Abstract
Background
Tendon adhesion is a serious problem and it affects tendon gliding and joint motion. Although recent studies have yielded promising results in developing anti-adhesion materials, there are still many problems. Polycaprolactone (PCL)-based polyurethane (PU) has good mechanical properties and biocompatibility, and it has a potential in anti-adhesion applications.
Materials and methods
In this study, a series of waterborne biodegradable polyurethane (WBPU) films with different ratios of ionic groups were synthesized. In order to select an effective anti-adhesion film, the WBPU films were cast and characterized for physicochemical properties and biocompatibility.
Results
All WBPU films were non-cytotoxic in the cell viability test and had suitable physicochemical and mechanical properties based on the measurement of zeta potential, water contact angle, mechanical properties, water absorption, thickness change, and gelatin test. To evaluate the anti-adhesion effect, severely injured tendons of rabbits were sutured with the modified Kessler core suture technique and WBPU films were then wrapped around the tendon. Implantation in rabbits showed that the WBPU film had better anti-adhesion effect than PCL films and the untreated control, and demonstrated no significant difference in the anti-adhesion performance from the commercial product Seprafilm based on gross evaluation, histological analysis, and biomechanical assessment.
Conclusion
Compared to Seprafilm and PCL applied in the tendon anti-adhesion, WBPU had better mechanical properties, low inflammatory reaction, and a proper degradation interval.
Keywords: adhesion, tendon, polyurethane, postoperative adhesion
Introduction
Postoperative adhesion is a natural process of surgical wound healing. However, unsuitable postoperative adhesion can cause adverse effects such as pain and loss of function.1 Tendons are the bridge that connects the muscles to a joint. The process of forces transmitting to bone through tendons leads to the joint and limb movement.2 The tendon sheath is a two-layer membrane-like structure surrounding the hand and foot. The outer fibrotic layer and the inner synovial layer form a closed duct, which contains the synovial fluid. The components of the fluid include hyaluronic acid (HA) and nutrients; the former serves as the lubricant that allows gliding of the tendon. As a biological barrier, the tendon sheath prevents the tendon from exogenous healing and provides nutrition to the tendon.3 Once the tendons and tendon sheath are injured, fibroblasts can migrate to the surrounding tissue. Fibroblasts play an important role in tendon repair. Without the protection of tendon sheet, tendon adhesion can occur, which restricts tendon movements such as tendon gliding and joint flexion.4,5 It is, therefore, essential to develop a tendon sheet-like material that promotes tendon healing and prevents unwanted adhesion.
When tendons are injured, surgical techniques are often necessary. The surgical technique is a factor that causes more serious tendon adhesion. A study compared the influence of modified Kessler (low-friction) and Becker (high-friction) suture techniques on the extent of tendon adhesion, and results showed that the adhesion caused by the low-friction suture technique was significantly lower than the one induced by the high-friction suture technique.6 The use of physical barriers is a feasible strategy to reduce adhesion. Nondegradable materials such as polyethylene, amnioplastin, and polytetrafluoroethylene have been effective in preventing adhesion at the initial period, but the foreign body reaction and subsequent adhesion remains a problem.7,8 Therefore, the films have to be removed after tendon recovery.1 Biodegradable materials such as poly(l-lactic acid)-polyethylene glycol (PELA), poly(l-lactic acid), and polycaprolactone (PCL) were examined for their anti-adhesion effects, but none of these materials showed significant anti-adhesion, unless combined with HA, chitosan, or polyethylene glycol.4,9–12 However, the acidic degradation products of these biodegradable materials could lead to further inflammation and other negative effects.13
Polyurethane (PU) elastomers are a family of synthetic compounds containing a soft segment and a hard segment that form a microphase-separated structure. The microphase separation provides PU with excellent flexibility and mechanical properties.14 Meanwhile, PUs are widely used in medical applications such as wound healing and cardiovascular devices due to their good biocompatibility.15 By adjusting the chemical structure of PU, it can be used in a variety of features that suit different tissues.15 Compared to traditional PU, waterborne biodegradable PU (WBPU) is more eco-friendly and can be dispersed in water by ionic groups in the form of homogeneous nanoparticles.16,17 Moreover, the ionic groups in WBPU could be regarded as physical cross-links. The mobility of the hard segment is restricted by the ionic groups, which causes a higher degree of microphase separation.18 In particular, anionic PU with carboxyl structure (COO) has demonstrated low inflammatory reaction.19,20 There have been few reports on uses of PU as an antiadhesive barrier. However, an early work by Rehman analyzed the antiadhesive effect of biodegradable PU.21 Results showed that the peritoneal adhesion was impacted by PU composition where aromatic hard segments had better effect than aliphatic ones, while different soft segments exhibited no significant difference in anti-adhesion effect.21
In this study, we prepared PCL-based WBPU with different amounts of ionic groups and investigated their physical and biological properties. Finally, the optimized formula of WBPU was applied in order to analyze its anti-adhesion effects on the healing of rabbit tendons.
