ABSTRACT
Lipid droplets (LD) are now-well recognized as playing a role in cancer progression, however their potential role in chemoresistance remains largely unknown, particularly in colorectal cancer (CRC). We recently highlighted that LD accumulate in CRC cells under the control of lysophosphatidylcholine acyltransferase 2 (LPCAT2) enzyme expression. We also showed that chemotherapy-induced LD accumulation counteracts intrinsic and extrinsic cancer cell death activation.
KEYWORDS: colorectal cancer, chemoresistance, immunogenic cell death, Lipid droplets, LPCAT2
Commentary
Increased de novo lipid biosynthesis characterized cancer cells, but lipid droplet (LD) just started being described in cancerous phenotypes. LDs are dynamic and functional organelles primarily described for their storage function. They are composed of a neutral lipid core (triglycerides and sterol-esters) surrounded by a phospholipid monolayer mainly composed of phosphatidylcholine (PC) and several types of proteins involved in their biogenesis, growth, stability or in fatty-acid (FA) lipolysis. Lipid and protein composition define a broad range of LD “species” changing among cell types and cell conditions. Mature LDs present membrane interactions with other organelles such as endoplasmic reticulum (ER) and mitochondria leading to lipid and protein exchanges. These contacts promote LD-growth especially via the production of PC by the enzymes of the Kennedy pathway, such as phosphocholine cytidylyltransferase alpha (CCTα) relocated in LD monolayer during LD-expansion.1 Remodeling of membranous FA-linked phospholipids is a major parameter in membrane-dependent cell functions. PC remodeling in LD occurs through the re-acylation of lysophosphatidylcholine (LPC) by the LD-localised enzymes of the Lands cycle: lysophosphatidylcholine acyltransferase LPCAT1 and LPCAT2 isoforms participating in LD expansion and limiting their coalescence.2 These organelles have been described in functions participating in cancer progression such as proliferation, survival under nutrient stress and lipotoxicity. In colorectal cancer (CRC) cells, they are now considered as a hallmark of stem cells and are involved in inflammation. Although LD accumulation was described in some chemoresistant cancer phenotypes, no direct mechanisms has been provided so far3 .
To explain differences in LD density among several CRC cell lines we analysed gene and protein expression profiles of PC synthesis enzymes. Data showed that only LPCAT2 expression was positively correlated with basal LD content in CRC cell lines and supported LD formation. Co-localisation and LD isolation experiments confirmed the location of LPCAT2 mainly in the LD monolayer in LD-rich cells at basal state. As evidenced by others, cell populations presented heterogeneity in LD content, both at resting and under chemotherapy treatments. High-LD cells within a same population may contribute to the sequestration of damaging molecules such as reactive oxygen species (ROS) or lipid peroxides and may thus protect whole cell populations from cytotoxic stress.4 Sequestration function may also be extended to chemotherapeutic drugs. Indeed, hydrophobic drugs could accumulate in LD during treatment limiting their actions and protecting sensitive cells among cell population.3
In high-LD cells, the sustained relocation of LPCAT2 to LD during a treatment with a combination of 5-fluorouracil and oxaliplatin (FOX) supported their induced-accumulation during treatment. Nevertheless, chemotherapy-induced LPCAT2/LD accumulation appeared critical for CRC cell response to treatment. CRC cells accumulating LD were able to inhibit cell death induction through the inhibition of caspase activation and ER stress pathways, which seems contradictory with some studies showing LD accumulation preceding apoptosis induction (Fig. 1).5 These discrepancies could be explained by the fact that mitochondrial dysfunction and subsequent lipotoxic mediators’ accumulation (i.e. free-fatty acids (FFAs)) might occur during chemotherapeutic treatment in low-LD/LPCAT2 sensitive cell populations. High-LPCAT2 cell populations displaying greater capacities in producing LD, could sequester damaging agents and limit cytotoxic FFA-release (known to induce apoptosis), which is supported by perilipin (PLIN) 2, 3 and 5 relocation to the LD fraction upon chemotherapy treatment.6 Nevertheless, lipid exchanges are essential for organelle functions and cell integrity. Several lipid exchanges occur in cancer cells between organelles especially between LD and ER, possibly maintaining ER homeostasis by limiting FFAs and cholesterol accumulation. PC is also important in this homeostasis, since inhibition of choline kinase alpha (CKα) was found to induce ER stress-mediated apoptosis through CHOP (CCAAT-enhancer-binding protein HOmologous Protein) protein expression.7 These membrane contacts may also be involved in calcium (Ca2+) homeostasis given that LPCAT2 overexpression decreased caspase-12 activation and calreticulin (CRT) exposure suggesting a reduced intracellular Ca2+ flux during chemotherapy.
