Abstract
A novel series of 11,12-cyclic carbonate azithromycin-3-O-descladinosyl-3-O-carbamoyl glycosyl derivatives were designed, synthesized, and evaluated for their antibacterial activities in vitro. Most of these compounds had significant antibacterial activity against seven kinds of susceptible strains. In particular, compound G1 exhibited the most potent activity against methicillin-resistant Streptococcus pneumoniae 943 (MIC: 1 μg/mL), Staphylococcus pneumoniae 746 (MIC: 2 μg/mL), Streptococcus pyogenes 447 (MIC: 8 μg/mL), and Escherichia coli 236 (MIC: 32 μg/mL), which were two-, four-, four-, four-, and eight-fold stronger activity than azithromycin, respectively. Additionally, compound G2 exhibited improved activity against methicillin-resistant Staphylococcus aureus MRSA-1 (MIC: 8 μg/mL), Streptococcus pneumoniae 943 (MIC: 2 μg/mL), Staphylococcus pneumoniae 746 (MIC: 2 μg/mL), and Escherichia coli 236 (MIC: 32 μg/mL), which were two-, two-, four-, and eight-fold better activity than azithromycin, respectively. As for methicillin-resistant Staphylococcus aureus MRSA-1, compound G6 presented the most excellent activity (MIC: 4 μg/mL), showing four-fold higher activity than azithromycin (MIC: 16 μg/mL) and erythromycin (MIC: 16 μg/mL). However, compared with other compounds, compounds G7 and G8 with the disaccharide side chain were observed the lower activity against seven strains.
Keywords: azithromycin, glycosyl derivatives, synthesis, antibacterial activity
1. Introduction
Macrolide antibiotics are polyketides composed of a 14-, 15-, or 16-membered macrocyclic lactone ring to which several sugars and/or side chains have been attached. They exhibit potent activity against human pathogens in clinical treatment of respiratory tract, genital, and skin infections, inhibiting bacterial protein synthesis by blocking the formation of peptide bond [1,2]. In the past five decades, the broad use of macrolide antibiotics has led to bacterial resistance that can even increase the likelihood of death from infection by common pathogens in developed and developing countries alike [3,4,5]. Therefore, it is still urgently necessary to seek and extend efficacious and safe chemotherapeutic agents to improve present situation. As is known to all, the most widespread approach with combating the resistance is to continue modifying existing classes of antibacterial agents to design and synthesize novel analogues.
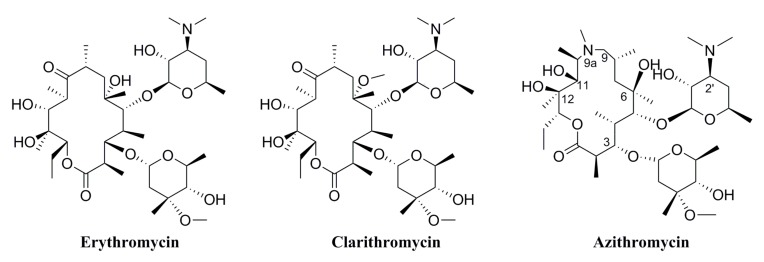
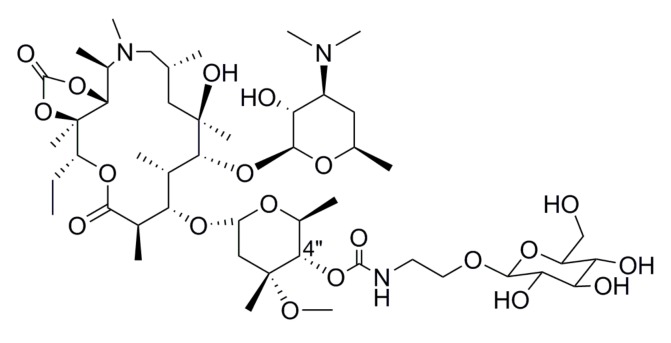
Commonly-used clinical macrolide antibiotics include erythromycin, clarithromycin, and azithromycin (Figure 1). The 14-membered macrolide erythromycin has been widely used for over five decades, but the extensive emergence of erythromycin resistance is extraordinarily high [6]. The 15-membered macrolide azithromycin, which is prepared from erythromycin by expanding semi-synthetically, is characterized by good oral bioavailability, high concentrations in tissues and fluids, wide spectrum of activity, and superior pharmacokinetic and safety properties in comparison to erythromycin [7,8,9,10]. Efforts to find macrolides that were active against macrolide-resistant strains led to the modifications of C-3, C-5, C-6 and C11/C12 positions of the azithromycin [11,12,13]. Additionally, 9a-N substituted 15-membered azalides with amide and amine functionalities also had marked advantages over azithromycin in terms of high selectivity for the bacteria and moderate oral bioavailability in vivo [9,14,15,16]. Besides, with intensive research of azithromycin, a novel modified measure of the 4″-position of the cladinose was potent against emergence of drug resistance [17,18,19,20,21]. To resolve these therapeutic problems of tolerance, we investigated and found that the 3-O-acylerythromycin derivatives, which were named ‘acylides’, not only recovered the abolished antibacterial activity but also enhanced pharmacological activity against resistant pathogens [22]. There have been few reports about the other glycosylation modification of the 3-O-position. Our previous studies have found that the hydroxy group of the glycosyl moiety was significant for hydrogen bond formation, greatly improving soluble in water [19]. Compound F1, the 4″-position of the cladinose modified with glucosyl group, was the best active glycosylated compound we reported (Figure 2). It showed improved activity against all the tested bacterial strains relative to the positive control erythromycin, even showed the same or higher antibacterial activities as azithromycin. Besides, we also found that compounds with disaccharide side chains expressed the lowest activity. We deduced that the monosaccharide side chain may help the target compounds fit into the binding pocket and interact with the bacterial ribosome, while the bulky disaccharide side chain might hinder the interactions of the derivative with the bacterial ribosome. Therefore, the group which connected to 3-O-position was very important for the activity. Considering these aspects, we hydrolyzed the cladinose of azithromycin and introduced different glycosyl moieties to the 3-O-position to examine the antibacterial activities of the derivatives in vitro and discussed the structure–activity relationship.