Materials and methods
Synthesis of PU
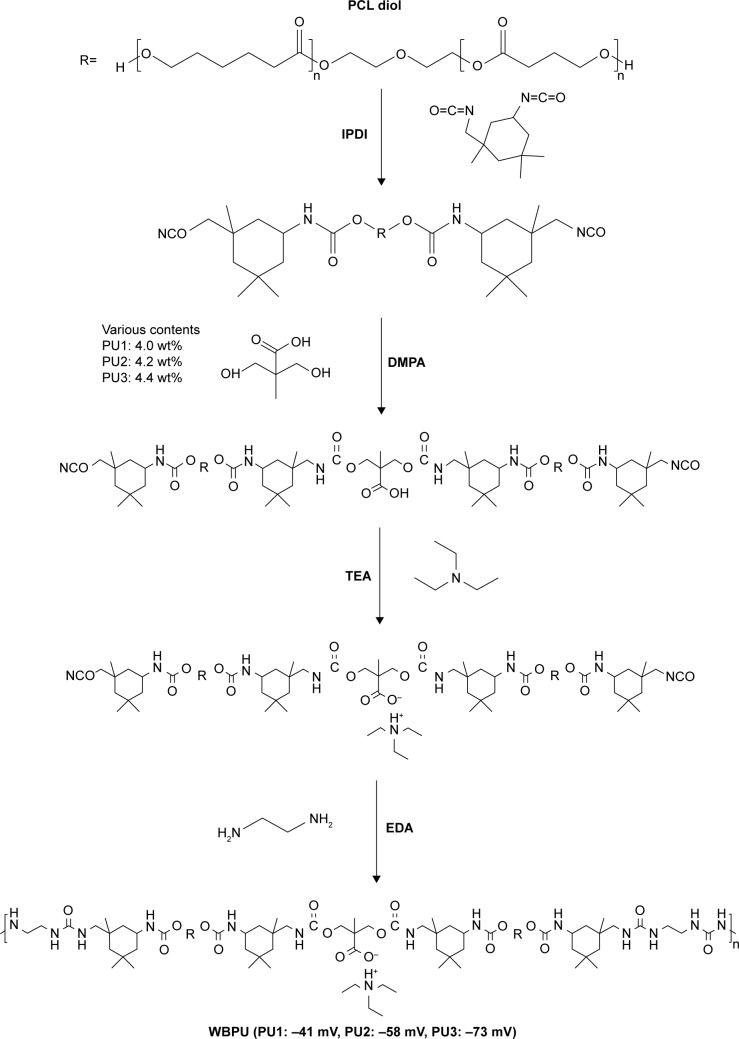
WBPUs were synthesized from an optimized and environmentally friendly process as shown in a previous study.22 Briefly, poly(ε-carpolactone) diol (PCL diol, molecular weight Mn=2,000 g mol−1; Sigma-Aldrich, St Louis, MI, USA) was used as the soft segment and was mixed with isophorone diisocyanate (IPDI, Sigma-Aldrich) and 0.03% tin(II)2-ethylhexanoate (T-9, Alfa Aesar) by stirring (180 rpm) and made to react under nitrogen at 75°C. After 3 hours, 2,2-bis(hydroxymethyl) propionic acid (DMPA, Sigma-Aldrich) was added to the reactor with appropriate methyl ethyl ketone (J. T. Baker) in order to reduce viscosity. The reaction was allowed to continue for another hour. After that, the temperature was decreased to 45°C and triethylamine (RDH, Seelze, Germany) was added as a neutralizer into the reactor and stirred for 30 minutes. The previous mixture was dispersed in water by high-speed stirring (1,100 rpm). Finally, ethylenediamine (EDA, Tedia) was added as the chain extender and the reaction was completed (Figure 1). The major difference among the WBPUs was in the amount of DMPA (the negatively charged component). The reactants IPDI, DMPA, and EDA formed the hard segment. In the chemical structure of WBPU, DMPA provided ionic groups. The three WBPUs used in the study were abbreviated as PU1, PU2, and PU3 and the DMPA contents were 4.0, 4.2, and 4.4 wt%, respectively, with respect to the whole WBPU molecule. The stoichiometric ratios of PU in this study are shown in Table 1. The zeta potential of PU1, PU2, and PU3 dispersion was characterized by dynamic light scattering (DLS; Delsa Nano; Beckman Coulter Inc., Fullerton, CA, USA.)
Figure 1.
Synthesis scheme of WBPU with PCL diol, IPDI, TEA, and various contents (4.0, 4.2, and 4.4 wt%) of DMPA. The zeta potential of three PUs was measured using dynamic light scattering.
Abbreviations: DMPA, 2,2-bis(hydroxymethyl) propionic acid; EDA, ethylenediamine; IPDI, isophorone diisocyanate; PCL diol, poly(ε-carpolactone)diol; PU, polyurethane; TEA, triethylamine; WBPU, waterborne biodegradable polyurethane.
Table 1.
The stoichiometric ratios of the PU chemical structure
| Sample | IPDI | PCL diol | DMPA | EDA |
|---|---|---|---|---|
| PU1 | 1.000 | 0.270 | 0.252 | 0.411 |
| PU2 | 1.000 | 0.265 | 0.265 | 0.404 |
| PU3 | 1.000 | 0.262 | 0.275 | 0.400 |
Abbreviations: DMPA, 2,2-bis(hydroxymethyl) propionic acid; EDA, ethylene-diamine; IPDI, isophorone diisocyanate; PCL diol, poly(ε-carpolactone)diol; PU, polyurethane.
Physical characterization of PU films
WBPUs were poured into a dish and dried at room temperature for 3 days. Then, moisture was removed from the WBPU films by vacuum distillation overnight. The zeta potential of the films was measured by DLS.
A Goniometer (FTA-1000B; First Ten Angstrom Company, Portsmouth, VA, USA) was used to test the water contact angle of the films at room temperature. Each film was dropped with 5 μL water drops. Water contact angles were measured after 30 seconds.
A universal testing system (HT-8504, Hung Ta) was used to determine the mechanical properties of WBPU films at a strain rate of 100 mm min−1. The properties included the Young’s modulus, 100% modulus, tensile strength, and elongation at break.
Water absorption and change in the thickness were also measured. WBPU films were cut into 1×1 cm2, and moisture was completely removed from them by vacuum distillation overnight. Afterward, the films were soaked in double-distilled water for 1 day at room temperature. The WBPU films were taken out and gently wiped to remove water from the surface. The ratios of water absorption and change in the thickness were calculated as follows:
| (1) |
| (2) |
In the equations, W and T indicate the weight and the thickness of the wet film, while W0 and T0 indicate the weight and the thickness of the dry film, respectively.
Gelatin test
T-peeling is an in vitro model that mimics the adhesion between hyperplasia and different materials. Gelatin was selected to mimic the extracellular matrix (ECM) in the early step at the adhesion site. A 60×15 mm polytetrafluoroethylene frame was created. All samples were cut into 80×35 mm segments. Gelatin solution (type A from porcine, ~300 bloom; Sigma-Aldrich) was prepared in double-distilled water at 40% w/w and kept at 60°C. The frame was placed on a sample, and then, the gelatin solution was poured into the frame. After 5 minutes at room temperature, the samples were transferred to a 37°C environment for 2 days. The frame was removed and gelatin on the sample was then peeled off at 180° peeling angle by a universal testing machine at 100 mm min−1. The average force was determined based on the data. The peeling energy (J m−2) was defined as E=(2P) b−1, where P was the average force and b was the width of gelatin that solidified on the sample.23
In vitro cell culture
Test films were prepared by coating the WBPU dispersion on coverslip glass (15 mm, Assistant; Glaswarenfabrik Karl Hecht KG, Sondheim vor der Rhön, Germany) by a spin-coater; a 100 μL solution was cast on coverslip glass and then spin-coated at 1,800 rpm for 20 seconds. The films were dried overnight and vacuum distillation was performed in order to completely remove the solvent. PCL (Mn=70,000–90,000, Sigma-Aldrich) films prepared from a 10% w/w solution in tetrahydrofuran (ECHO Chemical Co., Ltd) were used as the control samples. Test films were sterilized with ultraviolet light for 3 hours and placed in 24-well tissue culture polystyrene (TCPS) plates. Samples and TCPS were rinsed three times with PBS before cell culture.
Human foreskin fibroblast cells were obtained from the National Defense Medical Center and used at the passage numbers 4–6 in the study. The study protocol was reviewed and approved by the Institutional Review Board in the Tri-Service General Hospital, R.O.C. (TSGHIRB No: 100-05-251). A written informed consent was obtained from each donor. The culture medium was DMEM-high glucose (Gibco-BRL, Bethesda, MD, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin–streptomycin (Thermo Fisher Scientific). Each well was seeded with 1×104 fibroblasts on the films and incubated for 1 and 3 days in an incubator (37°C, 5% CO2).