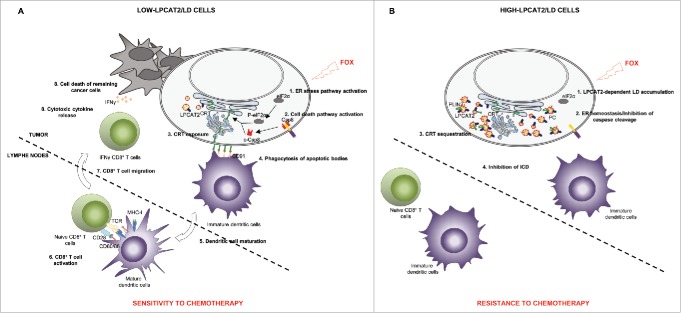
Figure 1.
LPCAT2-dependent lipid droplet accumulation drives chemotherapy response of colorectal cancer cells. A. In low LPCAT2 cells, FOX treatment promotes 1) ER stress leading to eIF2α-dependent activation of ICD signals with CRT-membrane exposure which binds to CD91 receptor initiating apoptotic cell uptake and maturation of dendritic cells. Mature DC in turn, could activate naïve CD8+ T cells with co-stimulatory factors CD80/86/MHC-I and CD28/TCR. This activation transduces signals for CD8+ T lymphocytes to migrate to the tumour site and produce cytotoxic cytokines such as IFNγ. B. In high LPCAT2 cells, FOX treatment induces LD production leading to 1) ER homeostasis 2) CRT sequestration into LD. Impairment of CRT membrane exposure is associated with the incapacity of cancer cells to induce ICD. Calreticulin: CRT; (cleaved-)caspase 8: (c-)Csp8; Dendritic cells: DC; (Phospho)-Eukaryotic translation initiation factor 2 alpha: (P-)eIF2α; Endoplasmic reticulum: ER; 5-Fluorouracil and Oxaliplatin combination: FOX; Immunogenic cell death: ICD; Interferon gamma: IFNγ; Lipid droplets: LD; lysophosphatidylcholine acyltransferase 2: LPCAT2; Major histocompatibility complex-I: MHC-I; Perilipin 2: PLIN2; Phosphatidylcholine: PC; T cell receptor: TCR.
The proteolytic activation of caspase 8 and the ER-stress induced phosphorylation of eIF2α (eukaryotic translation Initiation Factor 2 alpha) are both known as required parameters for the FOX-induced immunogenic cell death (ICD). ICD is characterized in part by the relocation of the ER-chaperone CRT to the plasma membrane, which allows dendritic cell (DC) recognizing the “eat me” signal of dying tumour cells required for phagocytosis and antigen uptake leading to an efficient activation of naïve CD8+ T cells and their migration to the tumour site where they participate to cancer surveillance through the secretion of cytotoxic cytokines such as interferon gamma (IFNγ) (Fig. 1A).8 In high-LD cells, FOX therapy failed to induce ICD due to the inhibition of caspases and eIF2α activation as well as to the sequestration of CRT in droplets, evidenced by immunofluorescence and LD fraction analysis. In vivo vaccination experiments and CD8+ T cell infiltration analyses further confirmed the impact of LPCAT2 expression on ICD (Fig. 1B). The type of chemotherapeutic drug used in our experiments differentially impact the degree of LD-accumulation and subsequently the cell-capacity to expose CRT to the plasma membrane. These mechanisms could be used as a predictive biomarker of chemotherapeutic response either impacting LD accumulation or limiting the utilization of non-ICD inducer drugs.
