Abstract
The fermentation and isolation of metabolites produced by an endophytic fungus, which was identified as Phomopsis sp. FJBR-11, based on phylogenetic analysis, led to the identification of six compounds, including dothiorelones A–C, and H, and cytosporones C and U. Among these compounds, cytosporone U exhibited potent inhibitory activity against Tobacco mosaic virus (TMV). Moreover, the crude and a purified exopolysaccharide were proved to possess strong inhibitory effects against the virus infection.
Keywords: Phomopsis, dothiorelone, cytosporone, Tobacco mosaic virus
1. Introduction
Tobacco mosaic virus (TMV) is a prevalent plant pathogen which brings about dramatic losses to modern agriculture. Over 885 plant species in 65 families could be infected by TMV, and up to U.S. $100 million is lost each year globally due to plant diseases caused by TMV. However, no effective chemical treatments appear capable, so far, of inhibiting virus replication once plants are infected [1,2,3,4]. As the type member of the genus Tobamovirus, TMV is a most commonly used target for discovery of bioactive antiviral natural products.
Plant-associated microorganisms are known to produce a variety of metabolites with novel structures and interesting biological activities [5,6,7]. We have recently reported the anti-TMV activity of malformin A1, which is a cyclic peptide produced by an endophytic fungus obtained from Brucea javanica (L.) Merr (Simaroubaceae) [8]. In our ongoing effort to identify antiviral natural products produced by endophytic fungus, a Phomopsis strain was isolated from this plant and selected for further study. Phomopsis spp. (teleomorph Diaporthe) figure among the most common endophytes of tropical plants and some species are the causal agents of important plant diseases. As dominant endophytes, Phomopsis spp. have been subjected to intense chemical investigation and various new secondary metabolites have been described, including polyketides, terpenes, and compounds of mixed origin, such as cytochalasins [9,10,11,12,13,14]. We report in this paper the identification of bioactive metabolites produced by an endophytic Phomopsis sp. FJBR-11 isolated from B. javanica, as well as their anti-TMV activity.
2. Results and Discussion
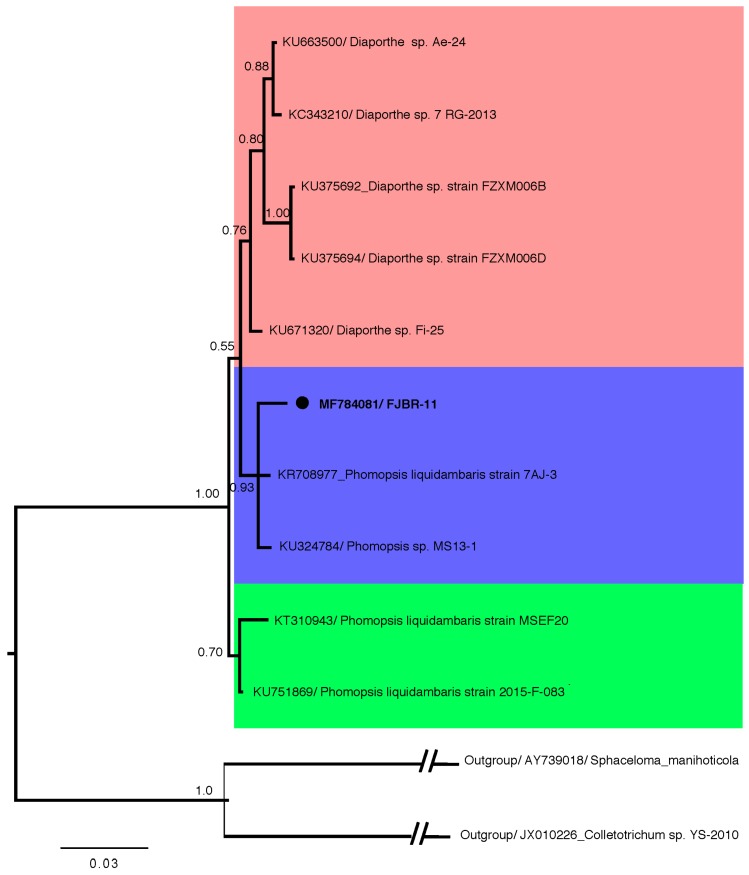
Fungal strain FJBR-11 was isolated from the healthy stem tissue of B. javanica. The result from Bayesian phylogenetic analysis (Figure 1), based on ITS sequences revealed that FJBR-11 (539 base pairs, GenBank accession no. MF784081) was clustered with high posterior probability (PP = 93) among members of the Phomopsis sp., including Phomopsis liquidambaris 7AJ-3 (GenBank accession no. KR708977) and Phomopsis sp. MS 13-1 (GenBank accession no. KU324784). This suggests that FJBR-11 should be classified as the genus Phomopsis.