Cell viability was evaluated by a commercial and nonradioactive cell proliferation reagent WST-1 (Roche Diagnostics GmbH, Mannheim, Germany) for quantification of cell proliferation. The medium was removed and samples were gently washed with PBS for three times. The test reagent (200 μL, 10% WST-1, and 90% medium) was dropped in each well and reacted for 90 minutes, and then, the absorbance at 450 nm was measured using a microplate reader (Spectra-Max® M5; Molecular Devices, Sunnyvale, CA, USA).
Cytoskeletal arrangements of cells were observed by β-actin staining on cells grown on films for 3 days. The samples were fixed by 4% paraformaldehyde for 15 minutes, permeabilized with 0.2% Triton X-100 in PBS for 10 minutes, washed with PBS, and blocked with 5% bovine serum albumin (Sigma-Aldrich) for 1.5 hours. The β-actin antibody (1:200 dilution; Santa Cruz Biotechnology Inc., Dallas, TX, USA) was added and placed overnight at 4°C, followed by addition of secondary antibody (1:200 dilution, Santa Cruz Biotechnology) at 4°C for 1 hour. Finally, the samples were stained with 4′,6-diamidino-2-phenylindole (1:500 dilution) for 5 minutes. Morphology and arrangements of cells were examined using an inverted fluorescent microscope (CKX-41, Olympus, Waltham, MA, USA).
WBPU films seeded with fibroblasts as described above were prepared for scanning electron microscopy (SEM) analysis. The specimens were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at room temperature for 5 hours, rinsed with phosphate buffer containing 50% ethanol (in triplicate for 5 minutes each), and postfixed in 1% osmium tetroxide in phosphate buffer for 1 hour. Subsequently, the samples were washed for 5 minutes in phosphate buffer and dehydrated in serially diluted ethanol solutions of 30%, 50%, 70%, 85%, 90%, 95%, 100%, and 100% acetone. Samples were dried in a Critical Point Dryer (CPD 030; Balzers Union Limited, Balzers, Liechtenstein) using liquid carbon dioxide. The dried cell/WBPU films were coated with gold and analyzed by SEM.
In vivo animal study
In this study, New Zealand white rabbits weighing 2.5–3.0 kg were used. All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee (IACUC: 15-286) of the National Defense Medical Center. A total of 30 rabbits were used. The rabbits were anesthetized by subjecting them to inhalation of 4% isofluorane with O2 at 2.5 L min−1 and maintained with 3% isofluorane. The hind paws were shaved completely and comprehensively disinfected by 75% alcohol and iodine. The operation room, surgical isolator, surgical hole towel, and instruments were disinfected according to standard procedures. Sterile drapes covered the nonsurgical area. At the hind paws, a 2 cm longitudinal midline incision was made on the proximal phalanx of the second and third toes. The tendon sheaths were exposed by careful dissection, and then, the tendon sheaths were incised to release flexor digitorum profundus (FDP) and flexor digitorum superficialis. After the FDP was isolated, it was cut transversely three quarters. Modified Kessler core suture with 4-0 Prolene suture was used to repair the injured tendon. At random, four toes of each rabbit were divided into the control group and three experimental groups including PCL (Mn=70,000–90,000 from gel permeation chromato graphy [GPC], Sigma-Aldrich), Seprafilm (Genzyme Corporation, Cambridge, MA, USA), and PU3 (molecular weight [MW]=600,000 from GPC) films. The control group received no treatment. Each film had a size of 1×1 cm2. PCL and PU3 films were soaked in PBS buffer for about 2 minutes, wrapped around the injured tendon, and fixed using 6-0 Prolene suture to ensure that the site of the repaired tendon had been covered. Seprafilm was wrapped around the injured tendon and some PBS was applied to snugly attach the film to the tendon. Finally, the skin was closed with 4-0 Nylon. Wound toes were appropriately fixed by plaster for 4 weeks.
Gross evaluation of tissue adhesion
After 4 weeks, the animals were anesthetized and sacrificed by injection of 15% KCl (1 mL kg−1). The skin suture of the toes was removed and the skin was opened along the original site of incision. The severity and extent of peritendinous adhesion was recorded by using the semi-quantitative grading scale based on surgical findings. The adhesion scale was divided into five levels: grade 1, no adhesion; grade 2, filmy (separated by blunt dissection); grade 3, mild (separated by sharp dissection); grade 4, moderate (35%–60% adhesion area); and grade 5, severe (>60% adhesion area). The scores were obtained from an investigator who was blinded to treatments and independently scored each rabbit.
Histological analysis
After 4 weeks, the animals were sacrificed; the toes were transected and collected for histological evaluation. The toes were fixed in 4% formalin for 24 hours and decalcified by Decalcifier II (Leica Microsystems, Milton Keynes, UK) for 7 days. Before embedding in paraffin, samples were dehydrated by an ascending alcohol series and xylene. Samples were cut into 4 μm sagittal slices and stained with H&E.
Biomechanical assessment of tendon healing
In order to evaluate the healing of the tendon after 4 weeks, a universal testing machine (AGS-J; Shimadzu, Shanghai, People’s Republic of China) was used. After gross evaluation, the ends of repaired FDP tendons were harvested and fixed by applying a stress of 0.3 MPa and then pulled at 5 mm min−1 until rupture. The maximum force recorded from a tension elongation curve was defined as the maximum tension force.
In vivo degradation
After gross evaluation, the residual of PCL or PU3 was collected by separating the adhesion from the tendon, gently removing the excess tissues, and washing with PBS. After drying at ambient temperature, films were dissolved in dimethylacetamide as the eluent (4 mg mL−1). GPC (PU-980, JASCO, Tokyo, Japan) with a refractive index detector (RI-930) was employed to analyze the MW after 4 weeks in vivo. The GPC system was calibrated with polystyrene standards.
Statistical analysis
Data were presented as mean±SD. Student’s t-test was used to evaluate the statistical difference between the two groups. Significant differences were considered for mean P-values <0.05. Multiple samples were used in each experiment.
Results
Characterization of PU
The synthesized PUs contained about 34% hard segment and 66% soft segment. The hard segment included IPDI, DMPA, and EDA and the soft segment was only PCL diol (Figure 1). In this study, the major difference in each PU was the amount of DMPA (the negatively charged component with ionic groups). The stoichiometric ratios of the three different PUs (PU1, PU2, and PU3) are shown in Table 1. The abbreviations PU1, PU2, and PU3 indicate that the DMPA component was 4.0, 4.2, and 4.4 wt%, respectively. The zeta potential of water-dispersed PU1, PU2, and PU3 nanoparticles was about −41, −58, and −73 mV, respectively, by DLS. Each PU dispersion was cast into films. The surface zeta potential of the cast films by DLS was also negative and was about −21, −36, and −38 mV for PU1, PU2, and PU3, respectively. The contact angles of different PUs are listed in Table 2. After water was dropped on the PU films for 30 seconds, the contact angles of PU1, PU2, and PU3 were about 74°, 73°, and 73°, respectively. There was no significant difference among the groups.
Table 2.
