (1H, qd, 6.8, 5.2) |
| 3 | 20.8 | 1.33 (3H, d, 6.8) | 20.8 | 1.32 (1H, d, 6.8) |
| 1′ | 128.6 | 128.0 | ||
| 2′ | 146.5 | 146.4 | ||
| 3′ | 116.4 | 7.20 (1H, dd, 7.5, 2.1) | 116.0 | 7.28 (1H, dd, 8.2, 1.1) |
| 4′ | 123.8 | 7.05 (1H, td, 7.5, 2.0) | 124.1 | 7.05 (1H, td, 7.8, 1.8) |
| 5′ | 122.6 | 7.02 (1H, td, 7.5, 1.8) | 122.2 | 6.99 (1H, td, 7.7, 1.5) |
| 6′ | 119.6 | 8.23 (1H, dd, 7.4, 2.3) | 119.6 | 8.19 (1H, dd, 8.0, 1.8) |
| Glc-1″ | 102.1 | 4.85 (1H, d, 7.5) | 101.5 | 4.86 (1H, d, 7.4) |
| 2″ | 73.3 | 3.27–3.32 (1H, overlap) | 73.3 | 3.25–3.34 (1H, overlap) |
| 3″ | 76.5 | 3.27–3.32 (1H, overlap) | 76.4 | 3.55–3.68 (1H, overlap) |
| 4″ | 69.6 | 3.18 (1H, td, 9.2, 5.4) | 69.7 | 3.18 (1H, td, 8.7, 5.3) |
| 5″ | 77.1 | 3.27–3.32 (1H, overlap) | 76.0 | 3.25–3.34 (1H, overlap) |
| 6″ | 60.7 | 3.69 (1H, ddd, 11.8, 5.4, 2.2) | 68.3 | 4.00 (1H, d, 10.3) |
| 3.49 (1H, dt, 11.8, 5.9) | 3.55–3.68 (1H, overlap) | |||
| Glc-1‴ | 103.2 | 4.22 (1H, d, 7.8) | ||
| 2‴ | 73.6 | 2.96 (1H, td, 8.3, 4.5) | ||
| 3‴ | 76.6 | 3.10 (1H, td, 8.4, 4.5) | ||
| 4‴ | 70.1 | 2.98–3.06 (1H, overlap) | ||
| 5‴ | 76.8 | 2.98–3.06 (1H, overlap) | ||
| 6‴ | 61.0 | 3.65 (ddd, 1H, 11.4, 5.8, 1.9) | ||
| 3.41 (1H, dt, 11.4, 5.8) | ||||
| NH | 9.42 (1H, s) | 9.36 (1H, s) | ||
| OH-2 | 5.99 (1H, d, 5.1) | 5.89 (1H, d, 5.2) | ||
| OH-6″ | 4.58 (1H, t, 5.9) | |||
| OH-6‴ | 4.46 (1H, t, 5.8) | |||
Figure 2.
Selected 1H-1H COSY and HMBC correlations of 1, 2, 4, and 5.
Compound 2 was obtained as a white amorphous powder. Its molecular formula was deduced to be C21H31NO13 by an HR-ESI-MS ion peak at m/z 528.1726 [M + Na]+ (Calcd. for C21H31NO13Na, 528.1688). The IR spectrum (Supplementary Materials) exhibited absorption bands due to the presence of hydroxyl, amide, and phenyl groups (3423, 3346, 1662, 1602, 1532, and 1454 cm−1). Analysis of the 1H- and 13C-NMR data (Table 1) suggested that Compound 2 possessed the same aglycone as that of 1, which was confirmed by the observed HMBC correlations as shown in Figure 2. The 1H-NMR spectra of 2 showed two anomeric proton signals at δH 4.86 (1H, d, J = 7.4 Hz, H-1″) and 4.22 (1H, d, J = 7.8 Hz, H-1‴), which correlated to the corresponding anomeric carbon signals at δC 101.5 (C-1″) and 103.2 (C-1‴) in the 13C-NMR spectra, respectively, indicating the presence of two glucopyranosyl units with β-form. The HMBC correlations from an anomeric proton at δH 4.22 (H-1‴) to δC 68.3 (C-6″) and from another anomeric proton at δH 4.86 (H-1″) to δC 146.4 (C-2′) indicated that two sugars were connected through 1→6 linkage, and the sugar chain was attached at C-2′ of the aglycone through an oxygen. Therefore, Compound 2 was determined as 2-hydroxy-N-[(2-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)phenyl]propionamide.
Compound 3 was obtained as a white amorphous powder, with a molecular formula of C9H12NO3 as indicated by an ion peak at m/z 182.0840 [M + Na]+ (Calcd. for C9H12NO3Na, 182.0812) in its HR-ESI-MS. Comparison of the 1H- and 13C-NMR data with that of Compounds 1 and 2, as well as with those reported in the literature [16], indicated that Compound 3 was the aglycone of Compounds 1 and 2 with a known structure, 2-hydroxy-N-(2-hydroxyphenyl)propionamide.
