Abstract
Acanthamoeba is free-living protist pathogen capable of causing a blinding keratitis and granulomatous encephalitis. However, the mechanisms of Acanthamoeba pathogenesis are still not clear. Here, our results show that cells co-cultured with pathogenic Acanthamoeba would be spherical and floated, even without contacting the protists. Then, the Acanthamoeba protists would contact and engulf these cells. In order to clarify the contact-independent pathogenesis mechanism in Acanthamoeba, we collected the Acanthamoeba-secreted proteins (Asp) to incubate with cells for identifying the extracellular virulent factors and investigating the cytotoxicity process. The Asps of pathogenic Acanthamoeba express protease activity to reactive Leu amino acid in ECM and induce cell-losing adhesion ability. The M20/M25/M40 superfamily aminopeptidase protein (ACA1_264610), an aminopeptidase be found in Asp, is upregulated after Acanthamoeba and C6 cell co-culturing for 6 h. Pre-treating the Asp with leucine aminopeptidase inhibitor and the specific antibodies of Acanthamoeba M20/M25/M40 superfamily aminopeptidase could reduce the cell damage during Asp and cell co-incubation. These results suggest an important functional role of the Acanthamoeba secreted extracellular aminopeptidases in the Acanthamoeba pathogenesis process. This study provides information regarding clinically pathogenic isolates to target specific molecules and design combined drugs.
Keywords: Acanthamoeba, pathogenesis, M20/M25/M40 superfamily aminopeptidase
1. Introduction
Acanthamoeba spp., a free-living amoeba, is found in different environments, including water and soil. In general, Acanthamoeba can cause human severe diseases, such as infections of the cornea and the central nervous system [1,2]. Acanthamoeba keratitis (AK) is an infection of the cornea caused by pathogenic Acanthamoeba that invades through injuries in the cornea. Most patients wearing contact lenses over a long period of time have a high risk to contract AK [3]. AK may result in lid edema, photophobia, epithelial defects and ring-like stromal infiltrates. Moreover, AK may cause serious consequences, such as permanent visual impairment, if patients are not treated appropriately and immediately [4,5,6,7,8]. However, there is little information that might allow us to comprehend the mechanism by which Acanthamoeba causes tissue invasion or infection in these patients. In parasites, the host cell is probably affected by the release of soluble parasite mediators which degrade or otherwise interact with target cells and molecules. In previous studies, researchers found that Entamoeba histolytica secretes products (Eh-SEC) or soluble components (Eh-SOL) that selectively damage enteric neurons to cause neurotoxicity [9]. As an example, E. histolytica secretes cysteine proteases and EhCP112, which is a protease that overcomes the protective mucus barrier and disrupts cell monolayers to cause invasive intestinal and extra-intestinal infections [10,11]. Acanthamoeba is also known to produce serine, cysteine and metalloproteases, and these extracellular protease activities are increased in pathogenic Acanthamoeba strains [12,13]. MIP133, a 133 kDa serine protease, has been identified as a principal virulence factor of pathogenic Acanthamoeba [14,15]. These studies showed that pathogenic Acanthamoeba exhibited diverse secreted proteases and kinases, which could play important roles in infections. However, the pathogenic mechanisms and influences between host cells and excretory-secretory products of Acanthamoeba remain undefined.
In our previous study, we reported that datasets of secreted proteomic Acanthamoeba strains, including the non-pathogenic ATCC_30010 strain and clinically pathogenic isolates, had been established established [16]. These clinically pathogenic Acanthamoeba isolates were collected by National Cheng Kung University (NCKU) Hospital from patients infected with AK [3]. These pathogenic Acanthamoeba isolates proved to have high resistance and tolerance to polyhexamethylene biguanide [17]. Furthermore, we investigated the strain-consensus and the pathogenic strain-specific secreted proteins from the comparative analysis for determining the role of these excretory-secretory products. In order to gain a deeper understanding of the contact-independent pathogenesis mechanism of Acanthamoeba, this study used an imaging system to investigate the effect on rat glial C6 cells of the Acanthamoeba secreted protein (Asp) and live pathogenic Acanthamoeba parasites.
Here, we show the cytopathic processes of Acanthamoeba and Asp on rat glial C6 cells by time-lapse observation. Acanthamoeba-secreted extracellular aminopeptidases play an important role in the Acanthamoeba pathogenesis process. Pre-treating the Asp with aminopeptidase inhibitors and specific Acanthamoeba aminopeptidase antibodies could delay the progression of cell disruption and reduce the cell damage during Asp and cell co-incubation.