The contact angles and swelling properties of PU films
| Samples | Contact angle (°) | Water absorption (%) | Change of thickness (%) |
|---|---|---|---|
| PU1 | 74.28±3.55 | 16.78±2.13 | 14.78±2.12 |
| PU2 | 72.50±3.88 | 12.25±7.69 | 13.40±5.21 |
| PU3 | 73.48±1.85 | 11.77±2.70 | 12.88±3.16 |
Note: There was no significant difference among the groups.
Abbreviation: PU, polyurethane.
Water absorption and change in the thickness of the different PU films are summarized in Table 2. When the content of DMPA (ionic groups) increased, the swelling ratio decreased from about 17% in PU1 to about 12% in PU3. Meanwhile, as the content of DMPA increased, change in the thickness decreased from ~15% in PU1 tô13% in PU3. Therefore, a higher DMPA content gave rise to lower water absorption and swelling of these PUs.
The mechanical properties of the PUs are displayed in Table 3. When the content of DMPA increased, the Young’s modulus decreased from ~38 MPa in PU1 to ~21 MPa in PU3. The 100% modulus of PU1, PU2, and PU3 was very close at 5.4, 5.4, and 5.7 MPa, respectively. Moreover, the tensile strength was about 32, 31, and 34 MPa for PU1, PU2, and PU3, respectively. There was no significant difference in 100% modulus and tensile strength among the groups. Outstanding elongation (>500%) was observed for all PUs.
Table 3.
The mechanical properties of PU films
| Samples | Young’s modulus (MPa) | 100% modulus (MPa) | Tensile strength (MPa) | Elongation (%) |
|---|---|---|---|---|
| PU1 | 37.7±3.9 | 5.4±0.12 | 31.6±2.4 | 544±30 |
| PU2 | 28.1±4.1 | 5.4±0.32 | 30.6±2.28 | 553±18 |
| PU3 | 21.1±2.1 | 5.7±0.19 | 34.1±1.9 | 547±11 |
Note: There was no significant difference among the groups.
Abbreviation: PU, polyurethane.
Gelatin test
The average peeling energies from the 180° peeling of different materials from gelatin are shown in Table 4. The average peeling energy of PUs was in the range of ~100−140 J m−2. No significant differences of the value were observed for PU1, PU2, and PU3. Meanwhile, the value obtained for PCL films peeled from gelatin was about 220 J m−2, which was significantly higher than those of PU1, PU2, and PU3.
Table 4.
The peeling force of samples stripped from gelatin
| Samples | Average peeling energy (J m−2) |
|---|---|
| PU1 | 101.52±75.72 |
| PU2 | 142.01±60.28 |
| PU3 | 114.53±58.58 |
| PCL | 220.08±67.22* |
Note:
P<0.05, with respect to the other groups.
Abbreviations: PCL, polycaprolactone; PU, polyurethane.
In vitro evaluation
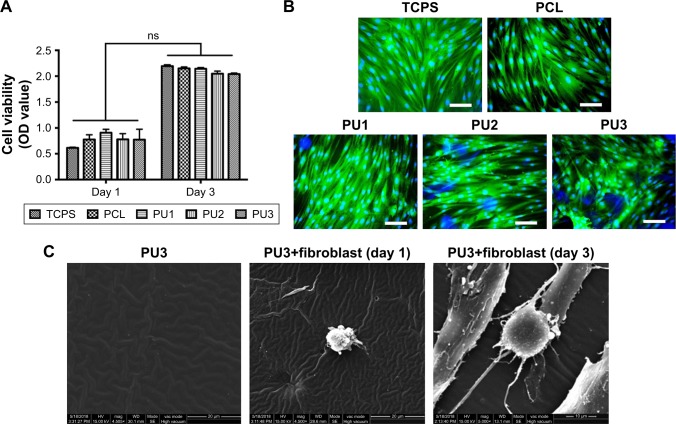
The viability and morphology of human foreskin fibroblasts seeded on PUs for 3 days are shown in Figure 2. When cells grew on different surfaces for 1 day, the cell viability in all PU groups was slightly better than that of TCPS group (Figure 2A). After 3 days, the TCPS group had slightly higher cell viability than the other groups. However, there was no significant difference among the groups either for 1 or 3 days. The cytoskeletal arrangement of cells grown on different materials for 3 days was examined by green fluorescence staining (Figure 2B). Human fibroblasts could adhere and proliferate on the surface in all groups. Cell spreading and distribution on PU films was similar to those on TCPS and PCL. The detailed morphological observations of SEM are shown in Figure 2C. Fibroblasts seeded on WBPU films were spherical at 1 day and remained round shaped with some protrusion after 3 days of incubation. The morphology of fibroblasts obtained from SEM was consistent with that obtained by immunostaining. There were no notable interactions between the cells and WBPU films.
Figure 2.
The viability and morphology of human foreskin fibroblasts seeded on PUs for 1 and 3 days.
Notes: (A) The viability of human fibroblasts cultured on TCPS, PCL, PU1, PU2, or PU3 after 1 and 3 days. (B) Immunofluorescence staining images showing the cytoskeletal arrangement of human fibroblasts grown on various materials for 3 days. Blue: DAPI; green: β-actin; scale bar: 100 μm. (C) The SEM analysis for the morphology of WBPU films and fibroblasts cultured on WBPU films at 1 and 3 days. Scale bar: 20 μm.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ns, nonsignificant; PCL, polycaprolactone; PU, polyurethane; SEM, scanning electron microscopy; TCPS, tissue culture polystyrene; WBPU, waterborne biodegradable polyurethane.
Animal study
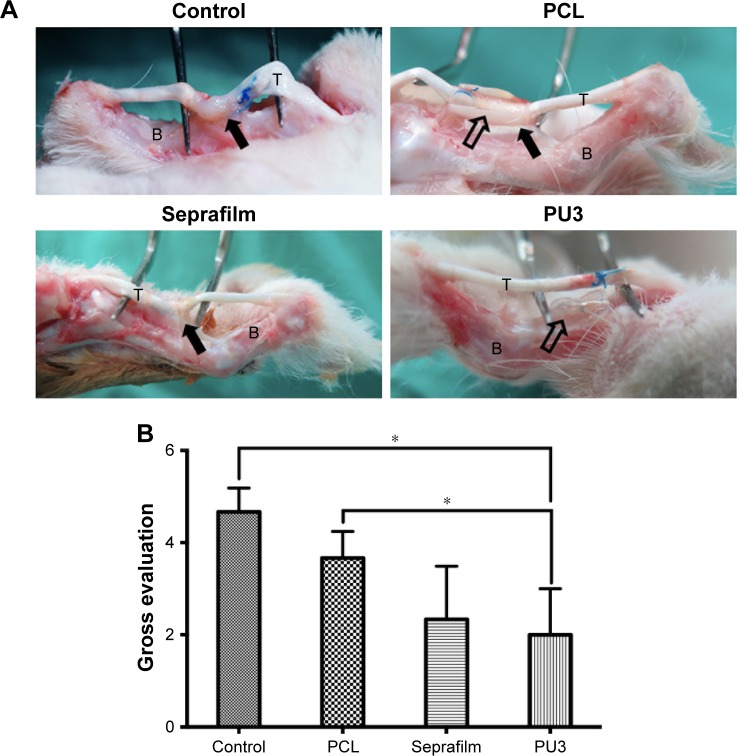
Direct observation of the repair sites was used to evaluate the situation of the peritendinous adhesion after 4 weeks (Figure 3A). For untreated control, severe peritendinous adhesion was observed at the repair site, and the adhesion site could only be separated from the surrounding tissue by sharp dissection. In the group treated with PU3 films, the adhesion area was reduced and no adhesion was observed between the PU3 film and the injured tendon. Compared to those treated with PU3 films, animals treated with PCL films still showed a wide adhesion area between the PCL films and the injured tendons. The adhesion area in the PCL-treated group was severe and could only be separated by sharp dissection. In the animals treated with commercial anti-adhesion Seprafilms, middle peritendinous adhesion at the repaired site was observed and the repaired tendon could be separated from the surrounding tissue by blunt dissection. Results of the semi-quantified evaluation of peritendinous adhesions are presented in Figure 3B. PCL films showed a significant difference with the untreated control, and PU3 films as anti-adhesion barriers performed better than PCL films and the untreated control (P<0.05).
