Abstract
Background
The prognostic value of neutrophil–lymphocyte ratio (NLR) in patients with castration-resistant prostate cancer (CRPC) had been investigated in previous studies; however, the results remain inconsistent. This study was aimed to investigate the prognostic value of NLR in CRPC patients.
Materials and methods
Literature was identified from PubMed, Embase, Web of Science, and Cochrane, which investigated the relationship between pretreatment NLR and prognosis in CRPC patients. HRs for overall survival (OS) and progression-free survival (PFS) were extracted from eligible studies. Heterogeneity was assessed using the I2 value. The fixed-effects model was used if there was no evidence of heterogeneity; otherwise, the random-effects model was used. Publication bias was evaluated using Begg’s funnel plot test.
Results
A total of 5,705 patients from 16 studies were included in this analysis. The pooled results showed that an elevated NLR predict poor OS (pooled HR = 1.52, 95% CI: 1.41–1.63, P<0.001) and PFS (pooled HR = 1.50, 95% CI: 1.21–1.85, P<0.001) in patients with CRPC. Subgroup analysis revealed that an elevated NLR significantly predicted poor OS in Asian studies group (HR = 2.43, 95% CI: 1.47–4.01, P=0.001). The elevated NLR also significantly predicted poor PFS in Asian studies group (HR = 1.99, 95% CI: 1.30–3.06, P=0.002).
Conclusion
This study suggests that an elevated NLR predict poor prognosis in patients with CRPC.
Keywords: neutrophil-lymphocyte ratio, castration-resistant prostate cancer, prognosis, meta-analysis
Introduction
Prostate cancer is one of the most common malignancies in Western males, which accounts for 9% of death in males, and the second leading cause of male cancer-related death.1 The standard treatment for advanced or metastatic prostate cancer is androgen deprivation therapy (ADT).2 Despite the high response rate of ADT, most prostate cancer patients progressed gradually and irreversibly to castration-resistant prostate cancer (CRPC).3 Docetaxel, the first-line use of chemotherapeutic agents to treat patients with CRPC, has been shown to confer a survival benefit in patients with CRPC.4,5 Several other agents, including cabazitaxel (CBZ),6 enzalutamide (ENZ),7,8 abiraterone acetate (AA),9,10 radium-223,11 and sipuleucel-T,12 also have been shown to confer a survival benefit in patients with metastatic CRPC (mCRPC). Although some prognostic factors and biomarkers have been reported, a more proper predictive and prognostic biomarker is required to predict the response of patients with CRPC-received chemotherapy accurately.
Cancer-related inflammatory response plays an important role in the progression of cancer development.13,14 Neutrophil-to-lymphocyte ratio (NLR), a marker of the systemic inflammatory response that can be easily measured from routine complete blood counts (CBCs) in the peripheral blood, has been reported to be an independent prognostic factor in cancers.15–18 NLR has shown to predict poor survival of patient with localized prostate cancer and CRPC.19–24 However, the results of these studies for the prognostic value of NLR are inconsistent. We conducted a comprehensive meta-analysis to drive a more precise estimate of the prognostic value of the NLR in patients with CRPC.
Materials and methods
Literature search
We comprehensively searched PubMed, Embase, Web of Science, and the Cochrane electronic databases for studies published before November 17, 2017. The search strategy combined key terms related to “castration resistant prostate cancer” or “hormone-refractory prostate cancer” or “androgen independent prostate cancer” or “metastatic prostate cancer” and “Neutrophil to Lymphocyte Ratio” or “NLR” and “prognosis” or “survival” or “outcome” in humans. Two reviewers (ZW and SP) independently screened the titles and abstracts of all initially identified studies according to the selection criteria. Full-text articles of studies that met the following selection criteria were retrieved.
Inclusion and exclusion criteria
Inclusion criteria for publication selection were as follows: 1) retrospective studies on the value of NLR in predicting prognosis in castration-resistant prostate cancer patients; 2) the HRs and their 95% CIs for overall survival (OS) or progression-free survival (PFS) analysis were reported in text or could be computed from given data; 3) the value of NLR was obtained for blood sample testing; 4) defined the cutoff value of increased NLR. When multiple reports describing the same population were published, the most recent or complete report was used.
The major exclusion criteria were as follows: abstract, review, case report or comment letter; laboratory studies; animal studies; duplicate publications; published not in English.