Figure 1.
Chemical structure of macrolide antibiotics.
Figure 2.
Figure 2. Chemical structure of compound F1.
2. Results and Discussions
2.1. Chemistry
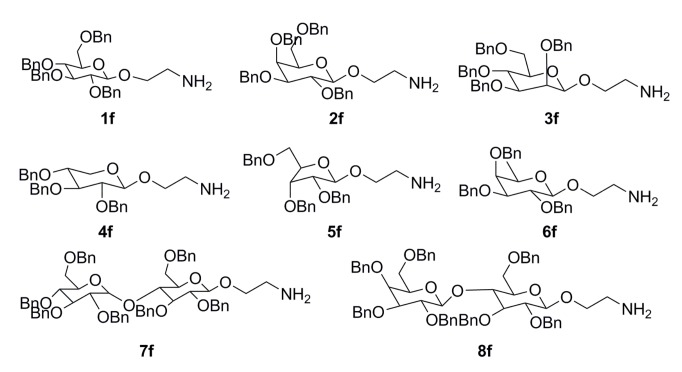
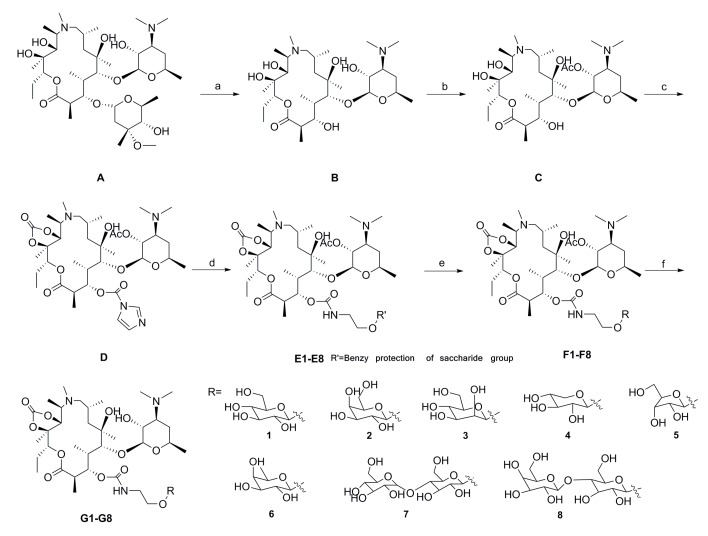
The eight glycosylated intermediate compounds 1f–8f were prepared from inexpensive and available carbohydrates with a series of reported synthetic methods (Figure 3) [19]. The general synthetic route of the target compounds G1–G8 was shown in Scheme 1. First, to remove the l-cladinose, azithromycin was stirred with aqueous HCl and compound B was obtained. Then, the 2′-hydroxyl group of compound B was protected with acetic anhydride to obtain compound C. 11,12-Cyclic carbonate azithromycin 3-O-acylimidazolide D was prepared by treatment of C with Et3N and CDI in toluene [23]. NaH and DMF were abandoned in this reaction, greatly improving yield in contrast to previous studies [19]. The compounds E1–E8 were obtained by benzyl protection of the saccharide group in the presence of DBU. The intermediates, E1–E8, were converted to compounds F1–F8 by Pd/CaCO3 under H2. Finally, the target compounds G1–G8 were prepared by deprotection of the 2′-O-acetyl group. Structures of all the objective compounds and intermediates were determined by 1H-NMR, 13C-NMR, and ESI-MS.
Figure 3.
Structure of the intermediates 1f–8f.
Scheme 1.
Scheme 1. The synthesis of the target compounds G1–G8. Reactions and conditions: (a) HCl/H2O, r.t., 5 h, in 91% yield; (b) Ac2O, DCM, Et3N, r.t., 20 h, in 88% yield; (c) CDI, Et3N, toluene, reflux, 48 h, in 93% yield; (d) 1f–8f, DBU, DMF, r.t., 24 h, in 17–25% yield; (e) Pd/C, H2, MeOH, r.t., in 70–80% yield; (f) CH3OH, 55 °C, 20 h, 62–75% yield.
2.2. Antibacterial Activity
The in vitro antibacterial activities were reported as minimum inhibitory concentrations (MICs), which were determined using a standard dilution assay as recommended by the CLSI [24].