Figure 3.
The observation and scoring of tendon adhesion in four experimental groups at 4 weeks postsurgery.
Notes: (A) Tendon adhesion in groups treated with PCL, commercial product (Seprafilm), or PU3 relative to untreated controls at 4 weeks. Solid arrows: the adhesion site; hollow arrows: the physical barrier. (B) Scoring based on gross evaluation of the implantation sites at 4 weeks. Scoring of PU3 films performed better than PCL films (P=0.024) and the untreated control (P=0.002). *P<0.05, with respect to the other groups. Data shown as mean values.
Abbreviations: B, bone; PCL, polycaprolactone; PU, polyurethane; T, tendon.
Histological sections
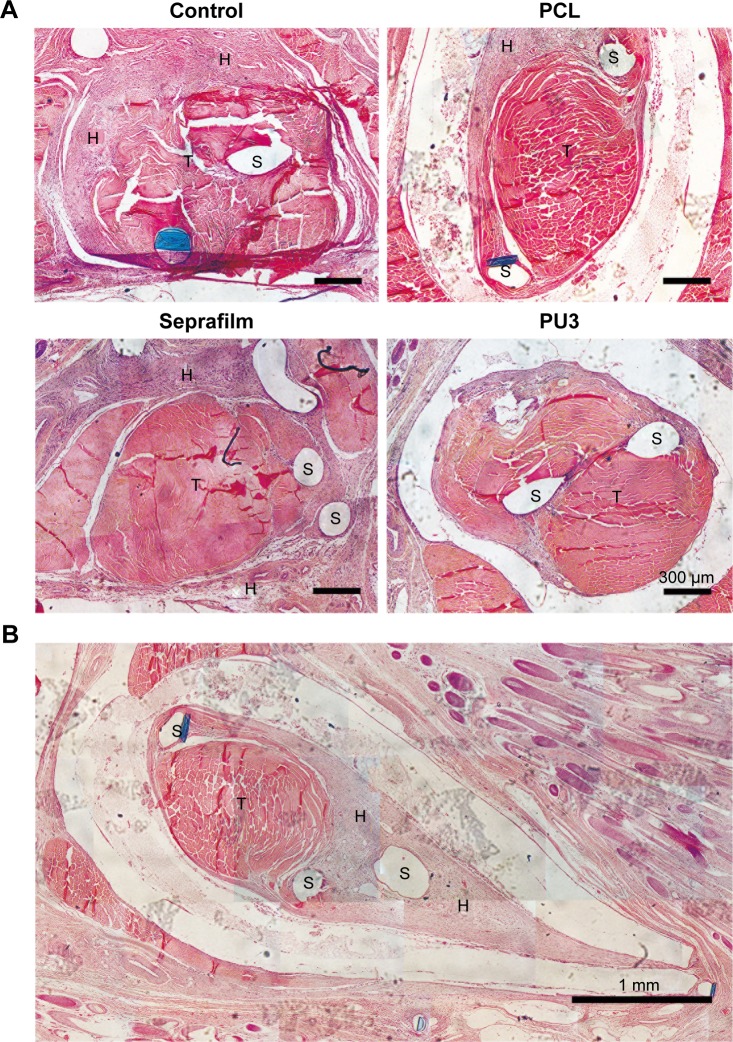
The histological sections obtained from animals after 4 weeks of receiving different treatments at the tendon repair site are presented in Figure 4A. Based on the histology, there was severe adhesion between tendons and the surrounding tissues in the untreated control. Hyperplasia tissues which formed during tendon recovery were numerous and linked the surrounding tissues to form adhesions. For PCL films, hyperplasia tissues did not penetrate the films. PCL films effectively separated the tendon and the surrounding tissues. In addition to the observations presented in Figure 3A, PCL films adhered with the tendon and the surrounding tissues. Thus, PCL did not provide an anti-adhesion function because the gliding of the repaired tendon was limited. Furthermore, many hyperplasia tissues filled inside the wrapped area of the PCL films. This gave rise to recovered tendons with significant adhesion to the PCL films (Figure 4B). Compared to the untreated control and PCL films, Seprafilm showed better anti-adhesion results. Hyperplasia tissues were one of the reasons for the adhesions in case of the Seprafilm-treated tendons, but the adhesion was concentrated on the area of the surgical sutures. For the group treated with PU3 films, there was no adhesion area and only slight hyperplasia tissues were found on repaired tendons.
Figure 4.
The histological analysis in four experimental groups at 4 weeks postsurgery.
Notes: (A) Histological analysis of the physical barrier for groups treated with PCL, commercial product (Seprafilm), or PU3 and untreated control by H&E stain for 4 weeks. (B) The enlarged image for the group treated with PCL showing the hyperplasia tissues filled inside the wrapped area of the PCL films at 4 weeks.
Abbreviations: H, hyperplasia; PCL, polycaprolactone; PU, polyurethane; S, suture; T, tendon.
Biomechanical evaluation
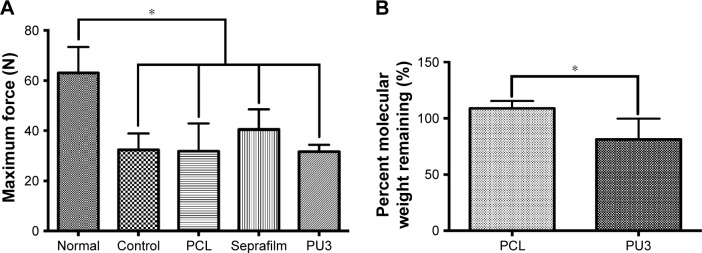
The maximum breaking forces for the repaired tendons wrapped with different physical barriers and for the untreated control after 4 weeks of recovery are reported in Figure 5A. The maximum force of repaired tendons for each group treated with different barriers was significantly lower than that of normal tendons (P<0.05). In the experimental group, the maximum force for the tendons wrapped with Seprafilm was slightly higher than those in the other groups, but there were no statistically significant differences among the experimental groups.