Figure 1.
Phylogenetic relationship of isolate FJBR-11 (accession no. MF784081) to representative sequences of known Phomopsis sp. and Diaporthe sp. Reference sequences were retrieved from GenBank and a Sphaceloma sp. and a Colletotrichum sp. served as an outgroup. The isolate FJBR-11 from the current study is marked with a black dot. The distance unit is substitutions/site.
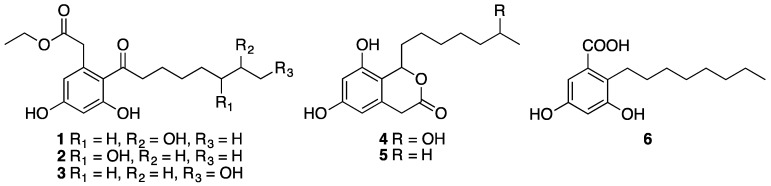
Fermentation of the fungus Phomopsis sp. FJBR-11 and chromatographic fractionation of the EtOAc extract resulted in the isolation of compounds 1–6. Their structures (Figure 2) were identified by comparison of the 1H- and 13C-NMR data with those previously reported in the literature as dothiorelone A (1) [15], dothiorelone B (2) [16], dothiorelone C (3) [15], dothiorelone H (4) [17], cytosporone C (5) [18,19], and cytosporone U (6) [20]. Compounds 1–6 were tested for their antiviral activity against Tobacco mosaic virus (TMV) at a concentration of 500 μg/mL using both the local lesion and leaf-disc methods. All the isolated compounds, except cytosporone U (6), showed only weak or no inhibitory effect against TMV (Table 1). Cytosporone U showed a dose-dependent inhibition against TMV, and its IC50 value was further determined as 144.6 μg/mL using leaf-disc method.
Figure 2.
Structure of compounds 1–6.
Table 1.
Antiviral activity of Compounds 1–6 (500 μg/mL) against TMV.
| Compound | Inhibition Rate (%) | |
|---|---|---|
| Local Lesion Assay | Leaf-Disc Method | |
| 1 | <10 | <10 |
| 2 | <10 | 10.9 ± 1.1 |
| 3 | <10 | 12.7 ± 1.0 |
| 4 | 11.3 ± 1.2 | 29.6 ± 2.1 |
| 5 | 22.9 ± 2.1 | 31.9 ± 2.0 |
| 6 | 39.2 ± 2.2 | 80.0 ± 3.0 |
| Ningnanmycin | 90.3 ± 3.4 | 91.4 ± 3.8 |
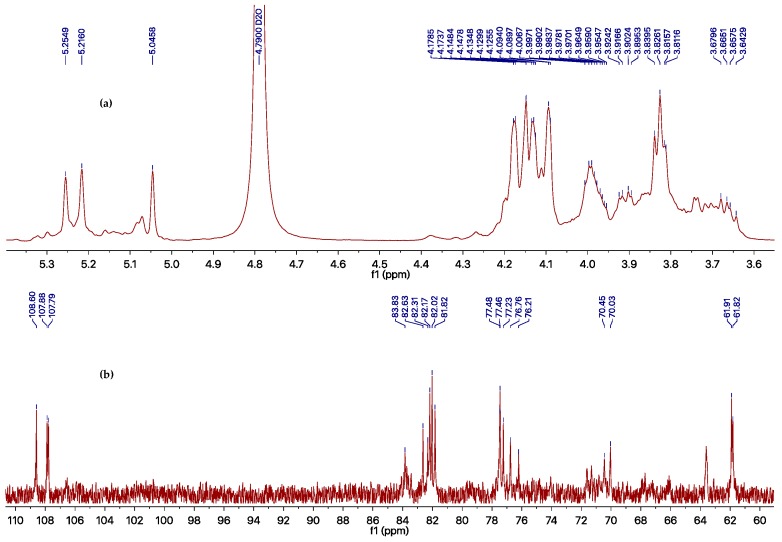
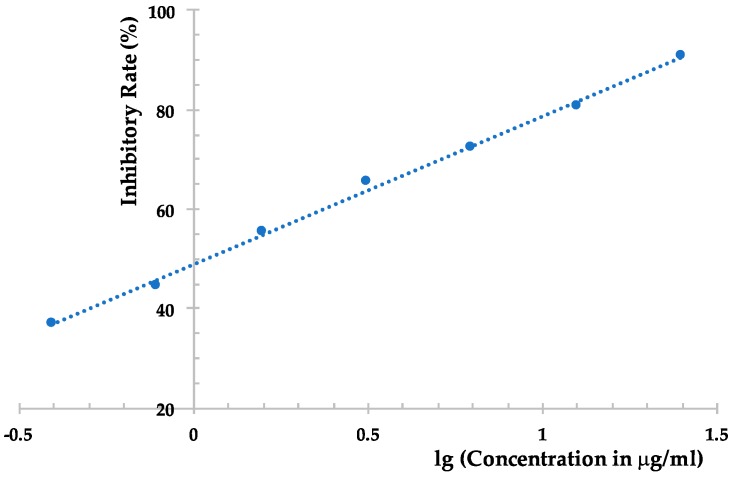
However, the EtOAc extract was more effective than the compounds obtained, which possesses an inhibition rate of >95% at 500 μg/mL against TMV, as determined using the local lesion method. The above results indicated that compounds 1–6 could not represent the major antiviral component produced by Phomopsis sp. FJBR-11. Our further effort led to the purification of an active exocellular polysaccharide (EP) fraction. The average molecular weight (Mw) was determined as 3.71 × 105 g/mol using gel permeation chromatography (GPC). The chemical structure of the more abundant polysaccharide of this fraction was characterized by means of 1H- and 13C-NMR, HSQC, TOCSY, as well as NOESY experiments. Its 13C-NMR spectra displayed three anomeric carbon signals with similar intensity at δH 