Abstract
Cyclodipeptides, called 2,5-diketopiperazines (2,5-DKPs), are obtained by the condensation of two amino acids. Fungi have been considered to be a rich source of novel and bioactive cyclodipeptides. This review highlights the occurrence, structures and biological activities of the fungal cyclodipeptides with the literature covered up to July 2017. A total of 635 fungal cyclodipeptides belonging to the groups of tryptophan-proline, tryptophan-tryptophan, tryptophan–Xaa, proline–Xaa, non-tryptophan–non-proline, and thio-analogs have been discussed and reviewed. They were mainly isolated from the genera of Aspergillus and Penicillium. More and more cyclodipeptides have been isolated from marine-derived and plant endophytic fungi. Some of them were screened to have cytotoxic, phytotoxic, antimicrobial, insecticidal, vasodilator, radical scavenging, antioxidant, brine shrimp lethal, antiviral, nematicidal, antituberculosis, and enzyme-inhibitory activities to show their potential applications in agriculture, medicinal, and food industry.
Keywords: cyclic dipeptides; 2,5-diketopiperazines; epipolythiodioxopiperazines; fungi; biological activities; occurrence; applications
1. Introduction
Cyclodipeptides (or cyclic dipeptides) are usually called 2,5-diketopiperazines (2,5-DKPs) or dioxopiperazines, and result from the condensation of two amino acids such as tryptophan, proline, alanine, histidine, leucine, isoleucine, phenylalanine, serine, and tyrosine [1]. They are the smallest cyclopeptides, and are distributed in many organisms including fungi, bacteria, plants and animals [1,2,3]. Since the first report in 1924, a large number of bioactive cyclodipeptides has been discovered to show cytotoxic, antitumor, antiviral, antifungal, antibacterial, antiprion, antioxidant, antihyperglycemic as well as biofilm and glycosidase inhibitory activities [2,3,4]. Some cyclodipeptides (e.g., tryprostatin A (103), tryprostatin B (104), FR106969 (590), and phenylahistin (392)) showed their potential applications [1].
Among the organisms, fungi have been considered as the most important sources of novel and bioactive cyclodipeptides. More and more cyclodipeptides with interesting biological activities have been isolated and characterized from fungi. However, no detailed and comprehensive summary of the fungal cyclodipeptides on their occurrence, structures and biological activities has been reported though chemistry and biology of the cyclodipeptides from either all organisms or a certain class of cyclodipeptides have been documented [3,5,6]. In this review, we aim to describe the diversity of chemical structures and biological activities of the fungal cyclodipeptides and their analogs. A total of 635 fungal cyclodipeptides have been discussed and reviewed with literature covered up to July 2017. According to their biosynthetic origins and structural characters, these cyclodipeptides are classified as tryptophan–proline, tryptophan–tryptophan, tryptophan–Xaa (Xaa is indicated as an unspecified amino acid), proline–Xaa, non-tryptophan–non-proline, and thio analogs. Some special cyclodipeptides (e.g., gunnilactams A–C (378–380)), which did not belong to 2,5-DKPs, were also included in the group of non-tryptophan-non-proline analogs.
2. Tryptophan–Proline Cyclodipeptides
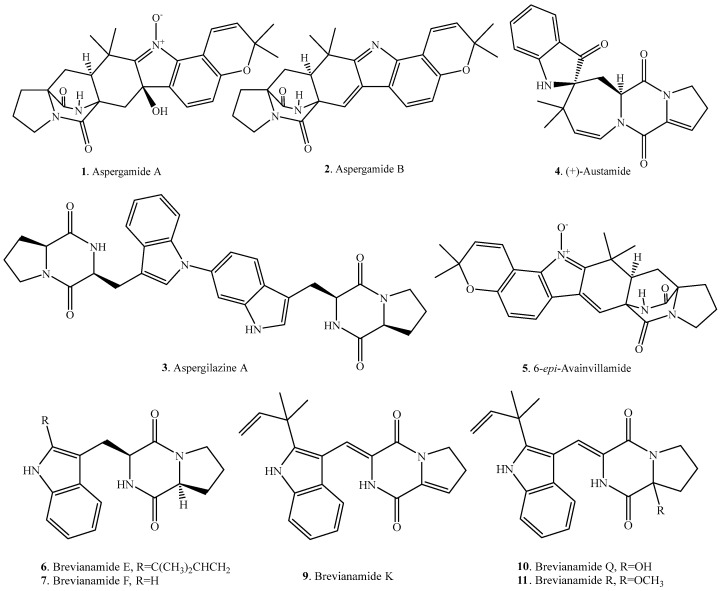
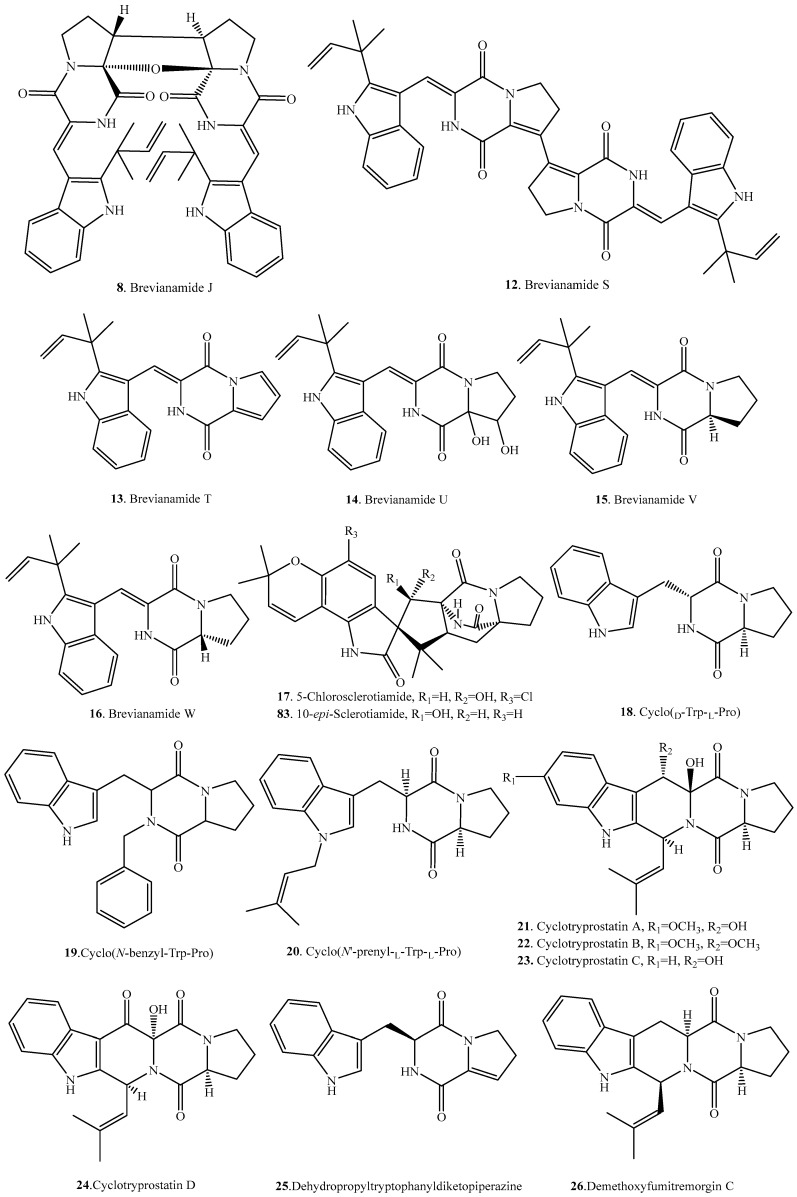
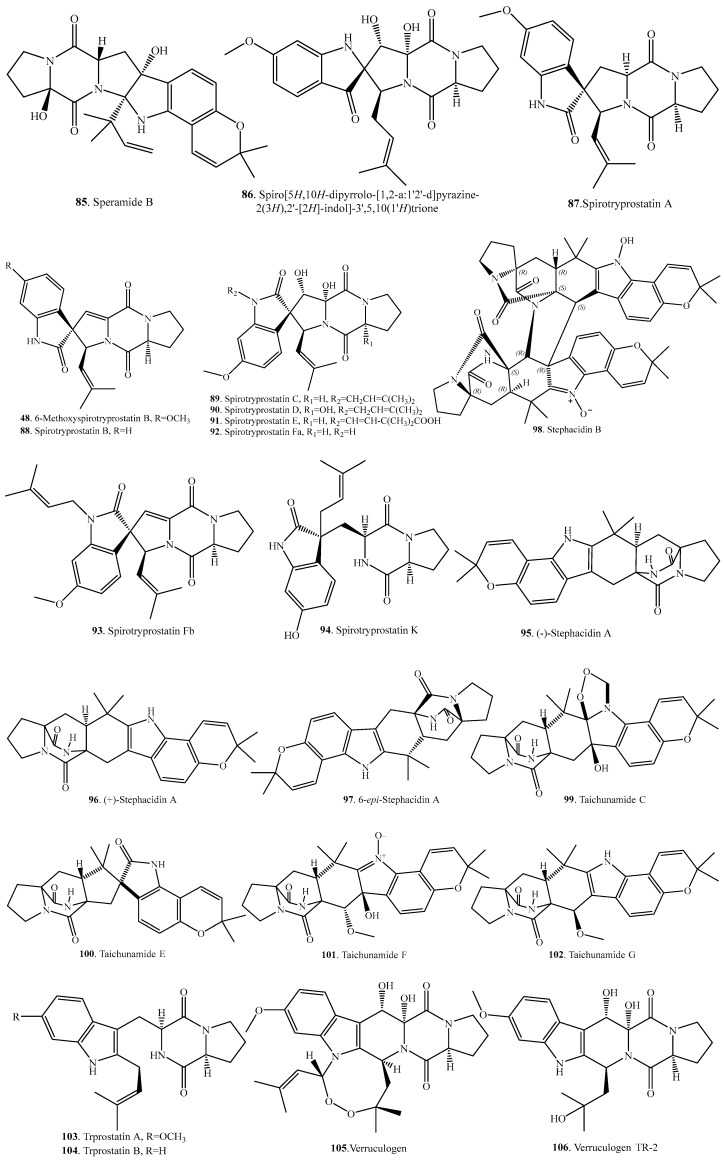
Tryptophan and proline are simultaneously incorporated into the cyclodipeptides in fungi. The proline residue adopts a cis-conformation about the Xaa–Pro tertiary amide bond and hence makes the Xaa–Pro sequence prone to cyclodipeptide formation. The tryptophan-proline cyclo(l-Trp–l-Pro) core was derived from condensation of tryptophan and proline residues, and this was often further modified by heterocyclization and isoprenyl addition [6]. The occurrence and biological activities of the tryptophan-proline cyclodipeptides from fungi are listed in Table 1, and their structures are provided in Figure 1.
Table 1.
Fungal tryptophan-proline cyclodipeptide analogs and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Aspergamide A (1) | Aspergillus ochraceus | - | [5] |
| Aspergamide B (2) | Aspergillus ochraceus | - | [5] |
| Aspergilazine A (3) | Marine-derived Aspergillus taichungensis ZHN-7-07 | Weak activity against influenza A (H1N1) virus | [18] |
| (+)-Austamide (4) | Aspergillus ustus | Acute toxicosis in day-old ducklings | [7] |
| 6-epi-Avrainvillamide (5) | Aspergillus taichungensis | - | [19] |
| Brevianamide E (6) | Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] |
| Brevianamide F = Cyclo(l-Trp–l-Pro) (7) | Endophytic Aspergillus fumigatus | - | [21] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant growth inhibitory activity | [11] | |
| Marine-derived Aspergillus taichungensis ZHN-7-07 | - | [18] | |
| Marine-derived Penicillium vinaceum | Antimicrobial activity | [22] | |
| Marine-derived Pseudallescheria sp. isolated from the surface of the drift wood | Antibacterial activity against Staphylococcus aureus | [23] | |
| Brevianamide J (8) | Aspergillus versicolor | - | [24] |
| Brevianamide K (9) | Aspergillus versicolor | - | [24] |
| Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] | |
| Aspergillus versicolor from the marine brown alga Sargassum thunbergii | - | [26] | |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide Q (10) | Aspergillus versicolor | - | [27] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide R (11) | Aspergillus versicolor | - | [27] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide S (12) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | Selective antibacterial activity | [25] |
| Brevianamide T (13) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Brevianamide U (14) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Brevianamide V (15) | Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] |
| Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] | |
| Brevianamide W (16) | Deep sea derived Aspergillus versicolor CXCTD-06-6a | Moderate radical scavenging activity against DPPH | [20] |
| 5-Chlorosclerotiamide (17) | Deep sea derived Aspergillus westerdijkiae | - | [28] |
| Cyclo(d-Trp–l-Pro) (18) | Marine-derived Penicillium vinaceum | Antimicrobial activity | [22] |
| Cyclo(N-benzyl-Trp–Pro) (19) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Cyclo(N’-prenyl-l-Trp–l-Pro) (20) | Endophytic Aspergillus fumigatus | - | [21] |
| Cyclotryprostatin A (21) | Endophytic Aspergillus fumigatus from Melia azedarach | - | [11] |
| Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin B (22) | Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] |
| Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin C (23) | Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Cyclotryprostatin D (24) | Aspergillus fumigatus | Inhibitory activity at G2/M-phase of the mammalian cell cycle | [30] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| 12,13-Dehydroprolyltryptopha-nyldiketopiperazine (25) | Penicillium piscarium | - | [31] |
| Demethoxyfumitremorgin C (26) | Aspergillus fumigatus | Cytotoxic activity | [9] |
| Deoxybrevianamide E (27) | Aspergillus sp. | - | [32] |
| Marine-derived Aspergillus versicolor from the sediment collected from the Bohai Sea of China | - | [25] | |
| (+)-Deoxyisoaustamide (28) | Aspergillus ustus | - | [7] |
| Dihydrocarneamide A (29) | Marine-derived Paecilomyces variotii | Weak cytotoxic activity | [33] |
| 8,9-Dihydroxyfumitremorgin C = 12,13-Dihydroxyfumitremorgin C (30) | Endophytic Aspergillus fumigatus | - | [21] |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Marine-derived Aspergillus sp. | Cytotoxic activity | [34] | |
| Deep-sea derived Aspergillus sp. SCSIO Ind09F01 | Anti-tuberculosis and cytotoxic activity | [35] | |
| Marine-derived Pseudallescheria sp. isolated from the surface of the drift wood | Antibacterial activity against Staphylococcus aureus | [23] | |
| rel-(8S)-19,20-Dihydro-9,20-dihydroxy-8-methoxy-9,18-di-epi-fumitremorgin C (31) | Endophytic Aspergillus fumigatus | - | [21] |
| rel-(8S,19S)-19,20-Dihydro-,19,20-trihydroxy-8-methoxy-9-epi-fumitremorgin C (32) | Endophytic Aspergillus fumigatus | - | [21] |
| (3S,8S,9S,18S)-8,9-Dihydroxyspirotryprostatin A (33) | Endophytic Aspergillus fumigatus | - | [21] |
| 9ξ-O-2(2,3-Dimethylbut-3-enyl) brevianamide Q (34) | Aspergillus versicolor from the marine brown alga Sargassum thunbergii | - | [26] |
| (-)-Enamide (35) | Marine-derived Aspergillus versicolor | - | [36] |
| Fumitremorgin A (36) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| Fumitremorgin B (37) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] | |
| Aspergillus fumigatus | - | [8] | |
| Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungal activity | [38] | |
| Derivative of fumitremorgin B (24R) (38) | Aspergillus fumigatus | Cytotoxic activity | [8] |
| Derivative of fumitremorgin B (24S) (39) | Aspergillus fumigatus | Cytotoxic activity | [8] |
| Fumitremorgin C (40) | Endophytic Aspergillus fumigatus | - | [21] |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Marine-derived Aspergillus sp. | Cytotoxic activity | [34] | |
| Marine-derived Pseudallescheria sp. from the surface of driftwood | Antibacterial activity against Staphylococcus aureus | [23] | |
| Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungi activity | [38] | |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] | |
| rel-(8R)-9-Hydroxy-8-methoxy-18-epi-fumitremorgin C (41) | Endophytic Aspergillus fumigatus | - | [21] |
| 12β-Hydroxy-13α-methoxyverruculogen TR-2 (42) | Endophytic Aspergillus fumigatus from Melia azedarach | Plant shoot elongation inhibitory activity | [11] |
| N-Hydroxy-6-epi-stephacidin (43) | Aspergillus taichungensis | - | [19] |
| 21-Hydroxystephacidin A (44) | Marine-derived Aspergillus ostianus | - | [39] |
| 12β-Hydroxyverruculogen TR-2 (45) | Endophytic Aspergillus fumigatus from Melia azedarach | - | [11] |
| 24-Hydroxyverruculogen (46) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| 26-Hydroxyverruculogen (47) | Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] |
| 6-Methoxyspirotryprostatin B (48) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxicity against HL-60 cells and A-549 cells | [10] |
| Endophytic Aspergillus fumigatus from Melia azedarach | Inhibition on elongation of lettuce shoots | [11] | |
| Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] | |
| Notoamide A (49) | Marine-derived Aspergillus sp. | Moderate cytotoxicity on Hela and L1210 cells | [32] |
| Notoamide B (50) | Marine-derived Aspergillus sp. | Moderate cytotoxicity on Hela and L1210 cells | [32] |
| (-)-Notoamide B (51) | Aspergillus protuberus MF297-2 | - | [32] |
| (+)-Notoamide B (52) | Aspergillus versicolor NRRL 35600 | - | [41] |
| iso-Notoamide B (53) | Marine-derived Paecilomyces variotii | Weak cytotoxic activity | [33] |
| Notoamide C (54) | Marine-derived Aspergillus sp. | - | [32] |
| 3-epi-Notoamide C (55) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide D (56) | Marine-derived Aspergillus sp. | - | [32] |
| Notoamide E (57) | Aspergillus versicolor NRRL 35600 | - | [43] |
| Notoamide E2 (58) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide E3 (59) | Marine-derived Aspergillus sp. | - | [42] |
| Notoamide F (60) | Marine-derived Aspergillus sp. | - | [44] |
| Marine-derived Aspergillus ostianus | - | [39] | |
| Notoamide G (61) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide H (62) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide I (63) | Marine-derived Aspergillus sp. | Weak cytotoxicity on HeLa cells | [44] |
| Notoamide J (64) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide K (65) | Marine-derived Aspergillus sp. | - | [44] |
| Notoamide L (66) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide M (67) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide N (68) | Marine-derived Aspergillus sp. | - | [45] |
| Notoamide O (69) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide P (70) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide Q (71) | Marine-derived Aspergillus sp. | - | [46] |
| Notoamide R (72) | Aspergillus ostianus | - | [39] |
| Marine-derived Aspergillus sp. | - | [46] | |
| Notoamide S (73) | Aspergillus amoenus | - | [47] |
| Notoamide T (74) | Marine-derived Aspergillus sp. | - | [48] |
| 6-epi-Notoamide T (75) | Marine-derived Aspergillus sp. | - | [48] |
| 13-Oxofumitremorgin B (76) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| 18-Oxotryprostatin A (77) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxicity against A-549 cells | [10] |
| Endophytic Aspergillus fumigatus | - | [21] | |
| Endophytic Aspergillus fumigatus from Melia azedarach | Plant growth inhibitory activity | [11] | |
| 13-Oxoverruculogen (78) | Aspergillus fumigatus | Moderate cytotoxic activity on four cancer cell lines | [8] |
| Piscarinine A (79) | Penicillium piscarium VKM F-691 | Cytotoxic and antimicrobial activities | [49] |
| Piscarinine B (80) | Penicillium piscarium VKM F-691 | Cytotoxic and antimicrobial activities | [49] |
| 13-O-Prenyl-26-hydroxyverruculogen (81) | Marine sediment-derived Penicillium brefeldianum SD-273 | Lethal activity against brine shrimp | [37] |
| Sclerotiamide (82) | Aspergillus sclerotiorum | Antiinsectan activity against the earworm Helicoverpa zea | [50] |
| 10-epi-Sclerotiamide (83) | Deep-sea-derived Aspergillus westerdijkiae | - | [28] |
| Speramide A (84) | Freshwater-derived Aspergillus ochraceus KM007 | Moderate activity against Pseudomonas aeruginosa | [51] |
| Speramide B (85) | Freshwater-derived Aspergillus ochraceus KM007 | - | [51] |
| Spiro[5H,10H-dipyrrolo-[1,2-a:1’2’-d]pyrazine-2(3H),2’-[2H]-indol]-3’,5,10(1’H) trione (86) | Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] |
| Spirotryprostatin A (87) | Aspergillus fumigatus | Inhibitory activity on mammalian cell cycle at G2/M phase | [12] |
| Endophytic Aspergillus fumigatus from Melia azedarach | The elongation of lettuce shoots inhibitory activity | [11] | |
| Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] | |
| Spirotryprostatin B (88) | Aspergillus fumigatus | Inhibitory activity on mammalian cell cycle at G2/M phase | [12] |
| Spirotryprostatin C (89) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin D (90) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin E (91) | Holothurian-derived Aspergillus fumigatus from Stichopus japonicus | Cytotoxic activity | [8] |
| Spirotryprostatin Fa (92) | Marine-derived Aspergillus fumigatus from soft coral Sinularia sp. | Stimulating action on the growth of sprout roots of soy, buckwheat and corn | [52] |
| Spirotryprostatin Fb (93) | Plant endophytic Penicillium brefeldianum from the rhizome of Pinellia ternata | Cytotoxic activity against HepG2 and MDA-MB-231 cells | [13] |
| Spirotryprostatin K (94) | Endophytic Aspergillus fumigatus from the stem of Erythrophloeum fordii | - | [40] |
| (-)-Stephacidin A (95) | Aspergillus amoenus (formerly A. versicolor) NRRL 35600 | - | [41] |
| (+)-Stephacidin A (96) | Aspergillus protuberus MF297-2 | - | [32] |
| 6-epi-Stephacidin A (97) | Aspergillus taichungensis | - | [19] |
| Stephacidin B (98) | Aspergillus ochraceus | Cytotoxic activity | [14] |
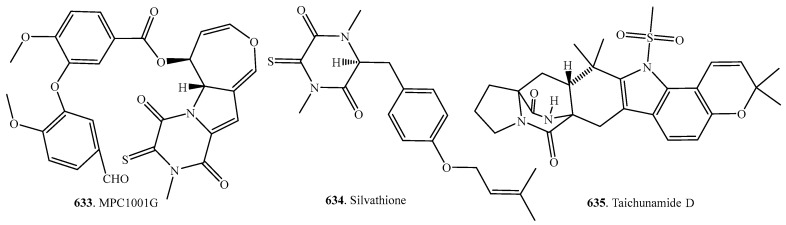
| Taichunamide C (99) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide E (100) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide F (101) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide G (102) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Tryprostatin A (103) | Endophytic Aspergillus fumigatus from Melia azedarach | Inhibitory activities on elongation of lettuce shoots, and on multidrug-resistance protein | [11,16] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Tryprostatin B (104) | Endophytic Aspergillus fumigatus | - | [21] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| - | Inhibitory activity on mammalian cell-cycle | [17] | |
| Verruculogen (105) | Endophytic Aspergillus fumigatus from Melia azedarach | The elongation of lettuce shoots inhibitory activity | [11] |
| Endophytic Aspergillus tamari from Ficus carica | - | [29] | |
| Marine sediment-derived fungus Penicillium brefeldianum SD-273 | - | [37] | |
| Verruculogen TR-2 = TR-2 (106) | Endophytic Aspergillus fumigatus from Melia azedarach | Inhibitory activity on elongation of lettuce shoots | [11] |
| Marine sediment-derived Penicillium brefeldianum SD-273 | - | [37] | |
| Versicamide A (107) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide B (108) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide C (109) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide D (110) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide E (111) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide F (112) | Marine-derived Aspergillus versicolor | - | [36] |
| Versicamide G (113) | Marine-derived Aspergillus versicolor | - | [36] |
| (−)-Versicolamide B (114) | Aspergillus sp. | - | [45] |
| (+)-Versicolamide B (115) | Aspergillus versicolor NRRL 35600 | - | [41] |
| (−)-Versicolamide C (116) | Aspergillus taichungensis | - | [19] |
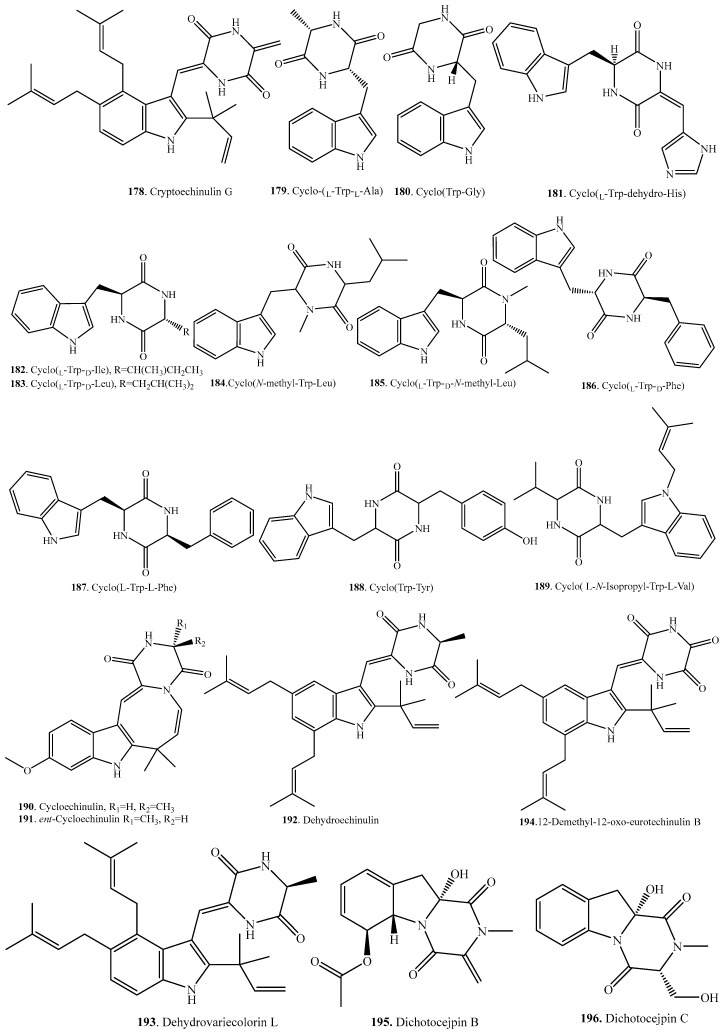
Figure 1.
Structures of the tryptophan-proline cyclodipeptide analogs isolated from fungi.
About 116 tryptophan-proline cyclodipeptides have been isolated from fungi so far. They are mainly distributed in the genera Aspergillus and Penicillium, and are also distributed in other genera such as Alternaria, Paecilomyces, and Pseudallescheria.
(+)-Austamide (4) and (+)-deoxyisoaustamide (28) were isolated from the maize meal cultures of the toxigenic fungus Aspergillus ustus, and (+)-austamide (4) caused acute toxicosis in day-old ducklings [7].
Fumitremorgin C (40) and its derivatives (38, 39) were identified in Aspergillus fumigatus from the holothurian Stichopus alternata. They displayed significant cytotoxic activity against MOLT-4 (human acute lymphoblastic leukemia cells), A-549 (human lung adenocarcinoma epithelial cells), and HL-60 (human promyelocytic leukemia cells), which speculated that this cytotoxic activity may be linked to hydroxyl groups in the side chains of the molecules [8]. Demethoxyfumitremorgin C (26) from marine-derived Aspergillus fumigatus showed inhibitory activity in the mouse cell cycle against tsFT210, and also inhibited tumor cell cycle arrest at G2/M with a minimum inhibitory concentration (MIC) value of 0.45 μM [9].
18-Oxotryprostatin A (77) was isolated from the marine-derived fungus Aspergillus sydowi and found to exhibit weak cytotoxic activity against A-549 cells with a median inhibitory concentration (IC50) value of 1.28 μM [10]. This compound was also obtained from the endophytic fungus Aspergillus fumigatus from Melia azedarach to display plant growth inhibitory activity [11].
Spirotryprostatins (87–94) were isolated from Aspergillus fumigatus. These compounds showed cytotoxic activity by inhibiting mammalian cell cycle at G2/M phase [8,12,13].
Stephacidin B (98) was isolated from Aspergillus ochraceus. This compound exhibited potent cytotoxic activity against LNCaP (a testosterone-dependent prostate cancer cell line), with IC50 values from 91 to 621 nM [14].
Both tryprostatins A (103) and B (104), which are prenylated, were isolated from the fermentation broth of the marine-derived fungus Aspergillus fumigatus [15]. Tryprostatin A (103) was an inhibitor of the multidrug-resistance breast cancer protein (BCRP) that mediated resistance to chemotherapeutics in breast cancer treatment [16], whereas tryprostatin B (104) was a mammalian cell-cycle inhibitor, attractive as a potential anticancer agent [17]. Furthermore, tryprostatin A (103) exhibited inhibitory activity on the elongation of lettuce shoots [11].
3. Tryptophan–Tryptophan Cyclodipeptides
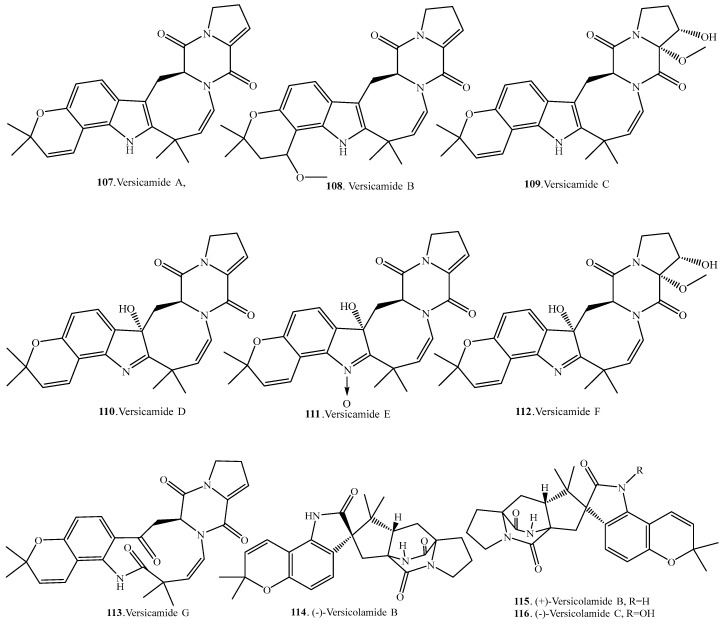
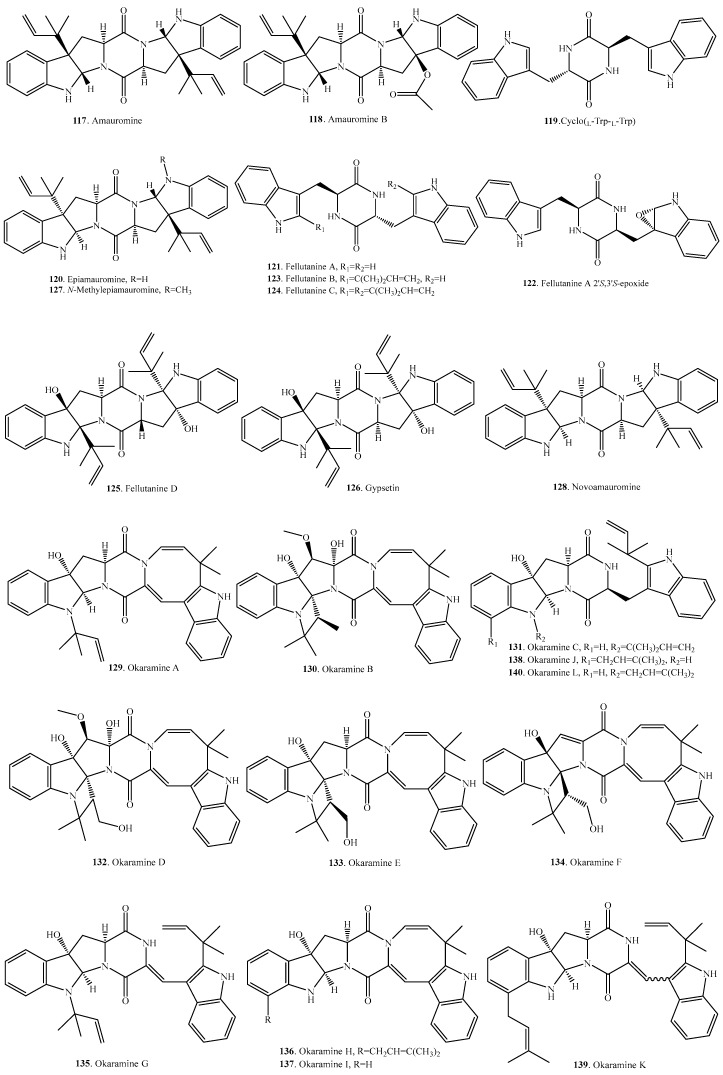
The ditryptophan cyclodipeptides, which have two tryptophan units, are widely distributed in filamentous fungi, especially in the genera Penicillium and Aspergillus. Their occurrence and biological activities are listed in Table 2, and the structures are provided in Figure 2.