2.3. Biological Evaluation
The results of the assays are summarized in Table 1. The data points express the mean of replicate experiments. All of our susceptibility tests were performed three times using each antibacterial agent.
Table 1.
Antibacterial activities of the title compounds in vitro (MIC, μg/mL).
| Compd. | S. aureus MSSA-1 | S. aureus MRSA-1 | S. pneumoniae 943 | S. pneumoniae 746 | S. pyogenes 447 | E. coli 236 | E. coli ATCC 25922 |
|---|---|---|---|---|---|---|---|
| G1 | 2 | 8 | 1 | 2 | 8 | 32 | 128 |
| G2 | 2 | 8 | 2 | 2 | 32 | 32 | 128 |
| G3 | 4 | 8 | 8 | 4 | 32 | 32 | 128 |
| G4 | 8 | 8 | 4 | 8 | 32 | 64 | 256 |
| G5 | 4 | 8 | 4 | 16 | 32 | 64 | 128 |
| G6 | 2 | 4 | 8 | 16 | 64 | 64 | 128 |
| G7 | 8 | 16 | 32 | 32 | 128 | 128 | 256 |
| G8 | 16 | 16 | 16 | 16 | 128 | 256 | 256 |
| Azi | 2 | 16 | 4 | 8 | 32 | 256 | 256 |
| Ery | 16 | 16 | 8 | 16 | 32 | 256 | 256 |
Abbreviations: Azi, azithromycin; Ery, erythromycin.
The antibacterial activities indicated that 11,12-cyclic carbonate azithromycin-3-O-descladinosyl-3-O-carbamoyl glycosyl derivatives exhibited apparent activity against seven strains compared with azithromycin and erythromycin. Noticeably, all the title compounds exhibited more potent against S. aureus and S. pneumoniae than the other strains. The MIC value indicated that the compounds G1 and G2 showed excellent activity against the other compounds, even more potent than the positive control erythromycin and azithromycin. The MIC value of compound G1 and G2 (2 µg/mL) was eight times lower than that of the positive control erythromycin (16 µg/mL) against S. aureus MSSA-1 in vitro and even the same as the positive control azithromycin (2 µg/mL). In particular, compound G1 exhibited the most potent activity against methicillin-resistant S. pneumoniae 943 (1 μg/mL), S. pneumoniae 746 (2 μg/mL), S. pyogenes 447 (8 μg/mL), and E. coli 236 (32 μg/mL), which were two-, four-, four-, four-, and eight-fold better activity than azithromycin, respectively. Additionally, compound G2 exhibited improved activity against methicillin-resistant S. aureus MRSA-1 (8 μg/mL), S. pneumoniae 943 (2 μg/mL), S. pneumoniae 746 (2 μg/mL), and E. coli 236 (32 μg/mL), which were two-, two-, four-, and eight-fold better activity than azithromycin, respectively. The compounds G3–G6 showed slightly higher activities than the positive control erythromycin and azithromycin, while compounds G7 and G8 presented the slightly less activities according to the MIC values. As for methicillin-resistant S. aureus MRSA-1, compound G6 presented the most excellent activity (4 µg/mL), showing four- and four-fold higher activity than azithromycin (16 μg/mL) and erythromycin (16 μg/mL). However, compared with other compounds, compounds G7 and G8 with the disaccharide side chain were observed the lower activity against seven strains. Activity of the tested compounds was either maintained or slightly improved against E. coli 236 and E. coli ATCC 25922. Unfortunately, all compounds exhibited similar or only slightly better activity than erythromycin and azithromycin against S. pyogenes 447, E. coli 236, and E. coli ATCC 25922.
Compared with the 11,12-cyclic carbonate azithromycin-4″-O-carbamoyl glycosyl derivatives we reported in the past [19], the title compounds displayed more potent activity against S. aureus MSSA-1 and S. aureus MRSA-1. Most of them exhibited 2–4 fold higher activity than that before. As for the other five strains, this series did not show notably improved antibacterial activity.
3. Materials and Methods
3.1. Chemistry
All reactions were monitored by thin-layer chromatography (Huanghai, Yantai, China). A part of each compound was visualized by UV light (Yukang, Shanghai, China). NMR spectra were recorded on a Bruker Avance II 600 spectrometer at 600 MHz for 1H-NMR and 150 MHz for 13C-NMR with TMS as the internal standard. Chemical shifts are expressed in δ (ppm). The HPLC-MS were recorded on Agilent 1100 series LC/MS. Column chromatography was carried out using silica gel (300–400 mesh, Huanghai, Yantai, China). Silica gel chromatography solvents were of analytical grade (Huanghai, Yantai, China). All reaction solvents were dried prior to use according to standard procedures (Taitan, Shanghai, China).
3.1.1. 3-O-descladinosyl Azithromycin (B)
We added 36% aqueous HCl dropwise to a solution of azithromycin (10 g, 13 mmol) in water (60 mL) at room temperature until the reaction mixture was adjusted to pH = 1.0–2.0. The resulting solution was allowed to stir for 5 h at the same temperature, and then it was adjusted to pH = 9 with 25% aqueous NH3. The precipitate was collected by filtration, and washed with cold water, crystallized from acetone to afford 7.17 g (91%) of 3-O-descladinosyazithromycin (B) as white solid, M.p. 139–141 °C.
