Abstract
The species of the genus Trillium (Melanthiaceae alt. Trilliaceae) include perennial herbs with characteristic rhizomes mainly distributed in Asia and North America. Steroids and saponins are the main classes of phytochemicals present in these plants. This review summarizes and discusses the current knowledge on their chemistry, as well as the in vitro and in vivo studies carried out on the extracts, fractions and isolated pure compounds from the different species belonging to this genus, focusing on core biological properties, i.e., cytotoxic, antifungal and anti-inflammatory activities.
Keywords: bioactive phytochemicals, cytotoxic activity, anti-inflammatory activity, analgesic activity, antifungal activity
1. Introduction
Natural products obtained from plants have played remarkable role in drug discovery and improvement of health care system [1,2,3,4,5,6]. According to the World Health Organization (WHO) estimate, about 80% of world population relies on natural sources for their primary health care need while the remaining 20% of the population uses integrated natural sources [7]. Even at the dawn of 21st century, 11% of the 252 drugs considered as basic and essential by the WHO were exclusively of flowering plants origin [8]. At present, the area of cancer and infectious diseases are mostly dependent on natural products, and among the 175 approved anti-cancer drug molecules, 85 (49%) are either natural products or their derivatives [3].
In scientific literature around the world, more than 35,000 plant species have been reported to be used in different human cultures for medicinal purposes [9]. Nevertheless, this number could be much higher, as knowledge of indigenous use of medicinal plants mainly passes verbally from one generation to another and largely remains undocumented. Among the 250,000 reported higher plant species, only 5–15% has been scrutinized for their bioactive molecules [10]. Therefore, medicinal plants represent an area under focus since their secondary metabolites encompass a significant number of drugs used in current therapeutics and their potential as source of new medicines is beyond any doubts.
Saponins are steroid or triterpene glycosides widely distributed in the plants that possess hemolytic properties and poisonous effects [11]. The aglycone part (sapogenin) of saponins may have steroid or triterpenoid nuclei, based on whom saponins are generally classified. Steroidal saponins are less common and usually found in monocotyledonous plants as compared to triterpenoid saponins, which are extensively distributed and found in dicotyledonous plants [12]. The basic skeleton of steroidal sapogenins (27C) may be either 6-ring spirostane or 5-ring furostane, while triterpenoid sapogenins (30C) are structurally different and often consist of five or rarely four units. In general, at C-3 of aglycone moiety, the glycone (sugar) is attached, including one to several monosaccharide units. The attached sugar chains may be from one to three, either straight, branched or both. The presence of different substituents in the sapogenin as well as the composition, linkage and number of sugar moieties account for structural diversity of saponins [13].
Similarly, the structural complexity of the saponins accounts for their diverse physicochemical, pharmacological and biological properties as well as their commercial relevance as promising molecules with several applications in food, cosmetic, pharmaceutical and health fields [14]. Indeed, saponins have been investigated for the development of new natural medicines and to prove the efficacy of traditional herbal medicines. Crude drugs containing saponins that have less irritating effects following oral administration are generally used as expectorant and antitussive agents [11]. It is worth mentioning that many saponins have been reported to exhibit significant anti-inflammatory, anti-nociceptive, antipyretic, anti-allergic and anti-cancer properties [15,16].
Steroids are a group of secondary metabolites derived from cholesterol, showing diverse chemical structure and biological functions. Almost all steroid molecules possess the same basic perhydroxyl cyclopentenophenanthrene skeleton. The differences in the basic skeleton and the attachment of different groups result in various classes of steroids [17]. Steroids have many pharmacological applications and the research is continuing to find out about these metabolites, as potential lead compounds in drug design and discovery [18]. For instance, ecdysteroids are polyhydroxy steroids produced by certain plants including those belonging to the genus Trillium. Plants containing ecdysteroids possess noticeable pharmacological properties as anabolic, antidiabetic, analgesic, anti-inflammatory and anthelmintic activities [18,19].
2. The Genus Trillium
Trillium is the most important genus belonging to the family Melanthiaceae alt. Trilliaceae. Steven Elliott in 1817 wrote: “The family Trilliaceae is an attractive one. A spiral of leaves at the peak of a stem, sustaining solitary flower; it encloses and covers numerous species”. The genus Trillium consists of perennial herbs with characteristic rhizomes that are horizontal or erect, semierect, branched or faintly unbranched, compressed to shortened, elongated to bulky and fleshy, distal end pointed or premorse; the apex bears large terminal shoot/bud. Stem has leaf-sheaths and brown scales at the base. Leaves are three located at the top of the main stem. Flowers are totally to partly pedicellate, sessile and syncarpous. The genus comprises about forty-eight interrelated species in eastern North America and temperate areas of Asia, as well as in western North America [20]. Most of the Trillium species are related with deciduous forests (Arcto-Tertiary flora), which have continued with remarkable changes in geographical ranges since the early Tertiary period in the northern hemisphere. At present, Trillium species are limited to one of three geographical areas: Asia, western North America and eastern North America [21]. Some important species of the genus Trillium with specific characteristics [22,23,24] are reported in Table 1.