Table 2.
Fungal tryptophan-tryptophan cyclodipeptide analogs and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amauromine = Nigriforine (117) | Amauroascus sp. | Hypotensive vasodilating activity | [54] |
| Penicillium nigricans | - | [55] | |
| Auxarthron reticulatum | Selective cannabinoid CB1 receptor antagonist | [56] | |
| Amauromine B (118) | Aspergillus terreus 3.05358 | Inhibitory activity on α-glucosidase | [68] |
| Cyclo(l-Trp–l-Trp) (119) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | - | [69] |
| Epiamauromine (120) | Aspergillus ochraceus | Moderate reduction in weight gain activity against the corn earworm | [57] |
| Fellutanine A (121) | Penicillium fellutanum | - | [58] |
| Marine sponge-derived Neosartorya glabra KUFA 0702 | - | [70] | |
| Fellutanine A 2’S,3’S-epoxide (122) | Marine sponge-derived Neosartorya glabra KUFA 0702 | - | [70] |
| Fellutanine B (123) | Penicillium fellutanum | - | [58] |
| Fellutanine C (124) | Penicillium fellutanum | - | [58] |
| Fellutanine D (125) | Penicillium fellutanum | Cytotoxic activity | [58] |
| Gypsetin (126) | Nannizzia gypsea var. incurvata | Inhibitory activity on acyl-CoA:cholesterol acyltransferase | [71] |
| N-Methylepiamauromine (127) | Aspergillus ochraceus | Moderate reduction in weight gain activity against the corn earworm | [57] |
| Novoamauromine (128) | Aspergillus novofumigatus | Inhibitory activity on the cell proliferation of A549, Hela, and LNCap cells | [59] |
| Okaramine A (129) | Penicillium simplicissimum AK-40 | Insecticidal activity | [62] |
| Okaramine B (130) | Penicillium simplicissimum AK-40 | Insecticidal activity | [62] |
| Okaramine C (131) | Penicillium simplicissimum | - | [65] |
| Penicillium simplicissimum | Oral insecticide activity against silkworms | [72] | |
| Okaramine D (132) | Penicillium simplicissimum | Insecticidal activity | [63] |
| Okaramine E (133) | Penicillium simplicissimum | - | [63] |
| Okaramine F (134) | Penicillium simplicissimum | - | [63] |
| Okaramine G (135) | Penicillium simplicissimum | Insecticidal activity | [73] |
| Okaramine H (136) | Aspergillus aculeatus | - | [60] |
| Okaramine I (137) | Aspergillus aculeatus | - | [60] |
| Okaramine J (138) | Penicillium simplicissimum | - | [64] |
| Okaramine K (139) | Penicillium simplicissimum | - | [64] |
| Okaramine L (140) | Penicillium simplicissimum | - | [64] |
| Okaramine M (141) | Penicillium simplicissimum | - | [64] |
| Okaramine N (142) | Penicillium simplicissimum | - | [65] |
| Okaramine O (143) | Penicillium simplicissimum | - | [65] |
| Okaramine P (144) | Penicillium simplicissimum | - | [65] |
| Okaramine Q (145) | Penicillium simplicissimum | - | [65] |
| Okaramine R (146) | Penicillium simplicissimum | - | [65] |
| Okaramine S (147) | Aspergillus taichungensis ZHN-7-07 | Cytotoxic activity against HL-60 cells with IC50 value of 0.78 μM | [61] |
| Okaramine T (148) | Aspergillus taichungensis ZHN-7-07 | - | [61] |
| Okaramine U (149) | Aspergillus taichungensis ZHN-7-07 | - | [61] |
Note: IC50, median inhibitory concentration.
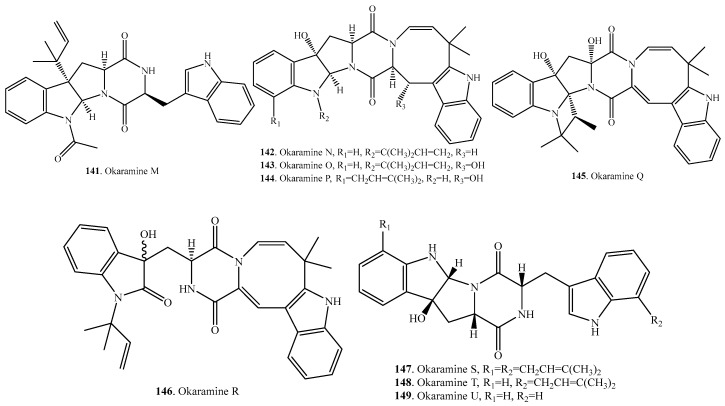
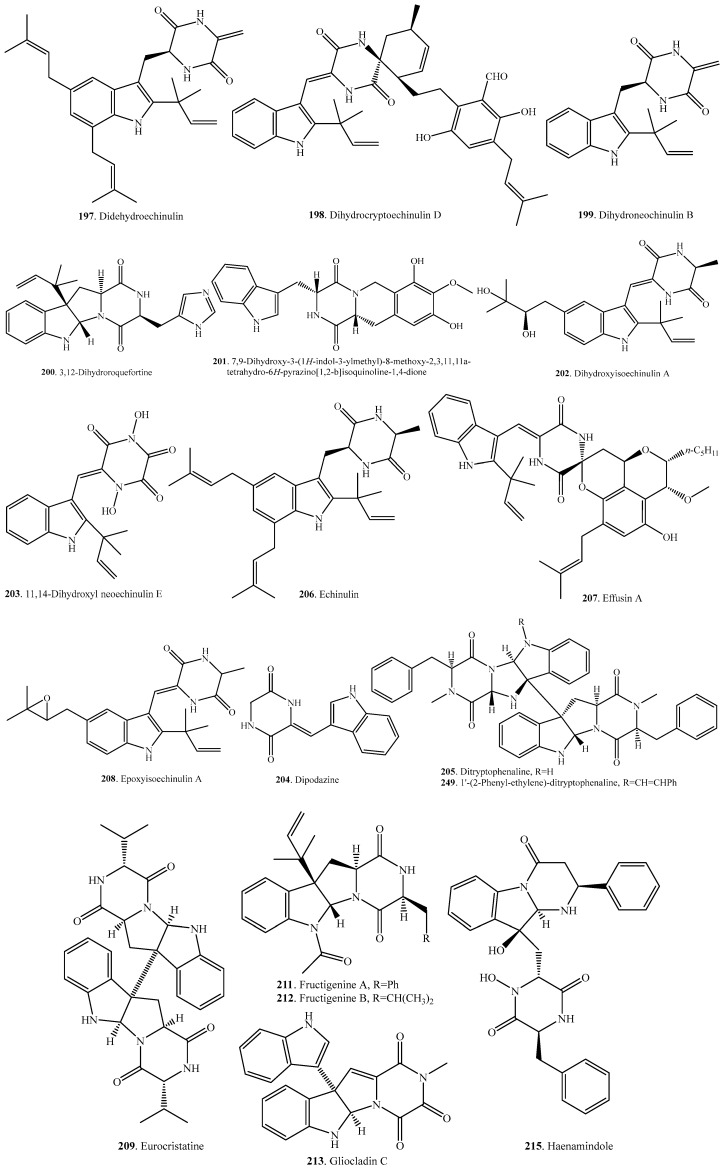
Figure 2.
Structures of the tryptophan-tryptophan cyclodipeptide analogs isolated from fungi.
Amauromine (117) from Amauroascus sp. [54] was identical with nigrifortine (117) from Penicillium nigricans. It is a diannulated DKP analog which shows hypotensive vasodilating activity [55]. This compound was later isolated from Auxarthron reticulatum, and identified as a selective cannabinoid CB1 receptor antagonist [56].
Epiamauromine (120) and N-methylepiamauromine (127) were isolated from the sclerotia of Aspergillus ochraceus. They caused moderate reduction in weight gain against the corn earworm Helicoverpa zea [57].
Fellutanines A–D (121, 123–125), the analogs of cyclo(l-Trp–d-Trp), were isolated from the cultures of Penicillium fellutanum. Among them, only fellutanine D (125) was diannulated and displayed cytotoxic activity against K-562 (human myeloid leukemia cells), L-929 (mouse fibroblastic cell), and HeLa (human epitheloid cervix carcinoma cells) with IC50 values of 9.5, 11.6 and 19.7 μg/mL, respectively [58].
Novoamauromine (128) was obtained from Aspergillus novofumitatus CBS117520. This compound had inhibitory activity on the cell proliferation of A549, HeLa, LNCap (human prostate carcinoma cells) [59].
Okaramines A–U (129–149) have been isolated from Aspergillus aculeatus [60], Aspergillus taichungensis [61], and Penicillium simplicissimum [62,63,64,65]. Structure–activity studies indicated the importance of the azetidine and azocine rings to okaramine insecticidal activity [66]. The action of okaramine B (130) on silkworm larval neurons using patch-clamp electrophysiology revealed that this compound activated the l-glutamate-gated chloride channel (GluCl) [67].
4. Tryptophan–Xaa Cyclodipeptides
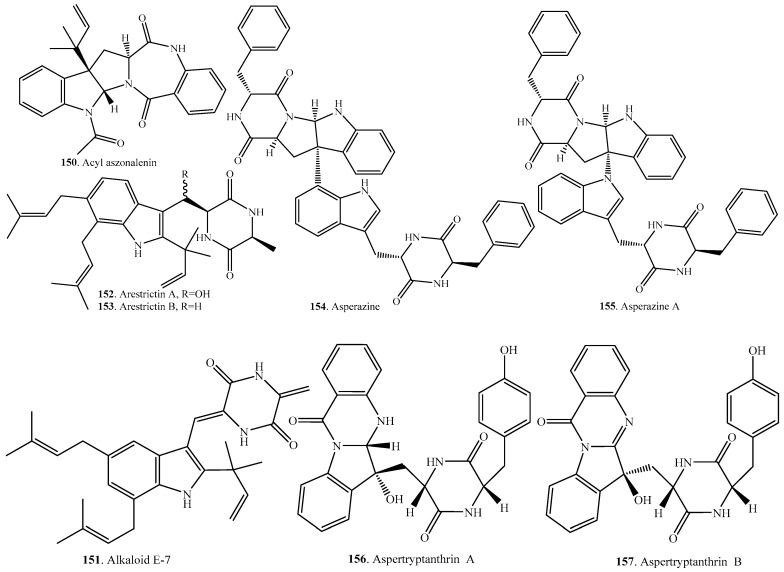
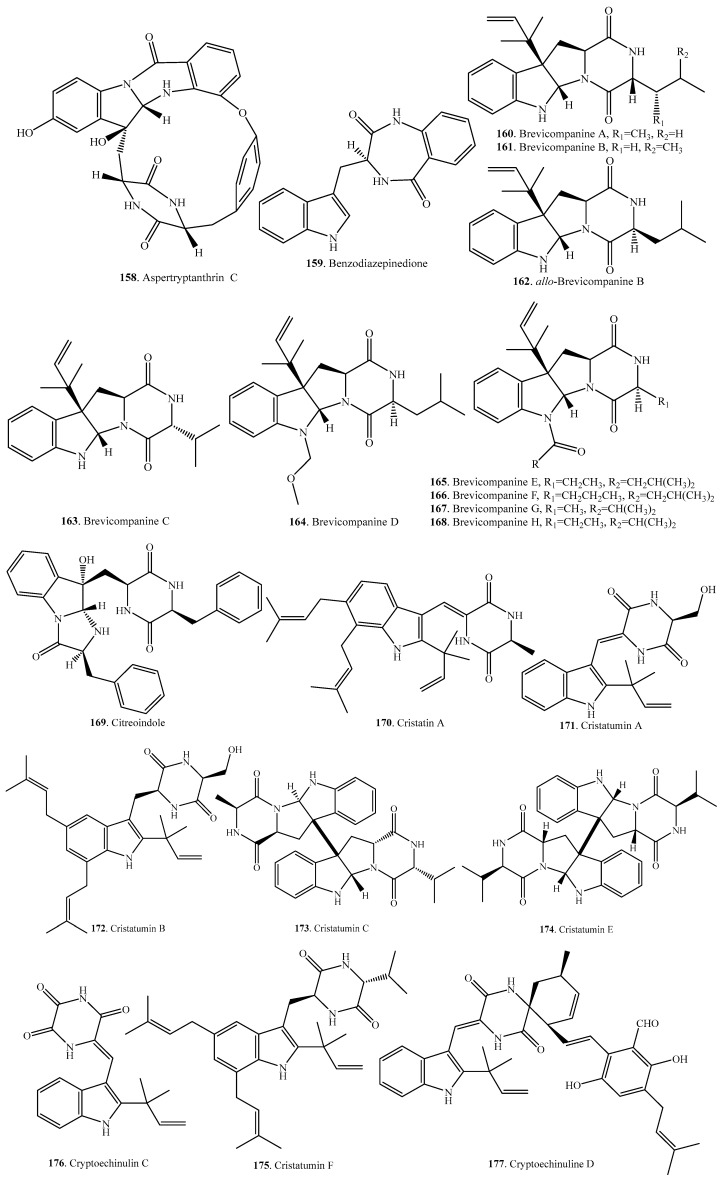
Apart from Trp–Pro and Trp–Trp cyclodipeptides, other tryptophan cyclodipeptides are also abundant in fungi and represent a structurally diverse group of natural products. Their occurrence and biological activities are shown in Table 3, and their structures are provided in Figure 3.
Table 3.
Fungal tryptophan-Xaa cyclodipeptide analogs and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Acyl aszonalenin (150) | Aspergillus flavipes | Substance P inhibitory activity | [88] |
| Alkaloid E-7 (151) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] | |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Arestrictin A (152) | Aspergillus restrictus | - | [91] |
| Arestrictin B (153) | Aspergillus restrictus | - | [91] |
| Aspergillus penicilloides | - | [91] | |
| Asperazine (154) | Marine-derived Aspergillus niger | Cytotoxic activity | [92] |
| Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | Weak cytotoxic activity | [69] | |
| Endophytic Aspergillus sp. KJ-9 from Melia azedarach | Antifungal and antibacterial activity | [93] | |
| Plant fungal pathogen Pestalotiopsis theae | Inhibitory effect on HIV-1 replication in C8166 cells | [94] | |
| Asperazine A (155) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | Weak cytotoxic activity | [69] |
| Aspertryptanthrin A (156) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Aspertryptanthrin B (157) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Aspertryptanthrin C (158) | Endophytic Aspergillus sp. from the stem bark of Melia azedarach | - | [74] |
| Benzodiazepinedione (159) | Aspergillus flavipes | - | [88] |
| Brevicompanine A (160) | Penicillium brevicompactum | Acceleration of the root growth of the lettuce seedlings | [95] |
| Brevicompanine B (161) | Penicillium brevicompactum | Acceleration of the root growth of the lettuce seedlings | [95] |
| Aspergillus janus | Inhibitory activity against the malaria parasite Plasmodium falciparum 3D7 | [96] | |
| allo-Brevicompanine B (162) | Deep ocean sediment derived fungus Penicillium sp. | - | [77] |
| Brevicompanine C (163) | Penicillium brevi-compactum | Acceleration of the root growth of the lettuce seedlings | [75] |
| Brevicompanine D (164) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine E (165) | Deep ocean sediment derived Penicillium sp. | Inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglial cells | [77,78] |
| Brevicompanine F (166) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine G (167) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Brevicompanine H (168) | Deep ocean sediment derived Penicillium sp. | Inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglial cells | [77] |
| Citreoindole (169) | Penicillium citreoviride | Cytotoxicity against HeLa cells | [97] |
| Cristatin A (170) | Aspergillus penicilloides | - | [91] |
| Cristatumin A (171) | Mangrove-derived endophytic Eurotium cristatum EN-220 | Antibacterial activity against Escherichia coli and Staphyloccocus aureus | [98] |
| Cristatumin B (172) | Mangrove-derived endophytic Eurotium cristatum EN-220 | Moderate lethal activity against brine shrimp | [98] |
| Cristatumin C (173) | Mangrove-derived endophytic Eurotium cristatum EN-220 | - | [98] |
| Cristatumin E (174) | Algal-derived Eurotium herbariorum HT-2 | Cytotoxic activity on K562 tumor cell line and antibacterial activity on Enterobacter aerogenes and Escherichia coli | [99] |
| Cristatumin F (175) | Eurotium cristatum isolated from Fuzhuan brick tea | Modest radical scavenging activity against DPPH radicals, and marginal attenuation of 3T3L1 pre-adipocytes | [100] |
| Cryptoechinuline C (176) | Marine-derived Eurotium rubrum | - | [84] |
| Cryptoechinuline D (177) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity | [101] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | Radical scavenging activity against DPPH | [102] | |
| Cryptoechinuline G (178) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against 1,1‘-diphenyl-1-picryhydrazyl (DPPH) | [87] |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activityagainst melanin synthesis | [90] | |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Cyclo(l-Trp–l-Ala) (179) | Marine-derived Eurotium rubrum | Weak antiviral effects | [84] |
| Marine-derived Aspergillus sp. | - | [103] | |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Cyclo(Trp–Gly) (180) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Cyclo(l-Trp–dehydro-His) (181) | Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| Cyclo(l-Trp–d-Ile) (182) | Penicillium brevi-compactum | Acceleration of root growth of lettuce seedlings | [75] |
| Cyclo(l-Trp–d-Leu) (183) | Penicillium brevi-compactum | Acceleration of root growth of lettuce seedlings | [75] |
| Cyclo(N-methyl-Trp–Leu) (184) | Endophytic Aspergillus tamari from Ficus carica | - | [29] |
| Cyclo(l-Trp-d-N-methyl-Leu) (185) | Aspergillus flavus | - | [107] |
| Cyclo(l-Trp–d-Phe) (186) | Endophytic Aspergillus niger from the liverwort Heteroscyphus tener | - | [69] |
| Cyclo(l-Trp–l-Phe) (187) | Aspergillus syndowi | - | [108] |
| Penicillium sp. | Acceleration of root growth of lettuce seedlings | [76] | |
| Cyclo(Trp–Tyr) (188) | Terrestrial Aspergillus oryzae | - | [109] |
| Cyclo(l-N-isopropyl-Trp–l-Val) (189) | An unidentified marine derived fungus M-3 from laver (Porphyra yezoensis) | Antifungal activity against the rice pathogen Pyricularia oryzae with MIC 0.36 μM | [110] |
| Cycloechinulin (190) | Aspergillus ochraceus | Insecticidal activity against the lepidopteran crop pest Helicoverpa zea | [57] |
| ent-Cycloechinulin (191) | Aspergillus novofumigatus CBS117520 | Antifungal activity against Aspergillus fumigatus, A. Niger, Candida albicans, and Cryptococcus neoformans | [59] |
| Dehydroechinulin (192) | Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Dehydrovariecolorin L (193) | Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] |
| 12-Demethyl-12-oxo-eurotechinulin B (194) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] |
| Dichotocejpin B (195) | Dichotomomyces cejpii FS110 | - | [112] |
| Dichotocejpin C (196) | Dichotomomyces cejpii FS110 | - | [112] |
| Marine-derived Dichotomomyces sp. L-8 | - | [113] | |
| Didehydroechinulin = Didehydroechinulin B (197) | Deep ocean sediment-derived Penicillium griseofulvum | - | [86] |
| Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity | [101] | |
| Dihydrocryptoechinulin D (198) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Cytotoxic activity against P388 cells | [114] |
| Dihydroneochinulin B (199) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | Weak cytotoxic activity against BEL-7402 and A-549 cell lines | [101] |
| 3,12-Dihydroroquefortine (200) | Permafrost sediment derived Penicillium aurantiogrieum | - | [115] |
| 7,9-Dihydroxy-3-(1H-indol--ylmethyl)-8-methoxy-2,3,11,11a-tetrahydro-6H-pyrazino[1,2-b]isoquinoline-1,4-dione (201) | Terrestrial Aspergillus oryzae | - | [109] |
| Dihydroxyisoechinulin A (202) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal and antibacterial activities | [104] | |
| 11,14-Dihydroxylneoechinulin E (203) | Mushroom Psilocybe merdaria from suburban district of Haikou of China | - | [116] |
| Dipodazine (204) | Penicillium dipodomyis | - | [117] |
| Ditryptophenaline (205) | Aspergillus flavus | - | [118] |
| Aspergillus flavus | Weak substance-P inhibitor activity | [119] | |
| Marine-derived Aspergillus flavus C-F-3 | - | [120] | |
| Terrestrial Aspergillus oryzae | - | [109] | |
| Echinulin (206) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Aspergillus chevalieri in rabbits | Toxic activity to rabbits by producing a significant degree of damage to lung and liver | [79] | |
| Soil-derived Chaetomium globosum | - | [121] | |
| Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] | |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Marine-derived Eurotium rubrum | Weak antiviral effect | [84] | |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Effusin A (207) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | - | [114] |
| Epoxyisoechinulin A (208) | Marine-derived Aspergillus ruber 1017 from a crinoid Himerometra magnipinna | - | [122] |
| Eurocristatine (209) | Sponge-associated Eurotium cristatum KUFC 7356 | - | [123] |
| Eurotechinulin B (210) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] |
| Fructigenine A = Rugulosuvine B (211) | Penicillium fructigenum | Inhibitory activity on the growth of Avena coleoptiles and L-5178Y cells | [80] |
| Penicillium rugulosum | Moderate cytotoxic activity | [81] | |
| Fructigenine B = Verrucofortine (212) | Deep ocean sediment derived Penicillium sp. | - | [77] |
| Penicillium verrucosum var. cyclopium | - | [124] | |
| Penicillium fructigenum | - | [80] | |
| Gliocladin C (213) | Gliocladium roseum PS-N132 | Significant cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Glioperazine C (214) | Bionectra byssicola F120 | - | [126] |
| Haenamindole (215) | Marine-derived Penicillium sp. KCB12F005 | - | [127] |
| 3-((1-Hydroxy-3-(2-methylbut- 3-en-2-yl)-2-oxoindilin- 3-yl)methyl)-1-methyl-3,4-dihydrobenzo[1,4] diazepine-2,5-dione (216) | Sponge-derived Aspergillus sp. from the Mediterranean sponge Tethya auranthium | Antibacteria activity on a few marine-derived Vibrio species | [128] |
| 16-Hydroxyroquefortine C (217) | Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| 14-Hydroxyterezine D (218) | Marine-derived Aspergillus sydowi from a driftwood sample | Weak cytotoxic activity against A-549 cells | [10] |
| (E)-3-(1H-Imidazole-4-yimethylene)-6-(1H-indl-3-ylmethyl)-2,5-piperazinediol (219) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| 3-[(1H-Indol-3-yl)methyl]-6-benzylpiperazine-2,5-dione (220) | Marine-derived Aspergillus flavus C-F-3 | - | [120] |
| (3S, 11aS)-3- [(1H-Indol-3-yl) methyl]-7,9- dihydroxy-8-methoxy- 2,3,11,11a-tetrahydro- 6H- pyrazino [1,2-b] isoquinoline-1,4-dione (221) | Algicolous Aspergillus flavus | Weak cytotoxicity against HL-60 cells | [130] |
| Isoechinulin A (222) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] | |
| Isoechinulin B (223) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Marine-derived Eurotium rubrum MPUC136 | Inhibitory activity against melanin synthesis | [90] | |
| Marine-derived Eurotium rubrum | - | [84] | |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Isoechinulin C (224) | Marine-derived Eurotium rubrum MPUC136 | - | [90] |
| Isoechinulin D (225) | Marine-derived Eurotium rubrum MPUC136 | - | [90] |
| Isopenilline A (226) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| 7-Isopentenylcryptoechinuline D (227) | Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] |
| Leptosin S (228) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Lumpidin (229) | Penicillium nordicum | - | [132] |
| 3-Methyl-6-[[(1-(3-methyl-2-butenyl)-1H-indol-3-yl)methyl)-2,5-piperazinediione (230) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| 7-O-Methylvariecolortide A (231) | Mangrove derived endophytic Eurotium rubrum from the inner tissue of the stems of Hibiscus tilliaceus | - | [133] |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | Inhibitory activity on caspase-3 | [111] | |
| (+)-(R)-7-O-Methylvariecolortide A (232) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (−)-(S)-7-O-Methylvariecolortide A (233) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| Neoechinulin A (234) | Aspergillus spp. | Scavenging, neurotrophic factor-like and antiapoptotic activities | [82] |
| Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] | |
| Marine-derived Aspergillus sp. | Ultraviolet-A (320-390 nm) protecting activity with IC50 value of 170 μM. | [103] | |
| Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with IC50 value of 24 μM | [134] | |
| Eurotium cristatum from Fuzhuan brick tea | - | [100] | |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] | |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Mushroom Psilocybe merdaria from suburban district of Haikou of China | - | [116] | |
| Marine-derived Eurotium rubrum | - | [84] | |
| Neoechinulin B (235) | Mangrove rhizosphere soil-derived Aspergillus effuses H1-1 | - | [101] |
| Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] | |
| Marine-derived Eurotium rubrum | Inhibition against H1N1 virus infected in MDCK cells, and a panel of influenza virus strains | [84] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Neoechinulin C (236) | Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] |
| Neoechinulin D (237) | Aspergillus amstelodami | - | [135] |