5.25, 5.22, and 5.05, which indicated that all these monosaccharides are β-d-Galf, and the polymer consists of a trisaccharide repeating unit of the polysaccharide. Assignment of the 1H- and 13C-NMR signals (Table 2) for different units was made by HSQC and TOCSY experiments. The observed deshielding of the C-6 of unit A allows its assignment as a 6-O-substituted β-Galf unit, while the C-5 chemical shift of units B and C are 6-O-substituted β-Galf units. The NOESY cross-peak observed between the anomeric proton of unit C with H-6 of unit A, between anomeric proton of unit A with H-6 of unit B, and between anomeric proton of unit B with H-6 of unit C indicating that unit C is (1→6)-linked to unit A, which is connected via a (1→5) linkage to unit B, which is in turn (1→5)-bonded to unit C. The full assigned 1H- and 13C-NMR data and the above analysis were identical to those described for a galactan isolated from the cell-wall of Neosartorya fungus [21]. These data indicated the more abundant polysaccharide of our purified EP fraction produced by Phomopsis sp. FJBR-11, a galactan structure with the repeating unit: [→6)-β-d-Galf-(1→5)-β-d-Galf-(1→5)-β-d-Galf-(1→]n. Moreover, the 1H- and 13C-NMR spectra (Figure 3, and Supplementary Materials) were identical with those of an impure phytotoxic exopolysaccharide isolated from the culture filtrate of Phomopsis foeniculi, as reported in the literature [22], whose chemical structure was also characterized with the same repeating unit described above. The purified EP fraction showed a dose-dependent inhibition (Figure 4) against TMV with an IC50 value of 1.08 μg/mL, as determined by the local lesion method, but no inhibitory effect was observed when tested using the leaf-disc method at a concentration of 500 μg/mL.
Table 2.
1H- and 13C-NMR chemical shifts for the impure galactan from Phomopsis sp. FJBR-11.
| Position | Unit A | Unit B | Unit C | |||
|---|---|---|---|---|---|---|
| δC (ppm) | δH (ppm) | δC (ppm) | δH (ppm) | δC (ppm) | δH (ppm) | |
| 1 | 107.8 | 5.25 | 107.9 | 5.22 | 108.6 | 5.05 |
| 2 | 82.2 | 4.17 | 82.0 | 4.18 | 81.8 | 4.15 |
| 3 | 77.5 | 4.09 | 77.5 | 4.13 | 77.2 | 4.13 |
| 4 | 83.8 | 4.09 | 82.3 | 4.15 | 82.6 | 4.13 |
| 5 | 70.4 | 4.02–3.95 | 76.2 | 4.02–3.95 | 76.8 | 4.02–3.95 |
| 6 | 70.0 | 3.93–3.89, 3.69–3.64 | 61.9 | 3.85–3.80 | 61.8 | 3.85–3.80 |
Figure 3.
1H- (a) and 13C- (b) NMR spectra of impure galactan in D2O.
Figure 4.
Dose-dependent inhibitory effect of the purified exocellular polysaccharide from the fermentation product of Phomopsis sp. FJBR-11 against TMV.