Data extraction and quality
Two investigators (ZW and SP) independently extracted data, and a consensus was reached in case of any inconsistency with the involvement of a third author (HX). The following data were extracted from the eligible studies: first author, year of publication, country of origin, median age (range), treatment, median follow-up time, number of elevated NLR, cutoff value, and HR for survival (OS and/or PFS). For articles that only provided survival data in a Kaplan–Meier curve, software designed by Jayne F Tierney and Matthew R Sydes was used to digitize and extract the relative risk and its 95% CI.25
Statistical analysis
Data were analyzed using Stata SE12.0 (StataCorp LP, College Station, TX, USA). HR with a 95% CI was selected as the effect to measure prognostic outcomes. Study heterogeneity was evaluated using the chi-squared test and I2 statistic (100%×[(Q–df)/Q]),26,27 the value of Pheterogeneity<0.1 and I2>50% represents significant heterogeneity. The fixed-effects model was used when the value of Pheterogeneity >0.1 and I2<50%, otherwise the random-effects model was applied. Subgroup analysis was performed for OS and PFS analysis. Begg’s funnel plot and Egger’s linear regression test were used to evaluate the potential for publication bias. Two-tailed P-value <0.05 was considered statistically significant.
Results
Features of included studies
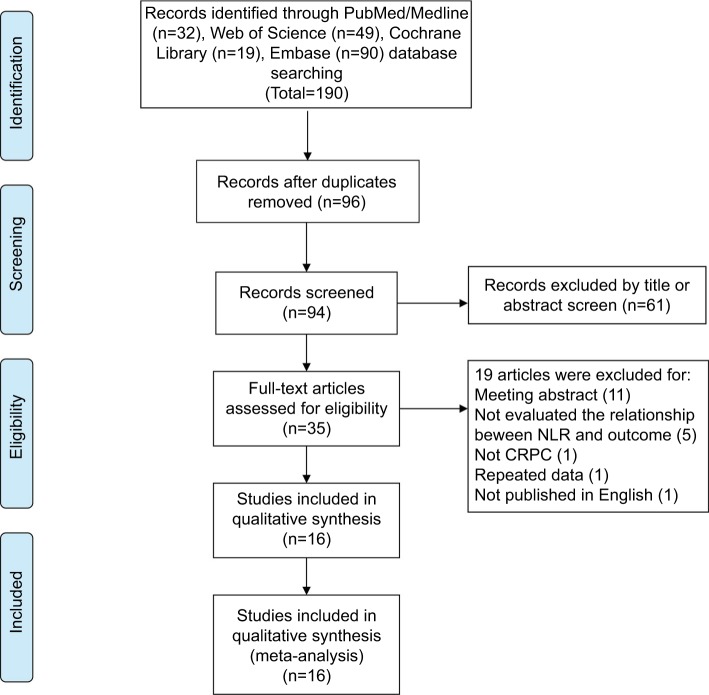
The selection process for this study is shown in Figure 1. Through systematic literature searching, a total of 190 potentially relevant studies were identified. Overall, 94 duplicated articles were removed. 61 articles were excluded after screening titles and abstracts, including reviews, letters, meeting abstracts, laboratory studies, and other articles irrelevant to our study. After assessing the full text, 19 additional articles were excluded. Finally, 16 retrospective studies were included in the following meta-analysis.
Figure 1.
Flow diagram of the study selection process.
Abbreviation: CRPC, castration-resistant prostate cancer.
Summary characteristics of these eligible studies are shown in Table 1. The 16 selected studies (17 cohorts) published between 2013 and 2017 were included in the meta-analysis.21–24,28–39 The sample size ranged from 33 to 1,224 patients, and a total of 5,705 patients were included. All trials were conducted in adult patients with CRPC. Three studies were conducted in Asian countries (Japan24,30 and China35). Thirteen studies were conducted in non-Asian countries, including Italy,34,36,39 UK,19,23 Australia,29,31 Germany,21,22 USA,37 Turkey,33 Canada,38 and the Netherlands.32 The NLR cutoff value ranged from 2.1 to 5. For the prognostic indicator of NLR in CRPC patients, six articles reported both OS and PFS, nine (10 cohorts) articles reported OS, and one article reported PFS.
Table 1.