| Neoechinulin E (238) | Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | DPPH radical scavenging activity | [102] |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Lethal activity on brine shrimp | [104] | |
| Neosartin A (239) | Marine-derived Neosartorya pseudofischeri from the inner tissue of starfish Acanthaster planci | - | [136] |
| Neosartin B (240) | Marine-derived Neosartorya pseudofischeri from the inner tissue of starfish Acanthaster planci | - | [136] |
| Oidioperazine B (241) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Oidioperazine C (242) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Oidioperazine D (243) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Penilline A (244) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| Penilline B (245) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
| Penilloid A (246) | Antarctic soil-derived Penicillium sp. SCSIO 05705 | - | [129] |
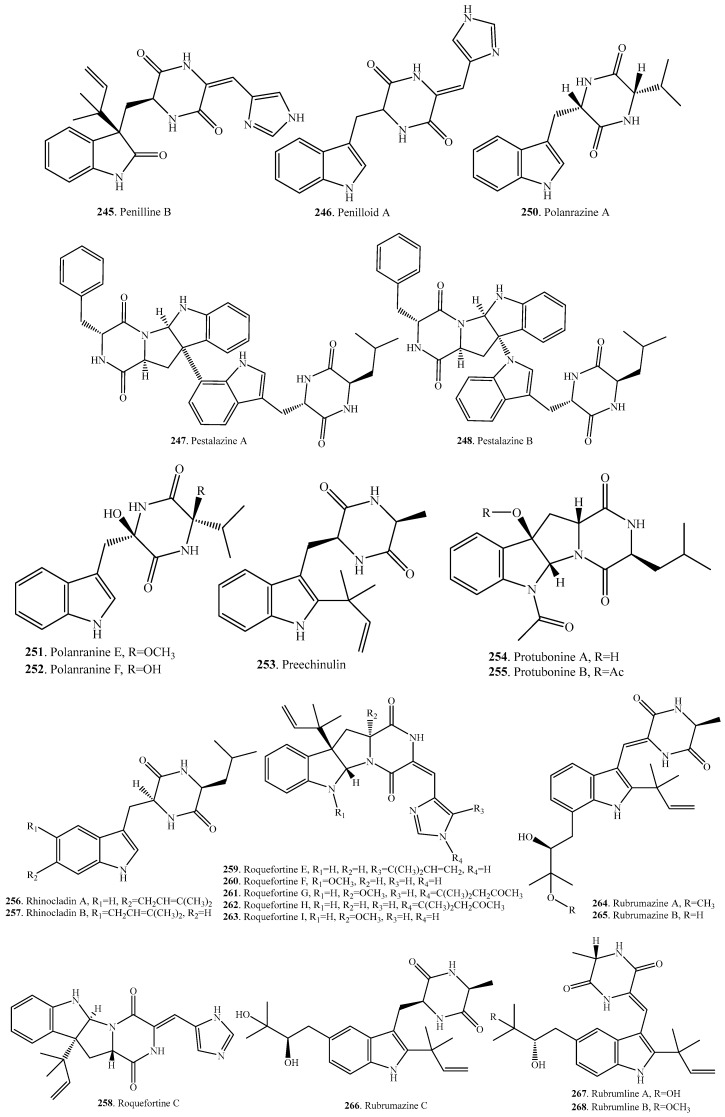
| Pestalazine A (247) | Plant pathogen Pestalotiopsis theae | Inhibitory activity on HIV-1 replication in C8166 cells | [94] |
| Pestalazine B (248) | Plant pathogen Pestalotiopsis theae | - | [94] |
| 1’-(2-Phenyl-ethylene)- ditryptophenaline (249) | Aspergillus flavus | Weak substance-P inhibitor activity | [119] |
| Polanrazine A = Cyclo(l-Trp–l-Val) (250) | Plant pathogen Phoma lingam | Phytotoxic activity | [83] |
| Polanranine E (251) | Plant pathogen Phoma lingam | Moderate and selective phytotoxicity by causing necrotic and chlorotic lesions | [138] |
| Polanranine F (252) | Plant pathogen Phoma lingam | - | [138] |
| Preechinulin (253) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Marine-derived Eurotium rubrum | Weak antiviral effect | [84] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Protubonine A (254) | Marine-derived Aspergillus sp. SF-5044 from an intertidal sediment sample | - | [139] |
| Protubonine B (255) | Marine-derived Aspergillus sp. SF-5044 from an intertidal sediment sample | - | [139] |
| Rhinocladin A (256) | Endophytic Rhinocladiella sp. lgt-3 from Tripterygium wilfordii | Weak inhibitory activity on monoamine oxidase | [140] |
| Rhinocladin B (257) | Endophytic Rhinocladiella sp. lgt-3 from Tripterygium wilfordii | Weak inhibitory activity on monoamine oxidase | [140] |
| Roquefortine C = Roquefortine (258) | Permafrost sediment derived Penicillium aurantiogriseum | - | [115] |
| Penicillium roqueforti from soil | - | [141] | |
| Endophytic Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] | |
| Roquefortine E (259) | Gymnoascus reessii | Weak cytotoxic activity to mammalian cells | [142] |
| Roquefortine F (260) | Marine-derived Penicillium sp. | - | [143] |
| Roquefortine G (261) | Marine-derived Penicillium sp. | - | [143] |
| Roquefortine H (262) | Deep ocean sediment-derived Penicillium sp. | - | [144] |
| Roquefortine I (263) | Deep ocean sediment-derived Penicillium sp. | - | [144] |
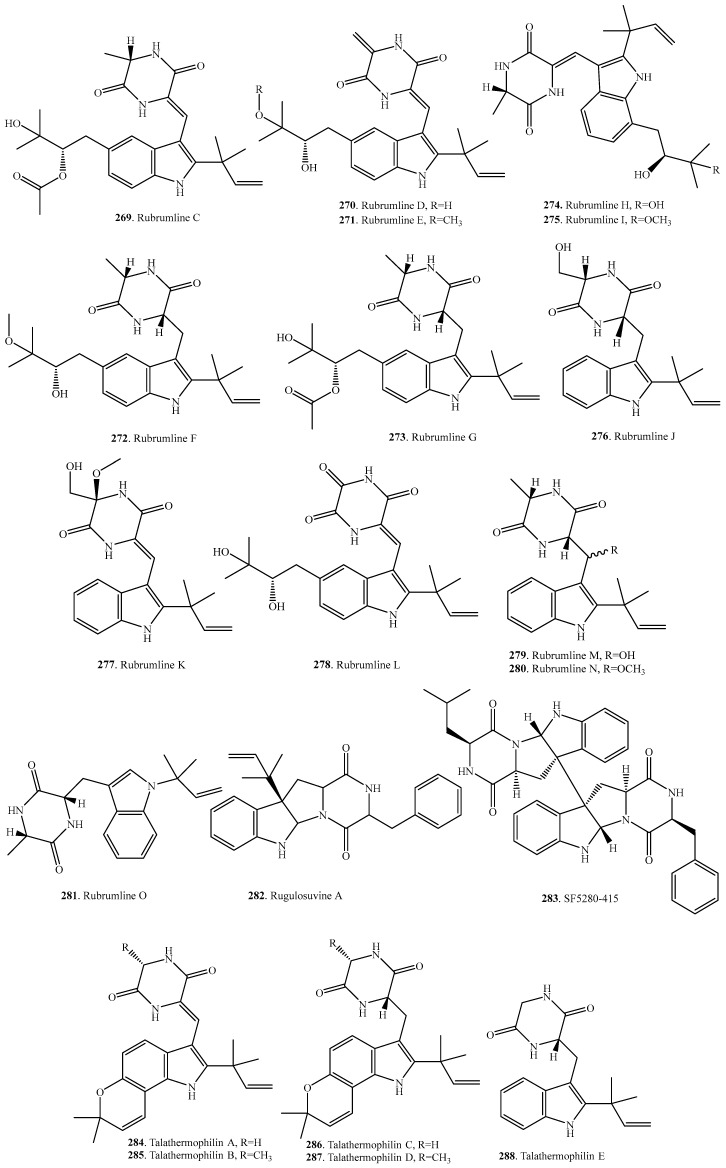
| Rubrumazine A (264) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] |
| Rubrumazine B (265) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Brine shrimp lethal activity | [104] |
| Rubrumazine C (266) | Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest brine shrimp lethal activity | [104] |
| Rubrumline A (267) | Marine-derived Eurotium rubrum | - | [84] |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Rubrumline B (268) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline C (269) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline D (270) | Marine-derived Eurotium rubrum | Inhibitory activity against influenza A/WSN/33 virus | [84] |
| Marine-derived Eurotium rubrum MPUC136 | - | [90] | |
| Rubrumline E (271) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline F (272) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline G (273) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline H (274) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline I (275) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline J (276) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline K (277) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline L (278) | Marine-derived Eurotium rubrum | - | [84] |
| Rubrumline M (279) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline N (280) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rubrumline O (281) | Marine-derived Eurotium rubrum | Weak antiviral effect | [84] |
| Rugulosuvine A (282) | Penicillium rugulosum | Moderate cytotoxic activity | [81] |
| SF5280-415 (283) | Marine-derived Aspergillus sp. SF-5280 | - | [145] |
| Talathermophilin A (284) | Thermophilic Talaromyces thermophilus YM1-3 | Nematicidal toxicity | [85] |
| Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] | |
| Talathermophilin B (285) | Thermophilic Talaromyces thermophilus YM1-3 | Nematicidal toxicity | [85] |
| Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] | |
| Talathermophilin C (286) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Talathermophilin D (287) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
| Talathermophilin E (288) | Thermophilic Talaromyces thermophilus YM3-4 collected in Tengchong hot spring, Yunnan of China | - | [105] |
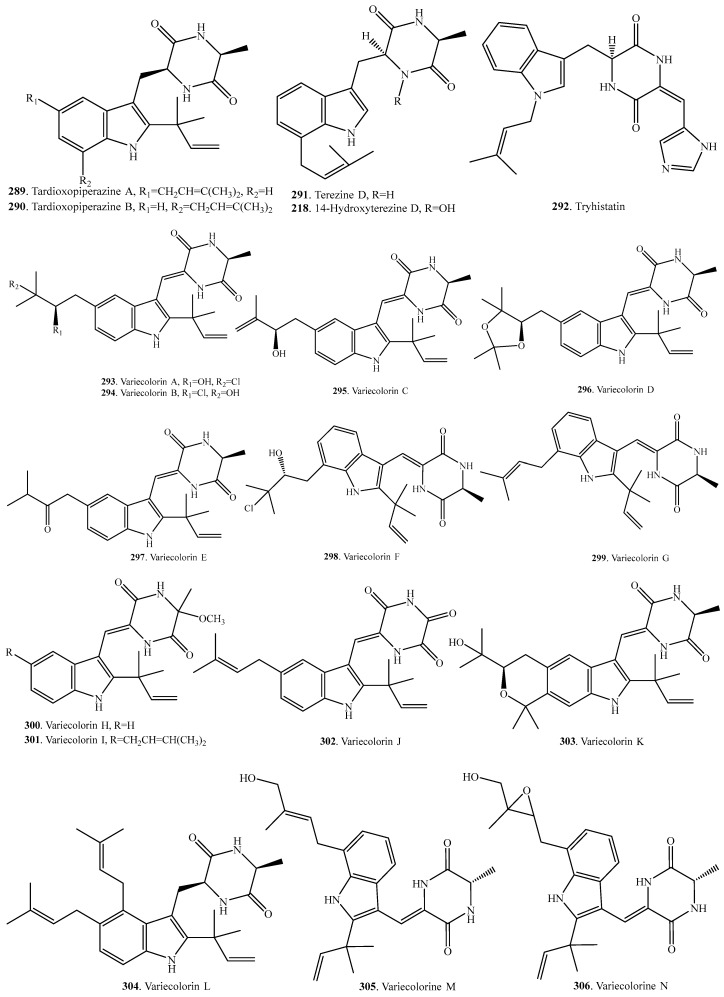
| Tardioxopiperazine A (289) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] | |
| Microascus tardifaciens | Moderate inhibition on con A and LPS mediated T cell proliferation | [146] | |
| Deep ocean sediment-derived Penicillium griseofulvum | - | [86] | |
| Tardioxopiperaine B (290) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Microascus tardifaciens | Weak inhibition on con A and LPS mediated T cell proliferation | [146] | |
| Terezine D (291) | Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] |
| Tryhistatin (292) | Endophytic fungus Penicillium sp. HS-3 from the stems of Huperzia serrata | - | [106] |
| Variecolorin A (293) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin B (294) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin C (295) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin D (296) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin E (297) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin F (298) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin G (299) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | Cytotoxic activity | [89] | |
| Eurotium rubrum MA-150 obtained from mangrove-derived rhizospheric soil | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin H (300) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Deep ocean sediment-derived fungus Penicillium griseofulvum | - | [86] | |
| Variecolorin I (301) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin J (302) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Mangrove-derived Eurotium rubrum from Hibiscus tiliaceus | - | [89] | |
| Variecolorin K (303) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | Weak radical scavenging activity against DPPH | [87] |
| Variecolorin L (304) | Halotolerant Aspergillus variecolor from sediments collected in the Jilantai salt field of China | - | [87] |
| Mangrove-derived Eurotium rubrum from the inner tissue of stems of Hibiscus tiliaceus | - | [102] | |
| Mangrove rhizosphere soil-derived Eurotium rubrum MA-150 | Modest lethal activity on brine shrimp | [104] | |
| Variecolorin M (305) | Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] |
| Variecolorin N (306) | Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] |
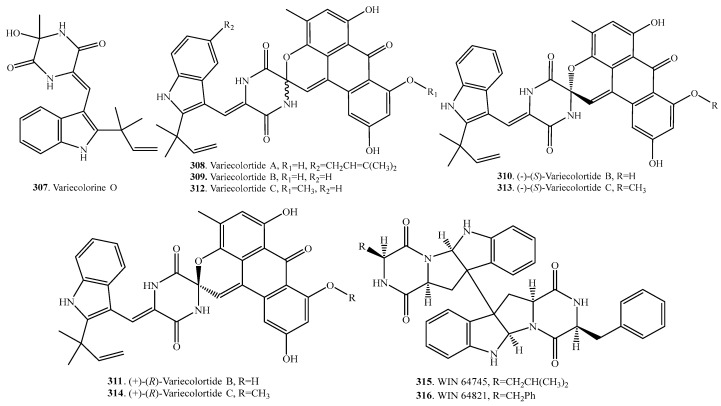
| Variecolorin O (307) | Eurotium cristatum isolated from Fuzhuan brick tea | - | [100] |
| Deep ocean sediment-derived Penicillium griseofulvum | Weak radical scavenging activity against DPPH | [86] | |
| Variecolortide A (308) | Halotolerant fungus Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| Variecolortide B (309) | Halotolerant Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| (−)-(S)-Variecolortide B (310) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (+)-(R)-Variecolortide B (311) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| Variecolortide C (312) | Halotolerant fungus Aspergillus variecolor B-17 | Weak cytotoxic and antioxidant activities | [147] |
| (−)-(S)-Variecolortide C (313) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| (+)-(R)-Variecolortide C (314) | Lichen-derived Eurotium sp. No. 17-11-8-1 from Cladina grisea collected in Changbaishan Mountain of China | - | [111] |
| WIN 64745 (315) | Aspergillus sp. SC319 | - | [148] |
| WIN 64821 (316) | Aspergillus sp. SC319 | - | [148] |
Note: IC50, median inhibitory concentration; MIC, minimum inhibitory concentration.
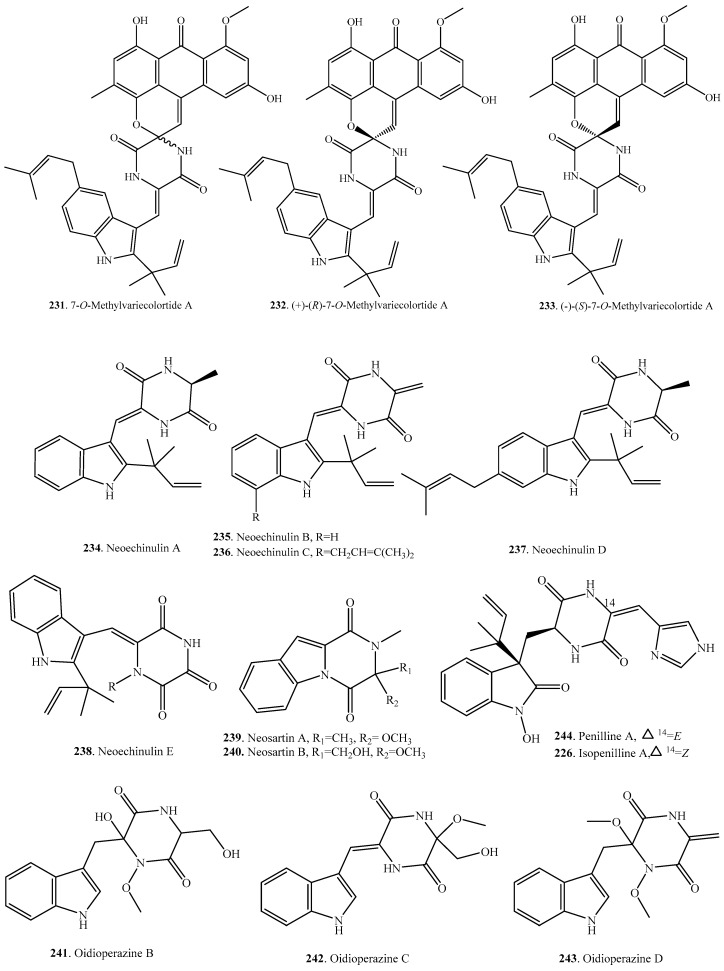
Figure 3.
Structures of the tryptophan-Xaa cyclodipeptide analogs isolated from fungi.
Aspertryptanthrins A–C (156–158) were obtained from a terrestrial-derived fungus Aspergillus sp. These compounds all contain an anthranilate unit and a tryptophan residue. In addition, aspertryptanthrin C (158) contains a rare 16-membered ring [74].
The cyclodipeptides brevicompanine C (163), cyclo(l-Trp–d-Ile) (182) and cyclo(l-Trp–d-Leu) (183) from Penicillium brevi-compactum [75], as well as cyclo(l-Trp–l-Phe) (187) from Penicillium sp. [76] accelerated the root growth of the lettuce seedlings in proportion to their concentrations from 1 to 100 mg/L.
Brevicompanines D–H (164–168) were isolated from a deep ocean sediment derived fungus Penicillium sp. [77]. Both brevicompanines E (165) and H (168) inhibited lipopolysaccharide (LPS)-induced nitric oxide production in BV2 microglial cells. Further studies showed that brevicompanine E (165) reduced lipopolysaccharide-induced production of pro-inflammatory cytokines and enzymes in microglia by inhibiting activation of activator protein-1 and nuclear factor κB and, hence, may be potentially useful for modulating neuroinflammation [78].
Cycloechinulin (190) was isolated from the sclerotia of Aspergillus ochraceus, and it showed moderate insecticidal activity against the lepidopteran crop pest Helicoverpa zea [57].
Echinulin (206) is one of the simplest classes of isoprenylated tryptophan cyclodipeptides. It was toxic to rabbits, producing a significant degree of damage to lung and liver [79].
Fructigenines A (211) and B (212) are annulated derivatives of cyclo(l-Trp–l-Phe) (187), and were isolated from Penicillium fructigenum. Only fructigenine A (211) inhibited growth of Avena coleoptiles and L-5178Y (mouse lymphoma cells), and was subsequently found to have more potent anti-inflammatory activity than indomethacin in the mouse ear edema model [80]. Fructigenine A (211) was also named rugulosuvine B (211) in Penicillium rugulosum, and showed potent anti-inflammatory and antitumor activities in vitro [81].
Neoechinulin A (234) had scavenging, neurotrophic factor-like and antiapoptotic activities. The protective properties of neoechinulin A (234) against SIN-1-induced neuronal cell death suggested that neoechinulin A (234) could protect against neuronal cell death in neurodegenerative diseases [82].
The valine analog polanrazine A (250) was isolated from the blackleg fungus Phoma lingam (teleomorph: Leptosphaeria maculans). This compound was toxic to canola (Brassica napus and B. rapa) [83].
Rubrumlines A–O (267–281) were isolated from the marine-derived fungus Eurototium rubrum. Among them, rubrumlines D (270), F (272), G (273), J (276), M (279), N (280), and O (281) showed inhibitory activity against influenza A/WSN/33 virus. Further analysis of the structure–activity relationship revealed that the analogs with an isoprenyl unit in indole ring displayed stronger cytotoxic effects than those linked by an oxygenated isoprenyl unit. Neoechinulin B (235) was also isolated from Eurototium rubrum. This compound was efficient in inhibiting influenza A/WSN/33 virus propagation even after the fifth passage. In addition, it exerted potent inhibition against H1N1 virus infected in MDCK cells, and was able to inhibit a panel of influenza virus strains including amantadine- and oseltamivir-resistant clinical isolates. The high potency and broad-spectrum activities against influenza viruses with less drug resistance made neoechinulin B (235) a new lead for the development of potential inhibitor of influenza viruses [84].
The prenylated pyranoindole derivatives talathermophilins A (284) and B (285) were isolated from a thermophilic fungus Talaromyces thermophilus strain YM1-3. Both talathermophilins A (284) and B (285) showed nematicidal activity toward the worms of the free-living nematode Panagrellus redivevus [85].
Variecolorins A–L (293–304) were isolated from halotolerant fungus Aspergillus variecolor [80], and variecolorins M–O (305–307) from deep ocean sediment-derived fungus Penicillium griseofulvum [86]. They all showed weak radical scavenging activity against DPPH [86,87].
5. Proline–Xaa Cyclodipeptides
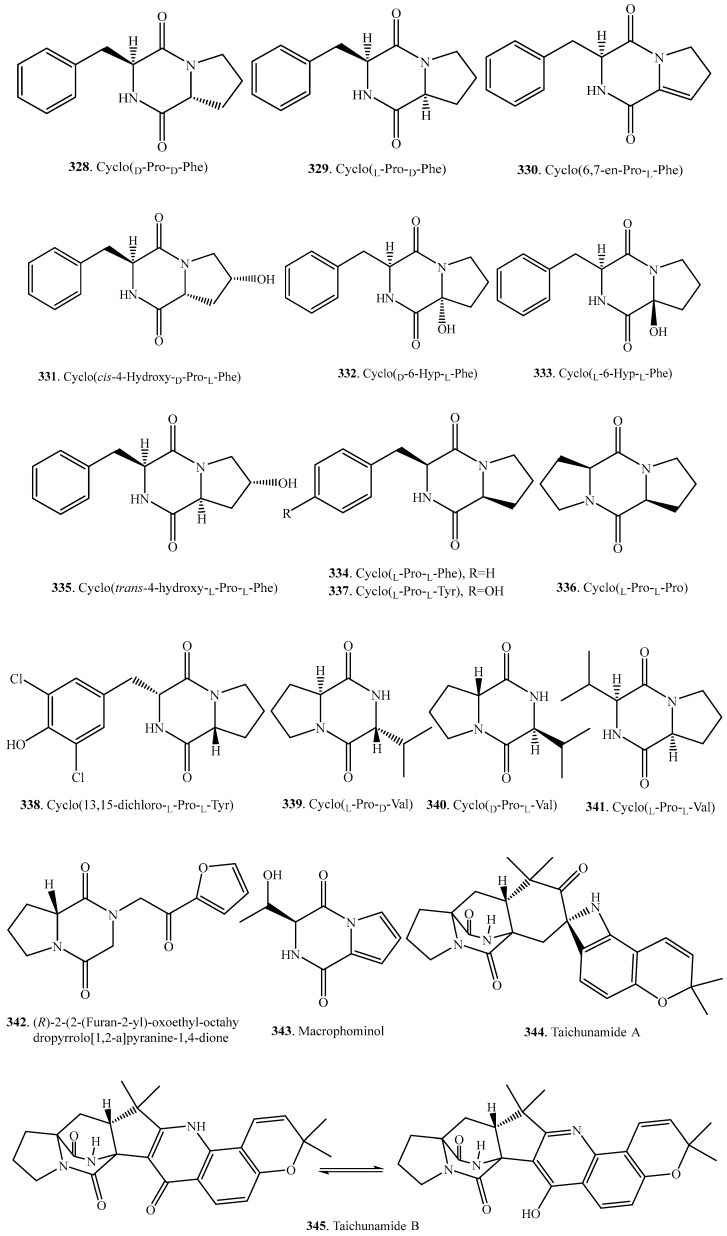
Except Trp–Pro cyclodipeptides, other proline containing cyclodipeptides (Pro–Xaa) are also abundantly distributed in fungi. Their occurrence and biological activities are shown in Table 4, and their structures are provided in Figure 4.
Table 4.
Proline-Xaa cyclodipeptide analogs and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
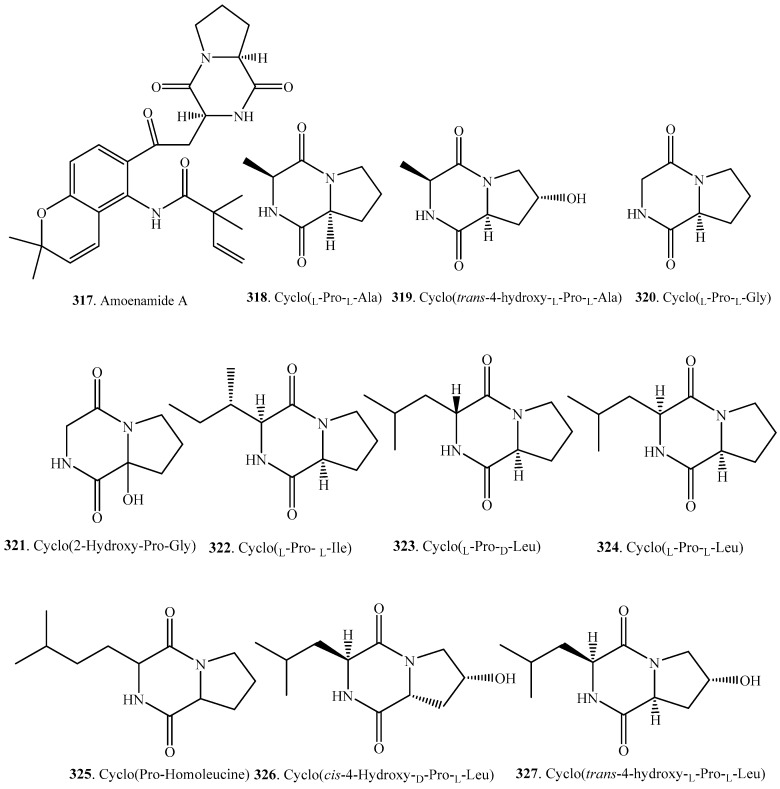
| Amoenamide A (317) | Aspergillus amoenus NRRL 35600 | - | [153] |
| Cyclo(l-Pro–l-Ala) (318) | Alternaria alternata | - | [149] |
| Phytopathogenic Colletotrichum gloesporoides | Inhibition of aflatoxin production in Aspergillus flavus | [150,151] | |
| Cyclo(trans-4-hydroxy-l-Pro–l-Ala) (319) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Cyclo(l-Pro–l-Gly) (320) | Phytopathogenic Colletotrichum gloesporoides | - | [150] |
| Cyclo(2-hydroxy-Pro–Gly) (321) | Simplicillium sp. YZ-11 | - | [155] |
| Cyclo(l-Pro–l-Ile) (322) | Endophytic fungus Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Endophytic Aspergillus fumigatus | - | [21] | |
| Rhizoctonia solani | - | [157] | |
| Cyclo(l-Pro–d-Leu) (323) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Cyclo(l-Pro–l-Leu) (324) | Endophytic Aspergillus fumigatus | Weak inhibitory activity of β-glucuronidase release | [21] |
| Phytopathogenic Colletotrichum gloesporoides | Phytotoxic, antitumoral and fungicide activity | [150] | |
| Rhizoctonia solani | - | [157] | |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(Pro–Homoleucine) (325) | Alternaria alternata | - | [149] |
| Cyclo(4-hydroxy-R-Pro–S-Leu) = Cyclo(cis-4-hydroxy-d-Pro–l-Leu) (326) | Marine-sponge derived yeast Aureobasidium pullulans at Okinawa of Japan | - | [159] |
| Mangrove-derived endophytic Pestalotiopsis vaccinii from a branch of Kandelia candel | - | [160] | |
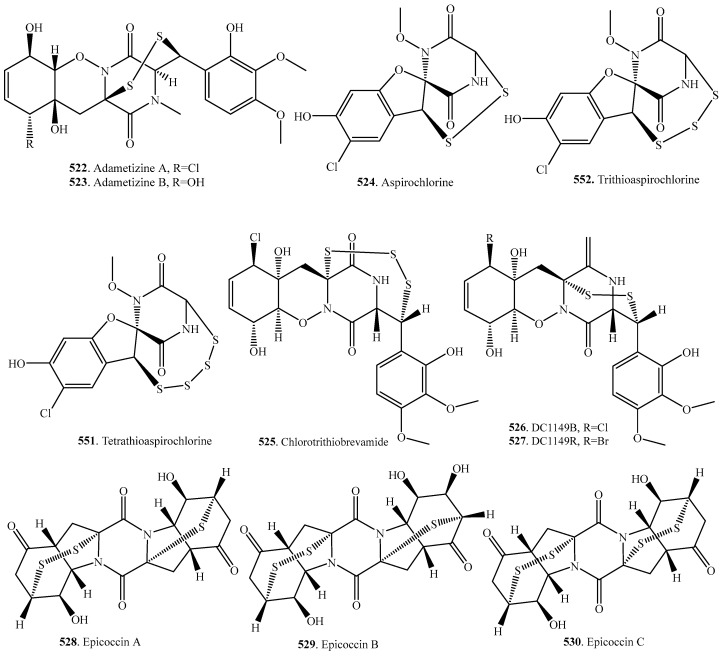
| Cyclo(trans-4-hydroxy-l-Pro–l-Leu) (327) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(d-Pro–d-Phe) (328) | Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Cyclo(l-Pro–d-Phe) (329) | Alternaria alternata | - | [149] |
| Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Cyclo(6,7-en-Pro–l-Phe) (330) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Cyclo(4-Hydroxy-R-Pro–S-Phe) = Cyclo-(cis-4-Hydroxy-d-Pro–l-Phe) (331) | Marine-sponge derived yeast Aureobasidium pullulans at Okinawa of Japan | - | [159] |
| Cyclo(d-6-Hydroxy-Pro–l-Phe) = Cyclo(d-6-Hyp–l-Phe) (332) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
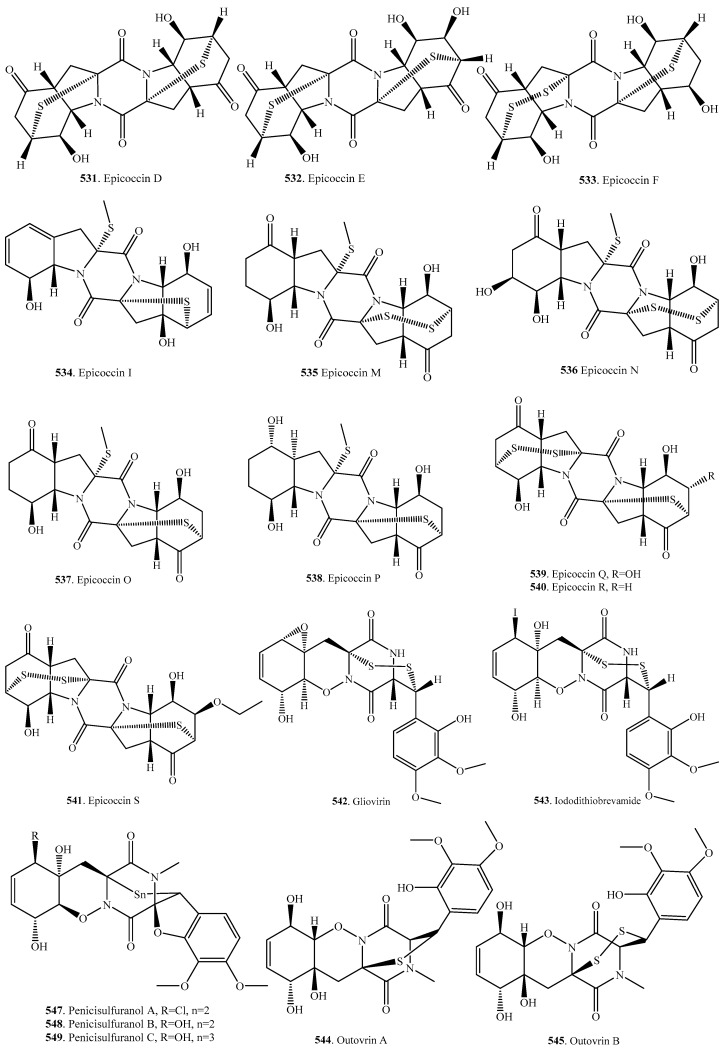
| Marine-derived Chromocleista sp. | - | [162] | |
| Cyclo(6-Hydroxy-l-Pro–l-Phe) = Cyclo(l-6-Hyp–l-Phe) (333) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
| Marine-derived Chromocleista sp. | - | [162] | |
| Cyclo(l-Pro–l-Phe) = Maculosin-2 (334) | Alternaria alternata from spotted knapweed (Centaurea maculosa) | Phytotoxic activity | [149,163] |
| Cyclo(trans-4-hydroxy-l-Pro–l-Phe) (335) | Endophytic Alternaria alternata from grapevine | Antifungal activity on Plasmopara viticola | [154] |
| Cyclo(l-Pro–l-Pro) (336) | Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] |
| Endophytic fungus Stagonosporopsis oculihominis from Dendrobium huoshanense | - | [164] | |
| Cyclo(l-Pro–l-Tyr) = Maculosin-1 (337) | Alternaria alternata from spotted knapweed (Centaurea maculosa) | Phytotoxic activity | [149,163] |
| Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Cyclo(13,15-dichloro-l-Pro–l-Tyr) (338) | Leptoxyphium sp. | Inhibitory activity on CCL2-induced chemotaxis | [152] |
| Cyclo(l-Pro–d-Val) (339) | Alternaria alternata | - | [149] |
| Endophytic Alternaria tenuissima from the bark of Erythrophleum fordii | - | [156] | |
| Cyclo(d-Pro–l-Val) (340) | Aspergillus sp. F70609 | Inhibitory activity on β-glucosidase | [165] |
| Cyclo(l-Pro–l-Val) (341) | Marine-derived Chromocleista sp. from a deep-water sediment sample collected in the Gulf of Mexico | - | [158] |
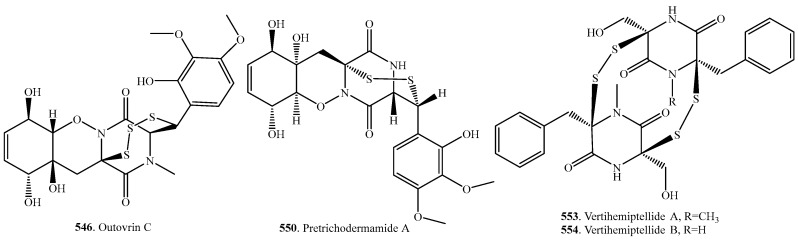
| Phytopathogenic Colletotrichum gloesporoides | Phytotoxic and antibiotic activities | [150] | |
| Phytopathogenic Fusarium oxysporum | - | [166] | |
| Marine-derived Penicillium bilaii | - | [161] | |
| Rhizoctonia solani | - | [157] | |
| (R)-2-(2-(Furan-2-yl)-oxoethyl- octahydropyrrolo[1,2-a] pyranine-1,4-dione (342) | Edible and medicinal Armillaria mellea | - | [167] |
| Macrophominol (343) | Phytopathogenic Macrophomina phaseolina | - | [168] |
| Taichunamide A (344) | Aspergillus taichungensis (IBT 19404) | - | [53] |
| Taichunamide B (345) | Aspergillus taichungensis (IBT 19404) | - | [53] |
Figure 4.