2. Results
2.1. Acanthamoeba castellanii Induces Cell Damage in C6 Cells
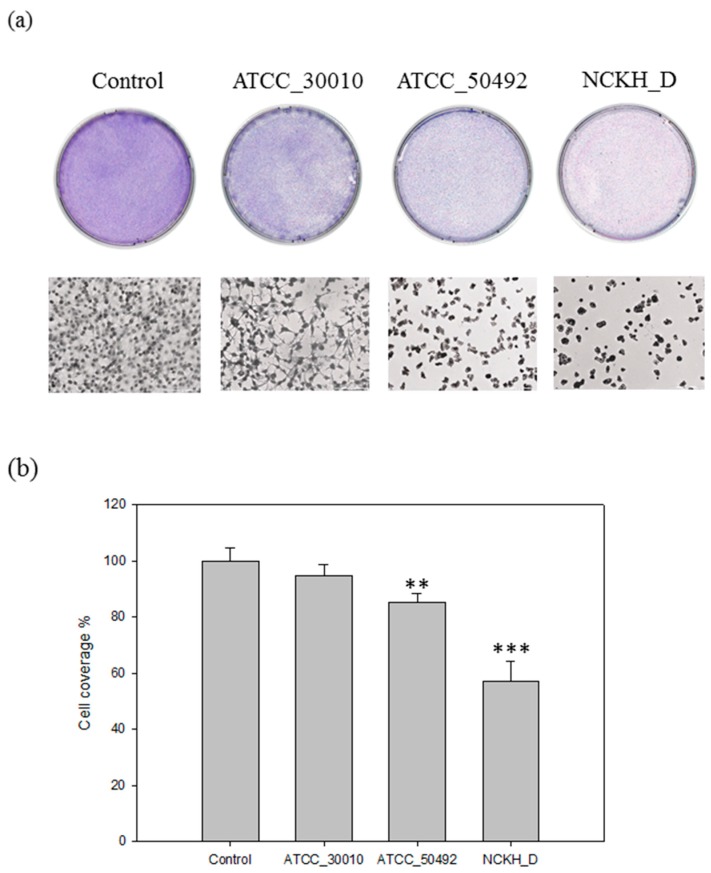
In a previous study, Lagmay et al. established the cytopathic effect (CPE) assay for evaluating and observing the effect of C6 cell co-incubation with different strains of Acanthamoeba castellani [18]. Here, in order to clarify the pathogenesis mechanisms of Acanthamoeba, we have to choose a suitable strain and the corrected damage-induced duration for observation by CPE assay. Results show that there was no significant change in C6 cell coverage area between C6 cells which were co-incubated with the non-pathogenic ATCC_30010 strain and control (mock) (Figure 1). The live pathogenic Acanthamoeba protists, NCKH_D and ATCC_50492, could cause C6 cell damage after co-incubation for 24 h. In our previous study, we have established an RNA-seq database and a comparative proteomic analysis of extracellular secreted proteins, both of them including the ATCC 30010 and the NCKH_D strains [16]. In order to gain a deeper understanding of the pathogenesis mechanism, we focus on the cytopathic effect process of NCKH_D.
Figure 1.
Acanthamoeba castellanii induced cell damage of C6 cells. (a) Demonstration of effect of different strains Acanthamoeba castellanii (ATCC_30010, NCKH_D, ATCC_50492) in the C6 cell line used for the CPE functional assays. The observation of C6 cell co-incubated with Acanthamoeba castellanii for 24 h; (b) Quantification of the coverage of C6 cells after co-incubated with different strains of Acanthamoeba castellanii for 24 h. Those results were means ± SD of triple independent experiments. ** p < 0.05, *** p < 0.01.
2.2. Cytopathic Effect Process of Acanthamoeba castellanii
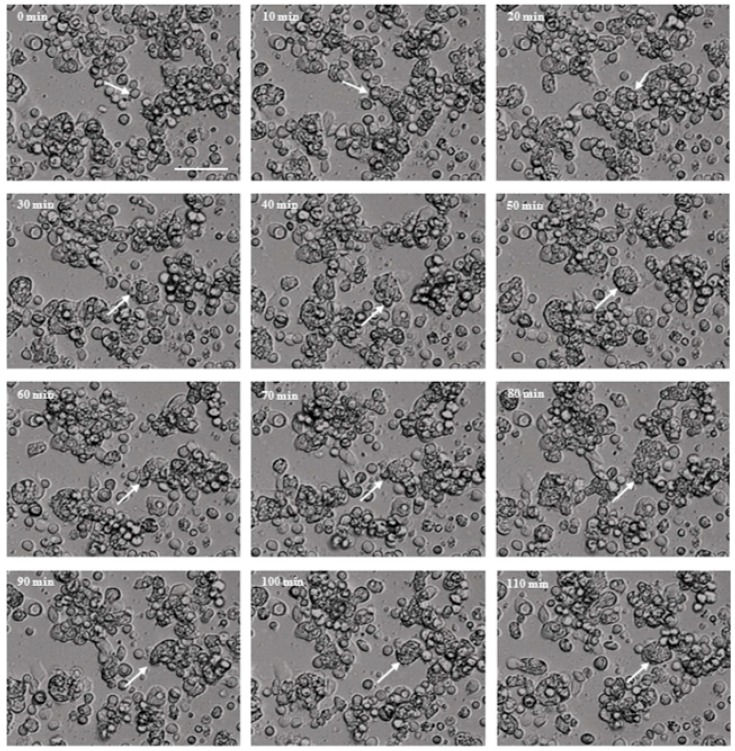
We observed the cytopathic effect process of NCKH_D-induced C6 cell damage by time-lapse microscopy. Furthermore, we observed that the co-incubated C6 cells would be spherical and floated, even without contacting the protists. Then, the NCKH_D protists attached to the disrupted cells for phagocytosis (Figure 2, supplemental video). From these results, we propose that Acanthamoeba releases secreted proteins (Asp) to induce cells to lose adhesion ability and the protists phagocytose these disrupted cells.
Figure 2.
Acanthamoeba castellanii NCKH_D induced cell damage by disruption of C6 cells. The observation of C6 cell co-incubated with Acanthamoeba castellanii NCKH_D in time-lapse microscopy every 10 min for 2 h. The white arrow: the disrupted C6 cell was attacked by Acanthamoeba castellanii NCKH_D. Scale bar = 50 μm.