3.1.2. 2′-O-Acetyl-3-O-descladinosyl Azithromycin (C)
Ac2O (2.3 mL, 49 mmol) and Et3N (6.7 mL, 49 mmol) were added to a solution of 3-O-descladinosylazithromycin (7.17 g, 12 mmol) prepared above in CH2Cl2 (80 mL) at room temperature. The resulting solution was allowed to stir for 24 h at the same temperature. The reaction was quenched with saturated NaHCO3 (60 mL) and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were washed with water and brine, then dried over anhydrous Na2SO4. The organic layer was filtered and concentrated in vacuum to afford 6.77 g (88%) of 2′-O-acetyl-3-O-descladinosy azithromycin (C) as white solid, M.p. 143–145 °C.
3.1.3. 2′-O-Acetyl-3-O-acylimidazolyl-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (D)
Et3N (2.2 mL, 14 mmol) and CDI (2.27 g, 14 mmol) were added to a solution of 2′-O-acetyl-3-descladinosy azithromycin (C, 3.59 g, 5.6 mmol) in toluene (30 mL) . The resulting solution was stirred for 48 h at 80 °C.The reaction was quenched with saturated NaHCO3 (30 mL) and the aqueous layer was extracted with toluene (2 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in a vacuum to afford 4.21 g (93%) of compound D as white solid, M.p. 149–151 °C.
3.1.4. 2′-O-Acetyl-3-O-amido-[ethyl-2-(2,3,4,6-tetra-O-benzyl-β-d-glucopyranoside)]-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (E1)
DBU (0.15 mL, 1.03 mmol) and 1f (0.47 g, 0.79 mmol) were added to a solution of D (0.4 g, 0.53 mmol) in DMF (15 mL). The resulting solution was stirred for 24 h at the room temperature. The reaction was quenched with saturated NaHCO3 (30 mL) and the aqueous layer was extracted with ethyl acetate (2 × 15 mL). The combined organic layers were washed with water and brine, and dried over NaSO4. After filtering and concentrating the reaction solution in vacuum to afford the crude compound E1 (0.21 g) as white solid. The syntheses of other intermediates (E2–E8) are carried out according to the above conditions.
3.1.5. 2′-O-Acetyl-3-O-amido-(ethyl-2-β-d-glucopyranoside)-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (F1)
A solution of E1 (140 mg, 0.11 mmol) Pd/C (50 mg) in methanol (15 mL) was adjusted to pH = 4–5 by acetic acid and stirred for 24 h under an atmosphere of hydrogen at the room temperature. After, filtering and concentrating the reaction solution in vacuum afforded the crude compound F1 as white solid. The syntheses of other intermediates (F2–F8) are carried out according to the above conditions.
3.1.6. General Procedure for the Preparation of Azithromycin Derivatives G1–G8
A solution of the above crude intermediate product in MeOH (15 mL) was heated to 55 °C and stirred for 36 h at the same temperature. After concentrating the reaction solution in vacuum, the residue was purified by flash chromatography (DCM/MeOH, 4:1, v/v) to afford the title compound.
3-O-Amido-(ethyl-2-β-d-glucopyranoside)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G1): White solid; Yield: 75%, M.p. 108–110 °C; 1H-NMR (600 MHz, CDCl3) δ 5.17 (1H, s), 5.05–5.04 (1H, m), 4.54 (2H, m), 4.38–4.36 (1H, m), 4.26–4.19 (2H, m), 3.99–3.97 (1H, m), 3.87 (1H, t), 3.82 H, m), 3.72–3.70 (1H, m), 3.68 (1H, d, J = 1.8 Hz), 3.66 (1H, d, J = 5.4 Hz) 3.64–3.61 (1H, m), 3.60 (1H, m), 3.57–3.55 (2H, m), 3.36 (2H, m), 3.27–3.23 (1H, m), 2.96 (1H, m), 2.93 (2H, m), 2.88–2.82 (2H, m), 2.67–2.62 (1H, m), 2.34 (3H, s), 2.52–2.49 (2H, m), 2.25 (9H, m), 1.98 (3H, m), 1.92–1.86 (6H, m), 1.31–1.26 (5H, m), 1.25–1.23 (7H, m), 0.94–0.90 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 176.7, 170.1, 153.7, l03.6, 100.2, 95.6, 85.9, 79.7, 76.8, 76.7, 76.2, 74.7, 73.4, 73.5, 70.8, 70.3, 68.8, 67.4, 65.3, 63.2, 61.4, 61.1, 50.4, 45.5, 44.2, 41.6, 40.4, 35.7, 34.2, 29.4, 26.7, 22.9, 22.1, 21.2, 15.1, l4.3, 12.2, 10.4, 5.7; MS (ESI) calcd. for C42H73N3O18 907.49; found [M + H]+ 908.51.