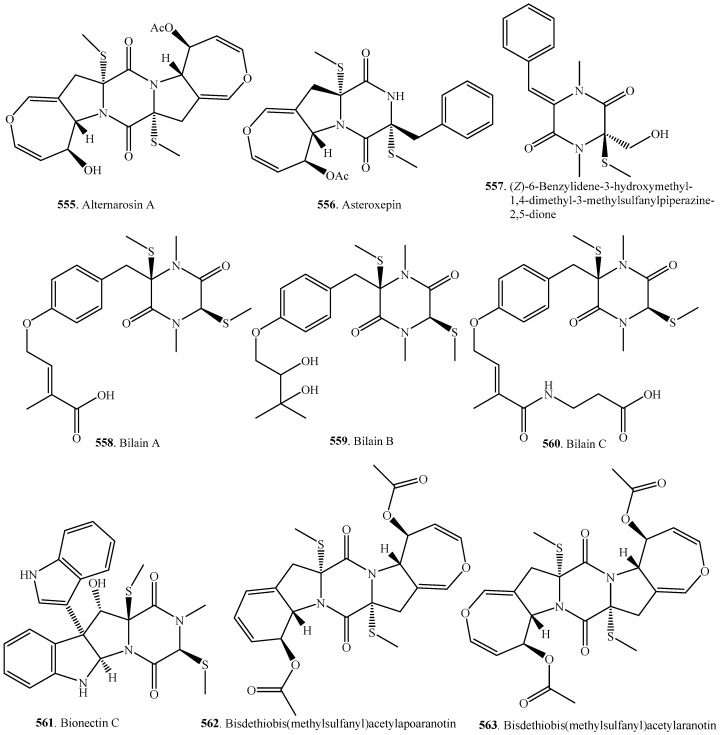
Structures of the proline-Xaa cyclodipeptide analogs isolated from fungi.
Cyclo(l-Pro–l-Ala) (318) was isolated from Alternaria alternata [149] and the phytopathogenic fungus Colletotrichum gloesporoides [150]. This compound inhibited aflatoxin production in aflatoxigenic fungi without affecting fungal growth. Further investigation on the mode of action suggested that this cyclodipeptide inhibited aflatoxin biosynthesis by affecting glutathione S-transferase (GST) function in Aspergillus flavus to show its potency as the biocontrol agent [151].
Cyclo(l-Pro–l-Phe) (334) and cyclo(l-Pro–l-Tyr) (337), which were also called maculosin-2 (334) and maculosin-1 (337), were host-specific fungal phytotoxins produced by Alternaria alternata on spotted knapweed (Centaurea maculosa) to show their protential as the bioherbicides [149].
The dechlorinated cyclodipeptide, cyclo(13,15-dichloro-l-Pro–l-Tyr) (338), isolated from the fungus Leptoxyphium sp. is considered as an inhibitor of monocyte chemotactic protein-1 (CCL2)-induced chemotaxis and was 10- to 20-fold more active than the nonchlorinated from maculosin-1 (337). In addition, no cellular toxicity was observed when cyclo(13,15-dichloro-l-Pro–l-Tyr) (338) at 100 μM was in contact with human monocyte culture for 24 h, suggesting its potential as a lead structure for the future development of anti-inflammatory compounds [152].
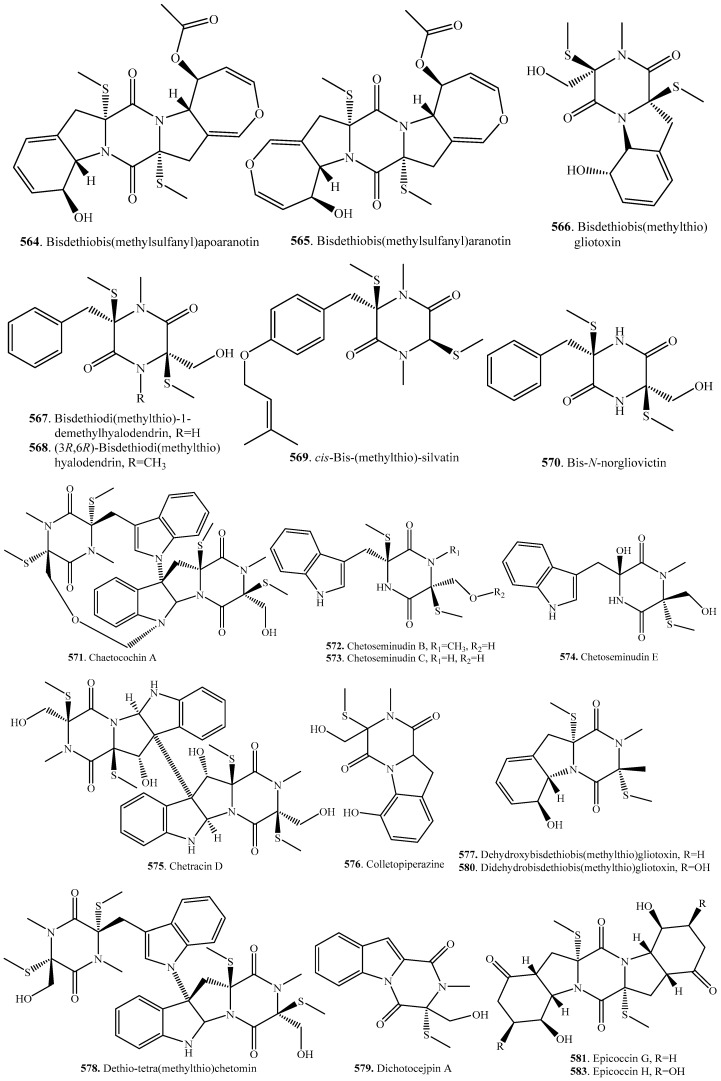
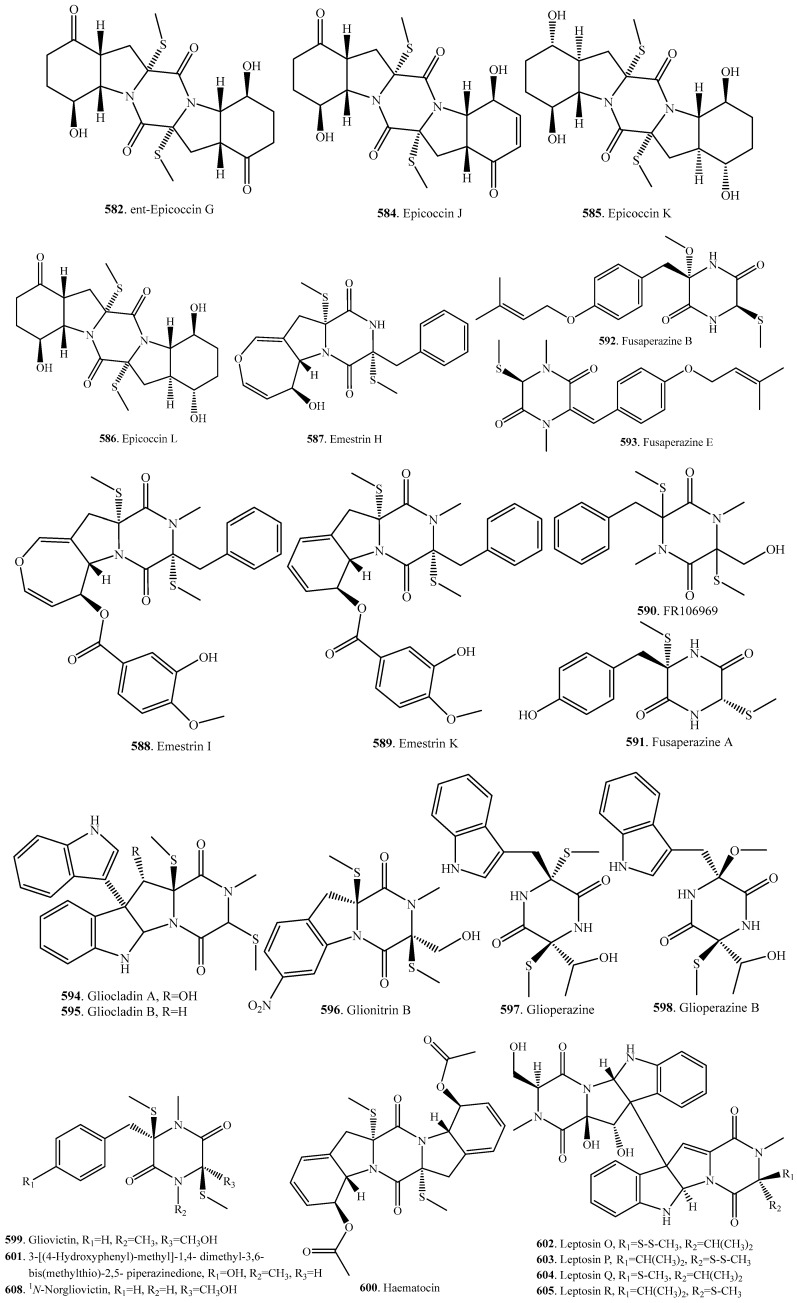
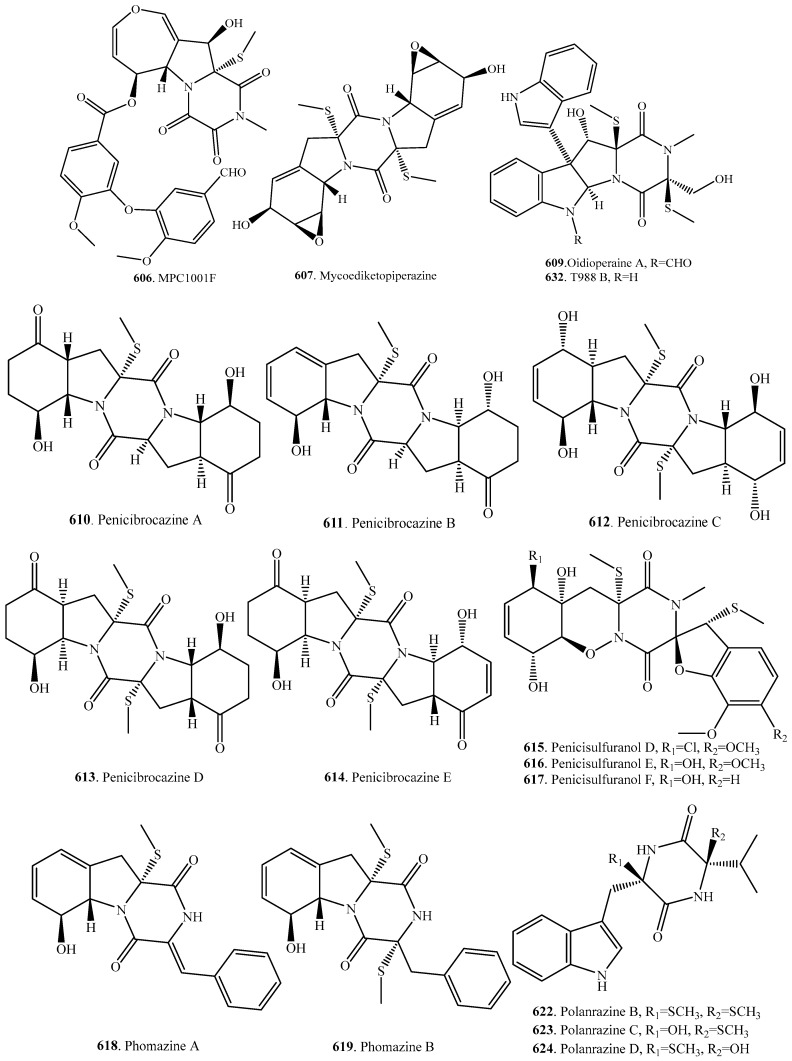
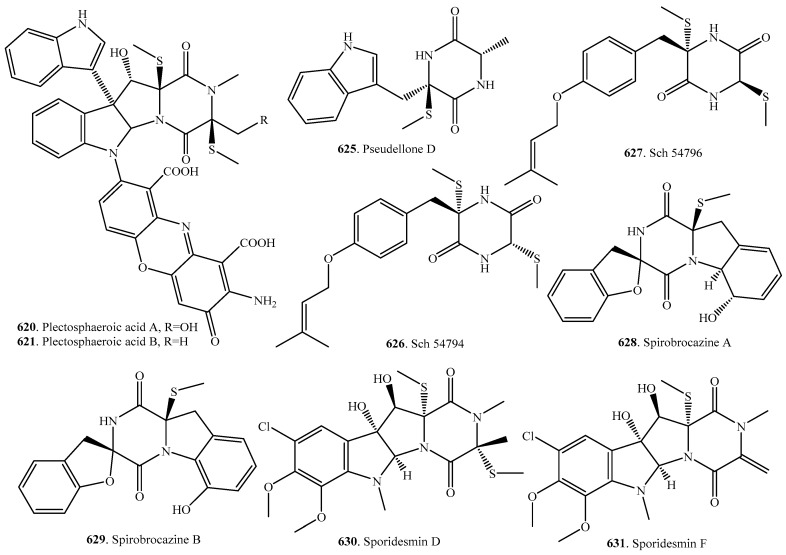
6. Non-Tryptophan–Non-Proline Cyclodipeptides
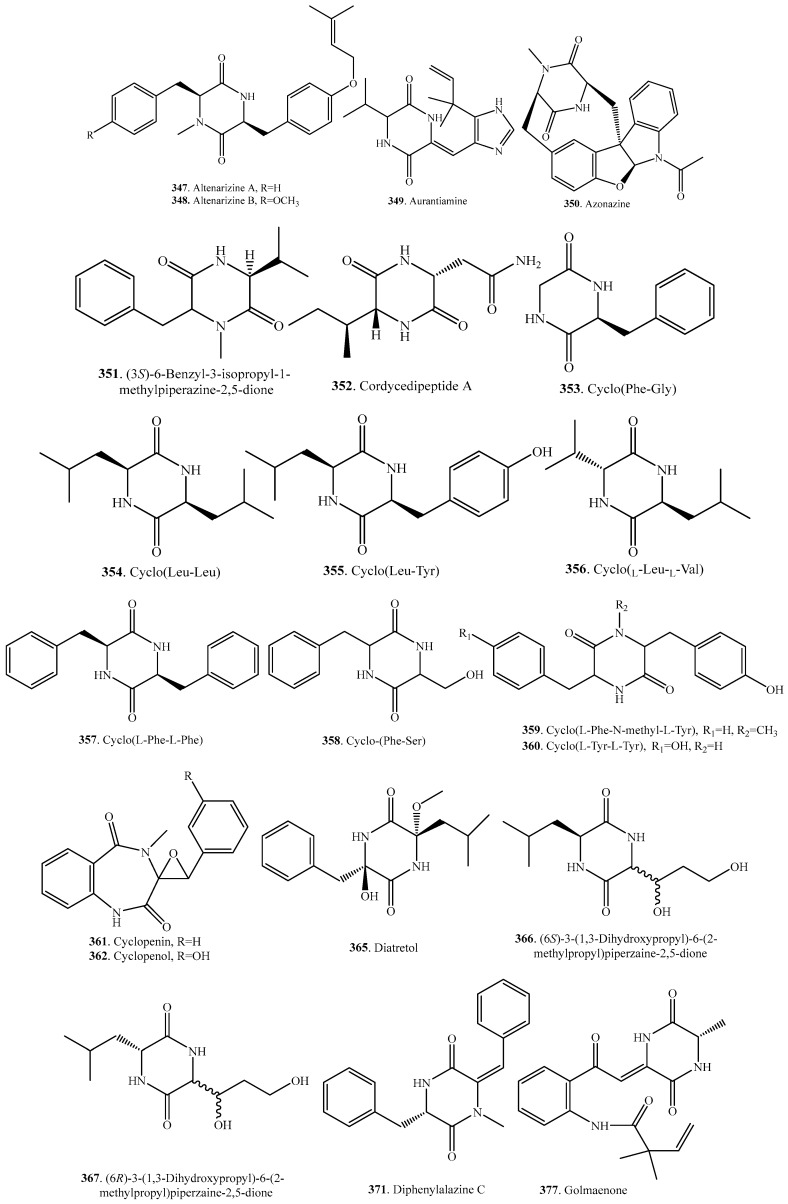
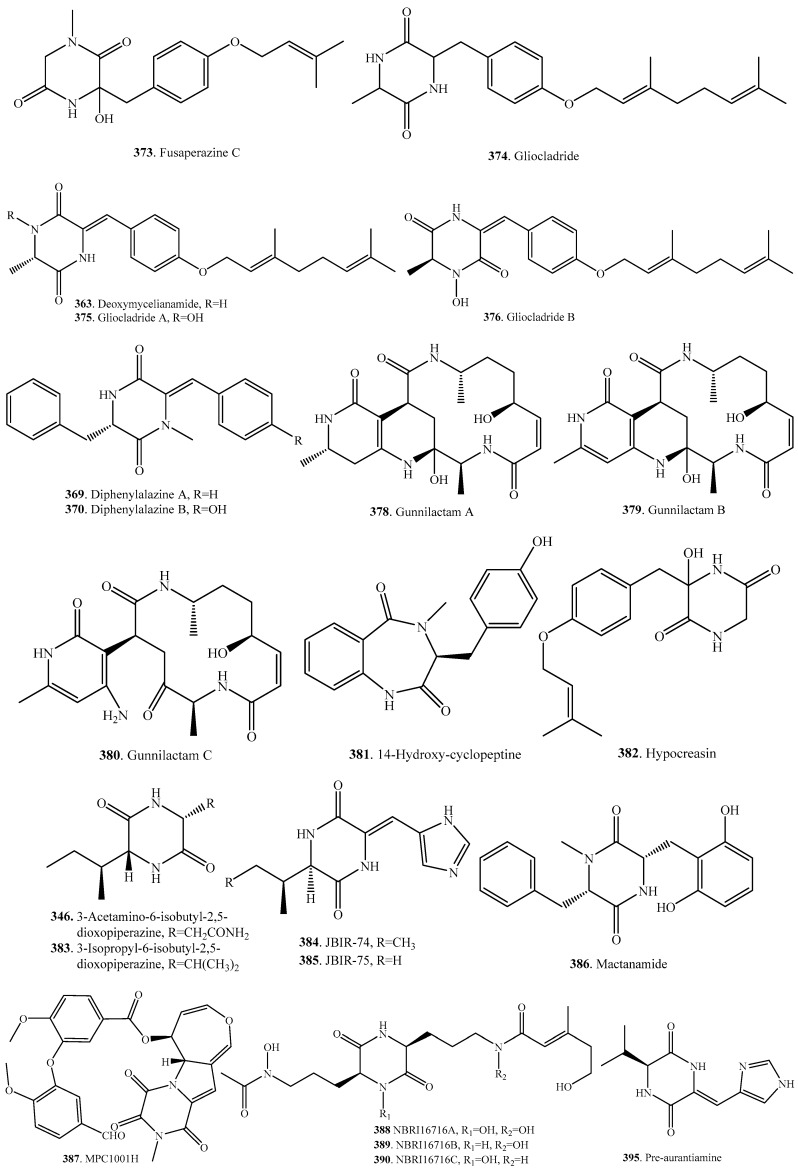
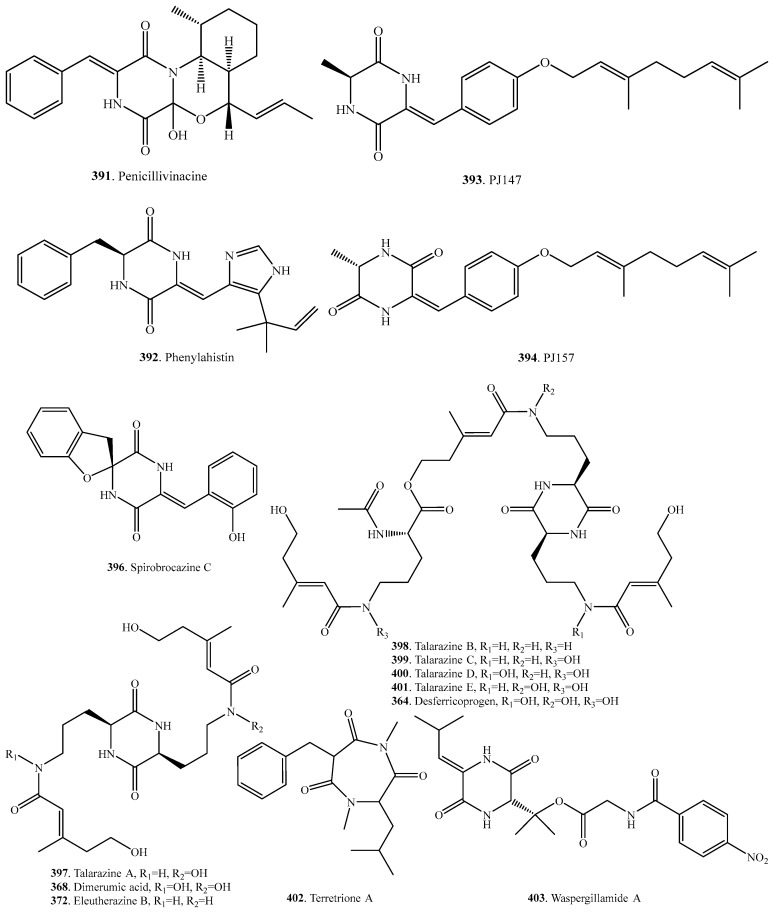
Non-tryptophan–non-proline cyclodipeptides mean neither tryptophan nor proline is incorporated into this group of cyclodipeptides in the fungi. Their occurrence and biological activities are shown in Table 5, and the structures are provided in Figure 5.
Table 5.
Non-tryptophan–non-proline cyclodipeptide analogs and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 3-Acetamino-6-isobutyl-2,5-dioxopiperazine (346) | Cordyceps sinensis | Cytotoxic activity | [169] |
| Altenarizine A (347) | Endophytic Alternaria alternata from the root of Ceratostigma griffithii | - | [183] |
| Altenarizine B (348) | Endophytic Alternaria alternata from the root of Ceratostigma griffithii | - | [183] |
| Aurantiamine (349) | Penicillium aurantiogriseum var. aurantiogriseum | - | [184] |
| Azonazine (350) | Aspergillus insulicola | Anti-inflammatory activity | [170] |
| (3S)-6-Benzyl-3-isopropyl-1-methylpiperazine-2,5-dione (351) | Entomogenous Paecilomyces tenuipes | Moderate cytotoxicity against prostate cancer cells 22RV1 and DU-145 | [185] |
| Cordycedipeptide A (352) | Cordyceps sinensis | Cytotoxic activity | [169] |
| Cyclo(Gly–Phe) (353) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(Leu–Leu) (354) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(Leu–Tyr) (355) | Unidentified fungus from Kandelia candel leaf | - | [186] |
| Cyclo(l-Leu–l-Val) (356) | Endophytic Aspergillus fumigatus from the stem of Erythrophleum fordii | - | [187] |
| Cyclo(l-Phe–l-Phe) (357) | Penicillium nigricans | Anthelmintic activity against Hymenolepis nana and Schistosoma mansoni in mice | [171,172] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Cyclo(Phe–Ser) (358) | Endophytic Alternaria sp. FL25 from Ficus carica | Antiphytopathogenic fungal activity | [38] |
| Insect pathogenic Verticillium hemipterigenum | Cytotoxic and antimicrobial activity | [173,174] | |
| Cyclo(l-Phe-N-methyl-l-Tyr) (359) | Geotrichum candidum | Inhibitory activity against Peronophythora litchii | [189] |
| Cyclo(l-Tyr–l-Tyr) (360) | Cordyceps sinensis | - | [169] |
| Cyclopenin (361) | Penicillium verrucosum var. cyclopium | - | [124] |
| Cyclopenol (362) | Penicillium verrucosum var. cyclopium | - | [124] |
| Mangrove endophytic Penicillium sclerotiorum from Bruguiera gymnorrhiza | - | [190] | |
| Deoxymycelianamide (363) | Marine-derived Gliocladium sp. | Strong cytotoxic activity | [179] |
| Desferricoprogen (364) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Diatretol (365) | Clitocybe diatreta | Weak antibacterial activity | [175] |
| (6S)-3-(1,3-Dihydroxypropyl)-6-(2-methylpropyl)piperzaine-2,5-dione (366) | Plant endophytic Trichosporum sp. from the seeds of Trigonella foenum-graecum | Antileishmanial activity against Leishmania donovani with IC50 value of 96.3 μg/mL | [192] |
| (6R)-3-(1,3-Dihydroxypropyl)-6-(2-methylpropyl)piperzaine-2,5-dione (367) | Plant endophytic Trichosporum sp. from the seeds of Trigonella foenum-graecum | Antileishmanial activity against Leishmania donovani with IC50 value of 82.5 μg/mL | [192] |
| Dimerumic acid (368) | Monascus anka | Antioxidant activity | [176,177] |
| Mud dauber wasp-derived Talaromyces sp. CMB-W045 | Demonstratinghigh affinity for Fe(III) | [191] | |
| Monascus anka | Antioxidant activity by inhibition on lipid peroxidation and hemeprotein-mediated oxidation | [193] | |
| Diphenylalazine A (369) | Epicoccum nigrum colonizing on Cordyceps sinensis | Inhibitory effects on HIV-1 replication in C8166 cells | [194] |
| Diphenylalazine B (370) | Epicoccum nigrum colonizing on Cordyceps sinensis | - | [194] |
| Diphenylalazine C (371) | Tin mine tailings-derived Schizophyllum commune | Weak antibacterial and cytotoxic activities | [195] |
| Eleutherazine B (372) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Fusaperazine C (373) | Endophytic Fusarium sp. from Viguiera arenaria | - | [196] |
| Gliocladride (374) | Marine-derived Gliocladium sp. | Cytotoxic activity | [178] |
| Gliocladride A (375) | Marine-derived Gliocladium sp. | Moderate cytotoxic activity | [179] |
| Gliocladride B (376) | Marine-derived Gliocladium sp. | Moderate cytotoxic activity | [179] |
| Golmaenone (377) | Marine-derived Aspergillus sp. | Radical scavenging activity against DPPH, UV-A protecting activity | [103] |
| Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with IC50 value of 20 μM | [134] | |
| Gunnilactam A (378) | Entomogenous Paecilomyces gunnii | Cytotoxic activity against human prostate cancer C42B cells | [197] |
| Gunnilactam B (379) | Entomogenous Paecilomyces gunnii | - | [197] |
| Gunnilactam C (380) | Entomogenous Paecilomyces gunnii | - | [197] |
| 14-Hydroxy-cyclopeptine (381) | Aspergillus sp. SCSIOW2 | Inhibition of nitric oxide production with IC50 value of 40.3 μg/mL in a lipopolysaccharide and recombinant mouse interferon-γ-activated macrophage-like cell line | [198] |
| Hypocreasin (382) | Hypocrea spp. | - | [199] |
| 3-Isopropyl-6-isobutyl-2,5-dioxopiperazine (383) | Cordyceps sinensis | - | [169] |
| JBIR-74 (384) | Marine-derived Aspergillus sp. fS14 from the unidentified marine sponge | - | [200] |
| JBIR-75 (385) | Marine-derived Aspergillus sp. fS14 from the unidentified marine sponge | - | [200] |
| Mactanamide (386) | Marine-derived Aspergillus sp. | Fungistatic activity to Candida albicans | [180] |
| MPC1001H (387) | Podospora australis | - | [201] |
| NBRI16716A (388) | Perisporiopsis melioloides Mer-f16716 | Cytotoxic activity | [181] |
| NBRI16716B (389) | Perisporiopsis melioloides Mer-f16716 | Cytotoxic activity | [181] |
| NBRI16716C (390) | Perisporiopsis melioloides Mer-f16716 | - | [181] |
| Penicillivinacine (391) | Marine-derived Penicillium vinaceum | Antimigratory activity | [22] |
| Phenylahistin (392) | Aspergillus ustus NSC-F038 | Growth inhibition of various tumor cell lines | [182] |
| PJ147 (393) | Marine-derived Gliocladium sp. YUP08 from soil | Cytotoxic activity on A375-S2, Hela, P388, A-549, HL-60, and BEL-7420 cell lines | [202,203] |
| PJ157 (394) | Marine-derived Gliocladium sp. YUP08 from soil | - | [202] |
| Pre-aurantiamine (395) | Marine-derived Aspergillus aculeatus CRI322-03 from the sponge Stylissa flabeliformis | - | [204] |
| Spirobrocazine C (396) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | Moderate cytotoxic and antibacterial activities | [205] |
| Talarazine A (397) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine B (398) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine C (399) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine D (400) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Talarazine E (401) | Mud dauber wasp-derived Talaromyces sp. CMB-W045 | - | [191] |
| Terretrione A (402) | Marine-derived Penicillium vinaceum | Antimigratory activity | [22] |
| Waspergillamide A (403) | Aspergillus sp. CMB-W031 | - | [206] |
Note: IC50, median inhibitory concentration.
Figure 5.
Structures of the non-tryptophan-non-proline cyclodipeptide analogs isolated from fungi.
3-Acetamino-6-isobutyl-2,5-dioxopiperazine (346) and 3-isopropyl-6-isobutyl-2,5-dioxopiperazine (383), belonging to the aliphatic isoleucine cyclodipeptide, were isolated from Cordyceps sinensis. Only 3-acetamino-6-isobutyl-2,5-dioxopiperazine (346) had cytotoxic activity against L-929, A375, and HeLa cells [169].
Azonazine (350) was isolated from a Hawaiian marine sediment-derived fungus Aspergillus insulicola, and exhibited anti-inflammatory activity by inhibiting NF-κB luciferase (IC50, 8.37 μM) and nitrate production (IC50, 13.7 μM) [170].
Cyclo(l-Phe–l-Phe) (357) originally isolated from Penicillium nigricans was also isolated from a marine mangrove endophytic fungus [171], and exhibited good anthelmintic activity against Hymenolepis nata and Schistosoma mansoni in mice [172].
Cyclic phenylalanyl serine cyclo(Phe–Ser) (358) was isolated from the insect pathogenic fungus Verticillium hemipterigenum. It exhibited concentration-dependent atypical intestinal absorption in the small intestine of rats, which consisted of passive transport, carrier-mediated absorptive transport by PEPT1, and carrier-mediated excretive transport. It also exhibited weak inhibition of several cancer cell lines and selected microorganisms [173,174].
The tyrosine analog cyclo(l-Tyr–l-Tyr) (360) isolated from the culture broth of Cordyceps sinensis reversibly blocked voltage-dependent L-type calcium channels [169].
Diatretol (365) from the fungus Clitocybe diatreta exhibited a weak antibacterial activity. A single-crystal X-ray analysis showed that diatretol (365) has a nearly planar boat conformation in the solid state [175].
Dimerumic acid (368) has been isolated from the fungus Monascus anka, traditionally used for fermentation of food, and shown to be an antioxidant with hepatoprotective actions against chemically induced liver injuries [176], as well as protecting against oxidative stress-induced cytotoxicity in the isolated rat hepatocytes [177].
Gliocladride (374) isolated from marine fungus Gliocladium sp. showed a cytotoxic effect with an IC50 value of 3.86 mg/mL against human A375-S2 melanoma cell line [178].
Gliocladrides A (375) and B (376) as well as deoxymycelianamide (363) were isolated from the marine fungus Gliocladium sp. to show cytotoxic activity against the three cell lines (HL-60, U937 and T47D) with IC50 values 11.6–52.8 μg/mL, while deoxymycelianamide (363) showed the strongest cytotoxic activity against U937 cell line with an IC50 value of 0.8 μg/mL [179].
Golmaenone (377) from the culture broth of the marine-derived fungus Aspergillus sp. exhibited a significant radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and showed UV-A (320–390 nm) protecting activity which was more active than oxybenzone currently used as a sunscreen [103].