Main characteristics of the included studies
| Authors | Year | Duration | Country | No. of patients | Median age (range) (years) | Treatment | Median follow-up (months) | NLR(+) No. (%) | NLR cutoff value | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Linton et al29 | 2013 | Australia | 182 | NA | Docetaxel | NA | 104 (56.5%) | ≥5 | OS | |
| Nuhn et al21 | 2014 | 1998-2010 | Germany | 238 | 68.3 (44.6-84.5) | Docetaxel | 15.0 (1.5-90.2) | 168 (70%) | >3 | OS |
| Sonpavde et al37 | 2014 | 2008-2010 | USA | 848 | 68 (NA) | Docetaxel | NA | NA | >3.4 | OS |
| Sümbül et al33 | 2014 | 2009-2013 | Turkey | 33 | 71.24* (NA) | Docetaxel | NA | 18(54.6%) | >3 | PFS |
| Templeton et al38 | 2014 | 2001-2011 | Canada | 357 | 71 (44-90) | Docetaxel | NA | 260 (73%) | >3 | OS |
| Lorente et al19 | 2015 | NA | UK | 755 | 67 (62-73) | Docetaxel or mitoxantrone | NA | NA | >3 | OS |
| McLachlan et al31 | 2015 | 2005-2012 | Australia | 42 | (50-84) | Docetaxel or Cabazitaxel | NA | 14(33.3%) | ≥5 | OS. PFS |
| van Soest et al32 | 2015 | NA | The Netherlands | 1,224 | 68 (40-88) | VENICE (Docetaxel) | NA | NA | ≥2 | OS |
| 1,006 | 68 (36-92) | TAX327 (D3+ Docetaxel) | NA | NA | ≥2.1 | OS | ||||
| Yao et al30 | 2015 | 2009-2014 | Japan | 57 | 74.0 (55-91) | Docetaxel | 19.0(1-61) | 27 (47.4%) | ≥3.5 | OS. PFS |
| Conteduca et al34 | 2016 | 2012-2014 | Italy | 193 | 73.1 (42.8-90.7) | Enzalutamide | 10.4 (NA) | 105 (54.4%) | >3 | OS. PFS |
| Lolli et al39 | 2016 | 2011-2015 | Italy | 230 | 74 (45-90) | Abiraterone | 29 (1-55) | 104 (45.2%) | ≥3 | OS |
| Boegemann et al22 | 2017 | NA | Germany | 96 | 70 (NA) | Abiraterone | 22 (NA) | 17(17.7%) | >5 | OS. PFS |
| Buttigliero et al36 | 2017 | 2004-2016 | Italy | 110 | 68 (48-85) | Docetaxel | 31.7 (NA) | 64 (58%) | >3 | OS. PFS |
| Mehra et al23 | 2017 | NA | UK | 75 | NA | Prednisolone or dexamethasone | NA | NA | ≥2.6 | OS |
| Pei et al35 | 2017 | 2009-2016 | China | 111 | 71(43-86) | Docetaxel | 16 (1-50) | 42 (37.8%) | >3.3 | OS. PFS |
| Uemura et al24 | 2017 | 2014-2016 | Japan | 47 | NA | Cabazitaxel | NA | 20 (42.6%) | ≥3.83 | OS |
Note:
mean age.
Abbreviations: NA, not available; NLR, neutrophil–lymphocyte ratio; OS, overall survival; PFS, progression-free survival.
Survival outcome
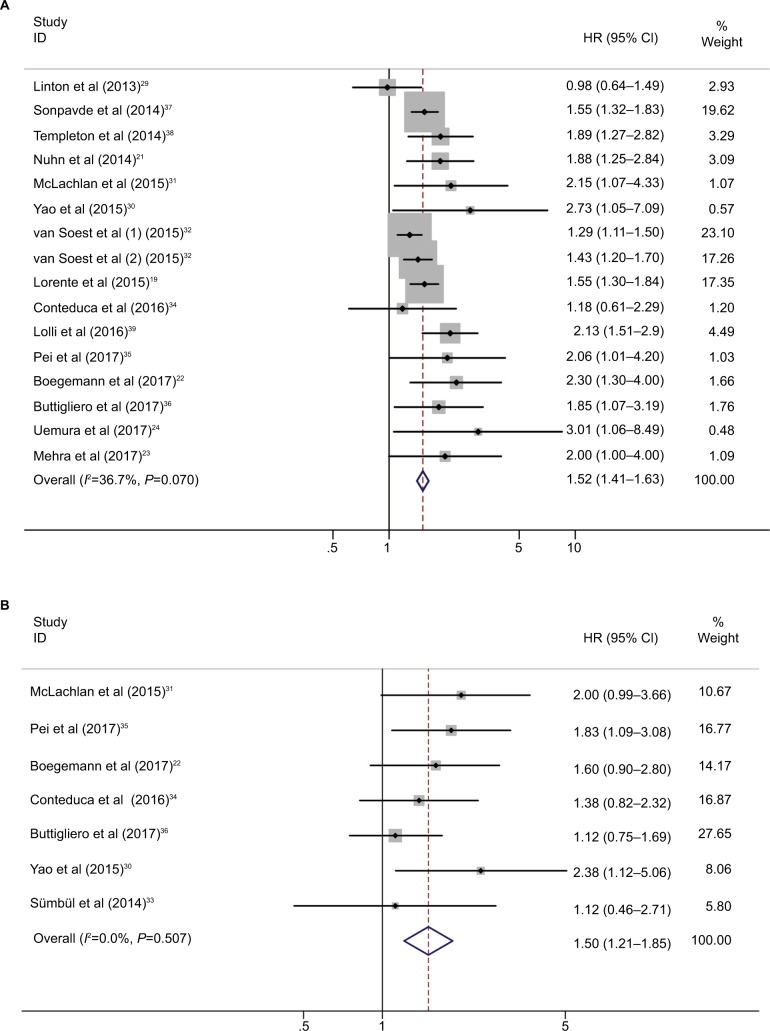
The relationship between cancer prognosis and NLR was detected in the included studies. OS and PFS were quantitatively synthesized. OS values were available from 16 cohorts with 5,571 patients with CRPC. The elevated NLR was significantly associated with poor OS (HR=1.52, 95% CI: 1.41–1.63, P<0.001; I2=37.7%, Pheterogeneity=0.07; Table 2, Figure 2), which meant that patients with a higher NLR had a greater mortality risk than those with a low NLR. Seven studies with 642 patients evaluated PFS outcome. The pooled results favored the patients with low NLR (HR=1.50, 95% CI: 1.21–1.85, P<0.001; I2=0.0%, Pheterogeneity =0.507).