Table 1.
Common species of the genus Trillium.
| No. | Species with Common Name | Occurrence | Flowering Period |
|---|---|---|---|
| 1 |
Trillium erectum L.
|
North America | April–June |
| 2 |
Trillium nivale Riddell
|
United States | March–April |
| 3 |
Trillium undulatum Willd.
|
Wisconsin (U.S.) | April–June |
| 4 |
Trillium pusillum Michx.
|
United States | March–May |
| 5 |
Trillium grandiflorum (Michx.) Salisb.
|
Mountains of Virginia (U.S.) | April–June |
| 6 |
Trillium ovatum Pursh
|
North America | March–May |
| 7 |
Trillium luteum Harb.
|
Joseph rivers and elsewhere in Michigan (U.S.) | April–May |
| 8 |
Trillium petiolatum Pursh
|
North America | April–May |
| 9 |
Trillium simile Gleason
|
North America | April–May |
| 10 |
Trillium lancifolium Raf.
|
North America | February–May |
| 11 | Trillium kamtschaticum Pall. Ex Miyabe | Korea, Japan, Russia, North America, China | April–June |
| 12 | Trillium tschonoskii Maxim. | Bhutan, Japan, Korea, China | July–August |
| 13 | Trillium taiwanense S.S.Ying | Taiwan, China | May–June |
| 14 |
Trillium parviflorum V.G.Sokup
|
North America | March–May |
| 15 | Trillium govanianum Wall. | Bhutan, India, Nepal, China, Pakistan | April–August |
3. Phytochemicals of the Genus Trillium
The genus Trillium is a rich source of bioactive phytochemicals as steroids, saponin derivatives and flavonoids [25,26,27,28,29]. Thus far, several steroidal saponins have been isolated and purified from the plants belonging to this genus and, at present, novel metabolites/phytochemicals from Trillium ssp. are investigating using the latest technologies [30,31,32,33,34]. The secondary metabolites/phytochemicals of species belonging to the genus Trillium are reported in Table 2.
Table 2.
List of phytochemicals isolated from genus Trillium.
| Source | Chemical Name | Chemical Structure | References |
|---|---|---|---|
| T. govanianum | Spirost-5-en-3-ol(diosgenin) (compound 1) |  |
[28] |
| T. erectum | (25S)-spirost-5-ene-3β,17α,27-triol (compound 2) |  |
[26] |
| T. erectum | (25S)-3β,17α-dihydroxyspirost-5-en-27-yl β-d-glucopyranoside (compound 3) | 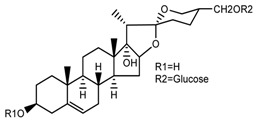 |
[26] |
| T. erectum | (25S)-17α,27-dihydroxyspirost-5-en-3 β-yl β-d-glucopyranoside (compound 4) | 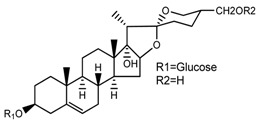 |
[26] |
| T. erectum | (25S)-27-[(β-d-glucopyranosyl)oxy]-17α-hydroxyspirost-5-en-3β-yl O α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 5) | 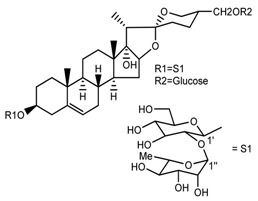 |
[26] |
| T. erectum | (25S)-27-[(β-d-glucopyranosyl)oxy]-17α,27-dihydroxyspirost-5-en-3-yl O-(4-O-acetyl-α-l-rhamnopyranosyl)-(1→2)-β-d-glucopyranoside (compound 6) |  |
[26] |
| T. erectum | (25S)-27-[(β-d-glucopyranosyl)oxy]-17α,27-dihydroxyspirost-5-en-3β-d-glucopyranosyl-(1→6)-O-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranoside (compound 7) | 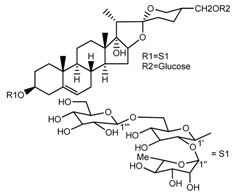 |
[26] |
| T. erectum | (25S)-17α,27-dihydroxyspirost-5-en-3β-yl O-(4-O-acetyl-α-l-rhamnopyranosyl)-(1→2)-β-d-glucopyranoside (compound 8) |  |
[26] |
| T. erectum | (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→4)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 9) |  |
[26] |
| T. erectum | (25R)-26-[(β-d-glucopyranosyl)oxy]-22α-methoxyfurost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 10) |  |
[26] |
| T. kamtschaticum | (25S)-17α,27-dihydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 11) |  |
[35] |