The marine-derived fungus Aspergillus sp. yielded mactanamide (386) containing an R-2,6-dihydroxyphenylalanine, which showed fungistatic activity to Candida albicans at nontoxic concentration [180].
Three siderophores NBRI16716A (388), NBRI16716B (389), and NBRI16716C (390) were isolated from the fungus Perisporiopsis melioloides Mer-f16716. Compounds NBRI16716A (388) and NBRI16716B (389) inhibited the growth of human prostate cancer DU-145 cells in the coculture with human prostate stromal cells (PrSCs) more strongly than that of DU-145 cells alone. Furthermore, both compounds showed antitumor effect against xenograft models of DU-145 cells and PrSCs in vivo [181].
Phenylahistin (392) from the culture broth of Aspergillus ustus NSC-F038 exhibited a strong growth inhibition on various tumor cell lines for its microtubule binding function to show its potency as the tubulin depolymerizing agent [182].
7. Thio-Cyclodipeptides
The thio-cyclodipeptides are 2,5-diketopiperazines containing thio functionality in bridged or open form, and their occurrence and activities have been reviewed in 2006 and 2014 [1,207]. According to the positions of the sulfur linkages, we divide thio cyclodipeptides into four subgroups: 1,4-bridged epipolythioxopiperazines (ETPs), derivatives with sulfur-bridge outside 2,5-DKP ring, nonbridged dimethylthio derivatives, and other sulfur-containing cyclodipeptides. About 232 thio-cyclodipeptides have been isolated from fungi.
7.1. 1,4-Bridged Epiplythiodioxopiperazine Analogs
The sulfur-bridged cyclodipeptides are a class of metabolites mainly dominated by the epipolythiodioxopiperazines (ETPs) [1]. The toxicity of ETPs is due to the presence of a sulfide bridge, which can inactivate proteins via reaction with thiol groups and by generation of reactive oxygen species by redox cycling [5]. The ETPs are known for their cytotoxic effect on cancer cell lines, and it has been shown mechanistically that ETPs were transcriptional antagonists that block the interaction of the p300/CBP coactivator with the hypoxia-inducible transcription factor HIF-1α by a zinc ejection mechanism, which resulted in rapid down regulation of hypoxia-inducible genes critical for cancer progression [208]. The occurrence and biological activities of 1,4-bridged epiplythiodioxopiperazine analogs from fungi are shown in Table 6, and their structures are provided in Figure 6.
Table 6.
Fungal 1,4-bridged epiplythiodioxopiperazine analogs and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 3822-A (404) | Stereum hirsutum HKI 0195 | - | [219] |
| A26771A (405) | Penicillium turbatum | Antiviral and antibacterial activity | [226] |
| A26771 C (406) | Penicillium turbatum | Antiviral and antibacterial activity | [226] |
| Acetylapoaranotin (407) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| Acetylaranotin (408) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| Apoaranotin (409) | Arachniotus aureus | - | [227] |
| Aranotin (410) | Arachniotus aureus | - | [227] |
| Bionectin A (411) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Bionectin B (412) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Brocazine A (413) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine B (414) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine C (415) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | - | [229] |
| Brocazine D (416) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | - | [229] |
| Brocazine E (417) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine F (418) | Endophytic Penicillium brocae MA-231 from mangrove Avicennia marina | Cytotoxic activity | [229] |
| Brocazine G (419) | Marine-derived Penicillium brocae MA-231 from the mangrove Avicennia marina | Cytotoxicity against both sensitive and cisplatin-resistant human ovrian cancer cells and strong antimicrobial activity on pathogenic Staphylococcus aureus | [205] |
| Chaetocin = Chaetocin A (420) | Chaetomium minutum | Antibacterial, cytostatic, inhibitory activity on lysine-specific histone methyltransferases | [209] |
| Chaetocin B (421) | Chaetomium sp. | Cytotoxic activity | [210] |
| Chaetocin C (422) | Chaetomium sp. | Cytotoxic activity | [210] |
| Chaetocochin B (423) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chaetocochin C (424) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chetomin (425) | Marine mudflat sediment derived Chaetomium cristatum collected at Suncheon Bay of Korea | Radical-scavenging activity against DPPH with an IC50 value of 15 μM | [134] |
| Chetoseminudin A (426) | Chaetomium globosum | Antibacterial activity | [230] |
| Chaetomium seminudum | Immunomodulatory activity | [231] | |
| Chetracin A (427) | Chaetomium spp. | Cytotoxic activity | [210] |
| Chetracin B (428) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Chetracin C (429) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Cristazine (430) | Mudflat-sediment-derived Chaetomium cristatum | Radical-scavenging activity, cytotoxic activity against human cervical carcinoma (HeLa) cells | [134] |
| Dehydrogliotoxin (431) | Gliocladium flavofuscum | Antituberculosis activity | [232] |
| Gliocladium virens | Antituberculosis activity | [233] | |
| Deoxyapoaranotin (432) | Marine-derived Aspergillus sp. KMD 901 | Directly cytotoxic and apoptosis inducing effects on HCT116 colon cancer cell lines | [212] |
| 11‘-Dexoyverticillin A (433) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| 11,11‘-Dihydroxychaetocin (434) | Verticillium tenerum | Antibacterial and antimitotic activities | [234] |
| Dithiosilvatin (435) | Aspergillus silvaticus | - | [235] |
| Emestrin (436) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Emestrin B (437) | Emericella striata | Antifungal activity | [237] |
| Emestrin C = MPC1001 (438) | Podospora australis | Antifungal activity | [201] |
| Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] | |
| Cladorrhinum sp. KY4922 | Antitumor and antibacterial activity | [220] | |
| Emestrin D = MPC1001D (439) | Podospora australis | Antifungal activity | [201] |
| Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] | |
| Emestrin E (440) | Podospora australis | Antifungal activity | [201] |
| Emestrin F (441) | Armillaria tabescens | Antifungal activity | [238] |
| Emestrin G (442) | Armillaria tabescens | - | [238] |
| Emestrin J (443) | Podospora australis | - | [201] |
| Emethallicin A (444) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [213] |
| Emethallicin B (445) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin C (446) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin D (447) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [214] |
| Emethallicin E (448) | Emericella heterothallica | Inhibitory activity on histamine release from mast cells | [215] |
| Emethallicin F (449) | Emericella heterothallica | Inhibitory activity on histamine release mast cells | [215] |
| Epicoccin T (450) | Endophytic fungus Epicoccum nigrum from the leaves Lysidice rhodostegia | - | [188] |
| Epicoccin U (451) | Tin mie tailings-derived Schizophyllum commune | Weak antibacterial and cytotoxic activities | [195] |
| Epicorazine A (452) | Capnodium sp. from palm leaf litter collected in Quetzalito, Guatemala | - | [239] |
| Epicoccum purpurascens | - | [240] | |
| Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | - | [229] | |
| Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] | |
| Podaxis pistillaris | Antibacterial and cytotoxic activities | [242] | |
| Basidiomycete Stereum hirsutum HKI 0195 | Cytotoxic activity against HeLa cells and antiproliferative effects against several mouse fibrobalst and cancer cell lines | [219] | |
| Epicorazine B (453) | Epicoccum purpurascens | - | [240] |
| Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove Kandelia candel | Cytotoxic activity | [241] | |
| Podaxis pistillaris | Antibacterial and cytotoxic activities | [242] | |
| Basidiomycete Stereum hirsutum HKI 0195 | Cytotoxic activity against HeLa cells and antiproliferative effects against several mouse fibroblast and cancer cell lines | [219] | |
| Epicorazine C (454) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] |
| Podaxis pistillaris | Antibacterial activity | [242] | |
| Stereum hirsutum HKI 0195 | Antibacterial, antifungal, antiproliferative and cytotoxic activities | [219] | |
| Gliocladine A (455) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine B (456) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine C (457) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine D (458) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Gliocladine E (459) | Gliocladium roseum from submerged wood | Atinematodal activity | [222] |
| Glionitrin A (460) | Coculture of the fungus Aspergillus fumigatus KMC-901 and the bacterium Sphingomonas sp. KMK-001 | Antibacerial and cytotoxic activities | [243] |
| Gliotoxin (461) | Aspergillus fumigatus | Cytotoxic activity | [217] |
| Gliocladium flavofuscum | - | [232] | |
| Deep-sea derived Aspergillus sp. SCSIO Ind09F01 | Anti-tuberculosis and cytotoxic activities | [35] | |
| Hyalodendrin (462) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Hyalodendrin-S3 (463) | Unidentified fungus NRRL 3888 | - | [245] |
| Hyalodendrin-S4 (464) | Hyalodendron sp. | - | [246] |
| Leptosin A (465) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [247] |
| Leptosin B (466) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin C (467) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [247] |
| Leptosin D (468) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin E (469) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin F (470) | Leptosphaeria sp. from a marine alga | - | [247] |
| Leptosin G (471) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin G1 (472) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin G2 (473) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin H (474) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [248] |
| Leptosin I (475) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [249] |
| Leptosin J (476) | Leptosphaeria sp. from a marine alga | Cytotoxicity | [249] |
| Leptosin K (477) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin K1 (478) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin K2 (479) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [250] |
| Leptosin M (480) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells; Inhibition on two protein kinases, PTK and CaMKIII, and human topoisomerase II | [251] |
| Leptosin M1 (481) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Leptosin N (482) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Leptosin N1 (483) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [251] |
| Melinacidin II (484) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Melinacidin III (485) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Melinacidin IV (486) | Acrostalagmus cinnabarinus var. melinacidinus | Antibacterial activity | [252] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] | |
| MPC1001B (487) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| MPC1001C (488) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Podospora australis | Antifungal activity | [201] | |
| MPC1001E (489) | Cladorrhinum sp. KY4922 | Antiproliferative activity | [236] |
| Phomalirazine (490) | Leptosphaeria maculans | Phytotoxic activity | [253] |
| Phomazine C (491) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | - | [241] |
| Plectosphaeroic acid C (492) | Marine-derived Plectosphaerella cucumerina | Inhibtion of indoleamine 2,3-dioxygenase | [254] |
| Rostratin A (493) | Endophytic Epicoccum nigrum from the leaves Lysidice rhodostegia | - | [188] |
| Exserohilum rostratum | Moderate cytotoxicity | [218] | |
| Rostratin B (494) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Rostratin C (495) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Rostratin D (496) | Exserohilum rostratum | Moderate cytotoxicity | [218] |
| Sch52900 (497) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| Sch52901 (498) | Gliocladium roseum from submerged wood | Antinematodal activity | [222] |
| Secoemestrin C (499) | Emericella foveolata | - | [255] |
| Secoemestrin D (500) | Podospora australis | - | [201] |
| Endophytic fungus Emericella sp. AST0036 from healthy leaf tissue of Astragalus lentiginosus | Cytotoxic activity | [221] | |
| Sirodesmin A (501) | Sirodesmium diversum | Antiviral activity | [256] |
| Sirodesmin B (502) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin C (503) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin G = Sirodesmin PL (504) | Leptosphaeria maculans | Phytotoxic activity | [257,258] |
| Sirodesmium diversum | Antiviral activity | [256] | |
| Sirodesmin H (505) | Leptosphaeria maculans | Phytotoxic activity | [257] |
| Sporidesmin A = Sporidesmin (506) | Pithomyces chartarum | Immunoregulatory activity | [259,260] |
| Delitschia corticola | Antibacterial and antifungal activities | [261] | |
| Sporidesmin B (507) | Pithomyces chartarum | - | [259] |
| Sporidesmin C (508) | Pithomyces chartarum | - | [262] |
| Sporidesmin E (509) | Penicillium terlikowskii | - | [263] |
| Sporidesmin G (510) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [264] |
| Sporidesmin H (511) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [265] |
| Sporidesmin J (512) | Pithomyces chartarum | Antiproliferative, cytotoxic, immunomodulatory, antiviral, antibacterial, antifungal activities | [265] |
| T988 A (513) | Tilachidium sp. | Cytotoxic activity | [266] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [211] | |
| T988 C (514) | Tilachidium sp. | Cytotoxic activity | [266] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [211] | |
| Verticillin A (515) | Gliocladium roseum from submerged wood | Antinematodal and cytotoxic activities | [222] |
| Verticillium sp. | - | [223] | |
| Verticillin B (516) | Verticillium sp. | - | [223] |
| Verticillin C (517) | Verticillium sp. | - | [223] |
| Verticillin D (518) | Bionectria byssicola | Antibacterial activity | [225] |
| Gliocladium catenulatum | Antibacterial activity | [224] | |
| Verticillin E (519) | Gliocladium catenulatum | Antibacterial activity | [224] |
| Verticillin F (520) | Gliocladium catenulatum | Antibacterial activity | [224] |
| Verticillin G (521) | Bionectra byssicola | Antibacterial activity | [225] |
Note: IC50, median inhibitory concentration.
Figure 6.
Structures of the 1,4-bridged epiplythiodioxopiperazine cyclodipeptide analogs isolated from fungi.
The dimeric ETP chaetocin (420) was isolated from the fungus Chaetomium minutum, and, in addition to its antibacterial and cytostatic activity, was reported to have inhibitory activity against lysine-specific histone methyltransferases (HMTs), which are key enzymes in the epigenetic control of gene expression [209]. Chaetocins B (421) and C (422) from a Chaetomium sp. fermentation broth were potent inhibitors of Staphylococcus aureus, and exhibited potent cytotoxic activity against HeLa cells with IC50 values of 0.03 and 0.02 μg/mL, respectively [210].
Chaetocochins B (423) and C (424), which were isolated from Chaetomium cochliodes, showed cytotoxic activity to the cells of Bre-04 (breast cancer cells), Lu-04 (big cell lung cells), and N-04 (glioma cells) [211].
Three ETPs deoxyapoaranotin (432), acetylaranotin (408) and acetylapoaranotin (407) were isolated from Aspergillus sp. KMD 901 found in the marine sediment obtained from the East Sea of Korea. They had directly cytotoxic and apoptosis inducing effects toward HCT116 colon cancer cell lines [212].
Emethallicins A–F (444–449) were obtained from Emericella heterothallica. These compunds all displayed inhibitory activity on histamine release from mast cells [213,214,215].
The best known ETP was the small lipid-soluble gliotoxin (461), which exerted toxic effects on phagocytic cells and T-lymphocytes at low concentrations in vitro. This compound was the first ETP to be obtained from fungi. It has been isolated from a variety of fungi including species in the genera of Penicillium, Aspergillus, Gliocladium, Thermoascus, and Candida [2]. High levels of gliotoxin (461) were produced by Aspergillus fumigatus in vivo, and it appeared to be a virulence factor associated with invasive aspergillosis of immunocompromised patients [216]. Gliotoxin (461) was also a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo [217].
Two bridged disulfides epicoccin T (450) and rostratin A (493) were isolated from the endophytic fungus Epicoccum nigrum obtained from the leaves of Lysidice rhodostegia. However, they did not show detectable cytotoxic activities toward six tumor cell lines in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [188]. In contrast, the rostratins A–D (493–496), which were extracted from Exserohilum rostratum, were found to be modestly cytotoxic against human colon carcinoma HCT-116 [218]. Similarly, the unsaturated analogs epicorazines A (452), B (453), and C (454) isolated from the culture broth of the basidiomycete Stereum hirsutum HKI 0195 were highly cytotoxic against HeLa cells and also exhibited antiproliferative effects against several mouse fibroblast and cancer cell lines [219].
The antifungal macrolide MPC1001 (438) possessing an ETP ring was isolated from the fungus Cladorrhinum sp. KY4922, which was found in a soil sample collected in Indonesia. It possessed antiproliferative activity against the human prostate cancer cell line (DU145) with an IC50 of 9.3 nM, and had antibacterial activity against Gram-positive bacteria [220].
Secoemestrin D (500) was obtained from the endophytic fungus Emericella sp. AST0036 isolated from a healthy leaf tissue of Astragalus lentiginosus. This compound showd cytotoxic activity with IC50 values ranging from 0.06 to 0.24 μM on tumor cell lines [221].
Verticillin A (515) was obtained from Gliocladium roseum derived from submerged wood. It showed antinematodal activity against Panagrellus redivivus and Caenorhabditis elegans, and cytotoxic activity against HeLa cells with an IC50 value of 0.2 μg/mL [222]. Verticillins B (516) and C (517) were isolated from Verticillium sp. [223]. Verticillin D (518), which was isolated from Bionectria byssicola [222] and Gliocladium catenulatum [224], showed antibacterial activity against Staphylococcus aureus. Verticillins E (519) and F (520) from Gliocladium catenulatum showed antibacterial activity [224]. Verticillin G (521) from Bionectra byssicola showed antibacterial activity against Staphylococcus aureus including methicillin-resistant and quinolone-resistant varieties with minimum inhibitory concentrations (MICs) of 3–10 μg/mL [225].
7.2. Analogs with Sulfur-Bridge outside 2,5-DKP Ring
There are a group of sulfur-bridged cyclodipeptides where the sulfur linkage is outside the 2,5-diketoperazine ring. The occurrence and biological activities of fungal analogs with sulfur-bridges outside 2,5-DKP ring are shown in Table 7, and their structures are provided in Figure 7.
Table 7.
Fungal analogs with sulfur-bridge outside 2,5-DKP ring and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Adametizine A = N-methyl pretrichodermamide B (522) | Marine sponge-derived Penicillium adametzioides AS-53 | Lethal activity against brine shrimp and antibacterial activity | [269] |
| Adametizine B = Pretrichodermamide C (523) | Marine sponge-derived Penicillium adametzioides AS-53 | - | [269] |
| Aspirochlorine (524) | Aspergillus flavus | Antifungal activity on azole-resitant Candida albicans | [107] |
| Chlorotrithiobrevamide (525) | Trichoderma cf. brevicompactum | Cytotoxic effects against Jurkat cells with IC50 values of 16 µM | [270] |
| DC1149B (526) | Trichoderma cf. brevicompactum | Cytotoxic effect against Jurkat cells with IC50 values of 5.1 µM | [270,271] |
| DC1149R(527) | Trichoderma cf. brevicompactum | - | [271] |
| Epicoccin A (528) | Cordyceps-colonizing Epicoccum nigrum | Moderate antimicrobial activity | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin B (529) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin C (530) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin D (531) | Cordyceps-colonizing Epicoccum nigrum | - | [268] |
| Epicoccin E (532) | Cordyceps-colonizing Epicoccum nigrum | - | [194] |
| Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] | |
| Epicoccin F (533) | Cordyceps-colonizing Epicoccum nigrum | - | [194] |
| Epicoccin I (534) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin M (535) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin N (536) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin O (537) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin P (538) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin Q (539) | Endophytic Epicoccum nigrum from the leaves of Lysidice rhodostegia | - | [188] |
| Epicoccin R (540) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin S (541) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Gliovirin (542) | Trichoderma cf. brevicompactum | - | [270,271] |
| Iododithiobrevamide (543) | Trichoderma cf. brevicompactum | - | [271] |
| Outovrin A (544) | Endophytic Penicillium raciborskii from Rhododendron tomentosum | - | [272] |
| Outovirin B (545) | Endophytivc Penicillium raciborskii from Rhododendron tomentosum | - | [272] |
| Outovirin C (546) | Endophytic Penicillium raciborskii from Rhododendron tomentosum | Antifungal activity | [272] |
| Penicisulfuranol A (547) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Penicisulfuranol B (548) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Penicisulfuranol C (549) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | Cytotoxic activity on the cell lines HeLa and HL-60 | [273] |
| Pretrichodermamide A (550) | Trichoderma cf. brevicompactum | - | [270,271] |
| Trichoderma sp. BCC 5926 | Activity against Mycobacterium tuberculosis H37Ra with an MIC value of 12.5 µg/mL | [274] | |
| Tetrathioaspirochlorine (551) | Aspergillus flavus | Antifungal activity on azole-resitant Candida albicans | [107] |
| Trithioaspirochlorine (552) | Aspergillus flavus | - | [107] |
| Vertihemiptellide A (553) | Insect pathogenic Verticillium hemipterigenum BBC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra, moderate cytotoxic activity | [174] |
| Vertihemiptellide B (554) | Insect pathogenic Verticillium hemipterigenum BBC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra, moderate cytotoxic activity | [174] |
Note: IC50, median inhibitory concentration; MIC, minimum inhibitory concentration.
Figure 7.
Structures of the analogs with sulfur-bridge outside 2,5-DKP ring isolated from fungi.
Both aspirochlorine (524) and tetrathioaspirochlorine (551) were isolated from Aspergillus flavus and were potent antifungals that inhibited azole-resistant Candida albicans [107]. Aspirochlorine (524) was a rather potent and selective inhibitor of fungal protein synthesis that did not inhibit bacterial or mammalian protein synthesis [267].
Epicoccins are diannulated 2,5-DKPs containing mono- or bis-cross ring sulfide/disulfide bridges. Epicoccins A–F (528–533) have been isolated from the solid-substrate fermentation culture of the Cordyceps-colonizing fungus Epicoccum nigrum [194,268]. Epicoccin A (528) showed modest antimicrobial activity against Bacillus subtilis [268].
The dimeric 2,5-DKPs vertihemiptellides A (553) and B (554) were isolated from the insect pathogenic fungus Verticillium hemipterigenum, and exhibited growth inhibitory activity against Mycobacterium tuberculosis H37Ra, and also showed moderate cytotoxic activity [174].
7.3. Nonbridged Methylthio-Containing Cyclodipeptide Analogs
Nonbridged methylthio-containing analogs were often isolated from fungi as co-metabolites with their sulfur-bridge 2,5-DKP parents. They had a related biosynthetic pathway [275]. The occurrence and biological activities of this group of fungal metabolites are shown in Table 8, and their corresponding structures are provided in Figure 8.
Table 8.
Nonbridged methylthio-containing cyclodipeptide analogs from fungi and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Alternarosin A (555) | Marine-derived Alternaria raphani | Weak antimicrobial activity | [276] |
| Asteroxepin (556) | Aspergillus terreus | - | [284] |
| (Z)-6-Benzylidene-3-hydroxymethyl-1,4-dimethyl-3-methylsulfanylpiperazine-2,5-dione (557) | Marine-derived unidentified strain CRIF2 of the order Pleosporales | Weak cytotoxic activity | [285] |
| Bilain A (558) | Marine-derived Penicillium bilaii | - | [161] |
| Bilain B (559) | Marine-derived Penicillium bilaii | - | [161] |
| Bilain C (560) | Marine-derived Penicillium bilaii | - | [161] |
| Bionectin C (561) | Bionectra byssicola F120 | Anti-MRSA activity | [228] |
| Bisdethiobis(methylsulfanyl)acetylapoaranotin (562) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylsulfanyl)acetylaranotin = Bisdethiodi(methylthio)-acetylaranotin (563) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylsulfanyl)apoaranotin = Bisdethiodi(methylthio)-acetylapoaranotin (564) | Aspergillus terreus BCC 4651 | Weak antimycobacterial activity | [286] |
| Bisdethiobis(methylsulfanyl)aranotin (565) | Aspergillus terreus BCC 4651 | - | [286] |
| Bisdethiobis(methylthio) gliotoxin (566) | Marine-derived Aspergillus fumigatus | - | [278] |
| Aspergillus fumigatus from saltwater | Moderate trypanocidal activity | [287] | |
| Endophytic Colletotrichum gloeosporioides from Viguiera robusta | Specific inhibitor of the platelet activating factor and antibacterial activity | [196] | |
| Marine-derived fungus Pseudallescheria sp. | Antibacterial activity | [280] | |
| Bisdethiodi(methylthio)-1-demethylhyalodendrin (567) | Insect pathogenic Verticillium hemipterigenum BCC 1449 | Inhibitory activity against Mycobacterium tuberculosis H37Ra; moderate cytotoxic activity | [174] |
| (3R,6R)-Bisdethiodi(methylthio)-hyalodendrin (568) | Marine-derived unidentified strain CRIF2 of the order Pleosporales | Weak cytotoxic activity | [285] |
| Cordyceps-colonizing fungus Isaria farinosa | - | [288] | |
| cis-Bis(methylthio)silvatin = cis-Bisdethiodi(methylthio) silvatin (569) | Fusarium chlamydosporum from the marine alga Carpopeltis affinis | Weak cytotoxic activity against P388 lymphocytic leukemia cells | [277] |
| Marine-derived Penicillium bilaii | Weak cytotoxicity against NS-1 cells | [161] | |
| Plant endophytic Penicillium sp. | Antibacterial activity against Staphylococcus aureus with an MIC value of 43.4 μg/mL | [289] | |
| Bis-N-norgliovictin (570) | Gliocladium virens | - | [233] |
| Marine-derived Aspergillus fumigatus | - | [278] | |
| Marine-derived fungus Neosartorya pseudofischeri | - | [136] | |
| Marine-derived Dichotomomyces sp. L-8 | - | [113] | |
| - | Inhibitory activity on LPS-induced inflammation in macrophages | [279] | |
| Chaetocochin A (571) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Chetoseminudin B (572) | Nectria inventa | Trypanocidal activity in the whole cell assay of Trypanosoma brucei | [287] |
| Chetoseminudin C (573) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Chaetomium seminudum | - | [231] | |
| Chetoseminudin E (574) | Endophytic fungus Chaetomium sp. 88194 | - | [290] |
| Chetracin D (575) | Antarctic psychrophilic fungus Oidiodendron truncatum | Cytotoxic activity | [137] |
| Colletopiperazine (576) | Endophytic Colletotrichum gloeosporioides from Viguiera robusta | - | [196] |
| Dehydroxybisdethiobis(methylthio) gliotoxin (577) | Marine-derived Pseudallescheria sp. | Antibacterial activity | [280] |
| Dethio-tetra(methylthio)chetomin (578) | Chaetomium cochliodes | Cytotoxic activity | [211] |
| Dichotocejpin A (579) | Dichotomomyces cejpii FS110 | Inhibitory activity against α-glucosidase | [112] |
| Didehydrobisdethiobis (methylthio) gliotoxins (580) | Marine-derived Aspergillus sydowi from a driftwood sample | - | [10] |
| Epicoccin G (581) | Cordyceps-colonizing Epicoccum nigrum | Inhibitory effect on HIV-1 replication in C8166 cells | [194] |
| ent-Epicoccin G (582) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | Inhibitory activity against the release of β-glucuronidase in rat polymorphonuclear leukocytes induced by platelet-activating factor | [188] |
| Epicoccin H (583) | Cordyceps-colonizing Epicoccum nigrum | Inhibitory effect on HIV-1 replication in C8166 cells | [194] |
| Epicoccin J (584) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin K (585) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Epicoccin L (586) | Endophytic Epicoccum nigrum from Lysidice rhodostegia | - | [188] |
| Emestrin H (587) | Podospora australis | - | [201] |
| Emestrin I (588) | Podospora australis | - | [201] |
| Emestrin K (589) | Podospora australis | - | [201] |
| FR106969 (590) | Penicillium citrinum | Inhibitory activity against PAF-induced rabbit platelet aggregation | [281] |
| Fusaperazine A (591) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | Weak cytotoxic activity | [277] |
| Fusaperazine B (592) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Fusaperazine E (593) | Endophytic Penicillium crustosum from Viguiera robusta | - | [196] |
| Gliocladin A (594) | Gliocladium roseum PS-N132 | Cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Gliocladin B (595) | Gliocladium roseum PS-N132 | Cytotoxicity against murine P388 lymphocytic leukemia cells | [125] |
| Glionitrin B (596) | Coculture of the fungus Aspergillus fumigatus KMC-901 and the bacterium Sphingomonas sp. KMK-001 | Suppression of DU145 cell invasion | [291] |
| Glioperazine (597) | Gliocladium roseum PS-N132 | Cytotoxic activity | [125] |
| Bionectra byssicola F120 | - | [126] | |
| Glioperazine B (598) | Bionectra byssicola F120 | Weak antibacterial activity | [126] |
| Gliovictin = (−)-Gliovictin = A26771E (599) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Marine-derived Asteromyces cruciatus | - | [292] | |
| Haematocin (600) | Phytopathogenic Nectria haematococca | Antifungal activity by inhibiting spore-germination and germ-tube elongation | [282] |
| 3-[(4-Hydroxyphenyl)-methyl]-1,4-dimethyl-3,6-bis(methylthio)-2,5-piperazinedione (601) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Leptosin O (602) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin P (603) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin Q (604) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| Leptosin R (605) | Leptosphaeria sp. from a marine alga | Cytotoxicity on P388 cells | [131] |
| MPC1001F (606) | Cladorrhinum sp. KY4922 | Antifungal activity | [236] |
| Podospora australis | - | [201] | |
| Mycoediketopiperazine (607) | Papularia sp. | Cytotoxicity on KB cells | [283] |
| 1N-Norgliovictin (608) | Marine-derived Asteromyces cruciatusis 763 from an unidentified decaying green alga | - | [244] |
| Oidioperazine A (609) | Antarctic psychrophilic fungus Oidiodendron truncatum | - | [137] |
| Penicibrocazine A (610) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | - | [293] |
| Penicibrocazine B (611) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine C (612) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine D (613) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicibrocazine E (614) | Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] |
| Penicisulfuranol D (615) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Penicisulfuranol E (616) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Penicisulfuranol F (617) | Mangrove-derived Penicillium janthinellum HDN13-309 from the roots of Sonneratia caseolaris | - | [273] |
| Phomazine A (618) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | - | [241] |
| Phomazine B (619) | Marine-derived Phoma sp. OUCMDZ-1847 from the mangrove plant Kandelia candel | Cytotoxic activity | [241] |
| Marine-derived Penicillium brocae MA-231 from the mangrove plant Avicennia marina | Antimicrobial activity | [293] | |
| Plectosphaeroic acid A (620) | Marine-derived Plectosphaerella cucumerina | Inhibition of indoleamine 2,3-dioxygenase | [254] |
| Plectosphaeroic acid B (621) | Marine-derived Plectosphaerella cucumerina | Inhibition of indoleamine 2,3-dioxygenase | [254] |
| Polanrazine B (622) | Plant pathogen Phoma lingam | Phytotoxic activity | [138] |
| Polanrazine C (623) | Plant pathogen Phoma lingam | Moderate and selective phytotoxicity by causing necrotic and chlorotic lesions | [138] |
| Polanrazine D (624) | Plant pathogen Phoma lingam | - | [138] |
| Pseudellone D (625) | Marine-derived Pseudallescheria ellipsoidea F42-3 associated with the soft coral Lobophytum crassum | - | [294] |
| Sch 54794 (626) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | Weak cytotoxic activity against P388 lymphocytic leukemia cells | [277] |
| Sch 54796 (627) | Fusarium chlamydosporum OUPS-N124 from the marine alga Carpopeltis affinis | - | [277] |
| Spirobrocazine A (628) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | Moderate antibacterial activity | [205] |
| Spirobrocazine B (629) | Mangrove-derived Penicillium brocae MA-231 from Avicennia marina | - | [205] |
| Sporidesmin D (630) | Pithomyces chartarum | - | [295] |
| Sporidesmin F (631) | Pithomyces chartarum | - | [296] |
| T988 B (632) | Tilachidium sp. | Cytotoxic activity | [225] |
| Antarctic psychrophilic fungus Oidiodendron truncatum | - | [211] |
Note: MIC, minimum inhibitory concentration.
Figure 8.
Structures of the nonbridged methylthio-containing cyclodipeptide analogs isolated from fungi.
Alternarosin A (555) from Alternaria raphanin, a halotolerant marine fungus obtained from the sediment of the Hongdao sea salt field, showed very weak antimicrobial activity against Escherichia coli, Bacillus subtilis, and Candida albicans with MIC values ranging from 200 to 400 μM [276].