3-O-Amido-(ethyl-2-galactosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G2): White solid; Yield: 68%, M.p. 113–115 °C; 1H-NMR (600 MHz, CDCl3) δ 5.40 (1H, d, J = 3.6 Hz), 5.11 (1H, m), 5.02 (1H, d, J = 10.5 Hz), 4.50–4.39 (2H, m), 4.34–4.21 (2H, m), 4.33–4.21 (2H, m), 4.18 (1H, d, J = 11.4 Hz), 4.12 (1H, m), 4.03 (1H, m), 3.91 (2H, m), 3.70 (2H, m), 3.68–3.54 (2H, m), 3.30 (2H, m), 3.27–3.22 (1H, m), 2.88–2.82 (1H, m), 2.69–2.60 (2H, m), 2.53–2.43 (1H, m), 2.37 (2H, m), 2.25 (9H, m), 2.21 (3H, s), 2.19 (1H, m), 1.91–1.85 (2H, m), 1.68–1.63 (4H, m), 1.45 (3H, m), 1.31–1.23 (4H, m), 1.06–1.01 (9H, m), 0.94–0.90 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 178.5, 171.1, 153.7, l03.2, 100.7, 94.9, 83.3, 78.4, 77.3, 76.8, 74.7, 74.2, 73.9, 73.7, 73.2, 71.1, 70.3, 70.1, 68.1, 65.5, 62.4, 61.7, 51.2, 45.3, 42.4, 42.1, 40.2, 36.1, 35.2, 29.1, 27.5, 26.8, 22.1, 21.1, 16.4, 14.7, 11.3, 9.2, 5.4; MS (ESI) calcd. for C42H73N3O18 907.49; found [M + H]+ 908.51.
3-O-Amido-(ethyl-2-mannosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G3): White solid; Yield: 64%, M.p. 114–116 °C; 1H-NMR (600 MHz, CDCl3) δ 5.35 (1H, m), 5.04 (1H, m), 4.88 (1H, d, J = 3.0 Hz), 4.60 (1H, s), 4.55 (1H, m), 4.40 (1H, d, J = 4.2 Hz), 4.30 (1H, d, J = 12 Hz), 4.13 (1H, m), 4.04 (1H, m), 3.95 (1H, m), 3.88 (2H, m), 3.82–3.80 (1H, m), 3.68 (2H, m), 3.66 (2H, m), 3.56 (1H, m), 3.47 (2H, m), 3.36–3.35 (2H, m), 3.00–2.95 (3H, m), 2.83 (4H, m), 2.68–2.60 (1H, m), 2.50–2.47 (1H, m), 2.34 (3H, s), 2.25 (6H, m), 2.19 (2H, m), 1.89 (2H, m), 1.66 (2H, m), 1.45–1.42 (5H, m), 1.23 (1H, m), 1.07–1.00 (12H, m), 0.94–0.89 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 177.6, 171.4, 157.6, 102.3, 101.6, 95.1, 83.7, 78.4, 76.5, 74.4, 74.1, 73.9, 73.2, 70.8, 70.1, 67.8, 65.5, 62.6, 62.1, 50.3, 45.2, 42.1, 40.3, 36.1, 35.1, 29.7, 27.3, 26.6, 21.9, 21.4, 21.2, 20.8, l6.2, l4.4, 11.5, 9.4, 5.3. MS (ESI) calcd. for C42H73N3O18 907.49; found [M + H]+ 908.51.
3-O-Amido-(ethyl-2-ribosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G4): White solid; Yield: 71%, M.p. 105–107 °C; 1H-NMR (600 MHz, CDCl3) δ 5.16 (1H, d, J = 4.8 Hz), 5.05–5.04 (1H, m), 4.54 (2H, m), 4.38–4.36 (1H, m), 4.26–4.19 (2H, m), 3.99–3.97 (1H, m), 3.72–3.70 (1H, m), 3.64–3.61 (1H, m), 3.59 (2H, m), 3.57–3.55 (2H, m), 3.53 (1H, d, J = 1.8 Hz), 3.51 (1H, d, J = 5.4 Hz), 3.47 (1H, m), 3.36 (2H, m), 3.27–3.23 (1H, m), 2.96 (1H, m), 2.91 (2H, m), 2.88–2.82 (2H, m), 2.67–2.62 (1H, m), 2.52–2.49 (2H, m), 2.25 (9H, m), 1.98 (3H, m), 1.92–1.86 (6H, m), 1.31–1.26 (5H, m), 1.25–1.23 (7H, m), 0.94–0.90 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 177.1, 153.4, 153.2, 103.5, 100.4, 95.6, 85.7, 79.6, 76.7, 76.4, 76.1, 75.1, 74.2, 73.6, 70.6, 68.2, 67.4, 64.8, 62.4, 61.1, 51.4, 45.3, 43.4, 40.0, 35.3, 34.3, 30.l, 26.7, 23.2, 22.0, 21.2, 15.1, 14.4, 11.3, 10.4, 5.8. MS (ESI) calcd.for C39H69N3O16 835.47; found [M + H]+ 836.54.
