ABSTRACT
Cancer genomics and mechanistic studies have revealed that heterogeneous mutations within a single kinase can result in a variety of activation mechanisms. The challenge has been to match these insights with tailored drug discovery strategies to yield potent, highly selective drugs. With optimized drugs in hand, physicians could apply the principles of personalized medicine with an increasing number of options to treat patients with improved precision according to their tumor's molecular genotype.
KEYWORDS: BLU-285, avapritinib, precision medicine, gastrointestinal stromal tumor, systemic mastocytosis
Small molecule inhibitors designed to target activated oncogenic kinases have had profound clinical benefit when given to genomically selected patient populations.1 As such, it is now becoming common practice to perform sequencing of tumor biopsies or circulating tumor DNA to inform a therapeutic course of action. Targeted kinase inhibitors designed to specifically interact with mutant epidermal growth factor receptor (EGFR) and B-Raf proto-oncogene serine/threonine kinase (BRAF) as well as activating fusions of ALK receptor tyrosine kinase (ALK), ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) or breakpoint cluster region protein–ABL proto-oncogene 1 non-receptor tyrosine kinase (BCR-ABL) have been successfully deployed in the clinic. However, in none of these cases has a single drug been successful at potently inhibiting all mutations of a particular gene (i.e., all primary and resistance mutations), leaving a gap in coverage for patients with certain driver mutations. One such example is KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet-derived growth factor receptor alpha (PDGFRA) activation loop mutations that drive systemic mastocytosis (SM) and gastrointestinal stromal tumors (GIST). Most SM patients and many advanced GIST patients have had no meaningful treatment options thus far.
80% of tumors from patients with advanced GIST have primary activating mutations in KIT outside of the kinase domain and receive imatinib as first line therapy.2 Imatinib is a highly selective, well tolerated drug that provides initial clinical benefit. However, most patients eventually progress due to the emergence of on-target resistance mutations within the KIT kinase domain, specifically in the adenosine 5′-triphosphate (ATP)-binding pocket or activation loop.2 Sunitinib is administered in the second line and is potent against the imatinib-resistant ATP-binding pocket mutations but not those found in the activation loop. Regorafenib, which is approved as third line therapy, has a limited activity profile against some but not all KIT activation loop mutations. After progression on these treatments, patients are enriched for KIT activation loop mutations that are resistant to all approved agents. The complex nature of the KIT mutational spectrum that drives advanced GIST exemplifies the difficulty for a single inhibitor to provide coverage across all disease-driving mutations, highlighting the need for other agents that specifically address mutations not covered with current therapies.
Beyond GIST, a D816V mutation in the activation loop of KIT is present in more than 90% of patients with SM.3 SM is driven by accumulation of neoplastic mast cells, which causes reduced quality of life and a shortened lifespan in the advanced setting. There are no targeted treatment options for these patients. The PDGFRA D842V activation loop mutation, which is structurally equivalent to KIT D816V, is a primary activating mutation seen in roughly 5–6% of advanced GIST patients for which there are no effective therapeutic options.4 Taken together, the lack of potent and selective inhibitors for KIT and PDGFRA activation loop mutations remains an unmet medical need in multiple malignancies.
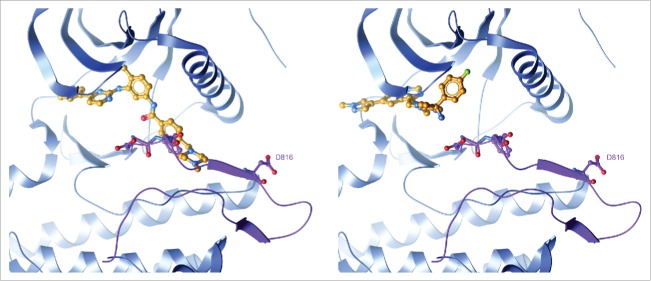
Blueprint Medicines' proprietary kinase-focused small molecule library was used to identify potential inhibitors of the KIT D816V activation loop mutant enzyme. Screening data from thousands of compounds across the human kinome and disease-relevant kinase mutants confirmed the hypothesis that KIT activation loop mutations destabilize the inactive kinase conformation, highly skewing the equilibrium to that of the active conformation and erodes the binding of type II inhibitors like imatinib (Fig. 1 left). We identified compounds that bound to the active conformation of the kinase, or type I inhibitors, which after optimization of physicochemical properties led to the discovery of avapritinib (formerly known as BLU-285). Avapritinib is the first potent and selective small molecule inhibitor of the previously unaddressed KIT and PDGFRA activation loop mutations,5 with biochemical half-maximal inhibitory concentration (IC50) = 0.27 nM and 0.24 nM for the KIT D816V and PDGFRA D842V mutant enzymes, respectively. Modeling studies confirmed that, distinct from type II inhibitors, avapritinib bound to the kinase in its active conformation (Fig. 1 right). Other type I inhibitors such as dasatinib and midostaurin are less potent than avapritinib against KIT activation loop mutations (IC50 = 1.6 nM and 2.9 nM for KIT D816V, respectively) and have broader kinome activity. In contrast, avapritinib's activity profile across the human kinome demonstrated that a type I inhibitor of KIT activation loop mutations can be exquisitely selective. This attribute of avapritinib may make it advantageous for combining with other agents to augment efficacy in KIT and PDGFRA-driven diseases.
Figure 1.