| Trillium kamtschaticum | (25R)-17α-hydroxyspirost-5-en-3 β-yl O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 12) | 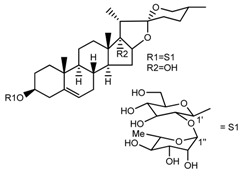 |
[36] |
| T. kamtschaticum | (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranoside (compound 13) | 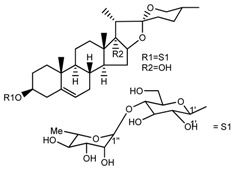 |
[36] |
| T. kamtschaticum | (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 14) | 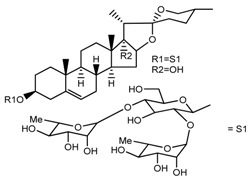 |
[36] |
| T. kamtschaticum | (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[O-α-l-rhamnopyranosyl-(1→4)-a-l-rhamnopyranosyl-(1→4)]-α-d-glucopyranoside (compound 15) | 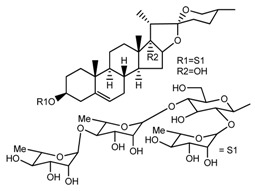 |
[36] |
| T. kamtschaticum | (25R)-spirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 16) |  |
[36] |
| T. kamtschaticum | (25R)-spirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 17) |  |
[36] |
| T. kamtschaticum | (25R)-spirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[O-α-l-rhamnopyranosyl-(1→4)-α-Lrhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 18) |  |
[36] |
|
T. kamtschaticum
T. erectum |
(25R)-26-[(β-d-glucopyranosyl)oxy]-17α-hydroxy-22β-methoxyfurost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 19) |  |
[26,37] |
|
T. kamtschaticum
T. erectum |
(25R)-26-[(β-d-glucopyranosyl)oxy]-17α-hydroxy-22-amethoxyfurost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 20) |  |
[26,37] |
| T kamtschaticum | (25R)-26-[(β-d-glucopyranosyl)oxy]-3β-[(O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl)oxy]-cholesta-5,17-diene-16,22-dione (compound 21) |  |
[36] |
| T. kamtschaticum | (25R)-27-hydroxypenogenin 3-O-α-l-rhamnopyranosyl-(1→2)-O-β-d-glucopyranoside (compound 22) |  |
[35] |
| T. kamtschaticum | Penogenin 3-O-α-l-rhamnopyranosyl-(1→2)-O-β-d-glucopyranoside (compound 23) |  |
[36] |
| T. kamtschaticum | Penogenin 3-O-β-d-glucopyranosyl-(1→6)-[O-α-l[-rhamnopyranosyl-(1→2)]-O-β-d-glucopyranoside] (compound 24) |  |
[35] |
| T. kamtschaticum | Penogenin 3-[O-β-[d-glucopyranoside] (compound 25) | 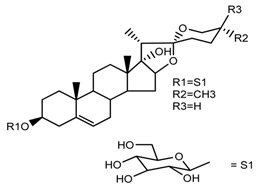 |
[36] |
| T. kamtschaticum | Deoxytrillenoside (compound 26) | 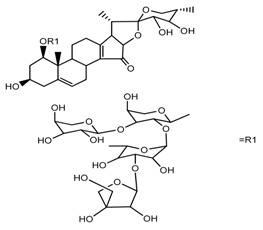 |
[35,38] |
| T. kamtschaticum | 26-O-β-d-glucopyranosyl (22,25R)-furost-5-eene-3β,17α,22,26-tetraol 3-O-α-l-rhamnopyranosyl-(1→2)-O-β-d-glucopyranoside (compound 27) |  |
[36] |
| T. kamtschaticum | 26-O-β-ad-23glucopyranosyl (22,25R)-furost-5-eene-3β,17α, 22,26-tetraol 3-O-α-l-rhamnopyranosyl-(1→42)-O-α-l-[rhamnopyranosyl-(1→04)]-O-β-d-glucopyranoside (compound 28) |  |
[35] |
| T. kamtschaticum | 26-O-β-d-glucopyranosyl 17(20)-dehydrokryptogenin 3-O-α-l-rhamnopyranosyl-(1→2)-[O-α-l-rhamnopyranosyl-(1→4)]-O-β-d-glucopyranoside (compound 29) |  |
[35] |
| T. kamtschaticum | 26-O-β-d-glucopyranosyl 17(20)-dehydrokryptogenin 3-O-α-l-rhamnopyranosyl-(1→2)-O-β-d-glucopyranoside (compound 30) |  |
[36] |
| T. govanianum | Govanoside A (compound 31) |  |
[28] |
| T. govanianum | Borassoside E (compound 32) |  |
[28] |
| T. tschonoskii | l-O-[2,3,4-tri-O-acetyl-α-l-rhamnopyranosyl-(1→2)4-O-acetyl-α-l-arabinopyranosyl]-21-O-acetyl-epitrillenogenin (compound 33) |  |
[39] |
| T. tschonoskii | (25S)-27-hydroxypenogenin-[3-O-α-l-rhamnopyranosyl-(1→2)-O-β-d-glucopyranoside] (compound 34) |  |
[39] |
|
T. govanianum
T. grandiflorum |
Spirost-5-ene-3,17-diol(pennogenin) (compound 35) |  |
[28,40] |
|
T. govanianum
T. kamtschaticum |
β-Ecdysone(20-hydroxyecdysone) (compound 36) |  |