Polysaccharides have many kinds of biological activities, such as anti-oxidation, hypoglycemic, enhancing immunity, anti-aging, anti-rheumatism, anti-cancer, and anti-viral properties. etc., which indicating a wide application prospect for their use in the field of medicine and pesticides [23,24]. The production of polysaccharides inducing phytotoxic effects was reported for several fungal pathogens, among which mycolaminarans from the cytoplasma of Phytophthora palmivora, P. cinnamomi, and P. megasperma var. sojae [25], and EP from a culture filtrate of P. cinnamomi, P. cryptogea, and P. nicotianae [26] could induce severe wilting on several hosts. More recently, the crude polysaccharide fraction produced by P. foeniculi and its components, galactan and manan, were reported showing phytotoxic effects, i.e., chlorosis, necrosis, and/or wilting, on fennel and on two non-host plants, tobacco and tomato. The macromolecules commonly appear to act by interfering with water movement in plant tissues due to mechanical plugging of the vessels which leads to wilt symptoms, and the phenomenon appears to be related to the size of the molecules and their viscosity rather than to their structure [21]. The exopolysaccharide produced by Phomopsis sp. FJBR-11 was found possessing a high inhibitory activity against TMV infection in our present study. A further investigation on the relationship between the antiviral effect and the size and viscosity of the polysaccharide will help us understand its role and behavior.
3. Materials and Methods
3.1. General Experimental Procedures
1H- and 13C-NMR spectra were obtained with a Bruker AVANCE III 500 spectrometer (Bruker BioSpin, Rheinstetten, Germany) and a JEOL JNM-ECA400 spectrometer (JEOL Ltd., Tokyo, Japan) using tetramethylsilane as an internal standard. Sephadex LH-20 (25–100 μm, Pharmacia Fine Chemical Co., Ltd., Uppsala, Sweden), Lichroprep RP-18 gel (40–63 μm, Merck, Darmstade, Germany), silica gel (200–300 mesh) and silica gel H (Qingdao Oceanic Chemical Co., Qingdao, China) were used for column chromatography. Thin-layer chromatography (TLC) was performed on glass-backed plates coated with 0.25 mm layers of silica gel H (Qingdao Oceanic Chemical Co., Qingdao, China). Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 5% H2SO4 in EtOH. All solvents and chemicals used were of analytical reagent grade (Sinopharm Chemical Reagent Co., Ltd., Beijing, China), and water was doubly distilled before use.
3.2. Fungal Strain
The endophytic fungus FJBR-11 was isolated from the healthy stems of B. javanica collected from the Xiamen Overseas Chinese Subtropical Plant Introduction Garden (Xiamen, China) in 2014. The pure culture is deposited in the Key Laboratory of Bio-Pesticide and Chemistry-Biology, Ministry of Education, Fujian Agriculture and Forestry University, Fuzhou, China. This fungal colony on PDA was white and did not produce any spores. Therefore, it was identified by molecular technique based on the DNA sequence data of the internal transcribed spacer (ITS1 and ITS4) regions of the ribosomal RNA gene using universal fungal primers.
The nucleotide (nt) sequences were aligned using the MUSCLE algorithm [27] implemented in MEGA5 [28]. The degree of mutational saturation in the aligned sequences was evaluated using the Iss statistic in DAMBE 6.0 [29]. Bayesian phylogenetic analysis using MrBayes 3.2.6 [30] under the K80 + G model, which was determined by jModelTest 2.17 [31]. Four Markov Monte Carlo (MCMC) chains were run for 2,000,000 generations, sampling every 100 generations, with the final standard deviation of split frequencies <0.01 and the first 25% of sampled trees discarded as burn-in. The Bayesian 50% majority rule consensus tree was visualized in FigTree 1.4.3.
3.3. Fermentation, Extraction, and Isolation
The Phomopsis fungus FJBR-11 was grown on potato dextrose agar (PDA) at 28 °C for seven days. Three pieces (0.5 × 0.5 cm) of mycelial agar plugs were inoculated into 500 mL Erlenmeyer flasks containing 200 mL of potato dextrose broth (PDB) at 28 °C on a rotary shaker at 120 rpm for seven days to obtain the seed culture. Cultivation was carried out in a 50 L fermentor containing 30 L of liquid PDB at 28 °C for three weeks. The fermentation broth was harvested and filtrated. The filtrate was evaporated in vacuo to give a crude solution of 2 L, which was then extracted with EtOAc (500 mL × 3) to give an EtOAc soluble fraction (19.8 g). The EtOAc fraction was dissolved with 60% MeOH in H2O, and separated by column chromatography over Lichroprep RP-18 and eluted with 60% MeOH to 100% MeOH in H2O to give 18 fractions (Fractions 1–18). Fraction 5 was subjected to Sephadex LH-20 column chromatography and eluted with a mixture of CHCl3 and MeOH (v/v, 1:1) to give compound 4 (14.0 mg). Fraction 11 (118 mg) was subjected to silica column chromatography using a mixture of petroleum ether and acetone (v/v, 70:30), and further purified with Sephadex LH-20 column chromatography to give compounds 1 (9.8 mg), 2 (24.3 mg), and 3 (6.4 mg). Fraction 14 (153 mg) was subjected to Sephadex LH-20 column chromatography and eluted with a mixture of CHCl3 and MeOH (v/v, 1:1) to give two subfractions, which were then subjected to silica column chromatography using a mixture of CHCl3 and MeOH (v/v, 98:2) and CHCl3 and MeOH (v/v, 95:5), respectively, to give compounds 5 (20.0 mg) and 6 (15.0 mg).