Figure 5.
The measurement of maximum tension force of the tenton and percent molecular weight remaining of PCL and PU at 4 weeks postsurgery.
Notes: (A) Maximum tension force of the tendon for the normal, the untreated control, and the group treated with PCL, commercial product (Seprafilm), or PU3 after 4 weeks in vivo. The maximum force of normal tendon was significantly higher than that of repaired tendons of untreated controls (P=0.001), PCL (P=0.016), Seprafilm (P=0.016), and PU3 (P=0.002), respectively. (B) Percent molecular weight remaining for PCL and PU3 of the physical barrier after 4 weeks in vivo. The degradation of PCL and PU3 showed a statistically significant difference (P=0.014). *P<0.05, with respect to the other groups.
Abbreviations: PCL, polycaprolactone; PU, polyurethane.
Biodegradation
The initial MWs of PCL and of PU3 obtained from GPC analysis were about 120,000 and 600,000, respectively. The degradation of films is displayed in Figure 5B. The MW of PCL after implantation for 4 weeks remained almost unchanged, but that of PU3 decreased to about 80%. The degradation of PCL and PU3 showed statistically significant differences.
Discussion
In this study, we synthesized waterborne PUs with different amounts of DMPA and used the PU films as physical anti-adhesion barriers to prevent postoperative adhesion. Compared to traditional biomaterials such as PELA and PCL, PUs have excellent elasticity and mechanical strength. For anti-adhesion application, traditional biomaterials often need to be modified to increase the hydrophilicity and elasticity. Moreover, PELA and PCL do not possess anti-adhesion properties and usually serve as the carrier for anti-adhesion materials.11,24 Compared to other biomedical polymers such as PCL, poly(lactic-co-glycolic acid), and polylactic acid, PU has elasticity and good mechanical properties.25 In addition, PU has been applied in tissue engineering, drug release, and medical devices due to cell and blood biocompatibility.13 PU is also used in vascular grafts and artificial hearts.26 The biocompatibility and mechanical properties of PU are candidate materials for anti-adhesion barriers. In the literature, nondegradable PUs as anti-adhesion barriers showed excellent anti-adhesion property, but foreign body reactions and limited nutrition diffusion posed potential risks and could lead to tissue necrosis.7 To date, the anti-adhesion function of PU at the tendon site has not been discussed.
Traditional PUs are hydrophobic. In order to improve the hydrophilicity, ionic groups were introduced to the chemical structure. During the synthesis process of PU, ionic groups can serve as the self-emulsifier that disperses the polymer and change the medium from solvent to water. Moreover, the ionic groups can act as physical crosslinkers that improve the mechanical and thermodynamic properties of PU.14,27 The isocyanate of PU provides the mechanical strength. Generally, aromatic isocyanates offer better mechanical strength than aliphatic ones, but aromatic isocyanate-based PUs can release aromatic urethane or urea groups during the degradation process.15 The aromatic groups are considered to have a greater risk of dermatitis and asthma.28 A report showed that 2,4-toluene diisocyanate-based PU could degrade to 2,4-toluene diamine (an acutely toxic substrate), but the decomposition products of 4,4′-methylenediphenyl diisocyanate-based PU had no toxicity in vivo.15 For anti-adhesion, the flexible aliphatic segment in the PU structure prevents damage from external or internal forces when PU films are implanted in vivo. In this study, we only found the Young’s modulus to be affected by the DMPA content in PU chains. When the DMPA content increased, the hard segment was more dispersed and more difficult to arrange, leading to less rigidity. The soft segment offered elasticity in PU and did not change among the PUs; therefore, the elongation of all PUs was similar.
An ideal anti-adhesion barrier should have biocompatibility, biodegradability, low inflammatory reaction, low swelling, and adhesion prevention until healing occurs without any side effect.29–31 The anti-adhesive physical barrier should keep its size and shape when implanted in the fluid-rich environment of the human body. When the barrier absorbs tissue fluid and becomes thicker, it may induce a more serious inflammatory reaction.32 As a practical anti-adhesion barrier, it is important to reduce the swelling phenomenon. If the barrier is not mechanically strong, the surrounding tissues might compress and induce the foreign body reaction. Therefore, it is very important to choose an appropriate PU structure. PUs with pure PCL diol as the soft segment are biodegradable and flexible.25 With a greater DMPA content, the swelling ratio of PU3 remains similar to those of PU1 and PU2.
The molecular structure of gelatin has C=O groups, and the gelatin test is a suitable way to evaluate the adherence to a hydrogen-bonding gel forming material.33 Compared to PCL, WBPU has more C=O groups (dipole–dipole) in the polymer chain and even has ionic groups (ionic bond), which may enable WBPU to decrease the surface tension.34 This structure of WBPU repels gelatin more powerfully than PCL.
Compared to the prevention of abdominal adhesion, prevention of tendon adhesion is more difficult due to gliding motion. Appropriate passive mobilization can reduce tendon adhesion and improve tensile strength in the recovery period, but the anti-adhesion barrier has better mechanical strength in order to prevent the damage caused by tendon gliding.35 Seprafilm is a useful film for anti-adhesion in abdominal adhesion, but it has a few drawbacks such as low mechanical strength, rapid degradation, and finite function when used in other sites such as the FDP and Achilles tendon. One of the reasons may be attributed to the components of Seprafilm – HA and carboxymethylcellulose. When Sepra-film is implanted in the tendon, the integrity of the film is hard to maintain due to film hydration and internal damage. The mechanical properties of the Seprafilm thus decrease, which leads to failure of the barrier. Moreover, rapid degradation of Seprafilm is not suitable for the tendon physical barrier because the healing time of the tendon is longer than that of the abdominal tissue. The effect of Seprafilm lasted for about 3 weeks only.8,11,36
The initiation of adhesion starts at the inflammatory stage after tendon injury occurs and inflammatory paracrines recruit inflammatory cells for the immune response and fibroblasts for fibrin deposition. Early adhesion forms as a result of inflammation and deposition.7,37 Therefore, inflammation is highly associated with adhesion. Many research studies have shown that anti-inflammation reagents are effective in reducing tissue adhesion.1,9,24 As physical barriers for the tendon sheath, it is essential to reduce inflammation or eliminate negative reaction in order to avoid more serious inflammatory reactions. Foreign body reaction causes long-term problems to the physical barriers because the inflammation lasts and deepens the adhesion.38 Materials loaded with nonsteroidal anti-inflammatory drugs were used for anti-adhesion application, but the slower degradation rate of the polymer could induce adhesion following complete release of the drug.39 Compared to other materials, PUs have a low inflammatory reaction.40
Regarding physical properties, PU1, PU2, and PU3 were appropriate for use in postoperative adhesion of the tendon. Different PU ionic groups did not have obvious effects on fibroblast morphology and viability, compared to TCPS. Many polymers with negatively charged functional groups could prevent the adhesion of macrophages, lymphocytes, and platelets.41 In a previous study, WBPU with a greater content of ionic groups showed better immunosuppressive activity.20 Based on these findings, we considered that PU3 was a better candidate for in vivo tests. Therefore, the lower immune reaction may account for the good outcomes in the animal studies. The PU3 films showed the smallest inflammatory area relative to the other groups, and the inflammation concentrated only on the suture site to fix the PU films.