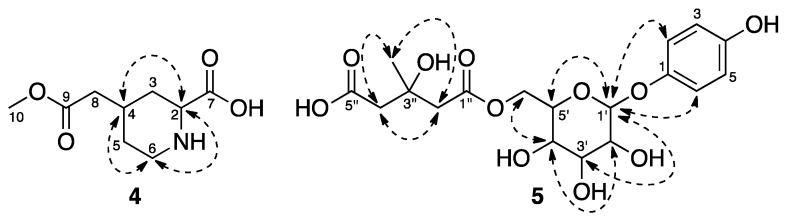
Compound 4 was isolated as a white amorphous powder. It was assigned with a molecular formula of C9H15NO4 by an HR-ESI-MS ion peak at m/z = 224.0907 [M + Na]+ (Calcd. for C9H15NO4Na, 224.0899). Its IR spectrum (Supplementary Materials) showed the presence of a secondary amide (1624 cm−1), a carbonyl (1729 cm−1), and a strong peak for a hydroxyl group (3428 cm−1). The 1H-NMR (Table 1) and HSQC spectrum of 4 revealed the presence of one methoxyl at δH 3.66 (3H, s), two methines at δH 3.47 (1H, dd, J = 12.8, 3.2 Hz) and 2.11 (1H, m), as well as four methylene protons. The 13C-NMR spectrum and DEPT showed the presence of two methine carbons at δC 60.6 and 32.9, one methoxyl carbon at δC 52.1, two carbonyl carbons at δC 173.7 and 173.8, as well as four methylene carbons. The key HMBC correlations (Figure 2) from H-2 to C-3, C-4, and C-6 and from H-4 to C-2, C-3, C-5, and C-6 revealed the presence of a 2,4-disubstituted piperidine ring, which was confirmed by 1H-1H COSY (correlated spectroscopy) correlations between H-2/H-3, H-3/H-4, H-4/H-5, H-5/H-6, and H-4/H-8. An acetic acid methyl ester group was established by the HMBC correlations from the methoxyl protons to C-8 and C-9, and it was attached to C-4 of the piperidine ring as indicated by the HMBC correlations from H-4 to C-8 and C-9. The HMBC correlation from H-2 to C-7 proved that a carbonyl group was located at C-2. The NOESY correlations (Figure 3) between H-2/H-4, H-2/H-6, and H-4/H-6 indicated that the two substituents were cis to each other. Thus, Compound 4 was determined as 2β-carboxyl-piperidine-4β-acetic acid methyl ester.
Figure 3.
Key NOESY correlations of 4 and 5.
Compound 5 was obtained as a pale-yellow solid. Its molecular formula C18H24O11 was established by HR-ESI-MS at m/z = 439.1234 [M + Na]+ (Calcd. for C18H24O11Na, 439.1211). The IR spectrum (Supplementary Materials) of 5 showed strong absorption bands at 3420 and 1718 cm−1, suggesting the presence of hydroxyl and carbonyl groups, respectively. Its 1H-NMR and HSQC spectrum (Supplementary Materials) showed signals indicating the presence of a 1,4-disubstituted benzene ring [δH 6.84 (2H, d, J = 9.0 Hz, H-2 and H-6) and 6.66 (2H, d, J = 9.0 Hz, H-3 and H-5)], a glucopyranosyl moiety [4.65 (1H, d, J = 7.7 Hz, H-1′), 4.32 (1H, dd, J = 11.8, 2.0 Hz, H-6′), 4.00 (1H, dd, J = 11.8, 7.0 Hz, H-6′), 3.50 (1H, ddd, J = 9.2, 7.0, 2.0 Hz, H-5′), 3.26 (1H, t, J = 8.8 Hz, H-3′), 3.19 (1H, dd, J = 8.8, 7.7 Hz, H-2′), and 3.14 (1H, t, J = 9.2 Hz, H-4′)], one methyl [1.22 (3H, s, H-6″)], and two methylene groups [2.57 (1H, d, J = 14.0 Hz, H-2″), 2.49 (1H, overlap, H-2″), 2.40 (1H, d, J = 15.0 Hz, H-4″), and 2.33 (1H, d, J = 15.0 Hz, H-4″)]. The 13C-NMR and DEPT of 5 displayed resonances of two carbonyls [δC 174.4 (C-5″) and 170.5 (C-1″)], one oxygenated quaternary carbon [δC 68.9 (C-3″)], two methylenes [δC 46.1 (C-4″) and 45.9 (C-2″)], and one methyl [δC 27.6 (C-6″)] carbons, besides signals for a typical glucopyranosyl moiety and a p-hydroxyphenyl group. The HMBC correlations (Figure 2) observed from H2-2″ to C-1″ and C-3″ and from H2-4″ to C-3″ and C-5″, as well as from H3-CH3 to C-2″, C-3″, and C-4″, allowed for the establishment of a 3-hydroxy-3-methyl glutaryl (HMG) group. The HMBC correlations from the anomeric proton H-1′ to C-1 (δC 150.1) and from H2-6′ to C-1″ indicated that the p-hydroxyphenyl and HMG group were connected with C-1′ and C-6′ of the glucopyranosyl moiety, respectively. The anomeric proton appearing as a doublet at δH 4.65 with a diaxial coupling constant of 7.7 Hz suggested that the glucopyranosyl moiety must be a β-anomer. Furthermore, the NOESY correlations (Figure 3) observed between H-1′ and H-5′, between H-3′ and H-5′, and between H-2′ and H-4′ indicated that H-1′, H-3′, and H-5′ are α-oriented and H-2′ and H-4′ are β-oriented. Therefore, Compound 5 was identified as 4-hydroxyphenyl-1-O-[6-(hydrogen 3-hydroxy-3-methylpentanedioate)]-β-d-glucopyranoside.
The known structures of 6–28 were identified by a comparison of their spectroscopic data with those reported in the literatures as arbutin (6) [17], β-d-glucopyranosyl-(1→6)-arbutin (7) [18], hydroquinone (8) [19], 2-(4-hydroxyphenyl)propane-1,3-diol (9) [20], 4-methoxyphenylacetic acid (10) [21], 4-hydroxybenzoic acid (11) [22], protocatechuic acid (12) [22], vanillic acid (13) [22], gallic acid (14) [23], methyl gallate (15) [24], 1-O-galloyl-β-d-glucose (16) [25], 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one (17) [26], corilagin (18) [27], astragalin (19) [28], kaempferol 3-O-rutinoside (20) [29], kaempferol 3-O-(2″-O-galloyl)-rutinoside (21) [30], quercetin (22) [29], isoquercitrin (23) [31], quercitrin (24) [32], quercetin 3-O-(2″-O-galloyl)-β-d-glucopyranoside (25) [33], quercetin 3-O-(6″-O-galloyl)-β-d-glucopyranoside (26) [34], rutin (27) [34], and quercetin 3-O-(2″-O-galloyl)-rutinoside (28) [33].