Another 15 L fermentation broth was obtained under the same culture conditions as described above. The filtrate was evaporated in vacuo to give a crude solution of 1 L, which was then subject to column chromatography over Diaion HP-20 and eluted with 0%, 25%, 50%, 75%, and 100% MeOH in H2O (v/v). The active fraction (25% MeOH elute), was subjected to silica gel column chromatography and eluted with a gradient EtOH in H2O (100%, 95%, 90%, 80%, 70%, 60%, and 50%). The 80% EtOH elute (280 mg) and 70% EtOH elute (45 mg) were both crude polysaccharide and showed >95% inhibition against TMV as tested using local lesion method under concentration of 500 μg/mL. The 70% EtOH elute was further purified using column chromatography over a Sephadex LH-20, eluted with H2O to give a polysaccharide fraction (18 mg) for NMR testing.
3.3.1. Dothiorelone A (1)
Colorless gum; 1H-NMR (400 MHz, CD3OD) δ 6.24 (1H, d, J = 2.3 Hz, H-6), 6.18 (1H, d, J = 2.3 Hz, H-4), 4.10 (2H, q, J = 7.1 Hz, H-17), 3.68 (1H, m, H-15), 3.57 (2H, s, H-2), 2.88 (2H, t, J = 7.5 Hz, H-10), 1.61 (2H, m, H-11), 1.40 (6H, m, H-12, 13, 14), 1.23 (3H, t, J = 7.1 Hz, H-18), 1.13 (3H, d, J = 6.2 Hz, H-16). 13C-NMR (100 MHz, CD3OD) δ 209.0 (C-9), 173.6 (C-1), 161.4 (C-5), 159.8 (C-7), 137.1 (C-3), 121.2 (C-8), 111.7 (C-4), 102.7 (C-6), 68.5 (C-15), 61.9 (C-17), 45.2 (C-10), 40.5 (C-2), 40.1 (C-14), 30.6 (C-13), 26.8 (C-12), 25.5 (C-11), 23.5 (C-16), 14.5 (C-18).
3.3.2. Dothiorelone B (2)
White solid; 1H-NMR (400 MHz, CD3OD) δ 6.23 (1H, d, J = 2.1 Hz, H-6), 6.16 (1H, d, J = 2.0 Hz, H-4), 4.08 (2H, q, J = 7.1 Hz, H-17), 3.55 (2H, s, H-2), 3.41 (1H, m, H-14), 2.90 (2H, t, J = 7.5 Hz, H-10), 1.60 (2H, m, H-11), 1.31–1.49 (6H, m, H-12, 13, 15), 1.22 (3H, t, J = 7.1 Hz, H3-18), 0.90 (3H, t, J = 7.4 Hz, H3-16). 13C-NMR (100 MHz, CD3OD) δ 208.9 (C-9), 173.6 (C-1), 161.4 (C-5), 159.8 (C-7), 137.1 (C-3), 121.2 (C-8), 111.7 (C-4), 102.7 (C-6), 73.8 (C-14), 61.9 (C-17), 45.2 (C-10), 40.6 (C-2), 37.8 (C-13), 31.0 (C-12), 26.6 (C-15), 25.7 (C-11), 14.5 (C-18), 10.4 (C-16).
3.3.3. Dothiorelone C (3)
White solid; 1H-NMR (400 MHz, CD3OD) δ 6.26 (1H, d, J = 2.3 Hz, H-6), 6.19 (1H, d, J = 2.3 Hz, H-7), 4.11 (2H, q, J = 7.1 Hz, H-17), 3.58 (2H, s, H-2), 3.54 (2H, t, J = 6.6 Hz, H-16), 2.89 (2H, t, J = 7.3 Hz, H-10), 1.61 (2H, m, H-11), 1.52 (2H, m, H-15), 1.35 (6H, m, H-12, H-13, and H-14), 1.24 (3H, t, J = 7.1 Hz, H-18). 13C-NMR (100 MHz, CD3OD) δ 209.0 (C-9), 173.6 (C-1), 161.3 (C-5), 159.9 (C-7), 137.0 (C-3), 121.2 (C-8), 111.8 (C-4), 102.7 (C-6), 63.0 (C-16), 61.9 (C-17), 45.2 (C-10), 40.5 (C-2), 33.6 (C-15), 30.4 (C-12, 14), 26.8 (C-13), 25.5 (C-11), 14.5 (C-18).