2.3. Acanthamoeba Secreted Proteins Involves the Co-Cultured Cell Damage Process
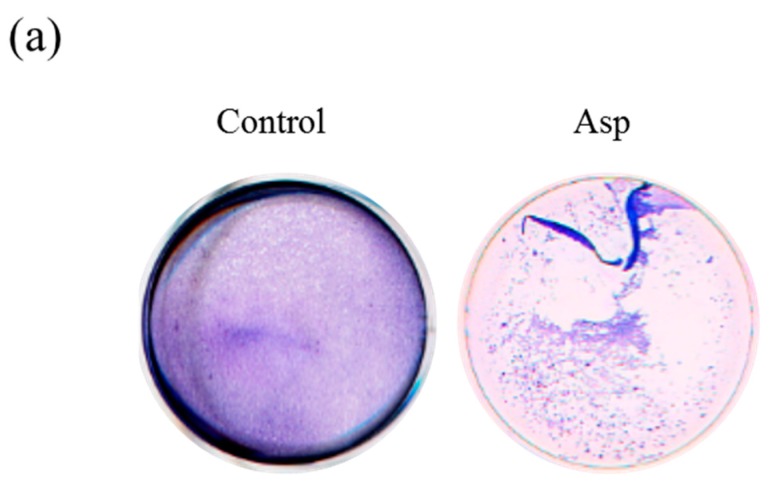
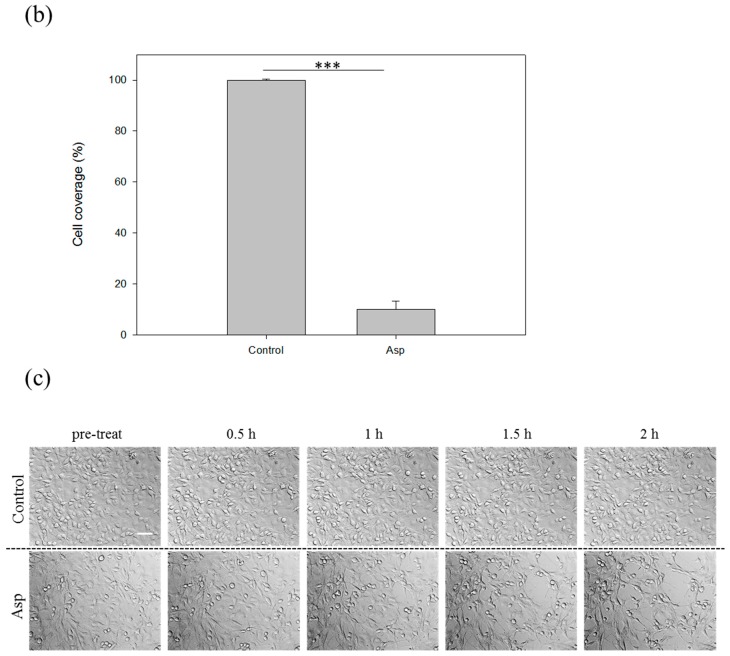
To investigative whether the Asp involved the cell disruption process of Acanthamoeba, we isolated the Asp of NCKH_D to treat the C6 cells and observed by CPE assay and time-lapse microscopy. The results of CPE assay were shown that the Asp of NCKH_D could disrupt the co-incubated C6 cells (Figure 3a,b). We observed that the Asp of Acanthamoeba NCKH_D induced the cell adhesion losing ability in a time-dependent manner (Figure 3c).
Figure 3.
Acanthamoeba castellanii NCKH_D secreted proteins induced cell damage by disruption of C6 cells. The observation of C6 cell co-incubated with Acanthamoeba castellanii NCKH_D secreted proteins for 2 h by CPE assay (a) and time-lapse microscopy (c); (b) Quantification of the coverage of C6 cells after co-incubated with secreted proteins for 2 h; (c) The morphology of C6 cells in control and Asp treated groups by time-lapse microscopy. Those results were means ± S.D. (standard deviation) of triplicate independent experiments. *** p < 0.01. Scale bar = 50 μm.
2.4. The Biochemical Characterization of Acanthamoeba Secreted Proteins
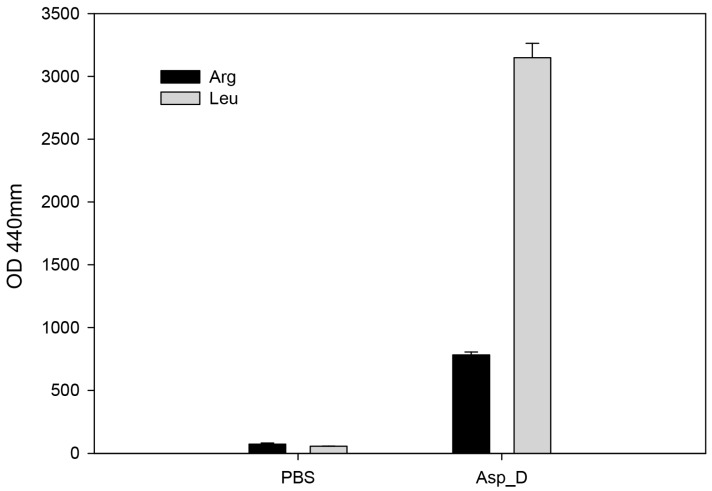
Cell adhesion mechanism is important for cell migration and cell-cell interactions according to the extracellular matrix (ECM) [19]. The epithelia in animals are surrounded by an extracellular matrix (ECM) of collagen fibers, proteoglycans, as well as multi-adhesive matrix proteins [20]. According to analysis of total protein synthesis in ECM, the amino acid leucine is produced not only in fibronectin but also in collagen [21]. We collected Asp of NCKH_D to treat Leu-AMC and Arg-AMC. Results show that Asp hydrolyzed Leu-AMC rapidly than Arg-AMC, a highly sensitive substrate for aminopeptidase (Figure 4). It suggested that Asp secreted by Acanthamoeba may have protease activity to peptides containing the amino acid Leu in ECM to cause cell damage.
Figure 4.
Aminopeptidase activity of Acanthamoeba castellanii NCKH_D secreted proteins. Asp_D is Asp from the pathogenic NCKH_D strain.