[29,35] |
| T. govanianum | 5 hydroxy, β-ecdysone(5,20 dihydroxyecdysone) (compound 37) |  |
[19] |
| Other non-steroidal compounds | |||
| T. kamtschaticum | (10R,6E)-7,11-dimethyl-3-mehyl3ene-6-dodecaene-1,2,10,11-tetraol 10-O-β-d-glucopyranoside (compound 38) |  |
[35] |
| T. kamtschaticum | (10R,6E)-3,7,11-trimethyl-1,6-dodecadien-3,10,11-triol 10-O-glucopyranoside (compound 39) |  |
[35] |
| T. kamtschaticum | (10R,6E)-3,7,11-trimethyl-1,6-dodecadien-3,10,11-triol 10-O-glucopyranoside (compound 40) |  |
[35] |
| T. tschonoskii | 7,11-dimethyl-3-methylene-1,6-dodecadien-10,11-diol 10-O-β-d-(1→4)glucopyranosyl-O-β-d-glucopyranoside (compound 41) |  |
[41] |
| T. tschonoskii | Methylferulorate (compound 42) |  |
[41] |
| T. kamtschaticum | Astragalin (compound 43) |  |
[35] |
| T. undulatum | 3,4,5,7-tetrahydroxyflavone (compound 44) |  |
[27] |
| T. undulatum | quercetin 3-O-rutinoside; [3-O-β-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside] (compound 45) |  |
[27] |
| T. undulatum | Kaempferol 3-O-α-rhamnosyl-(1→2)-O-[α-rhamnosyl-(l→6)]-β-glucoside (compound 46) |  |
[27] |
| T. tschonoskii | p-hydroxymethyl benzyl alcohol (compound 47) |  |
[42] |
| T. govanianum | Govanic acid (compound 48) |  |
[19] |
| T. tschonoskii | 3,7,11-trimethyl-3,9,11-trihydroxyl-1,6-dodecadiene glycerol (compound 49) |  |
[42] |
| T. tschonoskii | 2-methyl-3,4 dihydroxy-hexanedioic acid (compound 50) |  |
[42] |
4. Medicinal Importance and Biological Activities of the Genus Trillium
Plant species belonging to the genus Trillium have been extensively used as a remedy for various diseases in different traditional healing systems and several preclinical studies have corroborated these uses. The reported biological/pharmacological activities of different species indicate the promising potential of crude extracts, solvent fractions and isolated pure compounds.
The rhizomes of T. erectum, named beth roots, have been used in folk medicine for the treatment of hemorrhages from uterus, urinary tract and lungs [43]. T. tschonoskii has been traditionally used in China for at least one thousand years [44,45]. Dried rhizomes of this plant species have been used as herbal remedy for treatment of hypertension, neurasthenia, giddiness, headache, removing carbuncles and ameliorating pains [46,47]. The anticancer and pro-apoptotic activities of n-BuOH extract against human lung cancer cells have also been demonstrated [45].
The ethanol, ethyl acetate and butanol extracts of T. tschonoskii significantly suppressed the edema of rat hind paw swelling elicited by injection of carrageenan [48]. Finally, T. tschonoskii improved learning and memory in rats, by enhancing the expression of anti-oxidase enzymes [49].
Ethanol extract from rhizomes and aerial parts of T. grandiflorum exhibited antifungal activity [40]. The rhizome of T. govanianum is commonly known as “matar zela” or “teen patra” in Pakistan, and “nag chatri” in India [19]. In folk medicine, T. govanianum is used to cure dysentery and boils; in wound healing, and menstrual and sexual disorders; and as anti-inflammatory and antiseptic agent [50]. The powdered roots are used as body and sexual tonic [51]. Noteworthy, analgesic, anti-inflammatory, antifungal, free radical scavenging, β-glucuronidase inhibitory activities as well as cytotoxicity against prostate and cervical carcinoma cells of T. govanianum have been recently reported [19,29,50].