3.3.4. Dothiorelone H (4)
Pale yellow oil; 1H-NMR (500 MHz, CD3OD) δ 6.23 (1H, d, J = 2.2 Hz, H-6), 6.14 (1H, d, J = 2.1 Hz, H-4), 5.61 (1H, dd, J = 8.7, 4.9 Hz, H-9), 3.79 (1H, d, J = 19.6 Hz, H-2a), 3.70 (1H, m, H-15), 3.48 (1H, d, J = 19.6 Hz, H-2b), 1.91–1.75 (2H, m, H-10), 1.63–1.28 (8H, m, H-11, H-12, H-13, and H-14), 1.14 (3H, d, J = 6.2 Hz, H3-16). 13C-NMR (125 MHz, CD3OD) δ 174.1 (C-1), 159.5 (C-5), 155.2 (C-7), 132.8 (C-3), 113.9 (C-8), 106.1(C-4), 102.0 (C-6), 80.1 (C-9), 68.5 (C-15), 40.1 (C-14), 36.7 (C-10), 35.5 (C-2), 30.3 (C-12), 26.7 (C-13), 26.6 (C-11), 23.5 (C-16).
3.3.5. Cytosporone C (5)
White powder; 1H-NMR (500 MHz, CD3OD) δ 6.23 (1H, d, J = 2.3 Hz, H-6), 6.14 (1H, d, J = 2.2 Hz, H-4), 5.61 (1H, dd, J = 8.6, 5.2 Hz, H-9), 3.79 (1H, d, J = 19.6 Hz, H-2a), 3.48 (1H, d, J = 19.6 Hz, H-2b), 1.90–1.74 (2H, m, H-10), 1.53 (1H, m, H-11a), 1.43 (1H, m, H-11b), 1.40–1.24 (8H, m, H-12, H-13, H-14, and H-15), 0.90 (3H, t, J = 6.9 Hz, H3-16). 13C-NMR (125 MHz, CD3OD) δ 174.1 (C-1), 159.5 (C-5), 155.2 (C-7), 132.8 (C-3), 113.9 (C-8), 106.0 (C-4), 102.0 (C-6), 80.1 (C-9), 36.7 (C-10), 35.5 (C-2), 32.9 (C-14), 30.3 (C-13), 30.2 (C-12), 26.6 (C-11), 23.7 (C-15), 14.4 (C-16).
3.3.6. Cytosporone U (6)
White amorphous powder; 1H-NMR (500 MHz, CD3OD) δ 6.20 (1H, d, J = 2.4 Hz, H-6), 6.19 (1H, d, J = 2.4 Hz, H-4), 3.49 (2H, s, H-2), 2.50 (2H, t, J = 8.0 Hz, H-9), 1.43 (2H, m, H-13), 1.38–1.22 (10H, overlap, H-10, H-11, H-12, H-14, and H-15), 0.88 (3H, t, J = 7.5 Hz, H-16). 13C-NMR (125 MHz, CD3OD) δ 176.1 (C-1), 157.4 (C-5), 156.5 (C-7), 135.7 (C-3), 121.1 (C-8), 109.7 (C-4), 102.5 (C-6), 39.7 (C-2), 33.1 (C-14), 31.0 (C-13), 30.9 (C-10), 30.6 (C-12), 30.5 (C-11), 26.9 (C-9), 23.7 (C-15), 14.4 (C-16).
3.4. Determination of the Molecular Weight of the Polysaccharide
The molecular weight of the polysaccharide was determined by gel permeation chromatography (GPC) with a Waters 515 GPC system (Milford, MA, USA) equipped with light-scattering (LS) detector and differential refractive index (DRI) detector, and an OHpak SB-802.5HQ column. A 20 µL sample was injected, and the mobile phase consisting of 0.2 M NaNO3 was used at a flow rate of 0.5 mL/min. The column temperature was set as 25 °C and the operation time was 40 min. PEG was used as the standard sample and established the standard curve.
3.5. Anti-TMV Assay
3.5.1. Virus and Host Plant
Purified TMV (strain U1) was obtained from the Institute of Plant Virology, Fujian Agriculture and Forestry University, Fuzhou, China, and the concentration was determined as 15 mg/mL using the ultraviolet spectrophotometer method . The purified virus was kept at −20 °C and was diluted to 30 μg/mL with 0.01 M PBS before use. Nicotiana glutinosa and Nicotiana tabacum cv. K326, which were cultivated and grown to the 5–6 leaf stage in an insect-free greenhouse, were used for anti-TMV assay as the local lesion and systemic TMV infection hosts, respectively. Purified compounds were dissolved in DMSO and diluted with 0.01 M PBS to a certain concentration for testing. The final concentration of DMSO in the test solution (≤2%) was proved to show no adverse effect on the plants.