2.5. The Discovery of M20/M25/M40 Aminopeptidase by Comparative Analysis of RNA-seq
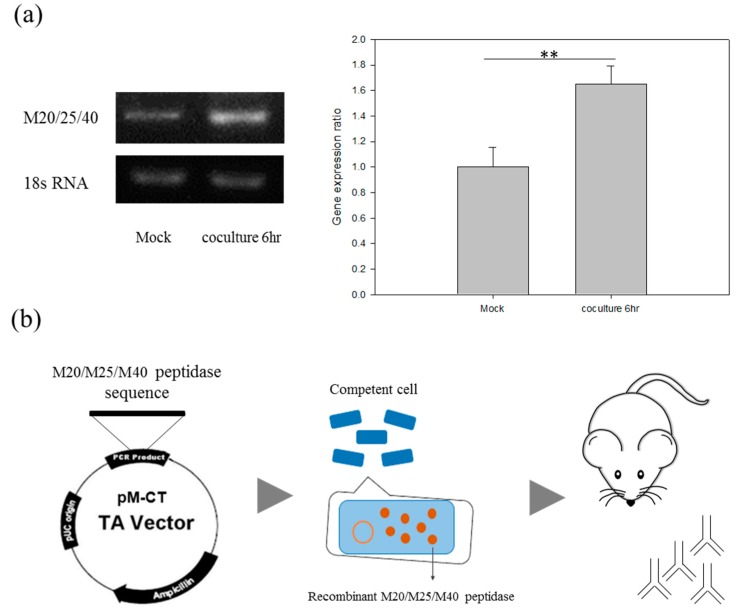
In order to clary the virulence factors in Asp, we combined the RNA-seq data and comparative proteomic analysis of extracellular secreted proteins, which were both collected from ATCC 30010 and NCKH_D Acanthamoeba cells to identify the candidate proteins [16,17]. From the analysis, we noticed that a protein, the M20/M25/M40 superfamily aminopeptidase protein (ACA1_264610), is expressed and secreted by NCKH_D but not ATCC 30010. Interestingly, we found that the gene expression level of M20/M25/M40 superfamily aminopeptidase protein is upregulated in NCKH_D after Acanthamoeba and C6 cell co-culturing for 6 h. The quantified RNA expression patterns of the M20/M25/M40 superfamily aminopeptidase protein are shown in Figure 5a.
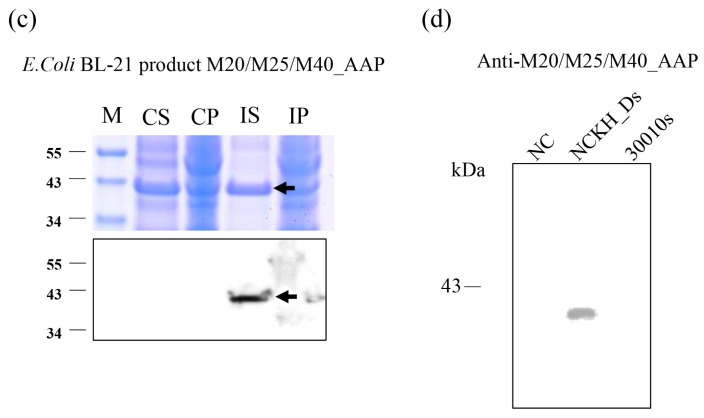
Figure 5.
M20/M25/M40 superfamily aminopeptidase gene expression and antibody production. (a) Reverse transcription PCR analysis of the M20/25/40 aminopeptidase gene, ACA1_264610, expression level in clinical isolate NCKH_D with or without C6 cells co-incubation. The 18S rRNA expression is used as a control. The production of anti-Acanthaamoeba M20/M25/M40 superfamily aminopeptidase antibody; (b) The flow chart of anti-Acanthaamoeba M20/M25/M40 superfamily aminopeptidase antibody production from recombinant protein to antibody; (c) Protein purification and Western blotting of M20/M25/M40 superfamily aminopeptidase recombinant proteins; (d) Western blotting of M20/M25/M40 superfamily aminopeptidase polyclonal antibody. M: Marker, CS: Control supernatant, CP: Control pellet, IS: Induce supernatant, IP: Induce pellet, NC: PBS control, NCKH_Ds: NCKH_D secreted protein, 30010s: ATCC_30010 secreted protein. Those results were means ± S.D. (standard deviation) of triplicate independent experiments. ** p < 0.05.
To further investigate the function of M20/M25/M40 superfamily aminopeptidase, we prepared an anti-Acanthamoeba M20/M25/M40 superfamily aminopeptidase antibody. First, we cloned the M20/M25/M40 superfamily aminopeptidase sequence to the pM-CT vector, then transferred it to the BL-21 E. coli strain for recombinant M20/M25/M40 superfamily aminopeptidase production (Figure 5b). The recombinant M20/M25/M40 superfamily aminopeptidase was detected by Coomassie Blue staining and anti-His antibody (Figure 5c). After the protein purification and molecular weight identification, the mouse polyclonal antibody production of M20/M25/M40 superfamily aminopeptidase was peformed by Leadgen Company (Tainan, Taiwan). From the result, we confirmed the specificity of NCKH_D secreted M20/M25/M40 superfamily aminopeptidase but not ATCC 30010 by western blot (Figure 5d).