5. Bioactivities of the Genus Trillium Phytochemicals
The isolated secondary metabolites of the genus Trillium mostly belong to the chemical class of steroidal saponins and steroids, including ecdysteroids, even if flavonoids and trihydroxy fatty acids have also been reported. The isolated compounds exhibited a remarkable potential when tested in different in vitro and in vivo assays. Thus far, among the tested steroidal saponins, both spirostanol and furostanol saponins, many of them exhibited relevant cytotoxicity against different cancer cell lines, few showed high potential against tested fungal strains, while some of them possessed antioxidant and COX-2 inhibitory activity, as reported in Table 3.
Table 3.
Bioactivities of the genus Trillium phytochemicals.
| Source | Compound | Reported Pharmacological Activity |
|---|---|---|
| T. tschonoskii | pennogenin 3-O-α-l-rhamnopyranosyl-(1→2) [α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 51) | Cytotoxic, anti-proliferative and morphological effects on lung cancer cell line [52]; cytotoxicity against malignant sarcoma cells [53] |
| 7-β-hydroxy trillenogenin 1-O-β-d-apiofuranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-α-l-arabinopyranoside, Trillenoside A (compound 52) | Inhibitory activity against COX-2 in in macrophagocytes of the mouse abdominal cavity stimulated by LPS [21] | |
| T. erectum | (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside (compound 53) | Cytotoxicity against HL-60 human promyelocytic leukemia cells. IC50 (μg/mL) = 6.10 ± 0.04 [26] |
| (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 9) | Cytotoxicity against HL-60 human promyelocytic leukemia cells, IC50 (μg/mL) = 3.58 ± 0.18 [26] | |
| (25R)-17α-hydroxyspirost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[O-α-l-rhamnopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→4)]-α-d-glucopyranoside (compound 54) | Cytotoxicity against HL-60 human promyelocytic leukemia cells, IC50 (μg/mL) = 2.65 ± 0.22 [26] | |
| (25R)-spirost-5-en-3β-yl O-α–l-rhamnopyranosyl-(1→2)-O-[O-α-l-rhamnopyranosyl-(1→4)-α–l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (compound 55) | Cytotoxicity against HL-60 human promyelocytic leukemia cells, IC50 (μg/mL) = 1.68 ± 0.11 [26] | |
| (25R)-26-[(β-d-glucopyranosyl)oxy]-22α-methoxyfurost-5-en-3β-yl O-α-l-rhamnopyranosyl-(1→2)-O-[α-l-rhamnopyranosyl-(1→4)]-β-d-glucopyranoside (methylprotodioscin) (compound 56) | Cytotoxicity against HL-60 human promyelocytic leukemia cells, IC50 (μg/mL) = 2.89 ± 0.24 [26] | |
| T. govanianum | (1β,3β,23S,24S)-1-[O-β–d-glucopyranosyl (1→3)-O-β-d-glucopyranosyl (1→6)-O-β-d-apiofuranosyl]-3,23 dihydroxyspirosta-5,25-dienyl-24-[O-α-l-rhamnopyranosyl (1→4)-β-d-6-deoxygulopyranoside] (govanoside A) (compound 31) boeassoside E (compound 32) 7, 8, 9-trihydroxy-(10Z)-10-octadecenoic acid (compound 48) | Antifungal activity against Aspergillus niger, A. flavus, Candida albicans, C. glabrata, Trichophyton rubrum [19,28] |
| pennogenin (compound 35), borassoside E (compound 32), diosgenin (compound 1) | ROS inhibitory activity [51] | |
| T. kamtschaticum | 21-O-acetyl-trillenogenin-1-O-β-d-apiofuranosyl-(1→3)-4′′-acetyl-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside (compound 56) | Cytotoxicity against human colorectal cancer cells (HCT116) IC50 (μM) = 4.92 ± 1.00 [34] |
| 24-O-acetyl-epitrillengenin-1-O-β-d-apiofuranosyl-(1→3)-α-Lrhamnopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-α-l-arabinopyranoside (compound 57) | Cytotoxicity against human colorectal cancer cells (HCT116) IC50 (μM) = 5.84 ± 1.05 [34] | |
| 26-O-β-d-glucopyranosyl-17(20)-dehydrokryptogenin-3-O-α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranoside (compound 58) | Cytotoxicity against human colorectal cancer cells (HCT116) IC50 (μM = 17.28 ± 2.69 [34] | |
| T. grandiflorum | (3β,25R)-spirost-5-en-3-yl O-6-deoxy-α-l-mannopyranosyl-(1→2)-O-[6-deoxy-α-l-mannopyranosyl-(1→4)]-β-d-glucopyranoside (compound 59) | Antifungal activity against Candida albicans MIC (μg/mL) = 1.56 [40] |
In addition, the biological activities of steroidal saponins mainly depend on their aglycone moieties (steroidal sapogenins) as well as the number and structure of monosaccharide units in their sugar chains. A slight structural diversity endorses a significant difference on their antifungal and cytotoxic properties, i.e., spirostanol saponins exhibit a higher antifungal potential in comparison to their analogous furostanol saponins. Therefore, it is worth mentioning that further detailed studies on the structure–activity relationships (SAR) and molecular/biochemical targets of steroidal saponins are required to explore the therapeutic potential of this important class of natural products as leads for new drug discovery.