2.2. Anti-Tobacco Mosaic Virus Activity
All the isolated compounds were tested for their inhibitory activity against TMV using the leaf-disc method at a concentration of 0.5 mM; however, only weak to moderate inhibitory activity were observed (Table 2). The IC50 values of arbutin (6), β-d-glucopyranosyl-(1→6)-arbutin (7), 4-methoxyphenylacetic acid (10), and corilagin (18) were determined as 0.49, 0.51, 0.27, and 0.45 mM, while the commercial antiviral agents, ningnanmycin and ribavirin, possessed an IC50 of 0.18 and 0.26 mM, respectively, during the test under the same condition.
Table 2.
Inhibitory activity against Tobacco mosaic virus (TMV) of 1–28.
| Compounds | Inhibitory Rate (%, Mean Value ± SD) | Compounds | Inhibitory Rate (%, Mean Value ± SD) |
|---|---|---|---|
| 1 | 12.2 ± 2.2 | 15 | – |
| 2 | 10.8 ± 2.5 | 16 | – |
| 3 | 24.6 ± 3.5 | 17 | 14.0 ± 4.3 |
| 4 | 42.0 ± 4.5 | 18 | 57.5 ± 4.4 |
| 5 | – | 19 | – |
| 6 | 50.5 ± 4.3 | 20 | 18.0 ± 4.8 |
| 7 | 49.8 ± 4.9 | 21 | 38.0 ± 6.5 |
| 8 | 47.0 ± 2.6 | 22 | 31.9 ± 6.3 |
| 9 | 12.7 ± 2.4 | 23 | – |
| 10 | 72.3 ± 4.9 | 24 | 13.2 ± 6.9 |
| 11 | – | 25 | 32.3 ± 5.3 |
| 12 | 43.2 ± 5.7 | 26 | 37.4 ± 5.5 |
| 13 | 16.3 ± 4.9 | 27 | 28.4 ± 1.9 |
| 14 | 37.0 ± 5.8 | 28 | 45.0 ± 6.3 |
| Ningnanmycin | 86.9 ± 3.6 | Ribavirin | 73.9 ± 4.5 |
– No inhibitory effect observed.
3. Discussion
Continuous efforts have been made since the 1980s in the phytochemical and biological study of secondary metabolites from the Chinese medicinal plant A. altissima. Phytochemical studies have led to the characterization of quassinoids, alkaloids, lipids, coumarins, and other phenolic derivatives, of which quassinoids are the major components, with antitumor, antimalarial, antifeedant, anti-inflammatory, and other activities [2,35]. Twenty-eight compounds, including four novel structures (1, 2, 4, and 5), were obtained from the fruit extract of A. altissima in our present study. Among the known structures, 12 compounds, including 3, 6–10, 15–16, 20–21, 25, and 28, were isolated from this plant for the first time.
Previous studies have revealed the presence of alkaloids with varying structural patterns, including indole, β-carboline, as well as canthinone types [7,8,36,37,38]. However, the nitrogenous compounds 1–4 obtained in our present study represent two novel types that have never been reported from the secondary metabolites of A. altissima. Phenylpropionamides 1–3 contain an α-hydroxyamide scaffold, which is present in a variety of compounds with confirmed biological activity, such as pantothenic acid (vitamin B5), the cholesterol-lowering drug bestatin, and the antibiotics amikacin and cefamandole [39]. 2-Hydroxy-N-(2-hydroxyphenyl)propionamide (3) has been previously reported to be isolated from the solid cultures of phytopathogen Peronophythora litchii, which is a major disease of lychee that causes twig withering, panicle shattering, fruit downfall, and rot [16]. To the best of our knowledge, this is the first report of 2-hydroxy-N-(2-hydroxyphenyl)propionamide (3) from plant secondary metabolites.
Piperidine alkaloids are among the most abundant metabolites of terrestrial plants [40]. Many of them, such as piperidine alkaloids identified from several Prosopis species, have been reported to possess diverse bioactivities, such as antibacterial, antifungal, and antiparasitic activities [41,42]. Recently, epidihydropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies), was reported to show promising antibacterial and anti-Candida activity [43]. 2β-Carboxyl-piperidine-4β-acetic acid methyl ester (4) was an unusual 2,4-disubstituted piperidine derivative of plant origin. Compounds 1–4 showed no potent anti-TMV activity in our present study, however, these unusual structures deserve further effort to evaluate their potential biological and pharmacological value.
Among the other known compounds obtained, corilagin (18) is a member of the tannin family, which has been discovered in a number of medicinal plants such as the Phyllanthus species. Corilagin was reported to possess diverse pharmacological activities such as antioxidative, anti-inflammatory, thrombolytic, antihypertensive, hepatoprotective, and antiatherogenic activities, as well as anti-tumor action in hepatocellular carcinoma, ovarian cancer, etc. [44,45]. Meanwhile, corilagin can reduce the cytotoxicity induced by human enterovirus 71 (EV71) and Coxsackie-virus A16 (CA16) on Vero cells and has been shown to protect against HSV1 encephalitis through inhibiting the TLR2 signaling pathways in vivo and in vitro [45,46]. Our present study indicated that corilagin (18) showed moderate antiviral activity against the positive single strand virus TMV, the type member of genus Tobamovirus.
4. Materials and Methods
4.1. General Experimental Procedures
IR spectra were obtained with a Thermo Scientific Nicolet iS50 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA). 1H- and 13C-NMR spectra were obtained with a Bruker AVANCE III 500 spectrometer (Bruker BioSpin, Fällanden, Switzerland) using tetramethylsilane as an internal standard. HR-ESI-MS were obtained with an Agilent 6520 Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Sephadex LH-20 (25–100 μm, Pharmacia Fine Chemical Co., Ltd., Uppsala, Sweden), Lichroprep RP-18 gel (40–63 μm, Merck, Darmstade, Germany), MCI gel CHP-20P (75–150 μm, Mitsubishi Chemical Co., Tokyo, Japan), Silica gel (200–300 mesh and 300–400 mesh), and Silica gel H (Qingdao Oceanic Chemical Co., Qingdao, China) were used for column chromatography. Thin-layer chromatography (TLC) was performed on glass-backed plates coated with 0.25 mm layers of Silica gel H (Qingdao Oceanic Chemical Co., Qingdao, China). Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 5% H2SO4 in EtOH. All solvents and chemicals used were of analytical reagent grade (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and water was doubly distilled before use.