2.6. Aminopeptidase Inhibitor and Antibodies Reduce the Cell Damage
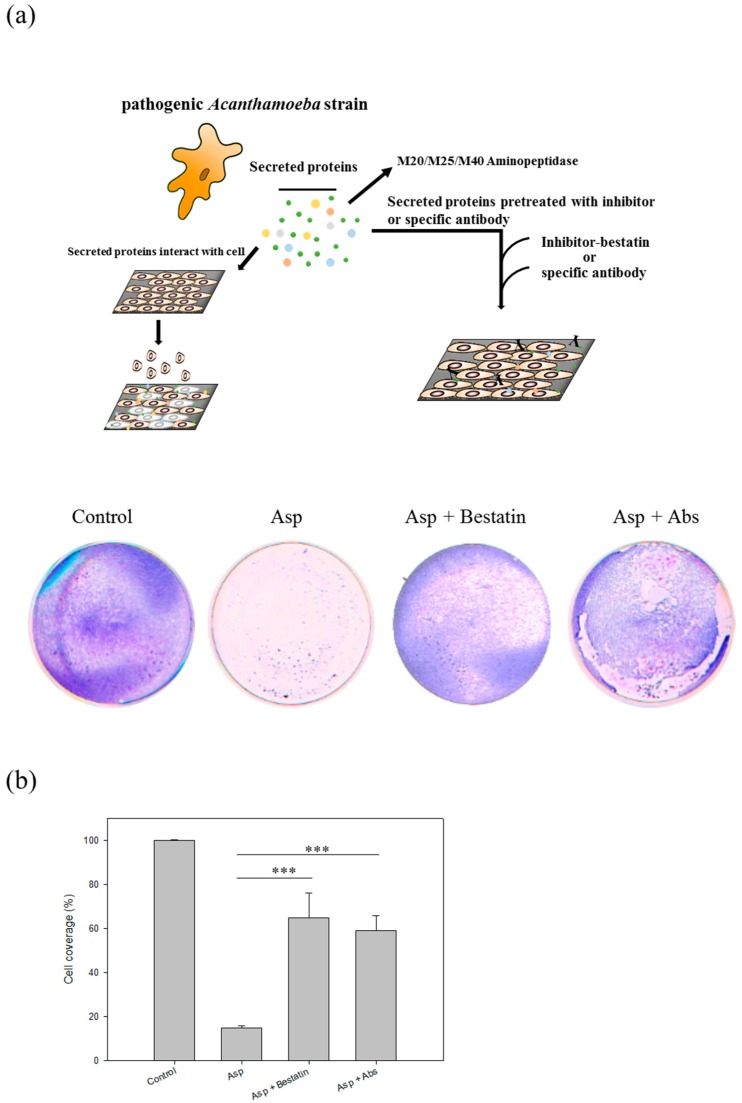
In our previous study, we noticed that a protein, the M20/M25/M40 superfamily aminopeptidase protein (ACA1_264610), is expressed and secreted by pathogenic Acanthamoeba castellanii strain (NCKH_D) but not the non-pathogenic ATCC 30010 strain [16]. To elucidate the importance of M20/M25/M40 superfamily aminopeptidase protein in the contact-independent pathogenesis mechanism of Acanthamoeba, we use the aminopeptidase inhibitor, bestatin, a competitive and specific inhibitor of aminopeptidase B, leucine aminopeptidase, and triamino peptidase [22,23], and the specific polyclonal antibodies to pre-treat the Asp of NCKH_D for knockout the aminopeptidase and then co-incubated them with cells. Results show that the cell disruption induced by Asp could be reduced by pre-treating with bestatin (0.42-fold change) and M20/M25/M40 aminopeptidase-specific antibodies (0.49-fold change) (Figure 6). Evaluation the effect of aminopeptidase inhibitor and antibody co-cultured by C6 cells are shown in Figure S1.
Figure 6.
Aminopeptidase of Acanthamoeba castellanii induced cell damage of C6 cells. (a) Evaluation the treatment effect of inhibitor and antibody of aminopeptidase in Acanthamoeba infection. The inhibitor and antibody reduced the cell disruption of C6 cells co-incubation with Asp for 2 h by CPE functional assays; (b) Quantification of the coverage of C6 cells after co-incubated with secreted proteins for 2 h. Bestatin: inhibitor. Abs: the specific antibody of M20/M25/M40 aminopeptidase. Those results were means ± SD of triplicate independent experiments. *** p < 0.01.
3. Discussion
The innate immune system is an important strategy to defend the host from infection by other organisms such as pathogens. The first line of defense, the epithelial surfaces forming a physical barrier, prevents most infectious agents from invading organisms. However, pathogens adopt different strategies to invade epithelial barriers and disseminate through the bloodstream to other organs. A previous study showed that the bacterium Vibrio vulnific expresses the MARTX toxin to induce intestinal epithelial dysfunction and increase its transmigration [24]. The pathogenesis of Acanthamoeba involves parasite-mediated phagocytosis and cytolysis of corneal epithelial cells and gives rise to disease [25]. The previous study showed that the Acanthamoeba isolates from AK patients, H-1 and IB-1-7, also produced CPE on rat glial C6 cells in a dose- and time-dependent manner [18]. It was proved that both the trophozoites and the culture cell-free supernatants from pathogenic Acanthamoeba strains could produce cytopathic effect on C6 cells. The report suggested a role of Acanthamoeba-secreted products as one of the pathogenic mechanisms on the target cells but they did not identify the pathogenic secreted proteins or other factors. Generally, there are contact-dependent and contact-independent mechanisms for host cell injury. In the life cycle of Acanthamoeba, the parasite adheres to the host cell when infecting through injury. The previous study showed that mannose-binding protein (MBP) expressed by pathogenic Acanthamoeba plays a major role in mediating host and parasite interactions [26]. It induces secondary incidents like interference with host intracellular signaling pathways and phagocytoses host cells by secreting toxins until cell death occurs. Follow this finding, Hurt found that when pathogenic Acanthamoeba castellanii trophozoites are grown in methyl-α-d-mannopyranoside, they are induced to secrete a novel 133-kDa protein that is cytolytic to corneal epithelial cells [15]. The secretion of a 133-kDa protein, mannose-induced protein (MIP-133), was significantly upregulated by mannose, but the supernatants from A. castellanii grown without mannose did not produce MIP-133. In the initial pathogenic cascade, before the binding, there must be some pathogenic secreted/released factors which play an important role. The purpose of this study was to examine the secretion of cytopathic factors by A. castellanii.