Tendon adhesion could be seen in about 7 days of the tendon trauma.42 A previous report evaluated the anti-adhesion function with different materials at 1 week. The result showed that there was no significant adhesion tissue, even in commercial Seprafilm.43 At this time, fibronectin could be detected, but it was in an initial stage of adhesion. Subsequently, mature adhesion included vascularization and tissue fiber connection.42 Thus, in order to observe the anti-adhesion function of different materials, we extended the recovery period to 1 month.
Appropriate degradation rate is an important issue to consider because it may prevent the recurrence of adhesion after tendon recovery. After the inflammatory stage of the tendon repair, the ECM starts to produce in the proliferation stage, which lasts for ~1 month. Following this, the ECM organizes to become tendon-like structures in the longitudinal axis and the mechanical strength of the tendon starts to recover. The remodeling stage lasts for about 1–2 years. In this study, the strength of the injured tendon did not fully reach that of the normal tendon. The total degradation period of the PCL iŝ2–4 years, depending on the MW. When PCL is implanted in vivo, hydrolytic degradation of ester groups occurs as the first step in PCL degradation. In the hydrolytic stage, the MW of PCL decreases rarely. The PCL degradation is not self-catalytic. In contrast, PCL undergoes bulk degradation over 6 months. When the MW is reduced to ~3,000, the PCL is subjected to intracellular degradation through enzyme.44 PU degradation is sensitive to temperature and liquids. As such, degradation of biodegradable PUs could undergo hydrolytic or enzymatic processes in vivo.45 PU degradation begins in the manufacturing process because moisture is not easily discharged. Hydrolysis starts at ester linkage and produces α-hydroxy acids and urethane or urea fragments.15 The degradation products of biodegradable PU synthesized from 4,4′-methylenediphenyl diisocyanate, aromatic diisocyanate, and PCL diol have been found to be non-cytotoxic.15 The microphase separation on the surface of PU can affect the surface organization and the rate of degradation. The hard segment (ionic groups) at the surface may have a higher proportion in a polar environment than in a non-polar environment. This phenomenon could minimize the free energy of the interface and increase the degradation.45 Based on a previous study, the amount of ionic groups (eg, DMPA) in WBPU ionomers critically determined the nanoparticle size of the dispersion as well as the mechanical properties of the cast (self-assembled) films.46 Moreover, the nanoparticles of PU3 did not provoke inflammation. Instead, they displayed immunosuppressive activities on the macrophages.47 While PU3 degradation may be slow in terms of MW, the nanoparticles eroded from the film surface may suppress the foreign body reaction. Consequently, PU3 may facilitate in vivo tissue repair/remodeling and prevent tendon postoperative adhesion.
In the future, we expect to further improve the surgical technique and the water-retaining properties of the materials to increase tendon gliding and healing.
Conclusion
The anti-adhesion function of PU at the tendon site has not been discussed in previous literature. In this study, a series of WBPUs with negative ionic groups were prepared and evaluated for the anti-adhesion performances. For the improvement of hydrophilicity, ionic groups were introduced to the chemical structure. Polymers with negatively charged functional groups could prevent adhesion of macrophages, lymphocytes, and platelets. In addition, PUs with pure PCL diol as the soft segment could improve biodegradability and flexibility. Compared to commercial anti-adhesion barriers such as PELA or PCL, WBPUs provided suitable mechanical strength and elasticity to prevent damage of anti-adhesion barriers from tendon gliding. The content of DMPA did not affect cytotoxicity and cell morphology. In the rabbit study, PU3 was effective in reducing the degree of tendon adhesion and appeared to be slightly better than Seprafilm due to excellent biocompatibility, restrained inflammatory reactions, and a proper degradation rate. The WBPU film is, thus, a potential anti-adhesion material and may be widely used in other sites of the human body. A more hydrophilic and water-retaining anti-adhesion barrier will be required to achieve the next goal for increased tendon gliding and healing.
Acknowledgments
The authors thank the Ministry of Science Technology, R.O.C (MOST105-2221-E-002–103 and MOST106-2221- E-002–079-MY2) for financial support. They also thank Mr Yao-Wen Hung in National Defense Medical Center, ROC for the assistance in Scanning Electron Microscopy analysis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee JH, Go AK, Oh SH, Lee KE, Yuk SH, Ak G, Sh O. Tissue anti-adhesion potential of ibuprofen-loaded PLLA-PEG diblock copolymer films. Biomaterials. 2005;26(6):671–678. doi: 10.1016/j.biomaterials.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Zhao J, Ruan H, et al. Biomimetic sheath membrane via electrospinning for antiadhesion of repaired tendon. Biomacromolecules. 2012;13(11):3611–3619. doi: 10.1021/bm301022p. [DOI] [PubMed] [Google Scholar]
- 4.Chen SH, Chen CH, Shalumon KT, Chen JP. Preparation and characterization of antiadhesion barrier film from hyaluronic acid-grafted electrospun poly(caprolactone) nanofibrous membranes for prevention of flexor tendon postoperative peritendinous adhesion. Int J Nanomedicine. 2014;9:4079–4092. doi: 10.2147/IJN.S67931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang S, Yan H, Fan D, Song J, Fan C. Multi-layer electrospun membrane mimicking tendon sheath for prevention of tendon adhesions. Int J Mol Sci. 2015;16(4):6932–6944. doi: 10.3390/ijms16046932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Amadio PC, Momose T, et al. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51(5):917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Meier Bürgisser G, Buschmann J. History and performance of implant materials applied as peritendinous antiadhesives. J Biomed Mater Res B Appl Biomater. 2015;103(1):212–228. doi: 10.1002/jbm.b.33182. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Skardal A, Shu XZ, Prestwich GD. Prevention of peritendinous adhesions using a hyaluronan-derived hydrogel film following partial-thickness flexor tendon injury. J Orthop Res. 2008;26(4):562–569. doi: 10.1002/jor.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Zheng X, Fan D, et al. Release of celecoxib from a bi-layer biomimetic tendon sheath to prevent tissue adhesion. Mater Sci Eng C Mater Biol Appl. 2016;61:220–226. doi: 10.1016/j.msec.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Hu C, Liu S, Zhang Y, et al. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater. 2013;9(7):7381–7388. doi: 10.1016/j.actbio.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Chen SH, Chen CH, Fong YT, Chen JP. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater. 2014;10(12):4971–4982. doi: 10.1016/j.actbio.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Chen SH, Shalumon KT, Chen JP. Prevention of peritendinous adhesions with electrospun polyethylene glycol/polycaprolactone nanofibrous membranes. Colloids Surf B Biointerfaces. 2015;133:221–230. doi: 10.1016/j.colsurfb.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Sartori S, Chiono V, Tonda-Turo C, Mattu C, Gianluca C, et al. Biomimetic polyurethanes in nano and regenerative medicine. J Mater Chem B. 2014;2(32):5128–5144. doi: 10.1039/c4tb00525b. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Xie D, Zhang X. The structure, microphase-separated morphology, and property of polyurethanes and polyureas. J Mater Sci. 2014;49(21):7339–7352. [Google Scholar]