4.2. Plant Material
The fruit of Ailanthus altissima was collected in Muyang City, Jiangsu Province, China, in October 2013. The plant was identified by associate Professor Chun-Mei Huang, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China. A voucher specimen (sample MF131001) was deposited at the Key Laboratory of Bio-Pesticide and Chemistry-Biology, Ministry of Education, Fujian Agriculture and Forestry University.
4.3. Extraction, Fraction, and Isolation
The air-dried and pulverized fruit (7.5 kg) of A. altissima Swingle was extracted with MeOH (25 L, 3 day) at room temperature for three times. After being concentrated in vacuo, the extract (390.0 g) was suspended in water and successively partitioned with n-hexane, CHCl3, and n-BuOH. The n-BuOH extract (90.0 g) was fractionated by silica gel (300–400 mesh) column chromatography (CC) and eluted with mixtures of CHCl3–MeOH–H2O (95:5:0, 90:10:0; 80:20:2; 70:30:5; 60:40:10; 0:100:0, each 6 L) to obtain 14 fractions (Fractions 1–14).
Fraction 2 (2.4 g) was subjected to Sephadex LH-20 CC and eluted with CHCl3–MeOH (1:1) to give five fractions (Fractions 2a–2e). Fraction 2d (292.0 mg) was separated using RP-18 gel CC and eluted with a gradient of MeOH–H2O (15:85 to 100:0) and chromatographed over a silica gel column eluted with CHCl3–MeOH (98:2) to yield 3 (33.0 mg) and 13 (54.0 mg). Fraction 3 (3.5 g) was separated by MCI gel CC eluted with a gradient of MeOH–H2O (15:85 to 100:0) to afford 16 fractions (Fractions 3a–3p). Fraction 3c (86.0 mg) was chromatographed over silica gel column and eluted with CHCl3–MeOH (96:4) to yield 8 (65.0 mg). Fraction 3e (460.0 mg) was subjected to RP-18 gel CC and eluted with MeOH–H2O (30:70) to give 15 (292.0 mg). Fraction 3k (81.0 mg) was subjected to RP-18 gel CC eluted with MeOH–H2O (60:40) to yield 10 (8.5 mg) and 11 (67.0 mg). Faction 4 (5.4 g) was chromatographed over MCI gel column and gradiently eluted with a mixture of MeOH–H2O (15:85 to 100:0) to afford 12 (90.5 mg) and 22 (37.5 mg). Fraction 6 (9.3 g) was first chromatographed over MCI gel column eluted with a gradient MeOH–H2O (15:85 to 100:0) and then purified using silica gel CC with CHCl3–MeOH (95:5) as eluate to give 9 (14.1 mg). Fraction 7 (6.3 g) was submitted to MCI gel CC and eluted with a gradient MeOH–H2O (15:85 to 100:0), affording 10 fractions (Fractions 7a–7j). Fraction 7b (2.6 g) was chromatographed over Sephadex LH-20 column and eluted with MeOH to yield 14 (2.5 g). Fraction 7c (314 mg) was subjected to RP-18 gel CC eluted with MeOH–H2O (30:70) and then purified by silica gel CC eluted with CHCl3–MeOH (90:10), yielding 1 (142.6 mg). Fraction 8 (10.1 g) was subjected to MCI gel CC, eluted with MeOH–H2O (5:95 to 100:0) to afford 11 fractions (Fractions 8a–8k). Fraction 8f (1.2 g) was subjected to Sephadex LH-20 CC eluted with MeOH to yield 17 (160.7 mg). Fraction 8g (1.0 g) was subjected to RP-18 gel CC eluted with MeOH–H2O (45:55), yielding 19 (50.8 mg), 23 (199.1 mg), and 24 (20.7 mg). Fraction 9 (8.9 g) was separated by MCI gel CC eluted with a gradient MeOH–H2O (0:100 to 100:0), chromatographed over RP-18 gel column eluted with MeOH–H2O (5:95), and then purified over silica gel CC with CHCl3–MeOH (90:10) as eluent to give 6 (97.0 mg). Fraction 10 (10.3 g) was subjected to MCI gel CC eluted with MeOH–H2O (5:95 to 100:0) and then chromatographed over an RP-18 gel column eluted with MeOH–H2O (45:55) to give 20 (33.3 mg). Fraction 11 (11.5 g) was separated using MCI gel CC eluted with a gradient mixture of MeOH–H2O (5:95 to 100:0), and eight fractions (Fractions 11a–11h) were obtained. Fraction 11a (3.2 g) was subjected to RP-18 gel CC eluted with MeOH–H2O (20:80), which was further purified by silica gel CC eluted with CHCl3–MeOH–H2O (80:20:2), to give 4 (22.8 mg) and 16 (36.8 mg). Fraction 11b (657.0 mg) was subjected to RP-18 gel CC eluted with MeOH–H2O (20:80) and further purified by silica gel CC with CHCl3–MeOH–H2O (80:20:2) to give 2 (11.5 mg) and 5 (10.8 mg). Fraction 11c (611.0 mg) was subjected to Sephadex LH-20 CC and eluted with MeOH to give 18 (171.0 mg). Fraction 11f (1.7 g) was subjected to RP-18 gel CC eluted with MeOH–H2O (45:55), affording 25 (31.3 mg), 26 (41.6 mg), and 27 (464.5 mg). Fraction 12 (7.8 g) was submitted to MCI gel CC eluted with a gradient mixture of MeOH–H2O (0:100 to 100:0), providing 11 fractions (Fractions 12a–12k). Fraction 12i (256.0 mg) and Fraction 12j (410.0 mg) were subjected to silica gel CC eluted with CHCl3–MeOH–H2O (70:30:5) followed by RP-18 gel CC with MeOH–H2O (45:55) as eluent to afford 28 (37.0 mg) and 21 (8.4 mg), respectively. Fraction 14 (1.1 g) was gradiently eluted with a mixture of MeOH–H2O (0:100 to 100:0) over MCI gel CC and then purified using silica gel CC with a mixture of EtOAc–acetone–MeOH (70:20:10) as eluent to afford 7 (21.7 mg).