Table 2.
NLR pooled HRs and 95% CIs in meta-analysis for OS and PFS
| Stratified analysis | OS
|
Begg’s test P-value | PFS
|
Begg’s test P-value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Chi-squared | Pheterogeneity | I2(%) | Pooled HR (95% CI)
|
No. of studies | Chi-squared | Pheterogeneity | I2 (%) | HR (95% CI)
|
|||||||||
| Fixed effect | P-value | Random effect | P-value | Fixed effect | P-value | Random effect | P-value | |||||||||||
| Overall | ||||||||||||||||||
| 16 | 23.7 | 0.07 | 37.7 | 1.52 (1.41–1.63) | <0.001 | 1.59 (1.42–1.77) | <0.001 | 0.096 | 7 | 5.29 | 0.507 | 0 | 1.50 (1.21–1.85) | <0.001 | 1.50 (1.21–1.85) | <0.001 | 0.23 | |
| Nation | ||||||||||||||||||
| Asian | 3 | 0.43 | 0.807 | 0 | 2.43 (1.47–4.01) | 0.001 | 2.43 (1.47–4.01) | 0.001 | 2 | 0.31 | 0.577 | 0 | 1.99 (1.30–3.06) | 0.002 | 1.99 (1.30–3.06) | 0.002 | ||
| Non- Asian | 13 | 19.82 | 0.071 | 39.5 | 1.50 (1.40–1.62) | <0.001 | 1.56 (1.39–1.74) | <0.001 | 5 | 2.72 | 0.606 | 0 | 1.36 (1.07–1.74) | 0.014 | 1.36 (1.07–1.74) | 0.014 | ||
| Treatment | ||||||||||||||||||
| First selected | 13 | 16.97 | 0.151 | 29.3 | 1.49 (1.38–1.60) | <0.001 | 1.53 (1.38–1.70) | <0.001 | 5 | 5.14 | 0.273 | 22.2 | 1.51 (1.17–1.95) | 0.002 | 1.55 (1.14–2.10) | 0.005 | ||
| Second selected | 3 | 2.8 | 0.247 | 28.5 | 1.97 (1.51–2.57) | <0.001 | 1.92 (1.37–2.69) | <0.001 | 2 | 0.14 | 0.706 | 0 | 1.48 (1.01–2.17) | 0.046 | 1.48 (1.01–2.17) | 0.046 | ||
| No. of patients | ||||||||||||||||||
| <182 | 7 | 1.04 | 0.984 | 0 | 2.16 (1.66–2.80) | <0.001 | 2.16 (1.66–2.80) | <0.001 | 6 | 5.18 | 0.394 | 3.4 | 1.52 (1.20–1.93) | <0.001 | 1.53 (1.20–1.94) | 0.001 | ||
| ≥182 | 9 | 15.15 | 0.056 | 47.2 | 1.47 (1/37–1.59) | <0.001 | 1.50 (1.34–1.69) | <0.001 | 1 | – | – | – | 1.38 (0.82–2.32) | 0.225 | 1.38 (0.82–2.32) | 0.225 | ||
| Cutoff | ||||||||||||||||||
| >3.3 | 7 | 10.68 | 0.099 | 43.8 | 1.58 (1.38–1.82) | <0.001 | 1.72 (1.32–2.25) | <0.001 | 4 | 0.73 | 0.867 | 0 | 1.87 (1.38–2.54) | <0.001 | 1.87 (1.38–2.54) | <0.001 | ||
| ≤3.3 | 9 | 12.52 | 0.13 | 36.1 | 1.49 (1.37–1.63) | <0.001 | 1.56 (1.38–1.76) | <0.001 | 3 | 0.41 | 0.814 | 0 | 1.20 (0.89–1.62) | 0.233 | 1.20 (0.89–1.62) | 0.233 | ||
Abbreviations: NLR, neutrophil–lymphocyte ratio; OS, overall survival; PFS, progression-free survival.
Figure 2.
Forest plot HR for the correlation between neutrophil–lymphocyte ratio and overall survival (A) and progression-free survival (B) in castration-resistant prostate cancer patients.