Avapritinib, a type I inhibitor, specifically binds the active conformation of the KIT kinase domain. Left: Structure of imatinib bound to KIT proto-oncogene receptor tyrosine kinase (KIT) with an open activation loop conformation. The open activation loop was modelled by superimposing the active, KIT:adenosine diphosphate (ADP) crystal structure (PDB code 1PKG) on the inactive, KIT:imatinib crystal structure (PDB code 1T46) and replacing the activation loop segment of 1T46 (residues 809–834) with that of 1PKG. The activation loop is colored purple with the Asp-Phe-Gly (DFG) motif and residue D816 shown in ball-and-stick. Steric clash with Phe811 highlights the inability of type II inhibitors like imatinib to bind to an active kinase conformation. Right: Avapritinib docked into the active 1T46/1PKG hybrid structure. Avapritinib readily fits into the adenosine 5′-triphosphate (ATP)-binding pocket with the activation loop in an open, DFG-in conformation.
Furthermore, avapritinib was active across KIT primary and resistance mutations beyond those in the activation loop. Avapritinib was potent against mutations in the juxtamembrane region (exon 11) alone and in tandem with ATP-binding pocket mutations (exons 11/13 and 11/14). Juxtamembrane (JM) mutations serve to release an autoinhibitory intramolecular interaction in KIT,6 allowing the kinase to more frequently interconvert between the inactive and active conformations. Imatinib potently inhibits this JM mutant kinase while in the inactive conformation6; avapritinib inhibits this same mutant kinase but does so by binding efficiently when in its active conformation. This work suggests that most activating KIT mutants may be amenable to avapritinib inhibition given that oncogenic mutations ultimately bias the kinase towards an active conformation.
Initial phase 1 testing of avapritinib demonstrated rapid proof of concept with decreased mutant allele burden and tumor volume reduction in patients with refractory KIT and PDGFRA activation loop mutant GIST.5 A high overall response rate was observed in PDGFRA D842V unresectable GIST, resulting in a breakthrough therapy designation for avapritinib in this indication. Similarly, treatment of advanced systemic mastocytosis patients with avapritinib showed marked decrease in serum tryptase levels reflective of a decrease in mast cell burden7 and significant reduction of malignant bone marrow mast cell infiltrates.5 These data, in two distinct malignancies driven by KIT and PDGFRA activation loop mutations, indicate promising initial clinical activity for avapritinib.
Beyond GIST and SM, several additional tumor types harbor KIT or PDGFRA activating mutations and may be indications for future investigation with avapritinib. KIT activation loop mutants have been observed in numerous germ cell tumors, melanomas that are BRAF wildtype or do not correlate with sun exposure and a subset of core binding factor acute myeloid leukemia (CBF-AML). Furthermore, surveying recent genomic sequencing efforts across tumor types8,9 indicates that KIT/PDGFRA mutations can be identified at low frequency across a broad range of tumors that may be responsive to avapritinib. Through the increased adoption of sequencing tumor and liquid biopsies, oncogenic kinase mutations can be paired with appropriately targeted agents, rather than administered solely by line of therapy. This will require a wealth of potent and selective kinase inhibitors, and suggests that in avapritinib, the practitioners of precision medicine may have an additional agent to deploy in the near future.
Funding Statement
This work was sponsored by Blueprint Medicines.
Disclosure of potential conflicts of interest
A.K.G., E.K.E., J.L.K., N.B., B.W. and C.L. are current employees and shareholders of Blueprint Medicines. B.L.H. is a shareholder of Blueprint Medicines. J.L.K. and B.L.H. are inventors on patent application WO2015/057873 held by Blueprint Medicines, which includes BLU-285 (avapritinib).
References
- 1.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al.. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. PMID:12181401. [DOI] [PubMed] [Google Scholar]
- 2.Antonescu CR. The GIST paradigm: lessons for other kinase-driven cancers. The Journal of Pathology. 2011;223(2):251–61. doi: 10.1002/path.2798 . PMID:21125679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, Aldanondo I, Sanchez L, Dominguez M, Botana LM, et al.. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366–72. doi: 10.1182/blood-2006-04-015545. PMID:16741248. [DOI] [PubMed] [Google Scholar]
- 4.Cassier PA, Fumagalli E, Rutkowski P, Schöffski P, Van Glabbeke M Debiec-Rychter M, Emile J-F, Duffaud F, Martin-Broto J, Landi B, et al.. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18(16):4458–64. doi: 10.1158/1078-0432.CCR-11-3025. PMID:22718859. [DOI] [PubMed] [Google Scholar]
- 5.Evans EK, Gardino AK, Kim JL, Hodous BL, Shutes A, Davis A, Zhu XJ, Schmidt-Kittler O, Wilson D, Wilson K, et al.. A precision therapy against cancers driven by KIT/PDGFRA mutations. Science Translational Medicine. 2017;9(414): 1–11. doi: 10.1126/scitranslmed.aao1690. PMID:29093181. [DOI] [PubMed] [Google Scholar]
- 6.Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, English JM, Greig MJ, He Y-A, Jacques SL, et al.. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106(5):1542–47. doi: 10.1073/pnas.0812413106. PMID:19164557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeAngelo Daniel J, Quiery Albert Thomas, Radia Deepti, Drummond Mark W, Gotlib Jason, Robinson William A, Hexner Elizabeth, Verstovsek Srdan, Shi Hongliang, Alvarez-Diez Terri, et al.. Clinical Activity in a Phase 1 Study of BLU-285, a Potent, Highly-Selective Inhibitor of KIT D816V in Advanced Systemic Mastocytosis (AdvSM). Blood. 2017;130:2. PMID:28684445.28684445 [Google Scholar]
- 8.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al.. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discovery. 2012;2(5):401–04. doi: 10.1158/2159-8290.CD-12-0095. PMID:22588877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The AACR Project GENIE Consortium AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discovery. 2017;7(8):818–31. doi: 10.1158/2159-8290.CD-17-0151. PMID:28572459. [DOI] [PMC free article] [PubMed] [Google Scholar]