6. Conclusions and Future Perspectives
Thus far, more than 40 steroidal saponins having spirostane and furostane type aglycons from the genus Trillium have been isolated. Their structure was determined by the use of spectroscopic techniques, including fast atom bombardment mass spectrometry (FABMS) and extensive 2D nuclear magnetic resonance (NMR) experiments (COSY, TOCSY, NOESY, HSQC, and HMBC). The existing data strongly suggest that plants belonging to the genus Trillium are rich source of steroids and saponins and possess a therapeutic potential in the management of cancers, fungal infections, inflammatory and painful disorders. Noteworthy, most of the plant species are still unexplored, and some are under investigation; therefore, further comprehensive studies are needed to screen new sources of phytochemicals to develop promising phytotherapeutics effective in the treatment of chronic degenerative and infectious diseases. Finally, the most promising Trillium secondary metabolites have to be investigated in humans, in properly designed randomized clinical trials, to reach the highest level of clinical evidence.
Acknowledgments
Authors did not receive any source of funding in support of this study.
Author Contributions
All the authors contributed equally to the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products, A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 4.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo L.T., Okogun J.I., Folk W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013;30:584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan H., Khan Z., Amin S., Mabkhot Y.N., Mubarak M.S., Hadda T.B., Maione F. Plant bioactive molecules bearing glycosides as lead compounds for the treatment of fungal infection: A review. Biomed. Pharmacother. 2017;93:498–509. doi: 10.1016/j.biopha.2017.06.077. [DOI] [PubMed] [Google Scholar]
- 7.Cragg G.M. Natural product drug discovery and development: The United States National Cancer Institute role. P. R. Health Sci. J. 2002;21:97–111. [PubMed] [Google Scholar]
- 8.Veeresham C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012;3:200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewington A. Medicinal Plants and Plant Extracts: A Review of Their Importation into Europe. Traffic International; Cambridge, UK: 1993. [Google Scholar]
- 10.Cragg G.M., Newman D.J. Natural product drug discovery in the next millennium. Pharm. Biol. 2001;39:8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 11.Shibata S. New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. Springer; Berlin, Germany: 1977. Saponins with biological and pharmacological activity; pp. 177–196. [Google Scholar]
- 12.Podolak I., Galanty A., Sobolewska D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincken J.P., Heng L., de Groot A., Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Netala V.R., Ghosh S.B., Bobbu P.U., Anitha D.A., Tartte V.I. Triterpenoid saponins: A review on biosynthesis, applications and mechanism of their action. Int. J. Pharm. Pharm. Sci. 2015;7:24–28. [Google Scholar]
- 15.Yassin N., Melek F., Selim M., Kassem I. Pharmacological activities of saponin containing fraction derived from Gleditsia caspica Desf. methanolic fruit extract. Der Pharm. Lett. 2013;5:247–253. [Google Scholar]
- 16.Yan L., Zhang Y., Gao W., Man S., Wang Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp. Oncol. 2009;31:27–32. [PubMed] [Google Scholar]
- 17.Marwat G.A., Khan A.R., Hussain I., Kalsoom S. A review on naturally occurring steroids. J. Chem. Soc. Pak. 2005;27:443–450. [Google Scholar]
- 18.Sultan A., Rauf Raza A. Steroids: A diverse class of secondary metabolites. Med. Chem. 2015;5:310–317. doi: 10.4172/2161-0444.1000279. [DOI] [Google Scholar]
- 19.Rahman S.U., Adhikari A., Ismail M., Shah M.R., Khurram M., Anis I., Ali F. A New Trihydroxylated Fatty Acid and Phytoecdysteroids from Rhizomes of Trillium govanianum. Rec. Nat. Prod. 2017;11:323–327. [Google Scholar]