3-O-Amido-(ethyl-2-xylosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G5): White solid; Yield: 65%, M.p. 107–109 °C; 1H-NMR (600 MHz, CDCl3) δ 5.21(1H, d, J = 4.8 Hz), 5.11 (1H, m), 4.50–4.39 (2H, m), 4.34–4.21 (2H, m), 4.33–4.21 (2H, m), 3.83 (1H, d, J = 12 Hz), 3.77 (2H, m), 3.74 (1H, d, J = 1.8 Hz), 3.71 (1H, d, J = 5.4 Hz), 3.68–3.54 (2H, m), 3.47 (2H, m), 3.27–3.22 (1H, m), 3.15(2H, m), 2.88–2.82 (1H, m), 2.69–2.60 (2H, m), 2.53–2.43 (1H, m), 2.37 (2H, m), 2.25 (9H, m), 2.19 (1H, m), 1.91–1.85 (2H, m), 1.68–1.63 (4H, m), 1.45 (3H, m), 1.31–1.23 (4H, m), 1.06–1.01 (9H, m), 0.94–0.90 (9H, m); 13C-NMR (150 MHz, CDCl3) 177.7, 153.4, 103.2, 101.2, 95.1, 83.6, 78.5, 75.7, 75.4, 74.4, 74.1, 73.5, 71.2, 70.3, 69.4, 68.3, 65.7, 62.3, 51.1, 45.1, 42.6, 40.4, 36.1, 35.4, 29.2, 27.5, 26.8, 21.9, 21.2, 16.2, 14.7, 11.3, 9.1, 5.4; MS (ESI) calcd. for C39H69N3O16 835.47; found [M + H]+ 836.54.
3-O-Amido-(ethyl-2-rhamnosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G6): White solid; Yield: 70%, M.p. 110–112 °C; 1H-NMR (600 MHz, CDCl3) δ 5.55 (1H, s), 5.03 (1H, m), 4.62–4.60 (2H, m), 4.44 (1H, m), 3.79 (1H, m), 3.65–3.55 (2H, m), 3.57 (2H, m), 3.53 (1H, d, J = 1.8 Hz), 3.52–3.44 (2H, m), 3.42 (1H, d, J = 5.4 Hz), 3.33 (1H, m), 3.28–3.25 (3H, m), 2.49 (1H, m), 2.39–2.37 (1H, m), 2.32–2.20 (10H, m), 2.18 (3H, m),1.91–1.86 (1H, m), 1.77–1.67 (4H, m), 1.48–1.43 (4H, m), 1.30–1.24 (4H, m), 1.04–1.00 (9H, m), 0.96–0.89 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 177.2, 171.3, 153.4, 145.5, 133.6, 103.1, 101.3, 95.6, 85.9, 79.6, 76.6, 76.1, 74.7, 73.5, 73.3, 71.2, 68.4, 67.5, 64.8, 61.2, 45.1, 43.4, 42.6, 40.2, 35.3, 34.2, 30.1, 26.8, 22.9, 22.0, 21.2, 16.7, l 5.1, l 4.2, l1.4, 10.3, 5.7; MS (ESI) calcd. for C42H73N3O17 891.49; found [M + H]+ 892.46.
3-O-Amido-(ethyl-2-maltosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G7): White solid; Yield: 62%, M.p. 117–119 °C; 1H-NMR (600 MHz, CDCl3) δ 5.41 (1H, d, J = 4.2 Hz), 5.03 (1H, m), 4.85 (2H, m), 4.62–4.60 (2H, m), 4.62 (3H, m), 4.53 (2H, m), 4.44 (1H, m), 3.96 (5H, m), 3.65–3.55 (2H, m), 3.33 (1H, m), 3.30 (2H, m), 3.28–3.25 (3H, m), 2.49 (1H, m), 2.39–2.37 (1H, m), 2.32–2.20 (10H, m), 2.14 (3H, m), 1.91–1.86 (1H, m), 1.77–1.67 (4H, m), 1.48–1.43 (4H, m), 1.30–1.24 (4H, m), 1.04–1.00 (9H, m), 0.96–0.89 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 177.6, 172.3, 153.4, 103.6, 103.1, 101.7, 95.2, 84.1, 78.4, 76.6, 76.3, 74.5, 74.2, 73.8, 73.9, 71.2, 70.1, 68.1, 65.5, 63.2, 62.4, 62.4, 62.3, 51.5, 45.2, 42.5, 40.3, 36.2, 35.2, 29.1, 27.5, 26.8, 22.1, 21.3, l6.3, 14.8, 11.1, 9.4, 5.2; MS (ESI) calcd. for C48H83N3O23 1069.54; found [M + H]+ 1070.51.