2-Hydroxy-N-[(2-O-β-d-glucopyranosyl)phenyl]propionamide (1). White amorphous powder. IR (KBr) υmax: 3478, 3388, 2907, 1674, 1603, 1534, 1456, 1256, 1118, 1074, 1023 cm−1; HR-ESI-MS m/z: 366.1185 (Calcd. for C15H21NO8Na, 366.1159); 1H-NMR (500 MHz, DMSO-d6) and 13C-NMR (125 MHz, DMSO-d6) data (see Table 1).
2-Hydroxy-N-[(2-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl) phenyl]propionamide (2). White amorphous powder. IR (KBr) υmax: 3423, 3346, 2928, 2891, 1662, 1602, 1532, 1454, 1260, 1070 cm−1; HR-ESI-MS m/z: 528.1726 (Calcd. for C21H31NO13Na, 528.1688); 1H-NMR (500 MHz, DMSO-d6) and 13C-NMR (125 MHz, DMSO-d6) data (see Table 1).
2-Hydroxy-N-(2-hydroxyphenyl)propionamide (3). White amorphous powder. HR-ESI-MS m/z: 182.0840 (Calcd. for C9H12NO3Na, 182.0812); 1H-NMR (DMSO-d6, 500 MHz) δ 10.06 (1H, s, OH-2′), 9.25 (1H, s, NH), 8.17 (1H, dd, J = 7.9, 1.3 Hz), 6.89 (2H, overlap, H-3′ and H-4′), 6.77 (1H, ddd, J = 7.9, 6.3, 2.5 Hz), 6.11 (1H, d, J = 4.9 Hz, OH-2), 4.14 (1H, qd, J = 6.8, 4.8 Hz, H-2), 1.32 (3H, d, J = 6.8 Hz). 13C-NMR (DMSO-d6, 125 MHz) δ 172.7 (C-1), 146.1 (C-2′), 126.2 (C-1′), 123.6 (C-4′), 119.1 (C-5′), 118.9 (C-6′), 114.7 (C-3′), 67.7 (C-2), 20.8 (C-3).
2β-Carboxyl-piperidine-4β-acetic acid methyl ester (4). White amorphous powder. IR (KBr) υmax: 3428, 2958, 1729, 1624, 1439, 1402, 1299, 1216, 1180, 1137, 1010 cm−1; HR-ESI-MS m/z: 224.0907 (Calcd. for C9H15NO4Na, 224.0899); 1H-NMR (methanol-d4, 500 MHz) δ 3.66 (3H, s, H-OCH3), 3.47 (1H, dd, J = 12.8, 3.2 Hz, H-2), 3.34 (1H, ddd, J = 12.8, 4.4, 2.2 Hz, H-6), 2.97 (1H, td, J = 13.2, 3.2 Hz, H-6), 2.34 (2H, d, J = 7.0 Hz, H-8), 2.34 (1H, overlap, H-3), 2.11 (1H, m, H-4), 1.90 (1H, brd, J = 14.1 Hz, H-5), 1.41 (1H, m, H-5), 1.32 (1H, dt, J = 14.1, 12.4 Hz, H-3). 13C-NMR (methanol-d4, 125 MHz) δ 173.8 (C-9), 173.7 (C-7), 60.6 (C-2), 52.1 (C-OCH3), 44.3 (C-6), 40.9 (C-8), 34.0 (C-3), 32.9 (C-4), 29.1 (C-5).
4-Hydroxyphenyl-1-O-[6-(hydrogen 3-hydroxy-3-methylpentanedioate)]-β-d-glucopyranoside (5). Pale-yellow solid. IR (KBr) υmax: 3420, 1718, 1510, 1399, 1216, 1073, 1042 cm−1; HR-ESI-MS m/z: 439.1234 (Calcd. for C18H24O11Na, 439.1211); 1H-NMR (DMSO-d6, 500 MHz) δ 6.84 (2H, d, J = 9.0 Hz, H-2 and H-6), 6.66 (2H, d, J = 9.0 Hz, H-3 and H-5), 4.65 (1H, d, J = 7.7 Hz, H-1′), 4.32 (1H, dd, J = 11.8, 2.0 Hz, H-6′), 4.00 (1H, dd, J = 11.8, 7.0 Hz, H-6′), 3.50 (1H, ddd, J = 9.2, 7.0, 2.0 Hz, H-5′), 3.26 (1H, t, J = 8.8 Hz, H-3′), 3.19 (1H, dd, J = 8.8, 7.7 Hz, H-2′), 3.14 (1H, t, J = 9.2 Hz, H-4′), 2.57 (1H, d, J = 14.0 Hz, H-2″), 2.49 (1H, overlap, H-2″), 2.40 (1H, d, J = 15.0 Hz, H-4″), 2.33 (1H, d, J = 15.0 Hz, H-4″), 1.22 (3H, s, H-6″). 13C-NMR (DMSO-d6, 125 MHz) δ 174.4 (C-5″), 170.5 (C-1″), 152.3 (C-4), 150.1 (C-1), 117.7 (C-2 and C-6), 115.5 (C-3 and C-5), 101.6 (C-1′), 76.3 (C-3′), 73.7 (C-5′), 73.2 (C-2′), 70.0 (C-4′), 68.9 (C-3″), 63.4 (C-6′), 46.1 (C-4″), 45.9 (C-2″), 27.6 (C-6″).