Subgroup analysis
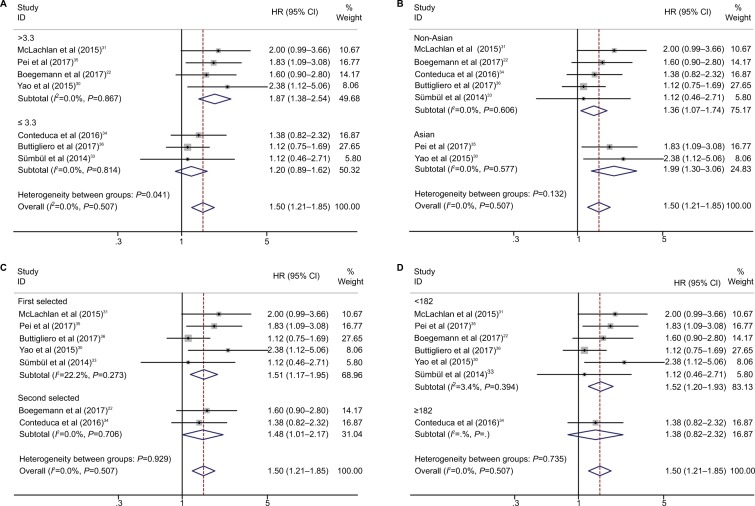
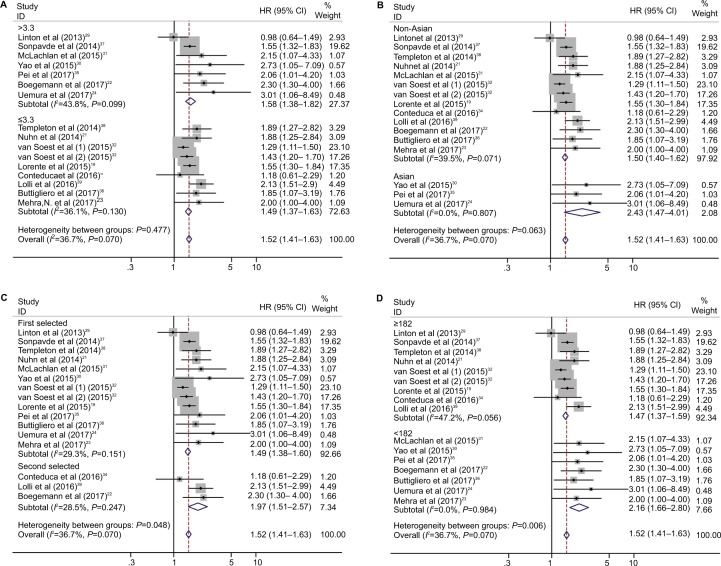
Subgroup analysis for OS and PFS was performed according to the cutoff value of NLR, nation, and treatment (Figures 3 and 4; Table 2).
Figure 3.
Subgroup analysis of pooled overall survival based on a neutrophil–lymphocyte ratio cutoff value (A), nation (B), treatment (C), and number of patients (D).
Figure 4.
Subgroup analysis of pooled progression-free survival based on neutrophil–lymphocyte ratio cutoff value (A), nation (B), treatment (C), and number of patients (D).
The pooled results showed that elevated NLR predicted worse OS in Asian studies (HR=2.43, 95% CI: 1.47–4.01, P<0.001; Table 2, Figure 3B) and second-line treatment studies (HR=1.97, 95% CI: 1.51–2.57, P<0.001; Table 2, Figure 3C). For PFS, elevated NLR predicted worse prognosis in cutoff value >3.3 studies (HR=1.87, 95% CI: 1.38–2.54, P<0.001; Table 2, Figure 4A) and Asian studies (HR=1.99, 95% CI: 1.30–3.06, P=0.002; Table 2, Figure 4B).