Three 2,5-DKPs, bilains A–C (558–560) and cis-bis(methylthio)silvatin (480) were isolated from the marine-derived fungus Penicillium bilaii collected in Tasmania. However, only cis-bis(methylthio)silvatin (569) showed weak cytotoxicity against NS-1 cells [161]. Both cis-bis(methylthio)silvatin (569) and its enantiomer Sch 54794 (524) were previously isolated from the fungus Fusarium chlamydosporum OUPS-N124 obtained from the marine alga Carpopeltis affinis. They exhibited weak cytotoxic activity against P388 lymphocytic leukemia cells [277].
Bis-N-norgliovictin (570) was first isolated from Gliocladium virens [233]. It was also isolated from three marine-derived fungi Aspergillus fumigatus [278], Neosartorya pseudosidcheri [136], and Dichotomomyces sp. L-8 [113]. Bis-N-norgliovictin (570) significantly inhibited lipopolysaccharide (LPS)-induced inflammation in macrophages and improved survival in sepsis, and it should be a therapeutic candidate for the treatment of sepsis and other inflammatory diseases [279].
Dehydroxybisdethiobis(methylthio)gliotoxin (577) has been isolated from the broth of a marine-derived fungus Pseudallescheria sp. and exhibited weak antibacterial activity against methicillin-resistant and multidrug-resistant Staphylococcus aureus with MIC values of 31.2 μg/mL [280].
Ent-epicoccin G (582) from the endophytic fungus Epicoccum nigrum showed potent in vitro activity (IC50, 3.07 μM) against the release of β-glucuronidase in rat polymorphonuclear leukocytes induced by platelet-activating factor [188].
The bis(methylthio)-2,5-DKP FR106969 (590) isolated from Penicillium citrinum showed high inhibitory activity against the platelet activating factor (PAF)-induced rabbit platelet aggregation to have its potency as the anti-inflammatory inhibitor [281].
The indole derivatives gliocladins A (594) and B (595) as well as glioperazine (597) have been obtained from a marine-derived fungus Gliocladium roseum PS-N132 isolated from the sea hare Aplysia qkurodai. All three 2,5-DKPs exhibited cytotoxicity against murine P388 lymphocytic leukemia cells [125].
Both haematocin (600) and mycoediketopiperazine (607) were dimethylthio 2,5-DKPs. Haematocin (600) was isolated from the phytopathogenic fungus Nectria haematococca and had inhibitory activity on the spore germination and germ-tube elongation of Magnarporte oryzae [282]. Mycoediketopiperazine (607) from the fungus Papularia sp. exhibited potent cytotoxic activity on KB cells with an IC50 value of 120 μg/mL [283].
Plectosphaeroic acids A (620) and B (621) were obtained from marine-derived fungus Plectosphaerella cucumerina. They were inhibitors of indoeamine 2,3-dioxygenase (IDO), which existed in primary tumor cells. IDO has been considered as an important molecular target for cancer therapy [254].
7.4. Other Sulfur-Containing Cyclodipeptide Analogs
Other sulfur-containing cyclodipeptide analogs included MPC1001G (633), silvathione (634) and taichunamide D (635) with their structures shown in Figure 9. MPC1001G (633) was isolated from Cladorrhinum sp. KY4922 [236], silvathione (634) from Aspergillus silvaticus [235], and taichunamide D (635) from Aspergillus taichungensis IBT 19404 [53]. Their biological activities have not been reported.
Figure 9.
Structures of the other sulfur-containing cyclodipeptide analogs isolated from fungi.
8. Conclusions and Future Perspectives
A large number of cyclodipeptides have been identified in fungi, and many have received attention not only as challenging synthetic targets but also because some of these compounds displayed diverse and interesting biological activities. Since then, interest has increased in the biosynthesis, genetics, total synthesis, biological activities, and medicinal properties of this class of natural products. Some cyclodipeptides such as tryprostatins A (103) and B (104), cyclo(l-Pro–l-Ala) (318), cyclo(l-Pro–l-Phe) (334), cyclo(l-Pro–l-Tyr) (337), phenylahistin (392), and FR106969 (590) have displayed their potential applications in agriculture and medicinal industry [16,17,149,151,182,281].
The fungal cyclodipeptides are mainly distributed in the genera of Aspergillus and Penicillium. However, the cyclodipeptides in the remaining genera seem to be less explored. Further identification and exploration of the cyclodipeptides from all of the fungal genera are needed. In recent years, more and more cyclodipeptides have been isolated from marine-derived and plant endophytic fungi [297,298,299,300]. These fungi inhabiting particular environments could be rich sources of biologically active cyclodipeptides that are indispensable for medicinal and agricultural applications.
The biological activities (shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8) of the cyclodipeptides reported by each investigator were random and limited. Systematical screening of biological activities for each cyclodipeptide should be necessary. In most cases, the biological activities as well as the mode of action of fungal cyclodipeptides have been investigated based on in vitro studies or animal modes. Few studies have been performed at the level of clinical trials in patients. Effective research and development methods for these compounds should be explored to maximize their usefulness in the drug discovery and development processes [6]. For the diverse biological activities, the cyclodipeptides from fungi are expected to inspire medicinal chemists in their search for better agents such as antitumors, antifungals, and antibacterials than existing ones [297].
It is very important to understand biosynthetic mechanisms of the cyclodipeptides in fungi. These need to combine their biochemical and genetic approaches. More and more designed biologically active cyclodipeptides will be expected to be produced by genetic manipulation. With a good understanding of the biosynthetic pathways of bioactive cyclodipeptides, we can not only increase outputs of the beneficial cyclodipeptides but also block biosynthesis of some harmful cyclodipeptides by specific interferences [6].
Acknowledgments
This work was co-financed by the grants from the National Key R&D Program of China (2017YFD0201105), and the National Natural Science Foundation of China (31271996).
Author Contributions
Xiaohan Wang performed bibliographic research, and drafted and corrected the manuscript. Yuying Li and Xuping Zhang retrieved literature, participated in the discussions and supported manuscript corrections. Daowan Lai reviewed the manuscript and helped to revise it. Ligang Zhou conceived the idea, designed the review structure, supervised manuscript drafting, and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang Y., Wang P., Ma H., Zhu W. Developments around the bioactive diketopiperazines: A patent review. Expert Opin. Ther. Pat. 2013;23:1415–1433. doi: 10.1517/13543776.2013.828036. [DOI] [PubMed] [Google Scholar]
- 2.Welch T.R., Williams R.M. Epidithiodioxopiperazines. Occurrence, synthesis and biogenesis. Nat. Prod. Rep. 2014;31:1376–1404. doi: 10.1039/C3NP70097F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witiak D.T., Wei Y. Dioxopiperazines: Chemistry and biology. Prog. Drug Res. 1990;35:249–363. doi: 10.1007/978-3-0348-7133-4_7. [DOI] [PubMed] [Google Scholar]
- 4.De Carvalho M.P., Abraham W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012;19:3564–3577. doi: 10.2174/092986712801323243. [DOI] [PubMed] [Google Scholar]
- 5.Borthwick A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012;112:3641–3716. doi: 10.1021/cr200398y. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y.-M., Liang X.-A., Kong Y., Jia B. Structural diversity and biological activities of indole diketopipezazine alkaloids from fungi. J. Agric. Food Chem. 2016;64:6659–6671. doi: 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- 7.Steyn P.S. The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron. 1973;29:107–120. doi: 10.1016/S0040-4020(01)99384-6. [DOI] [Google Scholar]
- 8.Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., Zhu W. Seven new prenylated indole diketoperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986–7991. doi: 10.1016/j.tet.2008.06.013. [DOI] [Google Scholar]
- 9.Cui C.B., Kakeya H., Okada G., Onose R., Osada H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996;49:527–533. doi: 10.7164/antibiotics.49.527. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M., Wang W.-L., Fang Y.-C., Zhu T.-J., Gu Q.-Q., Zhu W.-M. Cytotoxic alkaloids and antibiotic nordammarance triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q., Wang S.-Q., Tang H.-Y., Li X.-J., Zhang L., Xiao J., Gao Y.-Q., Zhang A.-L., Gao J.-M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method. J. Agric. Food Chem. 2013;61:11447–11452. doi: 10.1021/jf403200g. [DOI] [PubMed] [Google Scholar]
- 12.Cui C.-B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, spirotryprotatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1996;52:12651–12666. doi: 10.1016/0040-4020(96)00737-5. [DOI] [Google Scholar]
- 13.Gao N., Shang Z.-C., Yu P., Luo J., Jian K.-L., Kong L.-Y., Yang M.-H. Alkaloids from the endophytic fungus Penicillium brefelddianum and their cytotoxic activities. Chin. Chem. Lett. 2017;28:1194–1199. doi: 10.1016/j.cclet.2017.02.022. [DOI] [Google Scholar]
- 14.Qian-Cutrone J., Huang S., Shu Y.-Z., Yyas D., Fairchild C., Menendez A., Krampitz K., Dalterio R., Klohr S.E., Gao Q. Stephacidin A and B: Two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 15.Cui C.B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. II. Physico-chemical properties and structures. J. Antibiot. 1996;49:534–540. doi: 10.7164/antibiotics.49.534. [DOI] [PubMed] [Google Scholar]
- 16.Woehlecke H., Osada H., Herrmann A., Lage H. Reversal of breast cancer resistance protein-dediated drug resistance by tryprostatin A. Int. J. Cancer. 2003;107:721–728. doi: 10.1002/ijc.11444. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S., Smith K.S., Deveau A.M., Dieckhaus C.M., Johnson M.A., Macdonald T.L., Cook J.M. Biological activity of the tryprostatins and their diasteromers on human carcinoma cell lines. J. Med. Chem. 2002;45:1559–1562. doi: 10.1021/jm0155953. [DOI] [PubMed] [Google Scholar]
- 18.Cai S., Kong X., Wang W., Zhou H., Zhu T., Li D., Gu Q. Aspergilazine A, a diketopiperazine dimer with a rare N-1 to C-6 linkage, from a marine-derived fungus Aspergillus taichungensis. Tetrahedron Lett. 2012;53:2615–2617. doi: 10.1016/j.tetlet.2012.03.043. [DOI] [Google Scholar]
- 19.Cai S., Luan Y., Kong X., Zhu T., Gu Q., Li D. Isolation and photoinduced conversion of 6-epi-stephacidins from Aspergillus taichungensis. Org. Lett. 2013;15:2168–2171. doi: 10.1021/ol400694h. [DOI] [PubMed] [Google Scholar]
- 20.Kong X., Cai S., Zhu T., Gu Q., Li D., Luan Y. Secondary metabolites of a deep sea derived fungus Aspergillus versicolor CXCTD-06-6a and their bioactivity. J. Ocean Univ. China. 2014;13:691–695. doi: 10.1007/s11802-014-2216-2. [DOI] [Google Scholar]
- 21.Liu Y.-X., Ma S.-G., Wang X.-J., Zhao N., Qu J., Yu S.-S., Dai J.-G., Wang Y.-H., Si Y.-K. Diketopiperazine alkaloids produced by the endophytic fungus Aspergillus fumigatus from the stem of Erythrophloeum fordii Oliv. Helv. Chim. Acta. 2012;95:1401–1408. doi: 10.1002/hlca.201100521. [DOI] [Google Scholar]
- 22.Asiri I.A.M., Badr J.M., Youssef D.T.A. Penicillicinacine, antimigratory diketopiperazine alkaloid from the marine-drived fungus Penicillium vinaceum. Phytochem. Lett. 2015;13:53–58. doi: 10.1016/j.phytol.2015.05.014. [DOI] [Google Scholar]
- 23.Zhang D., Noviendri D., Nursid M., Yang X., Son B.W. 12,13-Dihydroxyfumitremorgin C, fumitremorgin C, and brevianamide F, antibacterial diketopiperazine alkaloids from the marine-derived fungus Pseudallescheria sp. Nat. Prod. Sci. 2007;13:251–254. [Google Scholar]
- 24.Li G.-Y., Yang T., Luo Y.-G., Chen X.-Z., Fang D.-M., Zhang G.-L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009;11:3714–3717. doi: 10.1021/ol901304y. [DOI] [PubMed] [Google Scholar]
- 25.Song F., Liu X., Guo H., Ren B., Chen C., Piggott A.M., Yu K., Gao H., Wang Q., Liu M., et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012;14:4770–4773. doi: 10.1021/ol302051x. [DOI] [PubMed] [Google Scholar]
- 26.Miao F.-P., Li X.-D., Liu X.-H., Cichewicz R.H., Ji N.-Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs. 2012;10:131–139. doi: 10.3390/md10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G.-Y., Li L.-M., Yang T., Chen X.-Z., Fang D.-M., Zhang G.-L. Four new alkaloids, brevianamides O-R, from the fungus Aspergillus versicolor. Helv. Chim. Acta. 2010;93:2075–2080. doi: 10.1002/hlca.201000053. [DOI] [Google Scholar]
- 28.Peng J., Zhang X.-Y., Tu Z.-C., Xu X.-Y., Qi S.-H. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 2013;76:983–987. doi: 10.1021/np400132m. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y.-M., Liang X.-A., Zhang H.-C., Liu R. Cytotoxic and antibiotic cyclic pentapeptide from an endiphytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 2016;64:3789–3793. doi: 10.1021/acs.jafc.6b01051. [DOI] [PubMed] [Google Scholar]
- 30.Cui C.-B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, cyclotryprostatins A-D produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1997;53:59–72. doi: 10.1016/S0040-4020(96)00978-7. [DOI] [Google Scholar]
- 31.Kozlovskii A.G., Vinokurova N.G., Adanin V.M. Diketopiperazine alkaloids from the fungus Penicillium piscarium Westling. Appl. Biochem. Microbiol. 2000;36:317–321. [PubMed] [Google Scholar]
- 32.Kato H., Yoshida T., Tokue T., Nojiri Y., Hirota H., Ohta T., Williams R.M., Tsukamoto S. Notoamides A-D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 33.Zhang P., Li X.-M., Wang J.-N., Li X., Wang B.-G. Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii. Chin. Chem. Lett. 2015;26:313–316. doi: 10.1016/j.cclet.2014.11.020. [DOI] [Google Scholar]
- 34.Saraiva N.N., Rodrigues B.S.F., Jimenez P.C., Guimaraes L.A., Torres M.C.M., Rodrigues-Filho E., Pfenning L.H., Abreu L.M., Mafezoli J., De Mattos M.C., et al. Cytotoxic compounds from the marine-derived fungus Aspergillus sp. revovered from the sediments of the Brazilian coast. Nat. Prod. Res. 2014;29:1545–1550. doi: 10.1080/14786419.2014.987772. [DOI] [PubMed] [Google Scholar]
- 35.Luo X., Zhou X., Lin X., Qin X., Zhang T., Wang J., Tu Z., Yang B., Liao S., Tian Y., et al. Antituberculosis compounds from a deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. Nat. Prod. Res. 2017;31:1958–1962. doi: 10.1080/14786419.2016.1266353. [DOI] [PubMed] [Google Scholar]
- 36.Peng J., Gao H., Li J., Ai J., Geng M., Zhang G., Zhu T., Gu Q., Li D. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014;79:7895–7904. doi: 10.1021/jo5010179. [DOI] [PubMed] [Google Scholar]
- 37.An C.Y., Li X.-M., Li C.-S., Xu G.-M., Wang B.-G. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs. 2014;12:746–756. doi: 10.3390/md12020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng C., Ma Y. Isolation and anti-phytopathogenic activity of secondary metabolites from Alternaria sp. FL25, an endophytic fungus in Ficus carica. Chin. J. Appl. Environ. Biol. 2010;16:76–78. doi: 10.3724/SP.J.1145.2010.00076. [DOI] [Google Scholar]
- 39.Kito K., Ookura R., Kusumi T., Namikoshi M., Ooi T. X-ray structure of two stehacidins, heptacyclic alkaloids from the marine-derived fungus Aspergillus ostianus. Heterocycles. 2009;78:2101–2106. [Google Scholar]
- 40.Shi Y.-S., Zhang Y., Chen X.-Z., Zhang N., Liu Y.-B. Metabolites produced by the endophytic fungus Aspergillus fumigatus from the stem of Erythrophloeum fordii Oliv. Moelcules. 2015;20:10793–10799. doi: 10.3390/molecules200610793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greshock T.J., Grubbs A.W., Jiao P., Wicklow D.T., Gloer J.B., Williams R.M. Isolation, structure elucidation, and biomaimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew. Chem. Int. Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukamoto S., Kato H., Greshock T.J., Hirota H., Ohta T., Williams R.M. Isolation of notoamide E, a key precursor in the biosynthesis of prenylated indole alkaloids in a marine-derived fungus, Aspergillus sp. J. Am. Chem. Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finefield J.M., Greshock T.J., Sherman D.H., Tsukamoto S., Williams R.M. Notoamide E: Biosynthetic incorporation into notoamides C and D in cultures of Aspergillus versicolor NRRL 35600. Tetrahedron Lett. 2011;52:1987–1989. doi: 10.1016/j.tetlet.2011.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukamoto S., Kato H., Samizo M., Nojiri Y., Onuki H., Hirota H., Ohta T. Notoamides F-K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]
- 45.Tsukamoto S., Kawabata T., Kat H., Greshock T.J., Hirota H., Ohta T., Williams R.M. Isolation of antipodal (−)-versicolamide B and notoamides L-N from a marine-derived Aspergillus sp. Org. Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukamoto S., Umaoka H., Yoshikawa K., Ikeda T., Hirota H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P-R from a marine-derived fungus, Aspergillus sp. J. Nat. Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 47.Kato H., Nakahara T., Sugimoto K., Matsuo K., Kagiyama I., Frisvad J.C., Sherman D.H., Williams R.M., Tsukamoto S. Isolation of notoamide S and enantiomeric 6-epi-stephacidin A form the fungus Aspergillus amoenus: Biogenetic implications. Org. Lett. 2015;17:700–703. doi: 10.1021/ol5037198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato H., Nakahara T., Yamguchi M., Kagiyama I., Finefield J.M., Sunderhau J.D., Sherman D.H., Williams R.M., Tsukamoto S. Bioconcersion of 6-epi-notoamide T produces metabolites of unprecedented structures in a marine-derived Aspergillus sp. Tetrahedron Lett. 2015;56:247–251. doi: 10.1016/j.tetlet.2014.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozlovsky A., Vinokurova N.G., Adanin V.M., Grafe U. Piscarinines, new polycyclic diketopiperazine alkaloids from Penicillium piscarium VKM F-691. Nat. Prod. Lett. 2000;14:333–340. doi: 10.1080/10575630008043765. [DOI] [PubMed] [Google Scholar]
- 50.Whyte A.C., Gloer J.B., Wicklow D.T., Dowd P.F. Sclerotiamide: A new member of the paraherquamide class with potent antiinsectan activity from the sclerotia of Aspergillus sclerotiorum. J. Nat. Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
- 51.Chang Y.-W., Yuan C.-M., Zhang J., Liu S., Cao P., Hua H.-M., Di Y.-T., Hao X.-J. Speramides A-B, two new prenylated indole aklkaloids from the freshwater-derived fungus Aspergillus ochraceus KM007. Tetrahedron Lett. 2016;57:4952–4955. doi: 10.1016/j.tetlet.2016.09.071. [DOI] [Google Scholar]
- 52.Afiyatullov S.S., Zhuravleva O.I., Chaikina E.L., Anisimov M.M. A new spirotryprostatin from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2012;48:95–98. doi: 10.1007/s10600-012-0166-8. [DOI] [Google Scholar]
- 53.Kagiyama I., Kato H., Nehira T., Frisvad J.C., Sherman D.H., Williams R.M., Tsukamoto S. Taichunamides: Prenylated indole alkaloids from Aspergillus taichungensis (IBT 19404) Angew. Chem. Int. Ed. 2016;55:1128–1132. doi: 10.1002/anie.201509462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takase S., Kawai Y., Uchida I., Tanaka H., Aoki H. Structure of amauromine, a new hypotensive vasodilator produced by Amauroascus sp. Tetrahedron. 1985;41:3037–3048. doi: 10.1016/S0040-4020(01)96656-6. [DOI] [Google Scholar]
- 55.Laws I., Mantle P.G. Nigrifortine, a diketopiperazine metabolite of Penicillium nigricans. Phytochemistry. 1985;24:1395–1397. doi: 10.1016/S0031-9422(00)81148-6. [DOI] [Google Scholar]
- 56.Elsbai M.F., Rempel V., Schnakenburg G., Stefan K., Muller C.E., Konig G.M. Identification of a potent and selective cannabinoid CB1 receptor antagonist from Auxarthron reticulatum. ACS Med. Chem. Lett. 2011;2:866–869. doi: 10.1021/ml200183z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Guzman F.S., Gloer J.B., Wicklow D.T., Dowd P.F. New diketopiperazine metabolites form the sclerotia of Aspergillus ochraceus. J. Nat. Prod. 1992;55:931–939. doi: 10.1021/np50085a013. [DOI] [PubMed] [Google Scholar]
- 58.Kozlovsky A.G., Vinokurova N.G., Adanin V.M., Burkhardt G., Dahse H.-M., Grafe U. New diketopiperazine alkaloids from Penicillium fellutanum. J. Nat. Prod. 2000;63:698–700. doi: 10.1021/np9903853. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa K., Hosoe T., Itabashi T., Wakana D., Takizawa K., Yaguchi T., Kawai K. Novoamauromine and ent-cycloechinulin: Two new diketopiperazine derivatives from Aspergillus novofumigatus. Chem. Pharm. Bull. 2010;58:717–719. doi: 10.1248/cpb.58.717. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi H., Furutsuka K., Shiono Y. Okaramines H and I, new okaramine congeners, from Aspergillus aculeatus. J. Nat. Prod. 1999;62:315–317. doi: 10.1021/np9802623. [DOI] [PubMed] [Google Scholar]
- 61.Cai S., Sun S., Peng J., Kong X., Zhou H., Zhu T., Gu Q., Li D. Okaramines S-U, three new indole diketopiperzine alkaloids from Aspergillus taichungensis ZHN-7-07. Tetrahedron. 2015;71:3715–3719. doi: 10.1016/j.tet.2014.09.019. [DOI] [Google Scholar]
- 62.Hayashi H., Takiuchi K., Murao S. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, form Penicillium simplicissimum AK-40. Agric. Biol. Chem. 1989;53:461–469. [Google Scholar]
- 63.Hayashi H., Asabu Y., Murao S., Arai M. New okaramine congeners, okaramines D, E, and F, form Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1995;59:246–250. doi: 10.1271/bbb.59.246. [DOI] [PubMed] [Google Scholar]
- 64.Shiono Y., Akiyama K., Hayashi H. New okaramine congeners, okamines J, K, L, M and related compounds from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1999;63:1910–1920. doi: 10.1271/bbb.63.1910. [DOI] [PubMed] [Google Scholar]
- 65.Shiono Y., Akiyama K., Hayashi H. Okaramines N, O, P, Q and R, new okaramine congeners, from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 2000;64:103–110. doi: 10.1271/bbb.64.103. [DOI] [PubMed] [Google Scholar]
- 66.Shiono Y., Akiyama K., Hayashi H. Effect of the azetidine and azocine rings of okaramine B on insecticidal activity. Biosci. Biotechnol. Biochem. 2000;64:1519–1521. doi: 10.1271/bbb.64.1519. [DOI] [PubMed] [Google Scholar]
- 67.Furutani S., Nakatani Y., Miura Y., Ihara M., Kai K., Hayashi H., Matsuda K. GluCl a target of indole alkaloid okaramines: A 25 year enigma solved. Sci. Rep. 2014;4:6190. doi: 10.1038/srep06190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shan W.-G., Wu Z.-Y., Pang W.-W., Ma L.-F., Ying Y.-M., Zhan Z.-J. α-Glucosidase inhibitors from the fungus Aspergillus terreus 3.05358. Chem. Biodivers. 2015;12:1718–1724. doi: 10.1002/cbdv.201500027. [DOI] [PubMed] [Google Scholar]
- 69.Li X.-B., Li Y.-L., Zhou J.-C., Yuan H.-Q., Wang X.-N., Lou H.-X. A new diketopiperazine heterodimer from an endophytic fungus Aspergillus niger. J. Asian Nat. Prod. Res. 2015;17:182–187. doi: 10.1080/10286020.2014.959939. [DOI] [PubMed] [Google Scholar]
- 70.Zin W.W.M., Buttachon S., Dethoup T., Fernandes C., Cravo S., Pinoto M.M.M., Gales L., Pereira J.A., Silva A.M.S., Sekeroglu N., et al. New cyclotetrapeptides and a new diketopiperazine derivative from the marine sponge-associated fngus Neosartorya glabra KUFA 0702. Mar. Drugs. 2016;14:136. doi: 10.3390/md14070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinohara C., Hasumi K., Takei Y., Endo A. Gpsetin, a new inhibitor of acyl-CoA: Cholesterol acyltransferase produced by Nannizzia gypsea var. incurvata IFO 9228. I. Fermentation, isolation, physico-chemical properties and biological activity. J. Antibiot. 1994;47:163–167. doi: 10.7164/antibiotics.47.163. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi H., Fujiwara T., Murao S., Arai M. Okaramine C, a new insecticidal indole alkaloid from Penicillium simpilicissimum. Agric. Biol. Chem. 1991;55:3143–3145. [Google Scholar]
- 73.Hayashi H., Sakaguchi A. Okaramine G, a new okaramine congener from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1998;62:804–806. doi: 10.1271/bbb.62.804. [DOI] [PubMed] [Google Scholar]
- 74.Lhamo S., Wang X.-B., Li T.-X., Wang Y., Li Z.-R., Shi Y.-M., Yang M.-H., Kong L.-Y. Three unusual indole diketopiperazine alkaloids from a terrestrial-derived endophytic fungus, Aspergillus sp. Tetrahedron Lett. 2015;56:2823–2826. doi: 10.1016/j.tetlet.2015.04.058. [DOI] [Google Scholar]
- 75.Kimura Y., Sawada A., Kuramata M., Kusano M., Fujioka S., Kawano T., Shimada A. Brevicompanine C, cyclo-(d-Ile-l-Trp), and cyclo-(d-Leu-l-Trp), plant growth regulators from Penicillium brevi-compactum. J. Nat. Prod. 2005;68:237–239. doi: 10.1021/np040178p. [DOI] [PubMed] [Google Scholar]
- 76.Kimura Y., Tani K., Kojima A., Sotoma G., Okada K., Shimada A. Cyclo-(l-tryptophyl-l-phenylalanyl), a plant growth regulator produced by the fungus Penicillium sp. Phytochemistry. 1996;41:665–669. doi: 10.1016/0031-9422(95)00693-1. [DOI] [Google Scholar]
- 77.Du L., Yang X., Zhu T., Wang F., Xiao X., Park H., Gu Q. Diketopiperazine alkaloids from a deep ocean sediment derived fungus Penicillium sp. Chem. Pharm. Bull. 2009;57:873–876. doi: 10.1248/cpb.57.873. [DOI] [PubMed] [Google Scholar]
- 78.Yang X., Du L., Tang X., Jung S.-Y., Zheng B., Soh B.Y., Kim S.-Y., Gu Q., Park H. Brevicompanine E reduces lipopolysaccharide-induced production of proinflammatory cytokines and enzymes in microglia by inhibiting activation of activator protein-1 and nuclear factor-κB. J. Neuoimmunol. 2009;216:32–38. doi: 10.1016/j.jneuroim.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Ali M., Mohammed N., Alnaqeeb M.A., Hassan R.A.H., Shmad H.S.A. Toxicity of echinulin from Aspergillus chevalieri in rabbits. Toxicol. Lett. 1989;48:235–241. doi: 10.1016/0378-4274(89)90049-0. [DOI] [PubMed] [Google Scholar]
- 80.Arai K., Kimura K., Mushiroda T., Yamamoto Y. Structures of fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem. Pharm. Bull. 1989;37:2937–2939. doi: 10.1248/cpb.37.2937. [DOI] [Google Scholar]
- 81.Kozlovsky A.G., Adanin V.M., Dahse H.M., Dahse H.M., Grafe U. Rugulosuvines A and B, diketopiperazine alkaloids of Penicillium rugulosum and Penicillium piscarium fungi. Appl. Biochem. Microbiol. 2001;37:253–256. doi: 10.1023/A:1010225017054. [DOI] [PubMed] [Google Scholar]
- 82.Maruyama K., Ohuchi T., Yoshida K., Shibata Y., Sugawara F., Arai T. Protective properties of neoechinulin A against SIN-1-induced neuronal cell death. J. Biochem. 2004;136:81–87. doi: 10.1093/jb/mvh103. [DOI] [PubMed] [Google Scholar]
- 83.Pedras M.S.C., Smith K.C., Taylor J.L. Production of 2,5-dioxopiperazine by a new isolate type of the blackleg fungus Phoma lingam. Phytochemistry. 1998;49:1575–1577. doi: 10.1016/S0031-9422(98)00271-4. [DOI] [PubMed] [Google Scholar]