LD accumulation is a characteristic of CRC stem cells, which suggest a potential implication of LD biogenesis in CRC relapse and its potential use as a biomarker in this cancer.3 More importantly, discriminating between high- and low-LPCAT2 patients, we confirmed a differential response to treatment and impact on the relapse-free survival rate after neoadjuvant chemotherapy, potentially through the modulation of CD8+ T cell infiltration in liver metastases. These data thus suggest that in addition to be a potential valuable prognostic factor, LD/LPCAT2 immunohistochemistry scoring could also constitute a promising clinical approach for the prediction of non-responding patients. LPCAT2 preferentially re-acylating LPC into arachidonic acid-enriched PC, could provide a local dynamic pool of proinflammatory metabolites such as prostaglandin E2, reinforcing the inflammatory and immunosuppressive tumor microenvironment, through the recruitment and activation of CD4+ Th17 cells which are associated with decreased CRC patient survival.2,9,10 Furthermore, given the role of LPCAT2 in phospholipid remodeling, it is feasible that the modulation of its expression level would also impact plasma membrane composition/dynamic and subsequently impact drug pharmacokinetics. Beyond these latter considerations and since tumor progression and invasion are closely related to structural changes in plasma membrane, LPCAT2-mediated pathways could also very possibly affect tumor cells capacity to metastasize. This could constitute future directions for untangling the intricate mechanisms of tumor resistance and progression.
Funding Statement
French National Research Agency (ANR), ANR-11-LABX-0021.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all of our colleagues and collaborators for their contribution to this work. This work was supported by a French Government grant managed by the French National Research Agency under the program “Investissements d'Avenir”, reference ANR-11-LABX-0021, and was supported by grants from the “Conseil Régional de Bourgogne” and the “Fonds Européen de Développement Régional” (FEDER).
References
- 1.Walther TC, Chung J, Farese RV Jr.. Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. PMID:28793795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–46. doi: 10.1083/jcb.201311051. PMID:24590170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koizume S, Miyagi Y. Lipid Droplets: A Key Cellular Organelle Associated with Cancer Cell Survival under Normoxia and Hypoxia. Yang L, ed. International Journal of Molecular Sciences. 2016;17(9):1430. doi: 10.3390/ijms17091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herms A, Bosch M, Ariotti N, Reddy BJ, Fajardo A, Fernandez-Vidal A, Alvarez-Guaita A, Fernandez-Rojo MA, Rentero C, Tebar F, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013;23:1489–96. doi: 10.1016/j.cub.2013.06.032. PMID:23871243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boren J, Brindle KM. Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ. 2012;19:1561–70. doi: 10.1038/cdd.2012.34. PMID:22460322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, Zhang L, Wu J, Hu P, Zhang X, et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015;61:870–82. doi: 10.1002/hep.27409. PMID:25179419. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Lopez E, Zimmerman T, Gomez del Pulgar T, Moyer MP, Lacal Sanjuan JC, Cebrian A. Choline kinase inhibition induces exacerbated endoplasmic reticulum stress and triggers apoptosis via CHOP in cancer cells. Cell Death Dis. 2013;4:e933. doi: 10.1038/cddis.2013.453. PMID:24287694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. PMID:23157435. [DOI] [PubMed] [Google Scholar]
- 9.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016;76, 5241–52. doi: 10.1158/0008-5472.CAN-15-3164. PMID:27496709. [DOI] [PubMed] [Google Scholar]
- 10.Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, Wang X, Murray AJ, Bellafante E, Virtue S, Vidal-Puig A, et al. Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology. 2017;65:1165–80. doi: 10.1002/hep.28953. PMID:27863448. [DOI] [PMC free article] [PubMed] [Google Scholar]