Publication bias
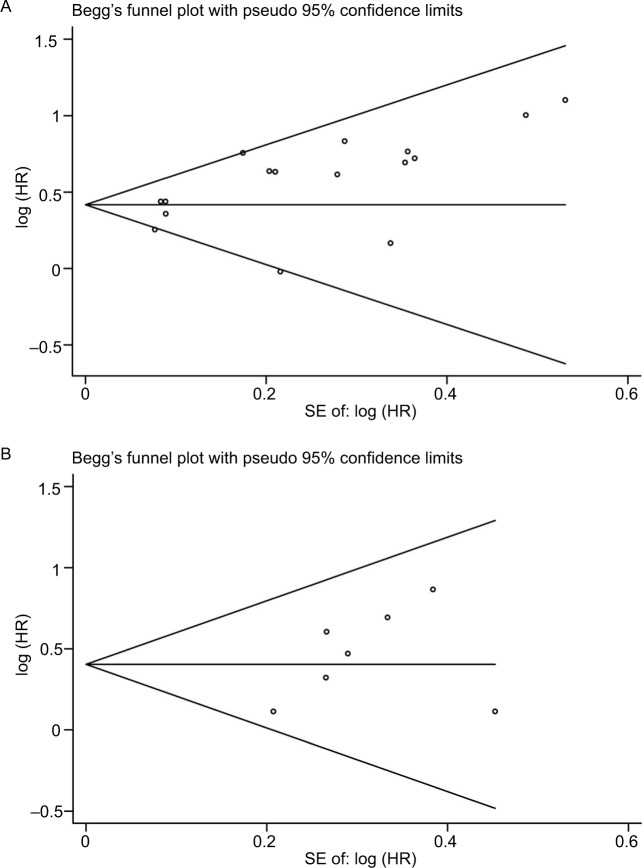
The publication bias in the meta-analysis was assessed by Begg’s funnel plots. Funnel plots for meta-analysis of elevated NLR and OS and PFS are shown in Figure 5. The Begg’s funnel plot test (OS: P=0.096, PFS: P=0.230; Figure 5) verified that there was no obvious publication bias.
Figure 5.
Funnel plots based on overall survival (A) and progression-free survival (B) (Begg’s test).
Abbreviation: SE, standard error.
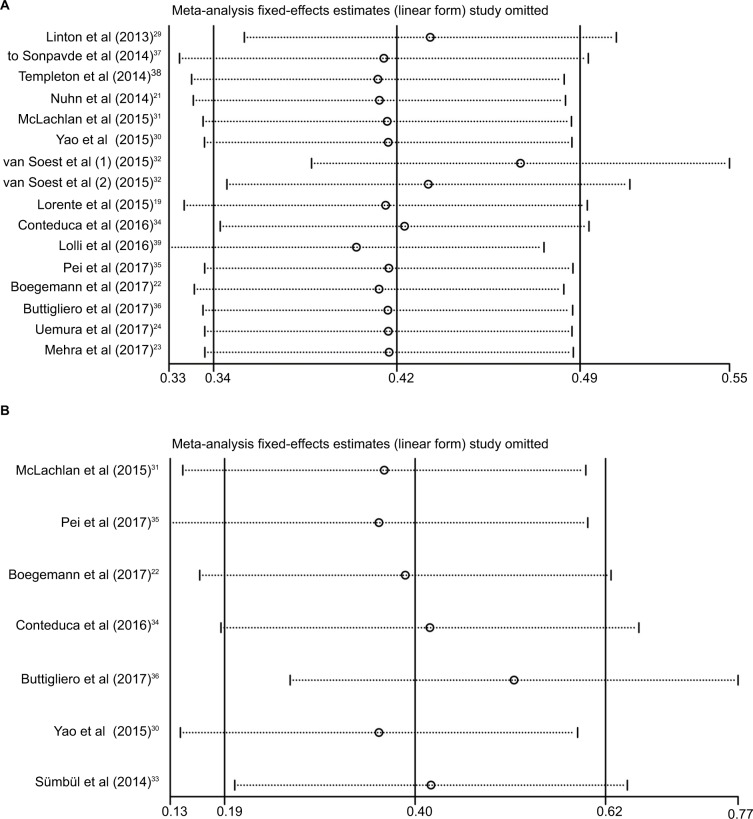
Sensitivity analysis
To evaluate the stability of the pooled results, a sensitivity analysis was performed. The result of the sensitivity analysis showed that for OS and PFS, the pooled result did not tend to exhibit alterations when an individual study was excluded (Figure 6).
Figure 6.
Sensitivity analysis for overall survival (A) and progression-free survival (B) in this meta-analysis.
Discussion
The incidence of prostate cancer has been increasing in the past few years.40 Most of the prostate cancer patients progressed to CRPC eventually.3 Several chemotherapeutic agents, including docetaxel, CBZ, ENZ, and AA, have been shown to confer a survival benefit in patients with mCRPC. A more proper predictive and prognostic biomarker is required to predict the response of patients with CRPC-received chemotherapy accurately. It is reported that inflammatory status is associated with the progression of cancer development.13,14 The marker of the systemic inflammatory response, NLR, suggests an independent prognostic factor in cancers.15–18 NLR has shown to predict poor survival in patients with CRPC. In this meta-analysis, we included 5,705 patients from 16 selected studies (17 cohorts) to evaluate the prognostic role of the NLR in CRPC patients. The pooled results indicated that an elevated NLR is an independent predictor of poor prognosis in CRPC patients.