4.4. Acid Hydrolysis
Compounds 1, 2, and 5 (2 mg each) were hydrolyzed in 1 M HCl (dioxane–H2O, 1:1, 2 mL) at 95 °C for 2 h, respectively. After evaporated to dryness, the reaction mixtures were diluted in H2O and extracted with Et2O (3 × 2 mL). The aqueous layer was neutralized with NaHCO3 and evaporated under vacuum to furnish a neutral residue, from which d-glucose was identified by TLC comparison with standard sugars.
4.5. Anti-TMV Assay
4.5.1. Virus and Host Plant
Purified TMV (strain U1) was obtained from Institute of Plant Virology, Fujian Agriculture and Forestry University, Fuzhou, China, and concentration was determined as 15 mg/mL using an ultraviolet spectrophotometer method [virus concentration = (A260 × dilution ration)/]. The purified virus was kept at −20 °C and diluted to 15 μg/mL with 0.01 M PBS before use. Nicotiana tabacum cv. K326, which were cultivated and grown to a 5–6-leaf stage in an insect-free greenhouse, were used for anti-TMV assay.
4.5.2. The Leaf-Disc Method
Pure compounds were dissolved in DMSO and diluted with 0.01 M PBS to a certain concentration for test. The final concentration of DMSO in the test solution (≤2%) showed no adverse effect on the plants. Anti-TMV assay was carried out using the leaf-disc method as described previously in our paper [47]. Growing leaves of N. tabacum cv. K326 were mechanically inoculated with TMV (15 μg/mL in 0.01 M PBS). After 6 h, three leaf discs (1 cm diameter) were punched and floated on solutions for test. Discs of healthy and inoculated leaves floated on a solution of 0.01 M PBS with 2% DMSO were used as a mock and control, respectively. Ningnanmycin and ribavirin were used as agent control. Three replicates were carried out for each sample. After incubating for 48 h at 25 °C in a culture chamber, the leaf discs were ground in a 0.01 M carbonate coating buffer (pH 9.6, 500 μL for each leaf disc) and centrifuged. The supernatant (200 μL) was transferred to a 96-well plate and used for an indirect enzyme-linked immunosorbent assay (ELISA). Indirect ELISA was performed as described in the literature [48,49]. Virus concentration was calculated from a standard curve constructed using OD405 values of purified TMV at concentrations of 1.0, 0.5, 0.25, 0.125, and 0.0625 μg/mL. The inhibition of test solutions on TMV was calculated as follows: inhibition rate = [1 − (virus concentration of treatment)/(virus concentration of control)] × 100%.
5. Conclusions
Two phenylpropionamides, 2-hydroxy-N-[(2-O-β-d-glucopyranosyl)phenyl]propionamide (1), and 2-hydroxy-N-[(2-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)phenyl]propionamide (2), one piperidine, 2β-carboxyl-piperidine-4β-acetic acid methyl ester (4), and one phenol derivative, 4-hydroxyphenyl-1-O-[6-(hydrogen 3-hydroxy-3-methylpentanedioate)]-β-d-glucopyranoside (5), as well as one known phenylpropionamide (3), 13 phenols (6–18), and 10 flavonoids (19–28), were identified from the n-BuOH-soluble fraction from MeOH extract of Ailanthus altissima fruit. All the compounds obtained were evaluated for their antiviral activity against TMV; however, only weak to moderate activity was observed. These results provide us with general knowledge of these diverse phenolic constituents and suggest the distribution of novel nitrogenous analogues in the metabolites of this Simaroubaceae plant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 31371987 and 31501687).
Supplementary Materials
The following are available online. HRESIMS, 1H- and 13C-NMR spectra of 1–5; IR, DEPT, COSY, HSQC, HMBC, and NOESY of 1, 2, 4, 5; 1H- and 13C-NMR data of 6–28.
Author Contributions
Q.-W.T. and Q.-J.C. conceived and designed the experiments; J.-C.N. and J.-T.S. performed the experiments; Q.-W.T., J.-C.N. and Q.-J.C. analyzed the data, and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–28 are available from the authors.
References
- 1.Kowarik I. Clonal growth in Ailanthus altissima on a natural site in West Virginia. J. Veg. Sci. 1995;6:853–856. doi: 10.2307/3236399. [DOI] [Google Scholar]
- 2.Dao T.T., Tran T.L., Kim J., Nguyen P.H., Lee E.H., Park J., Jang I.S., Oh W.K. Terpenylated coumarins as SIRT1 activators isolated from Ailanthus altissima. J. Nat. Prod. 2012;75:1332–1338. doi: 10.1021/np300258u. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Wang W.J., Su C., Zhang D.M., Xu L.P., He R.R., Wang L., Zhang J., Zhang X.Q., Ye W.C. Cytotoxic quassinoids from Ailathus altissima. Bioorg. Med. Chem. Lett. 2013;23:654–657. doi: 10.1016/j.bmcl.2012.11.116. [DOI] [PubMed] [Google Scholar]
- 4.De Feo V., De Martino L., Quaranta E., Pizza C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle) J. Agric. Food Chem. 2003;51:1177–1180. doi: 10.1021/jf020686+. [DOI] [PubMed] [Google Scholar]
- 5.Tamura S., Fukamiya N., Okano M., Koyama J., Koike K., Tokuda H., Aoi W., Takayasu J., Kuchide M., Nishino H. Three new quassinoids, ailantinol E, F, and G, from Ailanthus altissima. Chem. Pharm. Bull. 2003;51:385–389. doi: 10.1248/cpb.51.385. [DOI] [PubMed] [Google Scholar]