- 15.Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B Rev. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 16.Guo YH, Guo JJ, Miao H, et al. Synthesis and characterization of waterborne polyurethane emulsions based on poly(butylene itaconate) ester. Prog Org Coat. 2014;77:988–996. [Google Scholar]
- 17.Travinskaya T, Savelyev Y, Mishchuk E. Waterborne polyurethane based starch containing materials: preparation, properties and study of degradability. Polym Degrad Stab. 2014;101:102–108. [Google Scholar]
- 18.Cakić SM, Špírková M, Ristić IS, et al. The waterborne polyurethane dispersions based on polycarbonate diol: effect of ionic content. Mater Chem Phys. 2013;138(1):277–285. [Google Scholar]
- 19.Zhu Q, Wang Y, Zhou M, et al. Preparation of anionic polyurethane nanoparticles and blood compatible behaviors. J Nanosci Nanotechnol. 2012;12(5):4051–4056. doi: 10.1166/jnn.2012.5858. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y-J, Hung K-C, Hsieh F-Y, Hsu S-Hui, et al. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352–20364. doi: 10.1039/c5nr06379e. [DOI] [PubMed] [Google Scholar]
- 21.Rehman IU. Biodegradable polyurethanes: biodegradable low adherence films for the prevention of adhesions after surgery. J Biomater Appl. 1996;11(2):182–257. doi: 10.1177/088532829601100203. [DOI] [PubMed] [Google Scholar]
- 22.Hung KC, Hsu SH. Polymer surface interacts with calcium in aqueous media to induce stem cell assembly. Adv Healthc Mater. 2015;4(15):2186–2194. doi: 10.1002/adhm.201500374. [DOI] [PubMed] [Google Scholar]
- 23.Ning CC, Logsetty S, Ghughare S, Liu S. Effect of hydrogel grafting, water and surfactant wetting on the adherence of PET wound dressings. Burns. 2014;40(6):1164–1171. doi: 10.1016/j.burns.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Hu C, Li F, et al. Prevention of peritendinous adhesions with electrospun ibuprofen-loaded poly(l-lactic acid)-polyethylene glycol fibrous membranes. Tissue Eng Part A. 2013;19(3–4):529–537. doi: 10.1089/ten.TEA.2012.0208. [DOI] [PubMed] [Google Scholar]
- 25.Hsu S-Hui, Hung K-C, Lin Y-Y, et al. Water-based synthesis and processing of novel biodegradable elastomers for medical applications. J Mater Chem B. 2014;2(31):5083–5092. doi: 10.1039/c4tb00572d. [DOI] [PubMed] [Google Scholar]
- 26.de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67–68:1534. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Jaudouin O, Robin J-J, Lopez-Cuesta J-M, Perrin D, Imbert C, et al. Ionomer-based polyurethanes: a comparative study of properties and applications. Polym Int. 2012;61(4):495–510. [Google Scholar]
- 28.Henriks-Eckerman ML, Mäkelä EA, Laitinen J, et al. Role of dermal exposure in systemic intake of methylenediphenyl diisocyanate (MDI) among construction and boat building workers. Toxicol Lett. 2015;232(3):595–600. doi: 10.1016/j.toxlet.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Tuncay I, Ozbek H, Atik B, Ozen S, Akpinar F. Effects of hyaluronic acid on postoperative adhesion of tendo calcaneus surgery: an experimental study in rats. J Foot Ankle Surg. 2002;41(2):104–108. doi: 10.1016/s1067-2516(02)80033-3. [DOI] [PubMed] [Google Scholar]
- 30.Meier Bürgisser G, Calcagni M, Müller A, et al. Prevention of peritendinous adhesions using an electrospun DegraPol polymer tube: a histological, ultrasonographic, and biomechanical study in rabbits. Biomed Res Int. 2014;2014:656240. doi: 10.1155/2014/656240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson RE, Rinker B. The use of a hydrogel sealant on flexor tendon repairs to prevent adhesion formation. Ann Plast Surg. 2006;56(1):54–58. doi: 10.1097/01.sap.0000181666.00492.0e. [DOI] [PubMed] [Google Scholar]
- 32.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185–4192. doi: 10.1016/s0142-9612(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 33.Andrews EH, Kamyab I. Adhesion of surgical dressings to wounds. A new invitro model. Clin Mater. 1986;1(1):9–21. [Google Scholar]
- 34.Lei L, Xia Z, Cao G, Zhong L, et al. Synthesis and adhesion property of waterborne polyurethanes with different ionic group contents. Colloid Polym Sci. 2014;292(2):527–532. [Google Scholar]
- 35.Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 36.Kuo SM, Chang SJ, Wang HY, Tang SC, Yang SW. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohydr Polym. 2014;114:230–237. doi: 10.1016/j.carbpol.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37(6):865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015;8(9):5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Cui W. Hierarchical structure of electrospun composite fibers for long-term controlled drug release carriers. Adv Healthc Mater. 2012;1(6):809–814. doi: 10.1002/adhm.201200146. [DOI] [PubMed] [Google Scholar]
- 40.Sanders JE, Bale SD, Neumann T. Tissue response to microfibers of different polymers: polyester, polyethylene, polylactic acid, and polyurethane. J Biomed Mater Res. 2002;62(2):222–227. doi: 10.1002/jbm.10285. [DOI] [PubMed] [Google Scholar]
- 41.Verma D, Previtera ML, Schloss R, Langrana N. Polyelectrolyte complex membranes for prevention of post-surgical adhesions in neurosurgery. Ann Biomed Eng. 2012;40(9):1949–1960. doi: 10.1007/s10439-012-0564-x. [DOI] [PubMed] [Google Scholar]
- 42.Jaibaji M. Advances in the biology of zone II flexor tendon healing and adhesion formation. Ann Plast Surg. 2000;45(1):83–92. doi: 10.1097/00000637-200045010-00017. [DOI] [PubMed] [Google Scholar]
- 43.Lo HY, Kuo HT, Huang YY. Application of polycaprolactone as an anti-adhesion biomaterial film. Artif Organs. 2010;34(8):648–653. doi: 10.1111/j.1525-1594.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 44.Woodruff MA, Hutmacher DW. The return of a forgotten polymer – polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256. [Google Scholar]
- 45.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26(35):7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 46.Lin YY, Hung KC, Hsu SH. Stability of biodegradable waterborne polyurethane films in buffered saline solutions. Biointerphases. 2015;10(3):031006. doi: 10.1116/1.4929357. [DOI] [PubMed] [Google Scholar]
- 47.Huang YJ, Hung KC, Hsieh FY, Hsu SH. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352–20364. doi: 10.1039/c5nr06379e. [DOI] [PubMed] [Google Scholar]