According to the subgroup analysis, an NLR cutoff value >3.3 had a more significant prognostic value than a cutoff value <3.3 (Table 2), which indicated that a higher NLR cutoff is more specific to predict a poor prognosis in patients with CRPC. An elevated NLR in Asian group had a more significant prognostic value than that in non-Asian group, indicating that a higher NLR is more specific to predict a poor prognosis in Asian patients with CRPC. Given the limited number of the eligible studies in the meta-analysis, although the pooled results showed that an elevated NLR is associated with a poor prognosis, it will need further investigation to identify that NLR can serve as a clinical marker of prognosis in CRPC patients.
The heterogeneity was relatively small in the included studies. It may be partially explained by nation, sample size, cutoff value, and treatment. And the subgroup analysis showed that the prognostic value of NLR was not affected by the factors included in the analysis. The sensitivity analysis also indicated that the pooled results were relatively conclusive.
Both increased neutrophil-dependent systemic inflammatory response and a lower lymphocyte-mediated antitumor immune response will lead to an elevated NLR.41,42 As a predominant leukocyte subset in human peripheral blood, neutrophils play an important role in tumor progression.43 In comparison, lymphocytes are critical components of antitumor immunity.44,45 NLR is an indicator of systemic inflammatory and immune response of the host; it is also associated with the progression of cancers.15–18 NLR can be easily measured from routine CBCs in the peripheral blood, which is low cost, convenient, and reproducible. Moreover, NLR is closely related to the prognosis of CRPC. These reasons indicate that NLR could serve as a prognostic marker for patients with CRPC.
Several meta-analyses have been conducted on the prognosis of NLR for localized prostate cancer and mCRPC; however, the studies included in their meta-analysis were relatively small and the heterogeneity were high.46–48 We conducted a comprehensive meta-analysis for the prognostic value of NLR for CRPC, which included 5,705 patients from 16 selected studies (17 cohorts); the pooled results indicated that an elevated NLR is an independent predictor of poor prognosis in patients with CRPC.
Limitations
Several limitations should be acknowledged in the meta- analysis. First, the NLR cutoff value differed among the included studies, potential heterogeneity may exist. Therefore, more studies are required to identify the most suitable NLR cutoff value. Second, NLR could be affected by other diseases, such as inflammatory diseases, infection, renal diseases, and liver diseases.49,50 Third, the number of eligible studies in the meta-analysis is relatively small. Finally, studies with positive results are potentially more likely published than work with negative results, which could cause publication bias.51
Conclusion
Our analysis on the currently available clinical evidence suggests that elevated NLR predicted a poor OS and PFS in patients with CRPC. The NLR could serve as an indicator of the efficacy of the treatment of CRPC.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grants 81472682 and 81772756), and Natural Science Foundation of Tianjin (17JCZDJC35300, 15JCZDJC35400 and 15JCYBJC27200).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander TW, Ryan CJ. Targeting the androgen receptor. Urol Clin North Am. 2012;39(4):453–464. doi: 10.1016/j.ucl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Froehner M, Wirth MP. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(18):1755–1755. doi: 10.1056/NEJMc1410239. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalu-tamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 9.Berruti A, Pia A, Terzolo M. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;365(8):766–766. doi: 10.1056/NEJMc1107198. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 12.Pezaro C, Attard G. Prostate cancer in 2011: redefining the therapeutic landscape for CRPC. Nat Rev Urol. 2012;9(2):63–64. doi: 10.1038/nrurol.2011.235. [DOI] [PubMed] [Google Scholar]
- 13.Kowalewska M, Nowak R, Chechlinska M. Implications of cancer-associated systemic inflammation for biomarker studies. Biochim Biophys Acta. 2010;1806(2):163–171. doi: 10.1016/j.bbcan.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Kihara K. C-reactive protein as a biomarker for urological cancers. Nat Rev Urol. 2011;8(12):659–666. doi: 10.1038/nrurol.2011.145. [DOI] [PubMed] [Google Scholar]
- 15.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18(5):1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orditura M, Galizia G, Diana A, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open. 2016;1(2):e000038. doi: 10.1136/esmoopen-2016-000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Xu X, Liang D, Tian G, Song S, He Y. Prognostic value of blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in patients with gastric cancer. Zhonghua Zhong Liu Za Zhi. 2014;36(12):910–915. [PubMed] [Google Scholar]