The main fibrous ECM proteins which are collagens, elastins, fibronectins, and laminin provide tensile strength or regulate cell adhesion as well as migration [27]. The ECM proteases of some parasites in host cells have recently been reported. The majority of pathogens depend on contact-independent mechanisms to secrete hydrolytic enzymes like elastases [28], phospholipases [29], glycosidases and a variety of serine, cysteine, and metalloproteases for the host cell damage [6,7,25,30]. Amoebic collagenase activity was first described in 1982. It showed that E. histolytica produces membrane-bound enzyme to digest collagen type I and type III at neutral pH and 37 °C [31]. Furthermore, A. polyphaga binds to the ECM proteins such as collagen type IV, fibronectin and laminin [32]. Acanthamoeba has been shown to promote the cleavage of host plasminogen to plasmin activating host matrix metalloproteinases and resulting in degradation of the basement membranes [33]. The previous study has been showed that the secreted protein profiles of non-pathogenic and pathogenic Acanthamoeba strains [16]. However, the function of secreted proteins is still unknown.
Here, we observed that Acanthamoeba NCKH_D, secretes extracellular aminopeptidases to target cells for phagocytosis by disruption. Therefore, we suggest that it is possible that the disruption of cells via Asp is helpful to digestion by Acanthamoeba. In accordance with previous studies, trophozoites interacted with epithelia causing morphological changes and a rapid decrease of resistance and an increased paracellular permeability of epithelial cell layers [34,35,36]. Then, parasites invade dysfunctional epithelial barriers and disseminate through the bloodstream to other organs thus causing serious diseases. Overall, our evidence suggests that the secreted protein of Acanthamoeba plays an important role in causing host cell disruption in early steps. This study provides information regarding clinically pathogenic isolates to target specific molecules and design combined drugs.
4. Materials and Methods
4.1. Culture of Acanthamoeba Protozoa
Acanthamoeba species were axenically cultured in a protease peptone-yeast extract-glucose (PYG) medium and antibiotics (50 μg/mL streptomycin and 100 U/mL of penicillin), pH 6.5, at 28 °C in cell culture flasks. Trophozoites were harvested at the logarithmic growth phase after cultivation for 3–5 days. The clinical isolates were isolated from the corneal ulcers of patients who were diagnosed with AK in the Cheng Kung University Hospital [3,17,37].
4.2. Assay of Cytopathic Effects (CPE)
Rat C6 cells were plated 3 × 105 in a 24-well plate for incubating with Acanthamoeba and secreted proteins. After 2 h co-incubation, the cells were washed, paraformaldehyde-fixed, and then stained with Giemsa stain (Merck, Darmstadt, Germany) [18]. The quantitation of the cells coverage area is used by Image-Pro Plus analysis. The coverage area ratio of co-incubation compared to control.
4.3. Live Cell Imaging of C6 Cells Co-Incubation with Acanthamoeba
The live cell image analysis was used for the microscopy (Olympus CellR, Tokyo, Japan). The morphology of rat glial C6 cells was observed after co-incubating with Acanthamoeba and their secreted proteins for 24 h.
4.4. Isolation of Secreted Proteins
Acanthamoeba, at a density of 1.0 × 106 parasites/mL approximately, were washed three times and re-suspended in PBS buffer for 4 h, after the parasites were removed by centrifugation. The cell-free media containing secreted proteins was filtered through Amicon® Ultra-4 Centrifugal Filter Units (Merck Millipore, Darmstadt, Germany) and collected by centrifugation at 959× g for 75 min.
4.5. Rat Glial C6 Cell Treated with Acanthamoeba and Asp
To find out the probable mechanisms of virulence in vitro, the experiments following the previous study was conducted using a rat glial C6 cell line (ATCC® CCL-107™) to incubate Acanthamoeba and secreted proteins [18]. Rat glial C6 cells were cultured in 10 mL Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (50 μg/mL streptomycin and 100 U/mL of penicillin) at 37 °C incubate with 5% CO2. Rat glial C6 cells were cultured 3 × 105 in 24-well plate cell culture dishes. After 24 h of growth, Acanthamoeba live cells at a concentration of 3 × 105 were co-cultured with C6 cells. Acanthamoeba, at a density of 1.0 × 106 parasites/mL in PBS for 24 h, as well as their secreted proteins were collected. Acanthamoeba secreted proteins (150 μg) were co-incubated with rat glial C6 cell for 2 h followed by time-lapse microscopy observations and CPE assays. The effects of 100 μM aminopeptidase inhibitor (bestatin; Sigma-Aldrich, Darmstadt, Germany) and 15 μg antibody of M20/M25/M40 on C6 cell co-incubation with Asp for 2 h were monitored by CPE assay and time-lapse observation.
4.6. Biochemical Properties of Asp
Following the steps described in Section 4.4 Isolation of secreted proteins and using PBS buffer as the control the activity of Asp was assayed by the hydrolysis of l-leucine-7-amido-4-methylcoumarin hydrochloride (Leu-AMC) and l-arginine-AMC (Arg-AMC, Sigma-Aldrich). Five μL of Asp solution was added to 195 μL of assay buffer (50 mM Tris-HCl, pH 8.0) containing 10 μM Leu-AMC or Arg-AMC and incubated for 30 min at 37 °C. The release of fluorescence was measured at an excitation wavelength of 370 nm and an emission wavelength of 440 nm using a FlexStation 3 Multi-Mode microplate reader (Molecular Devices, Sunnyvale, CA, USA) [23].
4.7. Total RNA Isolation
The Total RNA Extraction Miniprep System (VIOGENE) was used. RNA was stored at −70 °C. The entire concentration and A260/A280 ratio of mRNA were measured with ND-1000 (NanoDrop, Thermo, Waltham, MA, USA).