- 6.Niimi Y., Tsuyuki T., Takahashi T., Matsushita K. Structure determination of shinjulactones M and N, new bitter principles from Ailanthus altissima Swingle. Bull. Chem. Soc. Jpn. 1986;59:1638–1640. doi: 10.1246/bcsj.59.1638. [DOI] [Google Scholar]
- 7.Kim H.M., Kim S.J., Kim H.Y., Ryu B., Kwak H., Hur J., Choi J.H., Jang D.S. Constituents of the stem barks of Ailanthus altissima and their potential to inhibit LPS-induced nitric oxide production. Bioorg. Med. Chem. Lett. 2015;25:1017–1020. doi: 10.1016/j.bmcl.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Ohmoto T., Koike K. Studies on the constituents of Ailanthus altissima Swingle. III. The alkaloidal constituents. Chem. Pharm. Bull. 1984;32:170–173. doi: 10.1248/cpb.32.170. [DOI] [Google Scholar]
- 9.Hong Z.L., Xiong J., Wu S.B., Zhu J.J., Hong J.L., Zhao Y., Xia G., Hu J.F. Tetracyclic triterpenoids and terpenylated coumarins from the bark of Ailanthus altissima (“Tree of heaven”) Phytochemistry. 2013;86:159–167. doi: 10.1016/j.phytochem.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X.J., Xu M., Li X.S., Wang Y.H., Gao Y., Cai R., Cheng Y.X. Triterpenoids and sterones from the stem bark of Ailanthus altissima. Bull. Korean Chem. Soc. 2011;32:127–130. doi: 10.5012/bkcs.2011.32.1.127. [DOI] [Google Scholar]
- 11.Tan Q.W., Ouyang M.A., Wu Z.J. A new seco-neolignan glycoside from the root bark of Ailanthus altissima. Nat. Prod. Res. 2012;26:1375–1380. doi: 10.1080/14786419.2011.587187. [DOI] [PubMed] [Google Scholar]
- 12.Tan Q.W., Wu Z.J., Ouyang M.A. Research progress in chemical constituents and bioactivities of Ailanthus ailanthus. Nat. Prod. Res. Dev. 2008;20:748–755. [Google Scholar]
- 13.Shin Y., Masami I., Takakiko T., Takahashi T., Matsushita K. Constituents of Ailanthus altissima Swingle. Isolation and structures of shinjuglycosides A, B, C, and D. Bull. Chem. Soc. Jpn. 1984;57:2496–2501. [Google Scholar]
- 14.Zhao C.C., Wang J.H., Li W., Sha Y., Li X. Studies on the chemical constituents of fructus Ailanthus altissima. Chin. J. Med. Chem. 2003;13:211–214. [Google Scholar]
- 15.Zhao C.C., Shao J.H., Li X., Xu J., Zhang P. Antimicrobial constituents from fruits of Ailanthus altissima Swingle. Arch. Pharm. Res. 2005;28:1147–1151. doi: 10.1007/BF02972977. [DOI] [PubMed] [Google Scholar]
- 16.Xie H., Liang Y., Xue J., Xu Q., Jiang Y., Wei X. Secondary metabolites of the phytopathogen Peronophythora litchii. Nat. Prod. Commun. 2010;5:245–248. [PubMed] [Google Scholar]
- 17.Pawlowska A.M., De Leo M., Braca A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006;54:10234–10238. doi: 10.1021/jf062230o. [DOI] [PubMed] [Google Scholar]
- 18.Park T.H., Choi K.W., Park C.S., Lee S.B., Kang H.Y., Shon K.J., Park J.S., Cha J. Substrate specificity and transglycosylation catalyzed by a thermostable beta-glucosidase from marine hyperthermophile Thermotoga neapolitana. Appl. Microbiol. Biotechnol. 2005;69:411–422. doi: 10.1007/s00253-005-0055-1. [DOI] [PubMed] [Google Scholar]
- 19.Joval E., Kroeger P., Towers N. Hydroquinone: The toxic compound of Agaricus hondensis. Planta Med. 1996;62:185. doi: 10.1055/s-2006-957852. [DOI] [PubMed] [Google Scholar]
- 20.Tyman J.H.P., Payne P.B. The synthesis of phenolic propane-1,2- and 1,3-diols as intermediates in immobilised chelatants for the borate anion. J. Chem. Res. 2006;2006:691–695. doi: 10.3184/030823406779173479. [DOI] [Google Scholar]
- 21.Sadeghian H., Attaran N., Jafari Z., Saberi M.R., Seyedi S.M., Eshghi H., Pordel M., Riazi M.M. Design and synthesis of 4-methoxyphenylacetic acid esters as 15-lipoxygenase inhibitors and SAR comparative studies of them. Bioorg. Med. Chem. 2009;17:2327–2335. doi: 10.1016/j.bmc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z., Liao L., Moore J., Wu T., Wang Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.) Food Chem. 2009;113:160–165. doi: 10.1016/j.foodchem.2008.07.061. [DOI] [Google Scholar]
- 23.Kamatham S., Kumar N., Gudipalli P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015;2:520–529. doi: 10.1016/j.toxrep.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X., Wu L., Ito Y., Tian W. Application of preparative high-speed counter-current chromatography for separation of methyl gallate from Acer truncatum Bunge. J. Chromatogr. A. 2005;1076:212–215. doi: 10.1016/j.chroma.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 25.Saijo R., Nonaka G.I., Nishioka I. Gallic acid esters of bergenin and norbergenin from Mallotus japonicus. Phytochemistry. 1990;29:267–270. doi: 10.1016/0031-9422(90)89047-D. [DOI] [Google Scholar]
- 26.Pfundstein B., El Desouky S.K., Hull W.E., Haubner R., Erben G., Owen R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Sudjaroen Y., Hull W.E., Erben G., Würtele G., Changbumrung S., Ulrich C.M., Owen R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry. 2012;77:226–237. doi: 10.1016/j.phytochem.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y., Xie Q.Q., Fisher D., Sutherland I.A. Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performanoe counter-current chromatography. J. Chromatogr. A. 2011;1218:6206–6211. doi: 10.1016/j.chroma.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.Y., Gao J.J., Lee W.C., Ryu K.S., Lee K.R., Kim Y.C. Antioxidative flavonoids from the leaves of Morus alba. Arch. Pharm. Res. 1999;22:81–85. doi: 10.1007/BF02976442. [DOI] [PubMed] [Google Scholar]