3.5.2. Local-Lesion Assay
A mixture of test solution with TMV (final concentration of 10 μg/mL) was made 30 min before inoculation. The mixture was inoculated on the left side of the leaves of N. glutinosa, while the right side of the same leaves were inoculated with a solution of the negative control mixed with an equal concentration of TMV. All assays were conducted in triplicate. The numbers of the local lesion were recorded 3–4 d after inoculation, and inhibition rates were calculated according to the following formula: Inhibition rate (%) = ((C − T)/C]) × 100%, where C is the average number of local lesions of the control, while T is the average number of local lesions of the treatment.
3.5.3. Leaf-Disc Method
Growing leaves of N. tabacum cv. K326 were mechanically inoculated with TMV (30 μg/mL in 0.01 M PBS). After 6 h, leaf discs (1 cm diameter) were punched and floated on solutions for testing. Discs of healthy and inoculated leaves floated on a solution of 0.01 M PBS with 2% DMSO were used as mock and negative controls, respectively. Three replicates were carried out for each sample. After incubating for 48 h at 25 °C in a culture chamber, the leaf discs were ground in 0.01 M carbonate coating buffer (pH 9.6) and OD405 values were measured using the TAS-ELISA method. TAS-ELISA was performed as described in the literature [32,33]. Virus concentration was calculated from a standard curve constructed using OD405 values of purified TMV at concentrations of 1.0, 0.5, 0.25, 0.125, and 0.0625 μg/mL. The inhibition of test solutions on TMV was calculated as follows: Inhibition rate = (1 − (virus concentration of treatment)/(virus concentration of negative control)) × 100%.
4. Conclusions
Six compounds, including dothiorelones A–C and H, and cytosporones C and U, were identified from the metabolites of an endophytic Phomopsis sp. FJBR-11 isolated from the stem of B. javanica. All the compounds, except cytosporone U, showed only weak or no inhibitory activity against TMV under test using the local lesion and leaf-disc methods. However, the crude and a purified polysaccharide produced by Phomopsis sp. FJBR-11 showed strong inhibitory effects against the virus infection as determined by using the local lesion assay.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 31371987 and 31501687).
Supplementary Materials
Supplementary materials are available online. 1H- and 13C-NMR spectra of compounds 1–6; 1H- and 13C-NMR spectra, as well as HSQC, TOCSY, and NOESY spectra of the purified polysaccharide.
Author Contributions
Qing-Wei Tan and Qi-Jian Chen conceived and designed the experiments. Jian-Cheng Ni and Pei-Hua Fang performed the experiments and test. Fangluan Gao was responsible for the fungal strain identification. Qing-Wei Tan analyzed the data and composed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–6 and the exopolysaccharide are available from the authors.
References
- 1.Chen L., Zhang L., Li D., Wang F., Yu D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2013;110:E1963–E1971. doi: 10.1073/pnas.1221347110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y., Mao X.Y., Huang L.J., Fan Y.M., Gu W., Yan C., Huang T., Zhang J.X., Yuan C.M., Hao X.J. Diterpene alkaloids and diterpenes from Spiraea japonica and their anti-Tobacco mosaic virus activity. Fitoterapia. 2016;109:8–13. doi: 10.1016/j.fitote.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Su B., Chen F., Wang L., Wang Q. Design, synthesis, antiviral activity, and structure-activity relationships (SARs) of two types of structurally novel phenanthroindo/quinolizidine analogues. J. Agric. Food Chem. 2014;62:1233–1239. doi: 10.1021/jf405562r. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Li P., Li Z., Yin J., He M., Xue W., Chen Z., Song B. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013;61:9575–9582. doi: 10.1021/jf403193q. [DOI] [PubMed] [Google Scholar]
- 5.Tan R.X., Zou W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 6.Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 7.Gunatilaka A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity and implications of their occurrence. J. Nat. Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Q.W., Gao F.L., Wang F.R., Chen Q.J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus Tubingensis FJBJ11. Int. J. Mol. Sci. 2015;16:5750–5761. doi: 10.3390/ijms16035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu L.M., Costa S.S., Pfenning L.H., Takahashi J.A., Larsen T.O., Andersen B. Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol. 2012;116:249–260. doi: 10.1016/j.funbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Wagenaar M.M., Clardy J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 2001;64:1006–1009. doi: 10.1021/np010020u. [DOI] [PubMed] [Google Scholar]