3-O-Amido-(ethyl-2-lactosyl)-3-O-descladinosyl azithromycin-11,12-cyclic carbonate (G8): White solid; Yield: 63%, M.p. 119–121 °C; 1H-NMR (600 MHz, CDCl3) δ 5.24 (1H, d, J = 4.8 Hz), 5.13 (1H, m), 4.53 (1H, m), 4.47–4.45 (1H, m), 4.38 (1H, m), 4.34–4.28 (2H, m), 3.92 (2H, d, J = 1.8 Hz), 3.83 (2H, m), 3.71 (2H, d, J = 10.4 Hz), 3.66 (4H, m), 3.63 (4H, m), 3.55 (2H, m), 3.51–3.47 (2H, m), 3.27–3.22 (2H, m), 3.18 (2H, m), 2.84–2.82 (1H, m), 2.67–2.63 (1H, m), 2.50–2.45 (1H, m), 2.28–2.20 (7H, m), 2.25–2.23 (6H, m), 2.18 (3H, s), 2.01 (1H, m), 1.89 (1H, m), 1.75 (m, 1H), 1.67–1.63 (2H, m), 1.46 (2H, m), 1.25–1.23 (4H, m), 1.05–1.01 (9H, m), 0.94–0.88 (9H, m); 13C-NMR (150 MHz, CDCl3) δ 177.3, 170.4, l56.7, l53.2, l03.1, 111.2, 101.5, 95.7, 86.1, 85.2, 80.4, 76.5, 76.3, 76.1, 73.5, 73.1, 70.9, 68.5, 67.6, 65.4, 63.1, 62.2, 61.8, 61.2, 53.5, 45.3, 43.5, 41.8, 40.3, 35.5, 34.3, 29.6, 26.8, 26.2, 22.1, 21.9, 21.2, 20.7, 20.7, 15.1, l4.1, l1.4, l0.2, 5.7; MS (ESI) calcd. for C48H83N3O23 1069.54; found [M + H]+ 1070.51.
3.2. Antibacterial Activity
The in vitro antibacterial activities were reported as minimum inhibitory concentrations (MICs), which were determined using a standard dilution assay as recommended by the CLSI [24]. For the assays, the title compounds to be tested were dissolved in dimethyl sulfoxide (DMSO), serially diluted in growth medium, inoculated and incubated at 35 °C. The selected strains evaluated were methicillin-susceptible Staphylococcus aureus MSSA-1 (S. aureus MSSA-1), methicillin-resistant Staphylococcus aureus MRSA-1 (S. aureus MRSA-1), Streptococcus pneumoniae 943 (S. pneumoniae 943), Staphylococcus pneumoniae 746 (S. pneumoniae 746), Streptococcus pyogenes 447 (S. pyogenes 447), Escherichia coli 236 (E. coli 236), and Escherichia coli ATCC 25922 (E. coli ATCC 25922). Erythromycin and azithromycin served as the positive control and were obtained from their respective manufacturers.
4. Conclusions
In summary, in our endeavor to develop promising antibacterial molecules against the strains based on the naturally occurring azithromycin, we have designed and synthesized a series of related glycosyl derivatives possessing potent in vitro antibacterial activity. The analysis of the in vitro results demonstrated that 11,12-cyclic carbonate azithromycin-3-O-descladinosyl-3-O-carbamoyl glycosyl derivatives expressed improved antibacterial activities compared with the parent compound. Above all, the compounds G1–G6 with monosaccharide side chain could be suitable to inhibit bacteria protein synthesis in the bacterial ribosome, while compounds G7 and G8 with disaccharide side chain exhibited the least activity, which could be due to the long side chain not binding well to the ribosomal RNA of bacteria. Besides, all the compounds showed significantly potent activity (2–16 μg/mL) against S. aureus than 11,12-cyclic carbonate azithromycin-4″-O-carbamoyl glycosyl derivatives reported in our published paper [19]. Maybe the hydrolyzation of l-cladinose is beneficial for activity improvement. This discovery, as well as further studies on azithromycin glycosyl derivatives, could develop a potent method to overcome the bacterial strain.
Acknowledgments
We thank Qiuye Wu for valuable scientific discussion. This work was supported by the National Natural Science Foundation of China (No. 81473104) and Shandong graduate education innovation program (SDYY16132).
Author Contributions
Chao-Ming Wang and Lei Zhang designed and carried out the experiments. Chao-Ming Wang and Feng-Lan Zhao wrote the paper. Xiao-Yun Chai and Qing-Guo Meng provided study design and guidance. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors have declared that no competing interests exist.
Footnotes
Sample Availability: Samples of the compounds G1–G8 are available from the authors.