- 30.Liu R.N., Wang W., Ding Y., Xie W.D., Ma C., Du L.J. A new flavonol glycoside and activity of compounds from the flower of Nymphaea candida. J. Asian Nat. Prod. Res. 2007;9:333–338. doi: 10.1080/10286020600727665. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.H., Ku C.H., Baek N.I., Kim S.H., Park H.W., Kim D.K. Phytochemical constituents from Diodia teres. Arch. Pharm. Res. 2004;27:40–43. doi: 10.1007/BF02980043. [DOI] [PubMed] [Google Scholar]
- 32.Fossen T., Larsen Å., Kiremire B.T., Andersen Ø.M. Flavonoids from blue flowers of Nymphaèa caerulea. Phytochemistry. 1999;51:1133–1137. doi: 10.1016/S0031-9422(99)00049-7. [DOI] [Google Scholar]
- 33.Hwang E.I., Ahn B.T., Lee H.B., Kim Y.K., Lee K.S., Bok S.H., Kim Y.T., Kim S.U. Inhibitory activity for chitin synthase II from Saccharomyces cerevisiae by tannins and related compounds. Planta Med. 2001;67:501–504. doi: 10.1055/s-2001-16487-2. [DOI] [PubMed] [Google Scholar]
- 34.Tran T.T.T., Nguyen T.Y., Tran M.H., Weon K.Y., Woo M.H., Min B.S. Compounds from the aerial parts of aceriphyllum rossii and their cytotoxic activity. Nat. Prod. Sci. 2014;20:146–151. [Google Scholar]
- 35.Okano M., Fukamiya N., Lee K.H. In: Studies in Natural Products Chemistry, Vol. 7, Biologically Active Compounds from Simaroubaceous Plants. Atta-ur-Rahman, editor. Elsevier; Amsterdam, The Netherlands: 1990. pp. 369–404. [Google Scholar]
- 36.Ohmoto T., Koike K., Sakamoto Y. Studies on the constituents of Ailanthus altissima Swingle. II. Alkaloidal constituents. Chem. Pharm. Bull. 1981;20:390–395. doi: 10.1248/cpb.29.390. [DOI] [Google Scholar]
- 37.Crespi-Perellino N., Guicciardi A., Malyszko G. Occurrence of indole alkaloids in Ailanthus altissima cell cultures. J. Nat. Prod. 1986;49:1010–1014. doi: 10.1021/np50048a007. [DOI] [Google Scholar]
- 38.Souleles C., Kokkalou E. A new β-carboline alkaloid from Ailanthus altissima. Planta Med. 1989;55:286–287. doi: 10.1055/s-2006-962006. [DOI] [PubMed] [Google Scholar]
- 39.Westerbeek A., Szymanski W., Feringa B.L., Janssen D.B. Dynamic kinetic resolution process employing haloalkane dehalogenase. ACS Catal. 2011;1:1654–1660. doi: 10.1021/cs2003565. [DOI] [Google Scholar]
- 40.Freyer A.J., Patil A.D., Killmer L., Troupe N., Mentzer M., Carte B., Faucette L., Johnson R.K. Threee new pseudodistomins, piperidine alkaloids from the ascidian Pseudodistoma megalarva. J. Nat. Prod. 1997;60:986–990. doi: 10.1021/np9701438. [DOI] [PubMed] [Google Scholar]
- 41.Reina L., Bennadji Z., Vinciguerra V., Ferreira F., Moyna G., Menendez P. Isolation and structural characterization of new piperidine alkaloids from Prosopis affinis. Phytochem. Lett. 2015;14:265–269. doi: 10.1016/j.phytol.2015.10.022. [DOI] [Google Scholar]
- 42.Rahman A.A., Samoylenko V., Jacob M.R., Sahu R., Jain S.K., Khan S.I., Tekwani B.L., Muhammad I. Antiparasitic and antimicrobial indolizidines from the leaves of Proscopsis glandulosa var. glandulosa. Planta Med. 2011;77:1639–1643. doi: 10.1055/s-0030-1270906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fyhrquist P., Virjamo V., Hiltunen E., Julkunen-Tiitto R. Epidihydropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies) shows promising antibacterial and anti-Candida activity. Fitoterapia. 2017;117:138–146. doi: 10.1016/j.fitote.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Du P., Ma Q., Zhu Z.D., Li G., Wang Y., Li Q.Q., Chen Y.F., Shang Z.Z., Zhang J., Zhao L. Mechanism of Corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model. Eur. J. Pharmacol. 2016;793:119–126. doi: 10.1016/j.ejphar.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y.J., Luo T., Wu F., Liu H., Li H.R., Mei Y.W., Zhang S.L., Tao J.Y., Dong J.H., Fang Y., et al. Corilagin protects against HSV1 encephalitis through inhibiting the TLR2 signaling pathways in vivo and in vitro. Mol. Neurobiol. 2015;52:1547–1560. doi: 10.1007/s12035-014-8947-7. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y.J., Zhao L., Li X.F., Mei Y.W., Zhang S.L., Tao J.Y., Zhou Y., Dong J.H. Effect of corilagin on anti-inflammation in HSV-1 encephalitis and HSV-1 infected microglias. Eur. J. Pharmacol. 2010;635:79–86. doi: 10.1016/j.ejphar.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 47.Tan Q.W., Ni J.C., Fang P.H., Chen Q.J. A new erythrinan alkaloid glycoside from the seeds of Erythrina crista-galli. Molecules. 2017;22:1558. doi: 10.3390/molecules22091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen J.G., Zhang Z.K., Wu Z.J., Ouyang M.A., Xie L.H., Lin Q.Y. Antiphytoviral activity of bruceine-D from Brucea javanica seeds. Pest Manag. Sci. 2008;64:191–196. doi: 10.1002/ps.1465. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z.J., Ouyang M.A., Wang C.Z., Zhang Z.K., Shen J.G. Anti-Tobacco mosaic virus (TMV) triterpenoid saponins from the leaves of Ilex oblonga. J. Agric. Food Chem. 2007;55:1712–1717. doi: 10.1021/jf062421r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.