- 84.Chen X., Si L., Liu D., Proksch P., Zhang L., Zhou D., Lin W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015;93:182–195. doi: 10.1016/j.ejmech.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Chu Y.-S., Niu X.-M., Wang Y.-L., Guo J.-P., Pan W.-Z., Huang X.-W., Zhang K.-Q. Isolation of putative biosynthetic intermediates of prenylated indole alkaloids from a thermophilic fungus Talaromyces thermophilus. Org. Lett. 2010;12:4356–4359. doi: 10.1021/ol101817g. [DOI] [PubMed] [Google Scholar]
- 86.Zhou L.-N., Zhu T.-J., Cai S.-X., Gu Q.-Q., Li D.-H. Three new indole-containing deketopiperaine alkaloids from a deep ocean sediment-derived fungus, Penicillium griseofulvum. Helv. Chim. Acta. 2010;93:1758–1763. doi: 10.1002/hlca.200900443. [DOI] [Google Scholar]
- 87.Wang W.-L., Lu Z.-Y., Tao H.-W., Zhu T.-J., Fang Y.-C., Gu Q.-Q., Zhu W.-M. Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007;70:1558–1564. doi: 10.1021/np070208z. [DOI] [PubMed] [Google Scholar]
- 88.Barrow C., Sun H.H. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J. Nat. Prod. 1994;57:471–476. doi: 10.1021/np50106a005. [DOI] [PubMed] [Google Scholar]
- 89.Yan H.-J., Li X.-M., Li C.-S., Wang B.-G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta. 2012;95:163–168. doi: 10.1002/hlca.201100255. [DOI] [Google Scholar]
- 90.Kamauchi H., Kinoshita K., Sugita T., Koyama K. Conditional changes enhanced production of bioactive metabolites of marine derived fungus Eurotium rubrum. Bioorg. Med. Chem. Lett. 2016;26:4911–4914. doi: 10.1016/j.bmcl.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Itabashi T., Matsuishi N., Hosoe T., Toyazaki N., Udagawa S., Imai T., Adachi M., Kawai K. Two new dioxopiperazine derivatives, arestrictins and A and B, isolated from Aspergillus restrictus and Aspergillus penicilloides. Chem. Pharm. Bull. 2006;54:1639–1641. doi: 10.1248/cpb.54.1639. [DOI] [PubMed] [Google Scholar]
- 92.Varoglu M., Corbett T.H., Valeriote F.A., Crews P. Asperazine, a selective cyotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997;62:7078–7079. doi: 10.1021/jo970568z. [DOI] [PubMed] [Google Scholar]
- 93.Xiao J., Zhang Q., Gao Y.-Q., Shi X.-W., Gao J.-M. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 2014;28:1388–1392. doi: 10.1080/14786419.2014.904308. [DOI] [PubMed] [Google Scholar]
- 94.Ding G., Jiang L., Guo L., Chen X., Zhang H., Che Y. Pestalazines and pestalamides, bioactive metabolites from the plant pathogenic fungus Pestalotiopsis theae. J. Nat. Prod. 2008;71:1861–1865. doi: 10.1021/np800357g. [DOI] [PubMed] [Google Scholar]
- 95.Kusano M., Sotoma G., Koshino H., Uzawa J., Chijimatsu M., Fujioka S., Kawano T., Kimura Y. Brevicompanines A and B: New plant growth regulators produced by the fungus, Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1. 1998;17:2823–2826. doi: 10.1039/a803880e. [DOI] [Google Scholar]
- 96.Sprogoe K., Manniche S., Larsen T.O., Christophersen C. Janoxepin and brevicompanine B: Antiplasmodial metabolites from the fungus Aspergillus janus. Tetrahedron. 2005;61:8718–8721. doi: 10.1016/j.tet.2005.06.086. [DOI] [Google Scholar]
- 97.Matsunaga K., Shizuri Y., Yamamura S., Kawai K., Furukawa H. Isolation and structure of citreoindole, a new metabolite of hybrid strain KO 0052 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 1991;32:6883–6884. doi: 10.1016/0040-4039(91)80433-7. [DOI] [Google Scholar]
- 98.Du F.-Y., Li X.-M., Li C.-S., Shang Z., Wang B.-G. Cristatumins A-D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012;22:4650–4653. doi: 10.1016/j.bmcl.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 99.Li Y., Sun K.-L., Wang Y., Fu P., Liu P.-P., Wang C., Zhum W.-M. A cytotoxic pyrrolidinoindoline diketopiperazine dimer from the algal fungus Eurotium herbaariorum HT-2. Chin. Chem. Lett. 2013;24:1049–1052. doi: 10.1016/j.cclet.2013.07.028. [DOI] [Google Scholar]
- 100.Zou X., Li Y., Zhang X., Li Q., Liu Q., Huang Y., Tang T., Zheng S., Wang W., Tang J. A new prenylated indole diketopiperazine alkaloid from Eurotium cristatum. Molecules. 2014;19:17839–17847. doi: 10.3390/molecules191117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao H., Zhu T., Li D., Gu Q., Liu W. Prenylated indole diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Arch. Pharm. Res. 2013;36:952–956. doi: 10.1007/s12272-013-0107-5. [DOI] [PubMed] [Google Scholar]
- 102.Li D.-L., Li X.-M., Li T.-G., Dang H.-Y., Wang B.-G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta. 2008;91:1888–1893. doi: 10.1002/hlca.200890202. [DOI] [Google Scholar]
- 103.Li Y., Li X., Kim S.-K., Kang J.S., Choi H.D., Rho J.R., Son B.W. Golmaenone, a new diketopiperazine alkaloid form the marine-derived fungus Aspergillus sp. Chem. Pharm. Bull. 2004;52:375–376. doi: 10.1248/cpb.52.375. [DOI] [PubMed] [Google Scholar]
- 104.Meng L.-H., Du F.-Y., Li X.-M., Pedpradab P., Xu G.-M., Wang B.-G. Rubrumazines A-C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015;78:909–913. doi: 10.1021/np5007839. [DOI] [PubMed] [Google Scholar]
- 105.Guo J.-P., Tan J.-L., Wang Y.-L., Wu H.-Y., Zhang C.-P., Niu X.-M., Pan W.-Z., Huang X.-W., Zhang K.-Q. Isolation of talathermophilins from the thermophilic fungus Talaromyces thermophilus YM3–4. J. Nat. Prod. 2011;74:2278–2281. doi: 10.1021/np200365z. [DOI] [PubMed] [Google Scholar]
- 106.Shan W.-G., Ying Y.-M., Yu H.-N., Liu W.-H., Zhan Z.-J. Diketopiperazine alkaloids from Penicillium spp. HS-3, an endophytic fungus in Huperzia serrata. Helv. Chim. Acta. 2010;93:772–776. doi: 10.1002/hlca.200900331. [DOI] [Google Scholar]
- 107.Klausmeyer P., McCloud T.G., Tucker K.D., Cardellina J.H., II, Shoemaker R.H. Aspirochlorine class compounds from Aspergillus flavus inhibit azole-resistant Candida albicans. J. Nat. Prod. 2005;68:1300–1302. doi: 10.1021/np050141k. [DOI] [PubMed] [Google Scholar]
- 108.Kaur A., Raja H.A., Darveaux B.A., Chen W.-L., Swanson S.M., Pearce C.J., Oberlies N.H. New diketopiperazine dimer from a filamentous fungal isolate of Aspergillus sydowii. Magn. Reson. Chem. 2015;53:616–619. doi: 10.1002/mrc.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaaban M., El-Metwally M.M., Nasr H. A new diketopiperazine alkaloid from Aspergillus oryzae. Nat. Prod. Res. 2014;28:86–94. doi: 10.1080/14786419.2013.841687. [DOI] [PubMed] [Google Scholar]
- 110.Byun H.-G., Zhang H., Mochizuki M., Adachi K., Shizuri Y., Lee W.-J., Kim S.-K. Novel antifungal diketopiperazine from marine fungus. J. Antibiot. 2003;56:102–106. doi: 10.7164/antibiotics.56.102. [DOI] [PubMed] [Google Scholar]
- 111.Chen G.-D., Bao Y.-R., Huang Y.-F., Hu D., Li X.-X., Guo L.-D., Li J., Yao X.-S., Gao H. Three pairs of variecolortide enantiomers from Eurotium sp. with caspase-3 inhibitory activity. Fitoterapia. 2014;92:252–259. doi: 10.1016/j.fitote.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 112.Fan Z., Sun Z.-H., Liu Z., Chen Y.-C., Liu H.-X., Liu H.-X., Li H.-H., Zhang W.-M. Dichotocejpins A-C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs. 2016;14:164. doi: 10.3390/md14090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang L.-H., Chen Y.-X., Yu J.-C., Yuan J., Li H.-J., Ma W.-Z., Watanapokasin R., Hu K.-C., Iram N.S., Yang D.-P., et al. Secondary metabolites from the marine-derived fungus Dichotomomyce sp. L-8 and their cytotoxic activity. Molecules. 2017;22:444. doi: 10.3390/molecules22030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao H., Liu W., Zhu T., Mo X., Mandi A., Kurtan T., Li J., Ai J., Gu Q., Li D. Diketopiperazine alkaloids from a mangrove rhzizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 2012;10:9501–9506. doi: 10.1039/c2ob26757h. [DOI] [PubMed] [Google Scholar]
- 115.Kozlovskii A.G., Zhelifonova V.P., Adanin V.M., Antipova T.V., Szerskaia S.M., Ivanushkina N.E., Grafe U. Penicillium aurantiogriseum Dierckx 1901: Producer of diketopiperazine alkaloids (roquefortine and 3,12-dihydroroquefortine), isolated from permafrost. Appl. Biochem. Microbiol. 2003;39:393–397. doi: 10.1023/A:1024572602458. [DOI] [PubMed] [Google Scholar]
- 116.Yang N.-N., Ma Q.-Y., Huang S.-Z., Kong F.-D., Dai H.-F., Yu Z.-F., Zhao Y.-X. Chemical study of the fungus Psilocybe merdaria. J. Asian Nat. Prod. Res. 2017;19:333–338. doi: 10.1080/10286020.2016.1205041. [DOI] [PubMed] [Google Scholar]
- 117.Sorensen D., Larsen T.O., Christophersen C., Nielsen P.H., Anthoni U. Dipodazine, a diketopiperazine from Penicillium dipodomyis. Phytochemistry. 1999;51:1181–1183. doi: 10.1016/S0031-9422(99)00014-X. [DOI] [Google Scholar]
- 118.Springer J.P., Buchi G., Kobbe B., Demain A.L., Clardy J. The structure of ditryptophenaline—A new metabolite of Aspergillus flavus. Tetrahedron Lett. 1977;18:2403–2406. doi: 10.1016/S0040-4039(01)83777-1. [DOI] [Google Scholar]
- 119.Barrow C.J., Sedlock D.M. 1′-(2-Phenyl-ethylene)-ditryptophenaline, a new dimeric diketoperazine from Aspergillus flavus. J. Nat. Prod. 1994;57:1239–1244. doi: 10.1021/np50111a008. [DOI] [PubMed] [Google Scholar]
- 120.Lin A.-Q., Du L., Fang Y.-C., Wang F.-Z., Zhu T.-J., Gu Q.-Q., Zhu W.-M. Iso-α-cyclopiazonic acid, a new natural product isolatated from the marine-derived fungus Aspergiluus flavus C-F-3. Chem. Nat. Compd. 2009;45:677–680. doi: 10.1007/s10600-009-9433-8. [DOI] [Google Scholar]
- 121.Kanokmedhakul S., Kanokmedhakul K., Phonkerd N., Soytong K., Kongsaeree P., Suksamrarn A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine aklakoid form the fungus Chaetomium globosum KMITL-N0802. Plant Med. 2002;68:834–836. doi: 10.1055/s-2002-34415. [DOI] [PubMed] [Google Scholar]
- 122.Li Y.-F., Wu X.-B., Niaz S.-I., Zhang L.-H., Huang Z.-J., Lin Y.-C., Li J., Liu L. Effect of culture conditions on metabolites produced by the crinoid-derived fungus Aspergillus ruber 1017. Nat. Prod. Res. 2017;31:1299–1304. doi: 10.1080/14786419.2016.1244200. [DOI] [PubMed] [Google Scholar]
- 123.Gomes N.M., Dethoup T., Singburaudom N., Gales L., Silva A.M.S., Kijjoa A. Euroscristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 2012;5:717–720. doi: 10.1016/j.phytol.2012.07.010. [DOI] [Google Scholar]
- 124.Hodge R.P., Harris C.M., Harris T.M. Verrucofortine, a major metabolite of Penicillium verrucosum var. cyclopium, the fungus that produces the mycotoxin verrucosidin. J. Nat. Prod. 1988;51:66–73. doi: 10.1021/np50055a008. [DOI] [PubMed] [Google Scholar]
- 125.Usami Y., Yamaguchi J., Numata A. Gliocladins A-C and glioperzaine; cytotoxic dioxo- or trioxopiperazine metabolites from a Gliocladium sp. separated from a sea hare. Heterocycles. 2004;63:1123–1129. doi: 10.3987/COM-04-10037. [DOI] [Google Scholar]
- 126.Zheng C.-J., Kim Y.-H., Kim W.-G. Glioperazine B, as a new antimicrobial agent against Staphylococcus aureus and glioperazine C: Two new dioxopiperazines from Bionectra byssicola. Biosci. Biotechnol. Biochem. 2007;71:1979–1983. doi: 10.1271/bbb.70167. [DOI] [PubMed] [Google Scholar]
- 127.Kim J.W., Ko S.-K., Son S., Shin K.-S., Ryoo I.-J., Hong Y.-S., Oh H., Hwang B.Y., Takahashi S., Kim B.Y., et al. Haenamindole, an unusual diketopiperazine derivative from a marine-derived Penicillium sp. KCB12F005. Bioorg. Med. Chem. Lett. 2015;25:5398–5401. doi: 10.1016/j.bmcl.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 128.Zhou Y., Debbab A., Wray V., Lin W., Schulz B., Trepos R., Pile C., Hellio C., Proksch P., Aly A.H. Marine bacterial inibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014;55:2789–2792. doi: 10.1016/j.tetlet.2014.02.062. [DOI] [Google Scholar]
- 129.Wang J., He W., Qin X., Wei X., Tian X., Liao L., Liao S., Yang B., Tu Z., Chen B., et al. Three new indolyl diketopiperazine metabolites from the Antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Adv. 2015;5:68736. doi: 10.1039/C5RA10828D. [DOI] [Google Scholar]
- 130.Lin A., Fang Y., Zhu T., Gu Q., Zhu W. A new diketopiperazine alkaloid isolated from an algicolous Aspergillus flavus strain. Pharmazie. 2008;63:323–324. [PubMed] [Google Scholar]
- 131.Yamada T., Iwamoto C., Yamagaki N., Yamanouchi T., Minoura K., Hagishita S., Numata A. Leptosins O-S, cytotoxic metabolites of a strain of Leptosphaeria sp. isolated from a marine alga. Heterocycles. 2004;63:641–653. doi: 10.3987/COM-03-9967. [DOI] [Google Scholar]
- 132.Larsen T.O., Petersen B.O., Duus J.O. Lumpidin, a novel biomarker of some ochratoxin A producing Penicillia. J. Agric. Food Chem. 2001;49:5081–5084. doi: 10.1021/jf010345g. [DOI] [PubMed] [Google Scholar]
- 133.Li D.-L., Li X.-M., Proksch P., Wang B.-G. 7-O-Methylvariecolortide A, a new spirocyclic deketopiperazine alkaloid from a marine mangrove derived endophytic fungus, Eurotium rubrum. Nat. Prod. Commun. 2010;5:1583–1586. [PubMed] [Google Scholar]
- 134.Yun K. Cristazine, a new cytotoxic dioxopiperazine alkaloid from the mudflat-sediment-derived fungus Chaetomium cristatum. Chem. Pharm. Bull. 2016;64:59–62. doi: 10.1248/cpb.c15-00525. [DOI] [PubMed] [Google Scholar]
- 135.Dossena A., Marchelli R., Pochini A. Neochinulin D, a new isoprenylated dehydrotryptophyl metabolite from Aspergillus amstelodami. Experienta. 1975;31:1249. doi: 10.1007/BF01945758. [DOI] [Google Scholar]
- 136.Liang W.-L., Le X., Li H.-J., Yang X.-L., Chen J.-X., Xu J., Liu H.-L., Wang L.-Y., Wang K.-T., Hu K.-C., et al. Exploring the chemodiversity and biological activities of the secondary metabolits from the marine fungus Neosartorya pseudofischeri. Mar. Drugs. 2014;12:5657–5676. doi: 10.3390/md12115657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li L., Li D., Luan Y., Gu Q., Zhu T. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012;75:920–927. doi: 10.1021/np3000443. [DOI] [PubMed] [Google Scholar]
- 138.Pedras M.S.C., Biesenthal C.J. Isolation, structure determination, and phytotoxicity of unusual dioxopiperazines form the phytopathogenic fungus Phoma lingam. Phytochemistry. 2001;58:905–909. doi: 10.1016/s0031-9422(01)00348-x. [DOI] [PubMed] [Google Scholar]
- 139.Lee S.U., Asami Y., Lee D., Jang J.-H., Ahn J.S., Oh H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. J. Nat. Prod. 2011;74:1284–1287. doi: 10.1021/np100880b. [DOI] [PubMed] [Google Scholar]
- 140.Zhang D.-B., Yang Z.-D., Xue P.-H., Xue P.-H., Zhi K.-K., Shi Y., Wang M.-G. Two new cyclic dipeptides from Rhinocladiella sp. lgt-3, a fungal endophyte isolated from Tripterygiun wilfordii Hook. Nat. Prod. Res. 2014;28:1760–1764. doi: 10.1080/14786419.2014.945176. [DOI] [PubMed] [Google Scholar]
- 141.Ohmomo S., Utagawa T., Abe M. Identification of roquefortine C produced by Penecillium roqueforti. Agric. Biol. Chem. 1977;41:2097–2098. [Google Scholar]
- 142.Clark B., Capon R.J., Lacey E., Tennant S., Gill J.H. Roquefortine E, a diketopiperazine from an Australian isolated of Gymnoascus reessii. J. Nat. Prod. 2005;68:1661–1664. doi: 10.1021/np0503101. [DOI] [PubMed] [Google Scholar]
- 143.Du L., Li D., Zhu T., Cai S., Wang F., Xiao X., Gu Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron. 2009;65:1033–1039. doi: 10.1016/j.tet.2008.11.078. [DOI] [Google Scholar]
- 144.Du L., Feng T., Zhao B., Li D., Cai S., Zhu T., Wang F., Xiao X., Gu Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot. 2010;63:165–170. doi: 10.1038/ja.2010.11. [DOI] [PubMed] [Google Scholar]
- 145.Cho K.-H., Sohn J.H., Oh H. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res. 2017;31 doi: 10.1080/14786419.2017.1346642. [DOI] [PubMed] [Google Scholar]
- 146.Fujimoto H., Fujimaki T., Okuyama E., Yamazaki M. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem. Pharm. Bull. 1999;47:1426–1432. doi: 10.1248/cpb.47.1426. [DOI] [PubMed] [Google Scholar]
- 147.Wang W.-L., Zhu T.-J., Tao H.-W., Lu Z.-Y., Fang Y.-C., Gu Q.-Q., Zhu W.-M. Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 2007;4:2913–2919. doi: 10.1002/cbdv.200790240. [DOI] [PubMed] [Google Scholar]
- 148.Barrow C.J., Cai P., Snyder J.K., Sedlock D.M., Sun H.H., Cooper R. WIN 64821, a new competitive antagonist to substance P, isolated from an Aspergillus species: Structure determination and solution conformation. J. Org. Chem. 1993;58:6016–6021. doi: 10.1021/jo00074a031. [DOI] [Google Scholar]
- 149.Stierle A.C., Cardellina J.H., II, Strobel G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA. 1988;85:8008–8011. doi: 10.1073/pnas.85.21.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trigos A., Reyna S., Gutierrez M.L., Sanchez M. Diketopiperazines from cultures of the fungus Colletotrichum gloesporoides. Nat. Prod. Lett. 1997;11:13–16. doi: 10.1080/10575639708043751. [DOI] [Google Scholar]
- 151.Iimura K., Furukawa T., Yamamoto T., Negishi L., Suzuki M., Sakuda S. The mode of action of cyclo(l-Ala-l-Pro) in inhibiting aflatoxin production of Aspergillus flavus. Toxins. 2017;9:219. doi: 10.3390/toxins9070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klausmeyer P., Howard O.M.Z., Shipley S.M., McCloud T.G. An inhibitor of CCL2-induced chemotaxis from the fungus Leptoxyphium sp. J. Nat. Prod. 2009;72:1369–1372. doi: 10.1021/np800745r. [DOI] [PubMed] [Google Scholar]
- 153.Sugimoto K., Sadahiro Y., Kagiyama I., Kato H., Sherman D.H., Williams R.M., Tsukamoto S. Isolation of amoenamide A and five antipodal prenylated alkaloids from Aspergillus amoenus NRRL 35600. Tetrahedron Lett. 2017;58:2797–2800. doi: 10.1016/j.tetlet.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Musetti R., Polizzotto R., Vecchione A., Borselli S., Zulini L., D’Ambrosio M., Di Toppi L.S., Pertot I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron. 2007;38:643–650. doi: 10.1016/j.micron.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 155.Yan B.-F., Fang S.-T., Li W.-Z., Liu S.-J., Wang J.-H., Xia C.-H. A new minor diketopiperzaine from the sponge-derived fungus Simplicillium sp. YZ-11. Nat. Prod. Res. 2015;29:2013–2017. doi: 10.1080/14786419.2015.1027890. [DOI] [PubMed] [Google Scholar]
- 156.Fang Z.F., Yu S.S., Zhou W.Q., Chen X.G., Ma S.G., Li Y., Qu J. A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nee & T. Nee: Fr) Wiltshire. Chin. Chem. Lett. 2012;23:317–320. [Google Scholar]
- 157.Pedras M.S.C., Yu Y., Liu J., Tandron-Moya Y.A. Metabolites produced by the phytopahtogenic fungus Rhizoctonia solani: Isolation, chemical structure determination, syntheses and bioactivity. Z. Naturforschung C. 2005;60:717–722. doi: 10.1515/znc-2005-9-1010. [DOI] [PubMed] [Google Scholar]
- 158.Park Y.C., Gunasekera S.P., Lopez J.V., McCarthy P.J., Wright A.E. Metabolites from the marine-derived fungus Chromocleista sp. isolated from a deep-water sediment sample collected in the Gulf of mexico. J. Nat. Prod. 2006;69:580–584. doi: 10.1021/np058113p. [DOI] [PubMed] [Google Scholar]
- 159.Shigemori H., Tenma M., Shimazaki K., Kobayashi J. Three new metabolites from the marine yeast Aureobasidium pullulans. J. Nat. Prod. 1998;61:696–698. doi: 10.1021/np980011u. [DOI] [PubMed] [Google Scholar]
- 160.Zhou X., Lin X., Ma W., Fang W., Chen Z., Yang B., Liu Y. A new aromatic amine from fungus Pestalotiopsis vaccinia. Phytochem. Lett. 2014;7:35–37. doi: 10.1016/j.phytol.2013.09.010. [DOI] [Google Scholar]
- 161.Capon R.J., Stewart M., Ratnayake R., Lacey E., Gill J.H. Citromycetins and bilains A-C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007;70:1746–1752. doi: 10.1021/np0702483. [DOI] [PubMed] [Google Scholar]
- 162.Prasad C. Nutritional Neuroscience. CRC Press LLC; Boca Raton, FL, USA: 2005. Food-derived neuroactive cyclic dipeptides; pp. 331–340. [Google Scholar]
- 163.Park S.H., Stierle A., Strobel G.A. Metabolism of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa) Phytochemistry. 1994;35:101–106. doi: 10.1016/S0031-9422(00)90516-8. [DOI] [Google Scholar]
- 164.Yu Y., Ma B., Liu J., Yue J., Chen H., Liang Y., Zhou Z., Wang G., Wang G. Two new alkaloid metabolites produced by endophytic fungus Stagonosporopsis oculihominis isolated from Dendrobium huoshanense. Phytochem. Lett. 2017;19:266–270. doi: 10.1016/j.phytol.2017.02.006. [DOI] [Google Scholar]
- 165.Kwon O.S., Park S.H., Yun B.-S., Pyun Y.R., Kim C.-J. Cyclo(d-Pro-l-Val), a specific β-glucosidase inhibitor produced by Aspergillus sp. F70609. J. Antibiot. 2001;54:179–181. doi: 10.7164/antibiotics.54.179. [DOI] [PubMed] [Google Scholar]
- 166.Trigos A., Reyna S., Cervantes L. Three diketopiperazines from the cultivated fungus Fusarium oxysporum. Nat. Prod. Lett. 1995;6:241–246. doi: 10.1080/10575639508043166. [DOI] [Google Scholar]
- 167.Wang Y.-C., Zhang Y.-W., Zheng L.-H., Bao Y.-L., Wu Y., Yu C.-L., Sun L.-G., Zhang Y., Huang Y.-X., Sun Y., et al. A new compound from liquid fermentation broth of Armillaria mellea and the determination of its absolute configuration. J. Asian Nat. Prod. Res. 2013;15:203–208. doi: 10.1080/10286020.2012.751977. [DOI] [PubMed] [Google Scholar]
- 168.Trigos A., Reyna S., Matamoros B. Macrophominol, a diketopiperazine from cultures of Macrophomina phaseolina. Phytochemistry. 1995;40:1697–1698. doi: 10.1016/0031-9422(95)00626-I. [DOI] [Google Scholar]
- 169.Jia J.-M., Ma X.-C., Wu C.-F., Wu L.-J., Hu G.-S. Cordycedipenptide A, a new cyclodipeptide from the culture liquid of Cordyceps sinensis (Berk.) Sacc. Chem. Pharm. Bull. 2005;53:582–583. doi: 10.1248/cpb.53.582. [DOI] [PubMed] [Google Scholar]
- 170.Wu Q.-X., Crews M.S., Draskovic M., Sohn J., Johnson T.A., Tenney K., Valeriote F.A., Yao X.-J., Bjeldanes L.F., Crews P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010;12:4458–4461. doi: 10.1021/ol101396n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Birkinshaw J.H., Mohemmed Y.S. Biochemistry of microorganisms. CXI. The production of l-phenylalanine anhydride (cis-l-3,6-dibenzyl-2,5-dioxopiperazine) by Penicillium nigricans. Biochem. J. 1962;85:523–527. doi: 10.1042/bj0850523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walchshofer N., Sarciron M.E., Garnier F., Delatour P., Petavy A.F., Paris J. Anthelmintic activity of 3,6-dibenzyl-2,5-dioxopiperazine cyclo(l-Phe-l-Phe) Amino Acids. 1997;12:41–47. doi: 10.1007/BF01373425. [DOI] [Google Scholar]
- 173.Mizuma T., Narasaka T., Awazu S. Concentration-dependent atypical intestinal absorption of cyclic phenylalanylserine: Small intestine acts as an interface between the body and ingested compounds. Biol. Pharm. Bull. 2003;26:1625–1628. doi: 10.1248/bpb.26.1625. [DOI] [PubMed] [Google Scholar]
- 174.Isaka M., Palasarn S., Rachtawee P., Vimuttipong S., Kongsaeree P. Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org. Lett. 2005;7:2257–2260. doi: 10.1021/ol0507266. [DOI] [PubMed] [Google Scholar]
- 175.Arnone A., Capelli S., Nasini G., Meille S.V., Vajna de Pava O. Structure elucidation of diatretol—A new diketopiperazine metabolite from the fungus Clitocybe diatreta. Liebigs Ann. Chem. 1996;11:1875–1877. doi: 10.1002/jlac.199619961123. [DOI] [Google Scholar]
- 176.Aniya Y., Ohtani I.I., Higa T., Miyagi C., Gibo H., Shimabukuro M., Nakanishi H., Taira J. Dimerumic acid as an antioxidant of the mold Monascus anka. Free Radic. Biol. Med. 2000;28:999–1004. doi: 10.1016/S0891-5849(00)00188-X. [DOI] [PubMed] [Google Scholar]
- 177.Yamashiro J., Shiraishi S., Fuwa T., Horie T. Dimerumic acid protected oxidative stress-induced cytotoxicity in isolated rat hepatocytes. Cell Biol. Toxicol. 2008;24:283–290. doi: 10.1007/s10565-007-9037-7. [DOI] [PubMed] [Google Scholar]
- 178.Yao Y., Tian L., Cao J.-A., Pei Y.-H. A new piperazine-2,5-dione from the marine fungus Gliocladium sp. Pharmazie. 2007;62:478–479. doi: 10.1002/chin.200741197. [DOI] [PubMed] [Google Scholar]
- 179.Yao Y., Tian L., Li J., Cao J., Pei Y. Cytotoxic piperazine-2,5-dione derivatives from marine fungus Gliocladium sp. Pharmazie. 2009;64:616–618. [PubMed] [Google Scholar]
- 180.Lorenz P., Jensen P.R., Fenical W. Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp. Nat. Prod. Lett. 1998;12:55–60. doi: 10.1080/10575639808048871. [DOI] [Google Scholar]
- 181.Kawada M., Someno T., Inoue H., Ohba S., Masuda T., Kato T., Ikeda D. NBRI16716A, a new antitumor compound against human prostate cancer cells, produced by Perisporiopsis melioloides Mer-f16716. J. Antibiot. 2010;63:319–323. doi: 10.1038/ja.2010.42. [DOI] [PubMed] [Google Scholar]
- 182.Kanoh K., Kohno S., Asari T., Harada T., Katada J., Muramatsu M., Kawashima H., Sekiya H., Uno I. (−)-Phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg. Med. Chem. Lett. 1997;7:2847–2852. doi: 10.1016/S0960-894X(97)10104-4. [DOI] [Google Scholar]
- 183.Guo D.-L., Zhao M., Xiao S.-J., Xia B., Wan B., Gu Y.-C., Ding L.-S., Zhou Y. Two new diketopiperazines and a new glucosyl sesterterpene from Alternaria alternata, an endophytic fungus from Ceratostigma griffithii. Phytochem. Lett. 2015;14:260–264. doi: 10.1016/j.phytol.2015.10.024. [DOI] [Google Scholar]
- 184.Vinokurova N.G., Baskunov B.P., Zelenkova N.F., Arinbasarov M.U. The alkaloids of Penicillium aurantiogriseum Dierckx (1901) var. aurantiogriseum VKM F-1298. Microbiology. 2004;73:414–419. doi: 10.1023/B:MICI.0000036986.02145.54. [DOI] [PubMed] [Google Scholar]
- 185.Zheng Y., Pang H., Wang J., Chen D., Shi G., Huang J. Novel diketopiperazine and ten-membered macrolides from entomogenous fungus Paecilomyces tenuipes. Chem. J. Chin. Univ. 2014;35:1665–1669. [Google Scholar]
- 186.Huang H., She Z., Lin Y., Vrijmoed L.L.P., Lin W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel) J. Nat. Prod. 2007;70:1696–1699. doi: 10.1021/np0605891. [DOI] [PubMed] [Google Scholar]
- 187.Ding G., Liu J., Wang J., Fang L., Yu S. Secondary metabolites from the endophytic fungi Penicillium polonicum and Aspergillus fumigatus. J. Asian Nat. Prod. Res. 2013;15:446–452. doi: 10.1080/10286020.2013.780349. [DOI] [PubMed] [Google Scholar]
- 188.Wang J.-M., Ding G.-Z., Fang L., Dai J.-G., Yu S.-S., Wang Y.-H., Chen X.-G., Ma S.-G., Qu J., Du D. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2010;73:1240–1249. doi: 10.1021/np1000895. [DOI] [PubMed] [Google Scholar]
- 189.Liu Y.N., Xue J.H., Feng N., Wu P., Liu X.Z., Wei X.Y. A new cyclodipeptide from the cultures of Geotrichum candidum. Chin. Chem. Lett. 2007;18:1081–1083. doi: 10.1016/j.cclet.2007.07.003. [DOI] [Google Scholar]
- 190.Li J., Wang J., Jiang C.-S., Li G., Guo Y.-W. (+)-Cyclopenol, a new naurally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2014;16:542–548. doi: 10.1080/10286020.2014.911290. [DOI] [PubMed] [Google Scholar]
- 191.Kalansuriya P., Quezada M., Esposito B.P., Capon R.J. Talarazines A-E: Noncytotoxic iron (III) chelators from an Australian mud dauber wasp-associated fungus, Talaromyces sp. (CMB-W045) J. Nat. Prod. 2017;80:609–615. doi: 10.1021/acs.jnatprod.6b00889. [DOI] [PubMed] [Google Scholar]
- 192.Metwaly A.M., Ghoneim M.M., Musa A. Two new antileishmanial diketopiperazine alkaloids form the endophytic fungus Trichosporum sp. Pharm. Chem. 2015;7:322–327. [Google Scholar]