References
- 1.Fyfe C., Grossman T.H., Kerstein K., Sutcliffe J. Resistance to Macrolide Antibiotics in Public Health Pathogens. Cold Spring Harb. Perspect. Med. 2016;6:1–37. doi: 10.1101/cshperspect.a025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deane C. Antibiotic discovery: Macrolides en masse. Nat. Chem. Biol. 2016;12:467. doi: 10.1038/nchembio.2120. [DOI] [Google Scholar]
- 3.Doern G.V., Heilmann K.P., Huynh H.K., Rhomberg P.R., Coffman S.L., Brueggemann A.B. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob. Agents Chemother. 2001;45:1721–1729. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamruzzaman M., Shoma S., Thomas C.M., Partridge S.R., Iredell J.R. Plasmid interference for curing antibiotic resistance plasmids in vivo. PLoS ONE. 2017;12:e0172913. doi: 10.1371/journal.pone.0172913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes P., Martens E., Pereira D. Nature nurtures the design of new semi-synthetic macrolide antibiotics. J. Antibiot. 2017;70:527–533. doi: 10.1038/ja.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song J.H., Jung S.I., Ko K.S., Kim N.Y., Son J.S., Chang H.H., Ki H.K., Oh W.S., Suh J.Y., Peck K.R., et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study) Antimicrob. Agents Chemother. 2004;48:2101–2107. doi: 10.1128/AAC.48.6.2101-2107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhanel G.G., Dueck M., Hoban D.J., Vercaigne L.M., Embil J.M., Gin A.S., Karlowsky J.A. Review of macrolides and ketolides: Focus on respiratory tract infections. Drugs. 2001;61:443–498. doi: 10.2165/00003495-200161040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Seiple I.B., Zhang Z.Y., Jakubec P., Mercier A.L., Wright P.M., Hog D.T., Yabu K., Allu S.R., Fukuzaki T., Carlsen P.N., et al. A platform for the discovery of new macrolide antibiotics. Nature. 2016;533:338–345. doi: 10.1038/nature17967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krajačić M.B., Perić M., Smith K.S., Schönfeld Z.I., Žiher D., Fajdetić A., Kujundžić N., Schönfeld W., Landek G., Padovan J., et al. Synthesis, structure-activity relationship, and antimalarial activity of ureas and thioureas of 15-membered azalides. J. Med. Chem. 2011;54:3595–3605. doi: 10.1021/jm2001585. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y., Wada H., Rossios C., Takagi D., Higaki M., Mikura S., Goto H., Barnes P.J., Ito K. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J. Pharmacol. Exp. Ther. 2013;345:76–84. doi: 10.1124/jpet.112.200733. [DOI] [PubMed] [Google Scholar]
- 11.Champney W.S., Tober C.L. Structure-activity relationships for six ketolide antibiotics. Curr. Microbiol. 2001;42:203–210. doi: 10.1007/pl00021055. [DOI] [PubMed] [Google Scholar]
- 12.Heggelund A., Rømming C., Undheim K. Preparation and antibacterial activity of cyclic 2′,3′-carbamate derivatives of azithromycin. Eur. J. Med. Chem. 2008;43:1657–1664. doi: 10.1016/j.ejmech.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Song L., Liu Z., Li H., Lu Y., Li Z., Ma S. Synthesis and antibacterial activity of novel 3-O-carbamoyl derivatives of clarithromycin and 11,12-cyclic carbonate azithromycin. Eur. J. Med. Chem. 2010;45:915–922. doi: 10.1016/j.ejmech.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Bukvić K.M., Novak P., Dumić M., Cindrić M., Paljetak H.C., Kujundzić N. Novel ureas and thioureas of 15-membered azalides with antibacterial activity against key respiratory pathogens. Eur. J. Med. Chem. 2009;44:3459–3470. doi: 10.1016/j.ejmech.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Perić M., Fajdetić A., Rupčić R., Alihodžić S., Žiher D., Krajačić M.B., Smith K.S., Schönfeld Z.I., Padovan J., Landek G., et al. Antimalarial activity of 9a-N substituted 15-membered azalides with improved in vitro and in vivo activity over azithromycin. J. Med. Chem. 2012;55:1389–1401. doi: 10.1021/jm201615t. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y., Choi J.Y., Fu H., Harvey C., Ravindran S., Roush W.R., Boothroyd J.C., Khosla C. Chemistry and biology of macrolide antiparasitic agents. J. Med. Chem. 2011;54:2792–2804. doi: 10.1021/jm101593u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlović D., Mutak S. Discovery of 4″-ether linked azithromycin-quinolone hybrid series: Influence of the central linker on the antibacterial activity. ACS Med. Chem. Lett. 2011;2:331–336. doi: 10.1021/ml100253p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stimac V., Škugor M.M., Jakopović I.P., Vinter A., Ilijaš M., Alihodžić S., Mutak S. Initial Scale-Up and Process Improvements for the preparation of a lead antibacterial macrolone compound. Org. Process Res. Dev. 2010;14:1401–1409. doi: 10.1021/op100199t. [DOI] [Google Scholar]
- 19.Zhang L., Chai X., Wang B., Yu S., Hu H., Zou Y., Zhao Q., Meng Q., Wu Q. Design, synthesis and biological evaluation of azithromycin glycosyl derivatives as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2013;23:5057–5060. doi: 10.1016/j.bmcl.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Ma S.T., Yan M., Wang Y.Z., Ma S.T. Synthesis and antibacterial evaluation of novel 11,4″-disubstituted azithromycin analogs with greatly improved activity against erythromycin-resistant bacteria. Eur. J. Med. Chem. 2013;59:209–217. doi: 10.1016/j.ejmech.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Ma S.T., Ma R.X., Xian R.Q., Hao B. Synthesis of novel 15-membered macrolide dimers. Chin. Chem. Lett. 2009;20:931–934. doi: 10.1016/j.cclet.2009.03.015. [DOI] [Google Scholar]
- 22.Tanikawa T., Asaka T., Kashimura M., Suzuki K., Sugiyama H., Sato M., Kameo K., Morimoto S., Nishida A. Synthesis and antibacterial activity of a novel series of acylides: 3-O-(3-pyridyl)acetylerythromycin A derivatives. J. Med. Chem. 2003;46:2706–2715. doi: 10.1021/jm020568d. [DOI] [PubMed] [Google Scholar]
- 23.Yan M., Ma R.X., Jia L., Venter H., Ma S.T. Synthesis and antibacterial activity of novel 3-O-descladinosylazithromycin derivatives. Eur. J. Med. Chem. 2017;127:874–884. doi: 10.1016/j.ejmech.2016.10.065. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2009. Approved Standard-Eight Edition: CLSI Document M07-A8. [Google Scholar]