- 20.Osaloo S.K., Utech F.H., Ohara M., Kawano S. Molecular systematics of Trilliaceae I. Phylogenetic analyses of Trillium using matK gene sequences. J. Plant Res. 1999;112:35–49. doi: 10.1007/PL00013853. [DOI] [Google Scholar]
- 21.Wang J., Zou K., Zhang Y., Liu C., Wu J., Zhou Y., Dan F., Zhang Y. An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem. Pharm. Bull. 2007;55:679–681. doi: 10.1248/cpb.55.679. [DOI] [PubMed] [Google Scholar]
- 22.Nasir E., Ali S. Flora of Pakistan. National Herbarium Publisher; Islamabad, Pakistan: 1989. [Google Scholar]
- 23.Torrey J., Gray A. A Flora of North America. Hafner Pub. Co.; New York, NY, USA: 1969. [Google Scholar]
- 24.Zhengyi W., Raven P.H., Deyuan H. Flora of China, Volume 7: Menispermaceae through Capparaceae. Science Press; Beijing, China: 2008. [Google Scholar]
- 25.Hayes P.Y., Lehmann R., Penman K., Kitching W., De Voss J.J. Steroidal saponins from the roots of Trillium erectum (Beth root) Phytochemistry. 2009;70:105–113. doi: 10.1016/j.phytochem.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Yokosuka A., Mimaki Y. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry. 2008;69:2724–2730. doi: 10.1016/j.phytochem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Yoshitama K., Oyamada T., Yahara S. Flavonoids in the leaves of Trillium undulatum Willdenow. J. Plant Res. 1997;110:379–381. doi: 10.1007/BF02524937. [DOI] [Google Scholar]
- 28.Ismail M., Shah M.R., Adhikari A., Anis I., Ahmad M.S., Khurram M., Govanoside A. A new steroidal saponin from rhizomes of Trillium govanianum. Steroids. 2015;104:270–275. doi: 10.1016/j.steroids.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ur Rahman S., Ismail M., Shah M.R., Iriti M., Shahid M. GC/MS analysis, free radical scavenging, anticancer and β-glucuronidase inhibitory activities of Trillium govanianum rhizome. Bangladesh J. Pharmacol. 2015;10:577–583. doi: 10.3329/bjp.v10i3.23446. [DOI] [Google Scholar]
- 30.Zhou Y., Gao X., Fu Q., Guo P., Xu X., Zhang T., Ge Y., Zhang B., Wang M., Zeng A., Luo Z. Enrichment of total steroidal saponins from the extracts of Trillium tschonoskii Maxim by macroporous resin and the simultaneous determination of eight steroidal saponins in the final product by HPLC. J. Sep. Sci. 2017;40:1115–1124. doi: 10.1002/jssc.201600884. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y.J., Sun X.G., Yang J., Li Q., Zhang J., Zhao Y., Ma B.P., Guo B.L. Determination of three saponins in rhizoma and fibrous root of Trillium tschonoskii and Trillium kamtschaticum. China J. Chin. Mater. Med. 2017;42:1146. doi: 10.19540/j.cnki.cjcmm.20170121.009. [DOI] [PubMed] [Google Scholar]
- 32.Gao X., Sun W., Fu Q., Niu X. Rapid Identification of Steroidal Saponins in Trillium tschonoskii Maxim by Ultra Performance Liquid Chromatography Coupled to Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. Phytochem. Anal. 2015;26:269–278. doi: 10.1002/pca.2560. [DOI] [PubMed] [Google Scholar]
- 33.Khan K.M., Nahar L., Mannan A., Ul-Haq I., Arfan M., Ali Khan G., Hussain I., Sarker S.D. Cytotoxicity, In vitro anti-Leishmanial and fingerprint HPLC-photodiode array analysis of the roots of Trillium govanianum. Nat. Prod. Res. 2017;10:1–9. doi: 10.1080/14786419.2017.1371164. [DOI] [PubMed] [Google Scholar]
- 34.Qin X.J., Si Y.A., Chen Y., Liu H., Ni W., Yan H., Shu T., Ji Y.H., Liu H.Y. Cytotoxic steroidal saponins from Trillium kamtschaticum. Bioorg. Med. Chem. Lett. 2017;27:2267–2273. doi: 10.1016/j.bmcl.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 35.Ono M., Takamura C., Sugita F., Masuoka C., Yoshimitsu H., Ikeda T., Nohara T. Two new steroid glycosides and a new sesquiterpenoid glycoside from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. 2007;55:551–556. doi: 10.1248/cpb.55.551. [DOI] [PubMed] [Google Scholar]