4.8. cDNA Synthesis
High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) were used in this study. The kit components were allowed to thaw on ice. Reverse transcription conditions were set at the following times and temperatures: 25 °C for 10 min, 37 °C for 120 min, 85 °C for 5 min, finally, the cDNA stored in 4 °C. The reaction volume was set to 20 μL.
4.9. Reverse Transcription PCR
One-step reverse transcription PCR was performed with SuperScript™ One-Step RT-PCR with a Platinum® Taq kit (Invitrogen, Waltham, Commonwealth of Massachusetts, USA) to investigate the gene expression of Acanthamoeba spp. of M20/M25/M40 superfamily aminopeptidase protein. All of the cDNA was synthesized from 1 μg of total RNA of Acanthamoeba. RT-PCR products were separated on the EtBr-stained gel after electrophoresis in 1% agarose. The partial M20/M25/M40 superfamily aminopeptidase forward primer was 5′-TGG ATC GAG TTC AAG GAG GG-3′; and reverse primer was 5′-GCC AGG TGT GCT CGA AGA A GT-3′ with a 364 bp amplification band. 18s rDNA forward primer was 5′-CCC AGA TCG TTT ACC GTG AA-3′ (AcantF900) and reverse primer was 5′-TAA ATA TTA ATG CCC CCA ACT ATC C-3′ (AcantR1100) with a 180 bp amplification band [38]. All experiments were performed independently in triplicate. Image analysis and quantification were performed by using Phoretix 1D (TotalLab, Newcastle upon Tyne, UK).
4.10. Protein Analysis
NCKH_D was washed with PAS twice and then scraped and collected. The pellet was incubated with lysis buffer for 5 min at 4 °C. Lysed samples were centrifuged at 15,900× g at 4 °C for 5 min. Protein samples were separated with 10% SDS-PAGE (T-Pro, Taipei, Taiwan) were used to separate total proteins. Proteins were then transferred onto the PVDF membranes (Merck Millipore, Darmstadt, Germany). 5% non-fat dry milk in 0.5% PBS-T was used to block the membranes. Immune complexes were formed by incubation of the proteins with our polyclonal antibody against M20/M25/M40 superfamily aminopeptidase overnight at 4 °C. After incubation with primary antibodies, membranes were washed and incubated for 1 h with HRP-tagged secondary antibody (1:10,000; Abcam, Cambridge, UK). Immunoreactive protein bands were detected using Biospectrum 600 Imaging System (UVP, Jena, Germany).
Supplementary Materials
Supplementary materials are available online.
Author Contributions
W.-C.L. conceived and designed the experiments; J.-M.H., C.-C.L. and C.-C.K. performed the experiments; W.-C.L., J.-M.H. and C.-C.L. analyzed the data; L.-R.C., L.L.H.H. and J.-W.S. contributed reagents/materials/analysis tools; J.-M.H., C.-C.L. and W.-C.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds the NCBI BioSample (accession number of NCKH_D is SAMN08174149) are available from the authors.
References
- 1.Illingworth C.D., Cook S.D. Acanthamoeba keratitis. Surv. Ophthalmol. 1998;42:493–508. doi: 10.1016/S0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 2.Martinez A.J., Visvesvara G.S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M.L., Shih M.H., Huang F.C., Tseng S.H., Chen C.C. Treatment of early Acanthamoeba keratitis with alcohol-assisted epithelial debridement. Cornea. 2012;31:442–446. doi: 10.1097/ICO.0b013e31823f091a. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D.W., Niederkorn J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22:175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui R., Khan N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors. 2012;5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006;30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 7.Marciano-Cabral F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynter-Allison Z., Lorenzo Morales J., Calder D., Radlein K., Ortega-Rivas A., Lindo J.F. Acanthamoeba infection as a cause of severe keratitis in a soft contact lens wearer in Jamaica. Am. J. Trop. Med. Hyg. 2005;73:92–94. [PubMed] [Google Scholar]
- 9.Lourenssen S., Houpt E.R., Chadee K., Blennerhassett M.G. Entamoeba histolytica infection and secreted proteins proteolytically damage enteric neurons. Infect. Immun. 2010;78:5332–5340. doi: 10.1128/IAI.00699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ocádiz R., Orozco E., Carrillo E., Quintas L.I., Ortega-López J., García-Pérez R.M., Sánchez T., Castillo-Juárez B.A., García-Rivera G., Rodríguez M.A. EhCP112 is an Entamoeba histolytica secreted cysteine protease that may be involved in the parasite-virulence. Cell. Microbiol. 2005;7:221–232. doi: 10.1111/j.1462-5822.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 11.Lidell M.E., Moncada D.M., Chadee K., Hansson G.C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. USA. 2006;103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo-Morales J., Ortega-Rivas A., Foronda P., Abreu-Acosta N., Ballart D., Martinez E., Valladares B. RNA interference (RNAi) for the silencing of extracellular serine proteases genes in Acanthamoeba: Molecular analysis and effect on pathogenecity. Mol. Biochem. Parasitol. 2005;144:10–15. doi: 10.1016/j.molbiopara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Mattana A., Cappai V., Alberti L., Serra C., Fiori P.L., Cappuccinelli P. ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines. Infect. Immun. 2002;70:4424–4432. doi: 10.1128/IAI.70.8.4424-4432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurtle W., Bode E., Kaplan R.S., Garrison J., Kearney B., Shoemaker D., Henchal E., Norwood D. Use of denaturing high-performance liquid chromatography to identify Bacillus anthracis by analysis of the 16S-23S rRNA interspacer region and gyrA gene. J. Clin. Microbiol. 2003;41:4758–4766. doi: 10.1128/JCM.41.10.4758-4766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurt M., Niederkorn J., Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2003;44:3424–3431. doi: 10.1167/iovs.03-0019. [DOI] [PubMed] [Google Scholar]