- 19.Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil–lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–755. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 20.Correa RG, Krajewska M, Ware CF, Gerlic M, Reed JC. The NLR-related protein NWD1 is associated with prostate cancer and modulates androgen receptor signaling. Oncotarget. 2014;5(6):1666–1682. doi: 10.18632/oncotarget.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuhn P, Vaghasia AM, Goyal J, et al. Association of pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int. 2014;114(6b):E11–E17. doi: 10.1111/bju.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boegemann M, Schlack K, Thomes S, et al. The role of the neutrophil to lymphocyte ratio for survival outcomes in patients with metastatic castration-resistant prostate cancer treated with abiraterone. Int J Mol Sci. 2017;18(2):380. doi: 10.3390/ijms18020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra N, Sharp A, Lorente D, et al. Neutrophil to lymphocyte ratio in castration-resistant prostate cancer patients treated with daily oral corticosteroids. Clin Genitourin Cancer. 2017;15(6):678–684. doi: 10.1016/j.clgc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Uemura K, Kawahara T, Yamashita D, et al. Neutrophil-to-lymphocyte ratio predicts prognosis in castration-resistant prostate cancer patients who received cabazitaxel chemotherapy. Biomed Res Int. 2017;2017:7538647–7538655. doi: 10.1155/2017/7538647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil. 2006;87(6):875. doi: 10.1016/j.apmr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–755. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 29.Linton A, Pond G, Clarke S, Vardy J, Galsky M, Sonpavde G. Glasgow prognostic score as a prognostic factor in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Clin Geni-tourin Cancer. 2013;11(4):423–430. doi: 10.1016/j.clgc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Yao A, Sejima T, Iwamoto H, et al. High neutrophil-to-lymphocyte ratio predicts poor clinical outcome in patients with castration-resistant prostate cancer treated with docetaxel chemotherapy. Int J Urol. 2015;22(9):827–833. doi: 10.1111/iju.12839. [DOI] [PubMed] [Google Scholar]
- 31.McLachlan JM, Chan DL, Crumbaker MA, Marx GM. Neutrophil– lymphocyte ratio prior to steroids as a prognostic marker in men with metastatic castration-resistant prostate cancer. Cancer Treat Commun. 2015;4:81–85. [Google Scholar]
- 32.van Soest RJ, Templeton AJ, vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol. 2015;26(4):743–749. doi: 10.1093/annonc/mdu569. [DOI] [PubMed] [Google Scholar]
- 33.Sümbül AT, Sezer A, Abalı H, et al. Neutrophil-to-lymphocyte ratio predicts PSA response, but not outcomes in patients with castration-resistant prostate cancer treated with docetaxel. Int Urol Nephrol. 2014;46(8):1531–1535. doi: 10.1007/s11255-014-0664-7. [DOI] [PubMed] [Google Scholar]
- 34.Conteduca V, Crabb SJ, Jones RJ, et al. Persistent neutrophil to lymphocyte ratio >3 during treatment with enzalutamide and clinical outcome in patients with castration-resistant prostate cancer. PLoS One. 2016;11(7):e0158952. doi: 10.1371/journal.pone.0158952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei XQ, He DL, Tian G, et al. Prognostic factors of first-line docetaxel treatment in castration-resistant prostate cancer: roles of neutrophil-to-lymphocyte ratio in patients from Northwestern China. Int Urol Nephrol. 2017;49(4):629–635. doi: 10.1007/s11255-017-1524-z. [DOI] [PubMed] [Google Scholar]
- 36.Buttigliero C, Pisano C, Tucci M, et al. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol. 2017;56(4):555–562. doi: 10.1080/0284186X.2016.1260772. [DOI] [PubMed] [Google Scholar]
- 37.Sonpavde G, Pond GR, Armstrong AJ, et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12(5):317–324. doi: 10.1016/j.clgc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Templeton AJ, Pezaro C, Omlin A, et al. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer. 2014;120(21):3346–3352. doi: 10.1002/cncr.28890. [DOI] [PubMed] [Google Scholar]
- 39.Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol. 2016;7:376. doi: 10.3389/fphar.2016.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishioka J, Saito K, Sakura M, et al. Development of a nomogram incorporating serum C-reactive protein level to predict overall survival of patients with advanced urothelial carcinoma and its evaluation by decision curve analysis. Br J Cancer. 2012;107(7):1031–1036. doi: 10.1038/bjc.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23(3):149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M, Immunity KM. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp. 2008;56(5):311–323. doi: 10.1007/s00005-008-0033-2. [DOI] [PubMed] [Google Scholar]
- 45.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J, Zhu X, Zhao X, Li XF, Xu R. Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis in prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158770. doi: 10.1371/journal.pone.0158770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu X, Gao X, Li X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Sci Rep. 2016;6:22089. doi: 10.1038/srep22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang L, Li X, Wang B, et al. Prognostic value of neutrophil-to-lymphocyte ratio in localized and advanced prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0153981. doi: 10.1371/journal.pone.0153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32(2):297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 50.Balta S, Cakar M, Demirkol S, Arslan Z, Akhan M. Higher neutrophil to lymphocyte ratio in patients with metabolic syndrome. Clin Appl Thromb Hemost. 2013;19(5):579. doi: 10.1177/1076029612475023. [DOI] [PubMed] [Google Scholar]
- 51.Sutton AJ, Song F, Gilbody SM, Abrams KR. Modelling publication bias in meta-analysis: a review. Stat Methods Med Res. 2000;9(5):421–445. doi: 10.1177/096228020000900503. [DOI] [PubMed] [Google Scholar]