- 36.Nohara T., Miyahara K., Kawasaki T. Steroid saponins and sapogenins of underground parts of Trillium kamtschaticum Pall. II. Pennogenin-and kryptogenin 3-O-glycosides and related compounds. Chem. Pharm. Bull. 1975;3:872–885. doi: 10.1248/cpb.23.872. [DOI] [Google Scholar]
- 37.Nakano K., Kashiwada Y., Nohara T., Tomimatsu T., Tsukatani H., Kawasaki T. Steroid saponins and sapogenins of underground parts of trillium-kamtschaticum pall. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1982;102:1031–1035. doi: 10.1248/yakushi1947.102.11_1031. [DOI] [Google Scholar]
- 38.Ono M., Yanai Y., Ikeda T., Okawa M., Nohara T. Steroids from the Underground Parts of Trillium kamtschaticum. Chem. Pharm. Bull. 2003;51:1328–1331. doi: 10.1248/cpb.51.1328. [DOI] [PubMed] [Google Scholar]
- 39.Ono M., Hamada T., Nohara T. An 18-norspirostanol glycoside from Trillium tschonoskii. Phytochemistry. 1986;25:544–545. doi: 10.1016/S0031-9422(00)85524-7. [DOI] [Google Scholar]
- 40.Hufford C.D., Liu S., Clark A.M. Antifungal activity of Trillium grandiflorum constituents. J. Nat. Prod. 1988;51:94–98. doi: 10.1021/np50055a013. [DOI] [PubMed] [Google Scholar]
- 41.Nakano K., Maruhashi A., Nohara T., Tomimatsu T., Imamura N., Kawasaki T. A flavonol glycoside and a sesquiterpene cellobioside from Trillium tschonoskii. Phytochemistry. 1983;22:1249–1251. doi: 10.1016/0031-9422(83)80233-7. [DOI] [Google Scholar]
- 42.Zhang Z., Zuo Y., Wang Y., Cai M., Li Y. Study on chemical constituents in the roots and rhizome of Trillium tschonoskii (III) Zhong yao cai Zhongyaocai. J. Chin. Med. Mater. 2013;36:1779–1782. [PubMed] [Google Scholar]
- 43.Deni B. The Royal Horticultural Society Encyclopedia of Herbs and Their Uses. Dorling Kindersley; London, UK: 1996. p. 424. [Google Scholar]
- 44.Li Y., Liu C., Xiao D., Han J., Yue Z., Sun Y., Fan L., Zhang F., Meng J., Zhang R., et al. Trillium tschonoskii steroidal saponins suppress the growth of colorectal Cancer cells in vitro and in vivo. J. Ethnopharmacol. 2015;168:136–145. doi: 10.1016/j.jep.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 45.Huang W., Zou K., Xiong B. The Rhizome of Trillium tschonoskii Maxim. Extract Induces Apoptosis in Human Lung Cancer Cells. Z. Naturforschung C. 2011;66:477–484. doi: 10.5560/ZNC.2011.66c0477. [DOI] [PubMed] [Google Scholar]
- 46.Fu L.-K., Jin J. China Plant Red Data Book-Rare and Endangered Plants. Volume 1. Science Press; Beijing, China: 1992. [Google Scholar]
- 47.Zhan Y. Resources of Medicinal Plants in Shennongjia of China. Hubei Scientific and Technologic Press; Wuhan, China: 1994. p. 249. [Google Scholar]
- 48.Yu L.-L., Zou K., Wang J.-Z., Zhu L.-B., Zhou Y., Yang J. Study on the anti-inflammatory, analgesic and thrombosis effects of extract of Trillium tschonoskii Maxim. Lishizhen Med. Mater. Med. Res. 2008;19:1178–1180. [Google Scholar]
- 49.Ai M.-X. Effects of Trillium tschonoskii Maxim on Learning and Memory and Anti-oxidase in Rats. Lishizhen Med. Mater. Med. Res. 2007;18 doi: 10.3969/j.issn.1008-0805.2007.03.059. [DOI] [Google Scholar]
- 50.Ur Rahman S., Adhikari A., Ismail M., Raza Shah M., Khurram M., Shahid M., Li F., Haseeb A., Akbar F., Iriti M. Beneficial effects of Trillium govanianum rhizomes in pain and inflammation. Molecules. 2016;21:1095. doi: 10.3390/molecules21081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S.M., Page S., Ahmad H., Shaheen H., Ullah Z., Ahmad M., Harper D.M. Medicinal flora and ethnoecological knowledge in the Naran Valley, Western Himalaya, Pakistan. J. Ethnobiol. Ethnomed. 2013;9:4. doi: 10.1186/1746-4269-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang W., Zou K. Cytotoxicity of a plant steroidal saponin on human lung cancer cells. Asian Pac. J. Cancer Prev. 2011;12:513–517. [PubMed] [Google Scholar]
- 53.Huang W., Zou K. Cytotoxicity of the saponin TTB2 on Ewing sarcoma cells. Exp. Ther. Med. 2015;10:625–628. doi: 10.3892/etm.2015.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]