- 193.Taira J., Miyagi C., Aniya Y. Dimerumic acid as an antioxidant from the mold, Monascus anka: The inhibition mechanisms against lipid peroxidation and hemeprotein-meidated oxidation. Biochem. Pharmacol. 2002;63:1019–1026. doi: 10.1016/S0006-2952(01)00923-6. [DOI] [PubMed] [Google Scholar]
- 194.Guo H., Sun B., Gao H., Chen X., Liu S., Yao X., Liu X., Che Y. Diketopiperazines from the Cordyceps-colonizing fungus Epicoccum nigrum. J. Nat. Prod. 2009;72:2115–2119. doi: 10.1021/np900654a. [DOI] [PubMed] [Google Scholar]
- 195.Chunyu W.-X., Ding Z.-G., Zhao J.-Y., Wang Y.-X., Han X.-L., Li M.-G., Wen M.-L. Two new diketopiperazines from the tin mine tailings-derived fungus Schizophyllum commune YIM DT 10058. Nat. Prod. Res. 2017;31:1566–1572. doi: 10.1080/14786419.2016.1274894. [DOI] [PubMed] [Google Scholar]
- 196.Guimaraes D.O., Borges W.S., Vieira N.J., De Oliveira L.F., Da Silva C.H.T.P., Lopes N.P., Dias L.G., Duran-Patron R., Collado I.G., Pupo M.T. Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochmistry. 2010;71:1423–1429. doi: 10.1016/j.phytochem.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 197.Zheng Y., Zhang J., Wei L., Shi M., Wang J., Huang J. Gunnilactams A-C, macrocyclic tetrlactams from the mycelial culture of the entomogenous fungus Paecilomyces gunnii. J. Nat. Prod. 2017;80:1935–1938. doi: 10.1021/acs.jnatprod.7b00060. [DOI] [PubMed] [Google Scholar]
- 198.Zhou X., Fang P., Tang J., Wu Z., Li X., Li S., Wang Y., He Z., Gou D., Yao X., et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016;30:52–57. doi: 10.1080/14786419.2015.1033623. [DOI] [PubMed] [Google Scholar]
- 199.Kawabata T., Uchida C., Kato H., Tsubata T., Takano F., Ohta T. Melanogenesis-modulating diketopiperazine derivatives from Hypocrea spp. Heterocycles. 2013;87:417–422. doi: 10.3987/COM-12-12633. [DOI] [Google Scholar]
- 200.Takagi M., Motohashi K., Shin-ya K. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, form the sponge-derived Aspergillus sp. fS14. J. Antibiot. 2010;63:393–395. doi: 10.1038/ja.2010.58. [DOI] [PubMed] [Google Scholar]
- 201.Li Y., Yue Q., Krausert N.M., An Z., Gloer J.B., Bills G.F. Emestrins: Anti-Cryptococcus epipolythiodioxopiperazines form Podospora australis. J. Nat. Prod. 2016;79:2357–2363. doi: 10.1021/acs.jnatprod.6b00498. [DOI] [PubMed] [Google Scholar]
- 202.Huang Y.-F., Tian L., Hua H.-M., Pei Y.-H. Two diketopiperazines from marine fungus Giocladium sp. YUP08. J. Aisan Nat. Prod. Res. 2007;9:197–201. doi: 10.1080/10286020500531878. [DOI] [PubMed] [Google Scholar]
- 203.Li X.-Z., Chen G., Wang H.-F., Hua H.-M., Pei Y.-H. Synthesis and bioactivity of diketopiperazine PJ147 and its derivatives from Gliocladium sp. YUP08. J. Asian Nat. Prod. Res. 2014;16:764–769. doi: 10.1080/10286020.2014.916283. [DOI] [PubMed] [Google Scholar]
- 204.Antia B.S., Aree T., Kasettrathat C., Wiyakrutta S., Ekpa O.D., Ekpe U.J., Mahidol C., Ruchirawat S., Kittakoop P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry. 2011;72:816–820. doi: 10.1016/j.phytochem.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 205.Meng L.-H., Wang C.-Y., Mandi A., Li X.-M., Hu X.-Y., Kassack M.U., Kurtan T., Wang B.-G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endiphytic fungus Penicillium brocae MA-231. Org. Lett. 2016;18:5304–5307. doi: 10.1021/acs.orglett.6b02620. [DOI] [PubMed] [Google Scholar]
- 206.Quezada M., Shang Z., Kalansuriya P., Salim A.A., Lacey E., Capon R.J. Waspergillamide A, a nitro depsi-tetrapeptide diketopiperazine from an Australian mud dauber wasp-associated Aspergillus sp. (CMB-W031) J. Nat. Prod. 2017;80:1192–1195. doi: 10.1021/acs.jnatprod.6b01062. [DOI] [PubMed] [Google Scholar]
- 207.Rezanka T., Sobotka M., Spizek J.S., Sigler K. Pharmacologically active sulfur-containing compounds. Anti Infect. Agents Med. Chem. 2006;5:187–224. doi: 10.2174/187152106776359002. [DOI] [Google Scholar]
- 208.Cook K.M., Hilton S.T., Mecinovic J., Motherweill W.B., Figg W.D., Schofield C.J. Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1α (HIF-1α) and p300 by a zinc ejection mechanism. J. Biol. Chem. 2009;284:26831–26838. doi: 10.1074/jbc.M109.009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Greiner D., Bonaldi T., Eskeland R., Roemer E., Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3–9. Nat. Chem. Biol. 2005;1:143–145. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]
- 210.Saito T., Suzuki Y., Koyama K., Natori S., Iitaka Y., Kinoshita T. Chetracin A and chaetocins B and C, three new epipolythiodioxopiperazines form Chaetomium spp. Chem. Pharm. Bull. 1988;36:1942–1956. doi: 10.1248/cpb.36.1942. [DOI] [Google Scholar]
- 211.Li G., Li B., Yang T., Yan J., Liu G., Zhang G. Chaetocochins A-C, epipolythiodioxopiperazines from Chaetomium cochliodes. J. Nat. Prod. 2006;69:1374–1376. doi: 10.1021/np0602970. [DOI] [PubMed] [Google Scholar]
- 212.Choi E.J., Park J.S., Kim Y.J., Jung J.H., Lee J.K., Kwon H.C., Yang H.O.J. Apoptosis-inducing effect of diketoperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. Appl. Microbiol. 2011;110:304–313. doi: 10.1111/j.1365-2672.2010.04885.x. [DOI] [PubMed] [Google Scholar]
- 213.Kawahara N., Nakajima S., Yamazaki M., Kawai K. Studies on fungal products. Part XXIX. Structure of a novel epidithiodioxopiperazine, emethallicin A, a potent inhibitor of histamine release, from Emericella heterothallica. Chem. Pharm. Bull. 1989;37:2592–2595. doi: 10.1248/cpb.37.2592. [DOI] [PubMed] [Google Scholar]
- 214.Kawahara N., Nozawa K., Yamazaki M., Nakajima S., Kawai K. Studies on fungal products. XXXI. Structures of novel epipolythiodioxopiperazines, emethallincins B, C, and D, potent inhibitors of histamine release, from Emericella heterothallica. Chem. Pharm. Bull. 1990;38:73–78. doi: 10.1248/cpb.38.73. [DOI] [PubMed] [Google Scholar]
- 215.Kawahara N., Nozawa K., Yamazaki M., Nakajima S., Kawai K. Studies on fungal products. Part 32. Novel epidithiodioxopiperazines, emethallicins E and F, from Emericella heterothallica. Heterocycles. 1990;30:507–515. [Google Scholar]
- 216.Hof H., Kupfahl C. Gliotoxin in Aspergillus fumigatus: An example that mycotoxins are potential virulence factors. Mycotoxin Res. 2009;25:123–131. doi: 10.1007/s12550-009-0020-4. [DOI] [PubMed] [Google Scholar]
- 217.Vigushin D.M., Mirsaidi N., Brooke G., Sun C., Pace P., Inman L., Moody C.J., Coombes R.C. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Oncol. 2004;21:21–30. doi: 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]
- 218.Tan R.X., Jensen P.R., Williams P.G., Fenical W. Isolation and structure assignments of rostratins A-D, cytotoxic disulfides produced by the marine-derived fungus Exserohilum rostratum. J. Nat. Prod. 2004;67:1374–1382. doi: 10.1021/np049920b. [DOI] [PubMed] [Google Scholar]
- 219.Kleinwachter P., Dahse H.-M., Luhmann U., Schlegel B., Dornberger K. Epicorazine C, an antimicrobial metabolite from Stereum hirsutum HKI 0195. J. Antibiot. 2001;54:521–525. doi: 10.7164/antibiotics.54.521. [DOI] [PubMed] [Google Scholar]
- 220.Tsumagari N., Nakai R., Onodera H., Hasegawa A., Rahayu E.S., Ando K., Yamashita Y. MPC1001, a new antitumor antibiotic produced by Cladorrhinum sp. J. Antibiot. 2004;57:532–534. doi: 10.7164/antibiotics.57.532. [DOI] [PubMed] [Google Scholar]
- 221.Xu Y., Espinosa-Artiles P., Liu M.X., Arnold A.E., Gunatilaka A.A.L. Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A-E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J. Nat. Prod. 2013;76:2330–2336. doi: 10.1021/np400762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dong J.-Y., He H.-P., Shen Y.-M., Zhang K.-Q. Nematicidal epipolysulfanyldioxopiperazine from Gliocladium roseum. J. Nat. Prod. 2005;68:1510–1513. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 223.Minato H., Matsumoto M., Katayama T. Studies on the metabolites of Verticillium sp. structures of verticillins A, B, and C. J. Chem. Soc. Perkin Trans. 1. 1973;17:1819–1825. doi: 10.1039/p19730001819. [DOI] [PubMed] [Google Scholar]
- 224.Joshi B.K., Gloer J.B., Wicklow D.T. New verticillin and glisoprenin analogues from Gliocladium catenulatum, a mycoparasite of Aspergillus flavus sclerotia. J. Nat. Prod. 1999;62:730–733. doi: 10.1021/np980530x. [DOI] [PubMed] [Google Scholar]
- 225.Zheng C.J., Park S.H., Koshino H., Kim Y.H., Kim W.G. Verticillin G, a new antibacterial compound from Bionectra byssicola. J. Antibiot. 2007;38:61–64. doi: 10.1038/ja.2007.8. [DOI] [PubMed] [Google Scholar]
- 226.Michel K.H., Chaney M.O., Jones N.D., Hoehn M.M., Nagarajan R. Epipolythiopiperazinedione antibiotics from Penicillium turbatum. J. Antibiot. 1974;27:57–64. doi: 10.7164/antibiotics.27.57. [DOI] [PubMed] [Google Scholar]
- 227.Neuss N., Nagarajan R., Molloy B.B., Huckstep L.L. Aranotin and related metabolites. II. Isoation, characterization, and structures of two new metabolites. Tetrahedron Lett. 1968;42:4467–4471. doi: 10.1016/S0040-4039(01)99162-2. [DOI] [Google Scholar]
- 228.Zheng C.J., Kim C.J., Bae K.S., Kim Y.H., Kim W.G. Bionectins A-C, epidithiodioxopiperazines with anti-MRSA activitiy, from Bionectra byssicola F120. J. Nat. Prod. 2006;69:1816–1819. doi: 10.1021/np060348t. [DOI] [PubMed] [Google Scholar]
- 229.Meng L.-H., Li X.-M., Lv C.-T., Huang C.-G., Wang B.-G. Brocazines A-F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014;77:1921–1927. doi: 10.1021/np500382k. [DOI] [PubMed] [Google Scholar]
- 230.Xu G., He G., Bai H., Yang T., Zhang G., Wu L., Li G. Indole alkaloids from Chaetomium globosum. J. Nat. Prod. 2015;78:1479–1485. doi: 10.1021/np5007235. [DOI] [PubMed] [Google Scholar]
- 231.Fujimoto H., Sumino M., Okuyama E., Ishibashi M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J. Nat. Prod. 2004;67:98–102. doi: 10.1021/np0302201. [DOI] [PubMed] [Google Scholar]
- 232.Avent A.G., Hanson J.R., Truneh A. Metabolites of Gliocladium flavofuscum. Phytochemistry. 1993;32:197–198. doi: 10.1016/0031-9422(92)80131-W. [DOI] [Google Scholar]
- 233.Kirby G.W., Rao G.V., Robins D. New co-metabolites of gliotoxin in Gliocladium virens. J. Chem. Soc. Perkin Trans. 1. 1988;2:301–304. doi: 10.1039/p19880000301. [DOI] [Google Scholar]
- 234.Hauser D., Loosli H.R., Niklaus P. Isolierung von 11α,11′α-dihydroxychaetocin aus Verticillium tenerum. Helv. Chim. Acta. 1972;55:2182–2187. doi: 10.1002/hlca.19720550639. [DOI] [PubMed] [Google Scholar]
- 235.Kawahara N., Nozawa K., Nakajima S., Kawai K. Studies on fungal products. Part 13. Isolation and structures of dithiosilvatin and silvathione, novel dioxopierazine derivatives from Aspergillus silvaticus. J. Chem. Soc. Perkin Trans. 1. 1987;1987:2099–2101. doi: 10.1039/p19870002099. [DOI] [Google Scholar]
- 236.Onodera H., Hasegawa A., Tsumagari N., Nakai R., Ogawa T., Kanda Y. MPC1001 and its analogues: New antitumor agents from the fungus Cladorrhinum species. Org. Lett. 2004;6:4101–4104. doi: 10.1021/ol048202d. [DOI] [PubMed] [Google Scholar]
- 237.Nozawa K., Udagawa S., Nakajima S., Kawai K. Studies on fungal products. XIV. Emestrin B, a new epitrithiodioxopiperazine, from Emericella striata. Chem. Pharm. Bull. 1987;35:3460–3463. doi: 10.1248/cpb.35.3460. [DOI] [Google Scholar]
- 238.Herath H.M.T.B., Jacob M., Wilson A.D., Abbas H.K., Nanayakkara N.P.D. New secondary metabolites from bioactive extracts of the fungus Armillaria tabescens. Nat. Prod. Res. 2013;27:1562–1568. doi: 10.1080/14786419.2012.738206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Herath K., Jayasuriya H., Zink D.L., Sigmund J., Vicente F., Cruz M., Basilio A., Bills G.F., Polishook J.D., Donald R., et al. Isolation, structure elucidation, and antibacterial activity of methiosetin, a teramic acid from a tropical sooty mold (Capnodium sp.) J. Nat. Prod. 2012;75:420–424. doi: 10.1021/np200857y. [DOI] [PubMed] [Google Scholar]
- 240.Brown A.E., Finlay R., Ward J.S. Antifungal compounds produced by Epicoccum purpurascens against soil-borne plant pathogenic fungi. Soil Biol. Biochem. 1987;6:657–664. doi: 10.1016/0038-0717(87)90044-7. [DOI] [Google Scholar]
- 241.Kong F., Wang Y., Liu P., Dong T., Zhu W. Thiodiketopeperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014;77:132–137. doi: 10.1021/np400802d. [DOI] [PubMed] [Google Scholar]
- 242.Al-Fatimi M.A.A., Julich W.D., Jansen R., Lindequist U. Bioactive components of the traditionally used mushroom Podaxis pistillaris. Evid. Based Complement. Altern. Med. 2006;3:87–92. doi: 10.1093/ecam/nek008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Park H.B., Kwon H.B., Lee C.-H., Yang H.O. Glionitrin A, an antibitotic-antitumor metabolite derived from competitive interaction between abandoned mine microbes. J. Nat. Prod. 2009;72:248–252. doi: 10.1021/np800606e. [DOI] [PubMed] [Google Scholar]
- 244.Gulder T., Hong H., Correa J., Egereva E., Wiese J., Imhoff J., Gross H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs. 2012;10:2912–2935. doi: 10.3390/md10122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Devault R.L., Rosenbrook W. Novel class of diketopiperazines. J. Antibiot. 1973;26:532–534. doi: 10.7164/antibiotics.26.532. [DOI] [PubMed] [Google Scholar]
- 246.Strunz G.M., Kakushima M., Stillwell M.A. Epitetrathiodioxopiperazine with 3S,6S configuration from Hyalodendron sp. Can. J. Chem. 1975;53:295–297. doi: 10.1139/v75-040. [DOI] [Google Scholar]
- 247.Takahashi C., Numata A., Ito Y., Matsumura E., Araki H., Kushida K. Leptosins, antitumor metabolites of a fungus isolated from a marine alaga. J. Chem. Soc. Perkin Trans. 1. 1994;13:1859–1864. doi: 10.1039/p19940001859. [DOI] [Google Scholar]
- 248.Takahashi C., Takai Y., Kimura Y., Numata A., Shigematsu N., Tanaka H. Cytotoxic metabolites from a fungal adherent of a marine alga. Phytochemistry. 1995;38:155–158. doi: 10.1016/0031-9422(94)00582-E. [DOI] [PubMed] [Google Scholar]
- 249.Takahashi C., Numata A., Matsumura E., Minoura K., Eto H., Shingu T., Ito T., Hasegawa T. Leptosins I and J, cytotoxic substances produced by a Leptosphaeria sp. physico-chemical properties and structures. J. Antibiot. 1994;47:1242–1249. doi: 10.7164/antibiotics.47.1242. [DOI] [PubMed] [Google Scholar]
- 250.Takahashi C., Minoura K., Yamada T., Numata A., Kushida K., Shingu T., Hagishita S., Nakai H., Sato T., Hiroshi H. Potent cytotoxic metabolites from a Leptosphaeria species structure determination and conformational analysis. Tetrahedron. 1995;51:3483–3498. doi: 10.1016/0040-4020(95)00102-E. [DOI] [Google Scholar]
- 251.Yamada T., Iwamoto C., Yamagaki N., Yamanouchi T., Minoura K., Yamori T., Uehara Y., Andoh T., Umemura K., Numata A. Leptosins M-N1, cytotoxic metabolites from a Leptosphaeria species separated from a marine alga. Structure determination and biological activities. Tetrahedron. 2002;58:479–487. doi: 10.1016/S0040-4020(01)01170-X. [DOI] [Google Scholar]
- 252.Argoudelis A.D., Mizsak S.A. Melinacidins II, III and IV structural studies. J. Antibiot. 1977;30:468–473. doi: 10.7164/antibiotics.30.468. [DOI] [PubMed] [Google Scholar]
- 253.Pedras M.S.C., Abrams S.R., Seguin-Swartz G., Quail J.W., Jia Z. Phomalirazine, a novel toxin from the phytopathogenic fungus Phoma lingam. J. Am. Chem. Soc. 1989;1:1904–1905. doi: 10.1021/ja00187a068. [DOI] [Google Scholar]
- 254.Carr G., Tay W., Bottriell H., Andersen S.K., Mauk A.G., Andersen R.J. Plectosphaeroic acids A, B, and C, indoleamine 2,3-dioxygenase inhibitors produced in culture by a marine isolate of the fungus. Org. Lett. 2009;11:2996–2999. doi: 10.1021/ol900972j. [DOI] [PubMed] [Google Scholar]
- 255.Ooike M., Nozawa K., Kawai K.-I. An epitetrathiodioxopiperazine related to emestrin from Emericella foveolata. Phytochemistry. 1997;46:123–126. doi: 10.1016/S0031-9422(97)00210-0. [DOI] [Google Scholar]
- 256.Curtis P.J., Greatbanks D., Hesp B., Cameron A.F., Freer A.A. Sirodesmins A, B, C, and G, antiviral epipolythiopiperazine-2,5-diones of fungal origin: X-ray analysis of sirodesmin A diacetate. J. Chem. Soc. Perkin Trans. 1. 1977;2:180–189. doi: 10.1039/p19770000180. [DOI] [PubMed] [Google Scholar]
- 257.Mitrovic P.M., Orcic D.Z., Sakac V.O., Marjanovic-Jeromela A.M., Grahovac N.L., Milosevic D.M., Marisavljevic D.P. Characerization of sirodesmins isolated from the phytopathogenic fungus Leptosphaeria macuans. J. Serbian Chem. Soc. 2012;77:1363–1379. doi: 10.2298/JSC111231048M. [DOI] [Google Scholar]
- 258.Elliott C.E., Gardiner D.M., Thomas G., Cozijnsen A., Van De Wouw A., Howlett B.J. Production of the toxin sirodesmin PL by Leptosphaeria maculans during infection of Brassica napus. Mol. Plant Pathol. 2007;8:791–802. doi: 10.1111/j.1364-3703.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 259.Ronaldson J.W., Taylor A., White E.P., Abraham R.J. Sporidesmins I, Isolation and characterization of sporidesmin and sporidesmin-B. J. Chem. Soc. 1963:3172–3180. doi: 10.1039/jr9630003172. [DOI] [Google Scholar]
- 260.Mullbacher A., Waring P., Tiwari-Palni U., Eichner R.D. Structural relationship of epipolythiodioxopiperazines and their immunomodulating activity. Mol. Immunol. 1986;23:231–235. doi: 10.1016/0161-5890(86)90047-7. [DOI] [PubMed] [Google Scholar]
- 261.Sun R., Gao Y., Shen K., Xu Y., Wang C., Liu H., Dong J. Antimicrobial metabolites from the aquatic fungus Delitschia corticola. Phytochem. Lett. 2011;4:101–105. doi: 10.1016/j.phytol.2010.12.001. [DOI] [Google Scholar]
- 262.Hodges R., Shannon J.S. The isolation and structure of sporidesmin C. Aust. J. Chem. 1966;19:1059–1066. doi: 10.1071/CH9661059. [DOI] [Google Scholar]
- 263.Rahman R., Safe S., Taylor A. Sporidesmins Part IX, Isolation and structure of sporidesmin E. J. Chem. Soc. C. 1969;12:1665–1668. doi: 10.1039/j39690001665. [DOI] [PubMed] [Google Scholar]
- 264.Francis E., Rahman R., Safe S., Taylor A. Sporidesmins XII, Isolation and structure of sporidesmin G, a naturally-occuring 3,6-epitetrathiopiperazine-2,5-dione. J. Chem. Soc. Perkin Trans. 1. 1972;6:470–472. doi: 10.1039/p19720000470. [DOI] [PubMed] [Google Scholar]
- 265.Rahman R., Safe S., Taylor A. Sporidesmins Part 17, Isolation of sporidesmin H and sporidesmin J. J. Chem. Soc. Perkin Trans. 1. 1978;12:1476–1479. doi: 10.1039/p19780001476. [DOI] [PubMed] [Google Scholar]
- 266.Feng Y.J., Blunt J.W., Cole A.L.J., Munro M.H.G. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004;67:2090–2092. doi: 10.1021/np030326w. [DOI] [PubMed] [Google Scholar]
- 267.Monti F., Ripamonti F., Hawser S.P., Islam K. Aspirochlorine: A highly selective and potent inhibitor of fungal protein synthesis. J. Antibiot. 1999;52:311–318. doi: 10.7164/antibiotics.52.311. [DOI] [PubMed] [Google Scholar]
- 268.Zhang Y., Liu S., Che Y., Liu X. Epicoccins A-D, epipolythiodioxopiperazines from a Cordyceps-colozizing isolate of Epicoccum nigrum. J. Nat. Prod. 2007;70:1522–1525. doi: 10.1021/np070239u. [DOI] [PubMed] [Google Scholar]
- 269.Liu Y., Li X.M., Meng L.H., Jiang W.L., Xu G.M., Huang C.G., Wang B.G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015;78:1294–1299. doi: 10.1021/acs.jnatprod.5b00102. [DOI] [PubMed] [Google Scholar]
- 270.Yamazaki H., Takahashi O., Murakami K., Namikoshi M. Induced production of a new unprecedented epitrithiodiketopiperazine, chlorotrithiobrevamide, by a culture of the marine-derived Trichoderma cf. brevicompactum with dimethyl sulfoxide. Tetrahedron Lett. 2015;56:6262–6265. doi: 10.1016/j.tetlet.2015.09.113. [DOI] [Google Scholar]
- 271.Yamazaki H., Rotinsulu H., Narita R., Takahashi R., Namikoshi M. Induced production of halogenated epidithiodiketopiperazines by a marine-derived Trichoderma cf. brevicompactum with sodium halides. J. Nat. Prod. 2015;78:2319–2321. doi: 10.1021/acs.jnatprod.5b00669. [DOI] [PubMed] [Google Scholar]
- 272.Kajula M., Ward J.M., Turpeinen A., Tejesvi M.T., Hokkanen J., Tolonen A., Hakkanen H., Picart P., Ihalainen J., Sahl H.-G., et al. Bridged epipolythiodiketopiperazines form Penicillium raciborskii, an endophytic fungus of Rhododendron tomentosum Harmaja. J. Nat. Prod. 2016;79:685–690. doi: 10.1021/np500822k. [DOI] [PubMed] [Google Scholar]
- 273.Zhu M., Zhang X., Feng H., Dai J., Li J., Che Q., Gu Q., Zhu T., Li D. Penicisulfuranols A-F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017;80:71–75. doi: 10.1021/acs.jnatprod.6b00483. [DOI] [PubMed] [Google Scholar]
- 274.Seephonkai P., Kongsaeree P., Prabpai S., Isaka M., Thebtaranonth Y. Transformation of an irregularly bridged epidithiodiketopiperazine to trichodermamide A. Org. Lett. 2006;8:3073–3075. doi: 10.1021/ol061046l. [DOI] [PubMed] [Google Scholar]
- 275.Guo C., Yeh H., Chiang Y., Sanchez J.F., Chang S., Bruno K.S., Wang C.C.C. Biosynthesis pathway for the epipopythiodioxopiperazine acetylaranotin in Aspergillus terreus revealed by genome-based deletion analysis. J. Am. Chem. Soc. 2013;135:7205–7213. doi: 10.1021/ja3123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wang W., Wang Y., Tao H., Peng X., Liu P., Zhu W. Cerebrosides of the halotolerant fungus Alternaria raphanin isolated from a sea salt field. J. Nat. Prod. 2009;72:1695–1698. doi: 10.1021/np9002299. [DOI] [PubMed] [Google Scholar]
- 277.Usami Y., Aoki S., Hara T., Numata A. New dioxopiperazine metabolites from a Fusarium species separated from a marine alga. J. Antibiot. 2002;55:655–659. doi: 10.7164/antibiotics.55.655. [DOI] [PubMed] [Google Scholar]
- 278.Zhao W.Y., Zhu T.J., Han X.X., Fan G.T., Liu H.B., Zhu W.M., Gu Q.Q. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2009;23:203–207. doi: 10.1080/14786410600906970. [DOI] [PubMed] [Google Scholar]
- 279.Song Y., Dou H., Gong W., Liu X., Yu Z., Li E., Tan R., Hou Y. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharmacol. 2013;705:49–60. doi: 10.1016/j.ejphar.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 280.Li X., Kim S.-K., Nam K.W., Kang J.S., Choi H.D., Son B.W. A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. 2006;59:248–250. doi: 10.1038/ja.2006.35. [DOI] [PubMed] [Google Scholar]
- 281.Yoshida K., Okamoto M., Shimazaki N., Hemmi K. PAF inhibitors of microbial origin. Studies on diketopiperazine derivatives. Prog. Biochem. Pharmacol. 1988;22:66–80. [PubMed] [Google Scholar]
- 282.Suzuki Y., Takahashi H., Esumi Y., Arie T., Morita T., Koshino H., Uzawa J., Uramoto M., Yamaguchi I. Haematocin, a new antifungal diketopiperazine produced by Nectria haematococca Berk. et Br. (880701a-1) causing nectria blight disease on ornamental plants. J. Anitbiot. 2000;53:45–49. doi: 10.7164/antibiotics.53.45. [DOI] [PubMed] [Google Scholar]
- 283.Fang M., Fang H., Huang Y., Zhao Y. Mycoediketopiperazine, a novel fungal metabolite from a Papularia sp. Tetrahedron Lett. 2005;46:2147–2148. doi: 10.1016/j.tetlet.2005.01.140. [DOI] [Google Scholar]
- 284.Kirby G.W., Robins D.J., Stark W.M. Asteroxiepin, a new sulfur-containing oxepine derivative from Aspergillus terreus. J. Chem. Res. S. 1986;8:302–303. [Google Scholar]
- 285.Prachyawarakorn V., Mahidol C., Sureram S., Sangpetsiripan S., Wiyakrutta S., Ruchirawat S., Kittakoop P. Diketopiperazines and phthalides from a marine derived fungus of the order Pleosporales. Planta Med. 2008;74:69–72. doi: 10.1055/s-2007-993783. [DOI] [PubMed] [Google Scholar]
- 286.Haritakun R., Rachtawee P., Komwijit S., Nithithanasilp S., Isaka M. Highly conjugated ergostane-type steroids and aranotin-type diketopiperazines from the fungus Aspergillus terreus BCC 4651. Helv. Chim. Acta. 2012;95:308–313. doi: 10.1002/hlca.201100335. [DOI] [Google Scholar]
- 287.Watts K.R., Ratnam J., Ang K.-H., Tenney K., Compton J.E., McKerrow J., Crews P. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines form two deep water marine-derived fungi. Bioorg. Med. Chem. 2010;18:2566–2574. doi: 10.1016/j.bmc.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang Y., Liu S., Liu H., Liu X., Che Y. Cycloaspeptides F and G, cyclic pentapeptides from a cordyceps-colonizing isolate of Isaria farinosa. J. Nat. Prod. 2009;72:1364–1367. doi: 10.1021/np900205m. [DOI] [PubMed] [Google Scholar]
- 289.Yang M.-H., Li T.-X., Wang Y., Liu R.-H., Luo J., Kong L.-Y. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia. 2017;116:72–76. doi: 10.1016/j.fitote.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 290.Wang F., Tong Q., Ma H., Xu H., Hu S., Ma W., Xue Y., Liu J., Wang J., Song H., et al. Indole diketopiperazines from endophytic Chaetomium sp. 88194 induce breast cancer cell apoptotic death. Sci. Rep. 2015;5:9294. doi: 10.1038/srep09294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Park H.B., Kim Y.-J., Park J.-S., Yang H.-O., Lee K.R., Kwon H.C. Glionitrin B, a cancer invasion inhibitory diketopiperazine produced by microbial coculture. J. Nat. Prod. 2011;74:2309–2311. doi: 10.1021/np200563x. [DOI] [PubMed] [Google Scholar]
- 292.Shin J., Fenical W. Isolation of gliovictin from the marine deuteromycete Asteromyces cruciatus. Phytochemistry. 1987;26:3347. doi: 10.1016/S0031-9422(00)82503-0. [DOI] [Google Scholar]
- 293.Meng L.-H., Zhang P., Li X.-M., Wang B.-G. Penicibrocazines A-E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs. 2015;13:276–287. doi: 10.3390/md13010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang K.-T., Xu M.-Y., Liu W., Li H.-J., Xu J., Yang D.-P., Lan W.-J., Wang L.-Y. Two additional new compounds from the marine-derived fungus Pseudallescheria ellipsoidea F42-3. Molecules. 2016;21:442. doi: 10.3390/molecules21040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ronaldson J.W. Sporidesmins XVIII, The infrared solution spectra (4000–1600 cm−1) of sporidesmin, sporidesmin-B, sporidesmin-D and sporidesmin-E. Aust. J. Chem. 1981;34:1215–1222. doi: 10.1071/CH9811215. [DOI] [Google Scholar]
- 296.Jamieson W.D., Rahman R., Taylor A. Sporidesmins VIII, Isolation and structure of sporidesmin-D and sporidesmin-F. J. Chem Soc. C Org. 1969;11:1564–1567. doi: 10.1039/j39690001564. [DOI] [Google Scholar]
- 297.Huang R., Zhou X., Xu T., Yang X., Liu Y. Diketopiperazines form marine organisms. Chem. Biodivers. 2010;7:2809–2829. doi: 10.1002/cbdv.200900211. [DOI] [PubMed] [Google Scholar]
- 298.Zhao J., Shan T., Mou Y., Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev. Med. Chem. 2011;11:159–168. doi: 10.2174/138955711794519492. [DOI] [PubMed] [Google Scholar]
- 299.Pejin B., Jovanovic K.K., Mojovic M., Savic A.G. New and highly antitumor natural products from marine-derived fungi: Covering the period from 2003 to 2012. Curr. Top. Med. Chem. 2013;13:2745–2766. doi: 10.2174/15680266113136660197. [DOI] [PubMed] [Google Scholar]
- 300.Nisa H., Kamili A.N., Nawchoo I.A., Shafi S., Shameem N., Bandh S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015;82:50–59. doi: 10.1016/j.micpath.2015.04.001. [DOI] [PubMed] [Google Scholar]