- 16.Huang J.M., Lin W.C., Li S.C., Shih M.H., Chan W.C., Shin J.W., Huang F.C. Comparative proteomic analysis of extracellular secreted proteins expressed by two pathogenic Acanthamoeba castellanii clinical isolates and a non-pathogenic ATCC strain. Exp. Parasitol. 2016;166:60–67. doi: 10.1016/j.exppara.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Huang F.-C., Shih M.-H., Chang K.-F., Huang J.-M., Shin J.-W., Lin W.-C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2015;50:570–577. doi: 10.1016/j.jmii.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Lagmay J.P., Matias R.R., Natividad F.F., Enriquez G.L. Cytopathogenicity of Acanthamoeba isolates on rat glial C6 cell line. Southeast Asian J. Trop. Med. Public Health. 1999;30:670–677. [PubMed] [Google Scholar]
- 19.Zaidel-Bar R., Cohen M., Addadi L., Geiger B. Hierarchical Assembly of Cell–Matrix Adhesion Complexes. Portland Press Limited; London, UK: 2004. [DOI] [PubMed] [Google Scholar]
- 20.Heickendorff L. Laminin, fibronectin and type IV collagen in BM-like material from cultured arterial smooth muscle cells. Int. J. Biochem. 1988;20:381–386. doi: 10.1016/0020-711X(88)90205-4. [DOI] [PubMed] [Google Scholar]
- 21.Sugrue S.P., Hay E.D. The identification of extracellular matrix (ECM) binding sites on the basal surface of embryonic corneal epithelium and the effect of ECM binding on epithelial collagen production. J. Cell Biol. 1986;102:1907–1916. doi: 10.1083/jcb.102.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T., Kanzaki H., Imai K., Narukawa S., Higuchi T., Katsuragawa H., Maeda M., Mori T. Bestatin, a potent aminopeptidase-N inhibitor, inhibits in vitro decidualization of human endometrial stromal cells. J. Clin. Endocrinol. Metab. 1994;79:171–175. doi: 10.1210/jcem.79.1.7913090. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.R., Na B.K., Moon E.K., Song S.M., Joo S.Y., Kong H.H., Goo Y.K., Chung D.I., Hong Y. Essential Role for an M17 Leucine Aminopeptidase in Encystation of Acanthamoeba castellanii. PLoS ONE. 2015;10:e0129884. doi: 10.1371/journal.pone.0129884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin H.E., Beubier N.T., Satchell K.J. The Effector Domain Region of the Vibrio vulnificus MARTX Toxin Confers Biphasic Epithelial Barrier Disruption and Is Essential for Systemic Spread from the Intestine. PLoS Pathog. 2017;13:e1006119. doi: 10.1371/journal.ppat.1006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niederkorn J.Y., Alizadeh H., Leher H., McCulley J.P. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999;1:437–443. doi: 10.1016/S1286-4579(99)80047-1. [DOI] [PubMed] [Google Scholar]
- 26.Garate M., Cao Z., Bateman E., Panjwani N. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J. Biol. Chem. 2004;279:29849–29856. doi: 10.1074/jbc.M402334200. [DOI] [PubMed] [Google Scholar]
- 27.Rozario T., DeSimone D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrante A., Bates E.J. Elastase in the pathogenic free-living amoebae Naegleria and Acanthamoeba spp. Infect. Immun. 1988;56:3320–3321. doi: 10.1128/iai.56.12.3320-3321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortazavi P.N., Keisary E., Loh L.N., Jung S.-Y., Khan N.A. Possible roles of phospholipase A 2 in the biological activities of Acanthamoeba castellanii (T4 genotype) Protist. 2011;162:168–176. doi: 10.1016/j.protis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Panjwani N. Pathogenesis of Acanthamoeba keratitis. Ocul. Surf. 2010;8:70–79. doi: 10.1016/S1542-0124(12)70071-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz M.D.L., Calderon J., Rojkind M. The collagenase of Entamoeba histolytica. J. Exp. Med. 1982;155:42–51. doi: 10.1084/jem.155.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon V.R., Asem E.K., Vodkin M.H., McLaughlin G.L. Acanthamoeba binds to extracellular matrix proteins in vitro. Investig. Ophthalmol. Vis. Sci. 1993;34:658–662. [PubMed] [Google Scholar]
- 33.Mitra M.M., Alizadeh H., Gerard R.D., Niederkorn J.Y. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol. Biochem. Parasitol. 1995;73:157–164. doi: 10.1016/0166-6851(94)00109-Z. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Palomo A., Gonzalez-Robles A., Chavez B., Orozco E., Fernandez-Castelo S., Cervantes A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J. Protozool. 1985;32:166–175. doi: 10.1111/j.1550-7408.1985.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 35.Leroy A., Lauwaet T., De Bruyne G., Cornelissen M., Mareel M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J. 2000;14:1139–1146. doi: 10.1096/fasebj.14.9.1139. [DOI] [PubMed] [Google Scholar]
- 36.Leroy A., Lauwaet T., Oliveira M.-J., De Bruyne G., Bracha R., Ankri S., Katz U., Mirelman D., Mareel M. Disturbance of tight junctions by Entamoeba histolytica: Resistant vertebrate cell types and incompetent trophozoites. Arch. Med. Res. 2000;31:S218–S220. doi: 10.1016/S0188-4409(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 37.Huang F.C., Liu T.S., Li S.C., Shih M.H., Shin J.W., Lin W.C. The effect of the disulfideisomerase domain containing protein in the defense against polyhexamethylene biguanide of highly tolerant Acanthamoeba at the trophozoite stage. Int. J. Parasitol. Drugs Drug Resist. 2016;6:251–257. doi: 10.1016/j.ijpddr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qvarnstrom Y., Visvesvara G.S., Sriram R., da Silva A.J. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 2006;44:3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.