- 11.Weber D., Sterner O., Anke T., Gorzalczancy S., Martino V., Acevedo C. Phomol, a new anti-inflammatory metabolite from an endophyte of the medicinal plant Erythrina crista-galli. J. Antibiot. 2004;57:559–563. doi: 10.7164/antibiotics.57.559. [DOI] [PubMed] [Google Scholar]
- 12.Dai J., Krohn K., Flörke U., Gehle D., Aust H., Draeger S., Schulz B., Rheinheimer J. Novel highly substituted biraryl ethers, phomosines D–G, isolated from the endophytic fungus Phomopsis sp. from Adenocarpus foliolosus. Eur. J. Org. Chem. 2005;23:5100–5105. [Google Scholar]
- 13.Dai J., Krohn K., Gehle D., Kock I., Flörke U., Aust H., Draeger S., Schulz B., Rheinheimer J. New oblongolides isolated from the endophytic fungus Phomopsis sp. from Melilotus dentate from the shores of the Baltic Sea. Eur. J. Org. Chem. 2005;18:4009–4016. [Google Scholar]
- 14.Pornpakakul S., Roengsumran S., Deechangvipart S., Petsom A., Muangsin N., Ngamrojnavanich N., Sriubolmas N., Chaichit N., Ohta T. Diaporthichalasin, a novel CYP3A4 inhibitor from an endophytic Diaporthe sp. Tetrahedron Lett. 2007;48:651–655. [Google Scholar]
- 15.Sommart U., Rukachaisirikul V., Sukpondma Y., Phongpaichit S., Towatana N.H., Graidist P., Hajiwangoh Z., Sakayaroj J. A cyclohexenone derivative from diaporthaceous fungus PSU-H2. Arch. Pharm. Res. 2009;32:1227–1231. doi: 10.1007/s12272-009-1907-5. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S., Akita Y., Nakamura T., Koshino H. Total synthesis of curvulone B and a proposed structure for dothiorelone B; determination of the configuration of curvulone B and structural revision of phomopsin A. Tetrahedron Asymmetry. 2012;23:952–958. [Google Scholar]
- 17.Du X.P., Su W.J. Two new polyketides from mangrove endophytic fungus Dothiorella sp. Chem. Nat. Compd. 2014;50:214–216. [Google Scholar]
- 18.Brady S.F., Wagenaar M.M., Singh M.P., Janso J.E., Clardy J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000;2:4043–4046. doi: 10.1021/ol006680s. [DOI] [PubMed] [Google Scholar]
- 19.Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R., Müller W.E.G., Bayer M., Lin W., Wu J., et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009;17:7362–7367. doi: 10.1016/j.bmc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q., Guo Y., Guo Z.K., Chu Y.L., Wang T., Tan R.X. Two new cytosporones from the culture of endophytic Phomopsis sp. Chem. Nat. Compd. 2013;48:938–941. [Google Scholar]
- 21.Leal J.A., Jiménez-Barbero J., Gómez-Miranda B., Parra E., Prieto A., Bernabé M. Structural investigation of cell-wall polysaccharides from Neosartorya: Relationships with their putative anamorphs of Aspergillus. Carbohydr. Res. 1995;273:255–262. doi: 10.1016/0008-6215(95)00085-8. [DOI] [PubMed] [Google Scholar]
- 22.Corsaro M.M., De Castro C., Evidente A., Lanzetta R., Molinaro A., Mugnai L., Parrilli M., Surico G. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydr. Res. 1998;308:349–357. doi: 10.1016/s0008-6215(98)00085-8. [DOI] [PubMed] [Google Scholar]
- 23.Jhamandas J.H., Wie M.B., Harris K., MacTavish D., Kar S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005;21:2649–2659. doi: 10.1111/j.1460-9568.2005.04111.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L., Hao X., Wu Y. Inhibitory effect of polysaccharide peptide (PSP) against Tobacco mosaic virus (TMV) Int. J. Biol. Macromol. 2015;75:474–478. doi: 10.1016/j.ijbiomac.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Keen N.T., Wang M.C., Bartnicki-Garcia S., Zentmyer G.A. Phytotoxicity of mycolaminarans—β-1,3-glucans from Phytophthora spp. Physiol. Plant Pathol. 1975;7:91–97. [Google Scholar]
- 26.Woodward J.R., Keane P.J., Stone B.A. Structures and properties of wilt-inducing polysaccharides from Phytophthora species. Physiol. Plant Pathol. 1980;16:439–454. [Google Scholar]
- 27.Edgar R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017;108:431–437. doi: 10.1093/jhered/esx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darriba D., Taboada G.L., Doallo R., Posada D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Method. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Yan X.H., Dong J.H., Sang P., Fang X., Di Y.T., Zhang Z.K., Hao X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009;57:6590–6595. doi: 10.1021/jf901632j. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y.S., Fan H.J., Li Y., Shi Z.L., Pan Y., Lu C.P. Development of a multi-mimotope peptide as a vaccine immunogen for infectious bursal disease virus. Vaccine. 2007;25:4447–4455. doi: 10.1016/j.vaccine.2007.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.