ABSTRACT
Altered pH homeostasis in cancer cells has been linked with essentially all classical hallmarks of cancer, including chemoresistance. We recently identified a conceptually novel mechanism for how dysregulated pH in hypoxic cells causes chemoresistance which is based on the aberrant cellular distribution of the endosomal pH regulator, the sodium/hydrogen exchanger 6 (NHE6).
KEYWORDS: NHE6, Solute Carrier Family 9 Member A6 (SLC9A6), chemoresistance, drug partitioning, pH-regulation, endosomes
The era of pH-based anti-cancer therapy
Resistance to drug therapies is the leading factor of cancer recurrence and a significant body of work is currently deployed investigating the underlying cellular and molecular mechanisms of resistance and finding predictive biomarkers to identify cancer patients more likely to respond to drug treatment. One important factor in drug resistance is tumor heterogeneity where during chemotherapy the majority of cancer cells are prone to die but selected tumor cell subpopulations can be predisposed to resistance through genetic alterations or adaptations to the tumor microenvironmental pressures. Gillies et al., elegantly illustrated how the combined genetic instability and pressure from the physical microenvironment such has hypoxia will eventually lead to the emergence of aggressive cancer cell subpopulations often resistant to multiple drug therapies.3 Multidrug resistance induced by hypoxia is caused by multi-factorial changes. For instance, a transcriptional program driven by hypoxia-inducible factors promotes the upregulation of various ATP-Binding Cassette (ABC) transporters, a group of cell-surface efflux pumps facilitating the efflux of intracellular cytotoxic drugs. Besides, hypoxic tumor cells adapt to survive in this poorly-oxygenated microenvironment through metabolic reprogramming that causes acidification of the extracellular space. Poor tumor perfusion and extracellular acidification are two important physiological barriers to chemotherapy that lead to exposure of tumor cell to suboptimal concentrations of anti-cancer drugs. In acidic pH, weak-base chemotherapeutic drugs such as anthracyclines, vinca alcaloïds and some tyrosine kinase inhibitors are ionized in interstitial fluid and uptake through cell membranes is decreased resulting in drug resistance. This phenomenon has been referred to as ion trapping. The use of alkalinizers (i.e NaHCO3, chloroquine) or proton pump inhibitors (PPIs) has thereby being tested to improve the response to weak-base drugs. Preclinical and clinical trials in companion animals have demonstrated that PPIs, that are widely used in the treatment of peptic diseases and gastroprotection, act as chemosensitizer suggesting that they should be included in new anti-cancer strategies.9 However, increasing data indicate that the prolonged use of PPI can lead to severe adverse effects including bone fractures and susceptibility to infections so there is a need to develop other approaches for pH-based therapy.8
Drug sequestration through ion-trapping can also occur intracellularly due to the presence of a pH gradient between the alkaline cytosol and acidic endosomal/lysosomal compartments of cancer cells that promotes the sequestration of weak-base drugs in these organelles.10 Such drug confinement can result in a markedly reduced concentration of drugs at their cellular target sites leading to diminished cytotoxic drug activity. Characterization of the molecular regulators involved in this mechanism is a major goal to further support the use of pH-based therapy as an option to outsmart pH-associated resistance and improve patient response to chemotherapy.
A new mechanism behind ion trapping in hypoxic tumor cells
Our group has a strong interest in studying the functional roles of hypoxia and pH in cancer progression and we have recently found that hypoxia promotes endosomal hyperacidification and chemoresistance through pH-dependent weak-base drug sequestration within endosomes.7 One of the key findings of that work was the observation of endosomal lumen hyperacidification in hypoxic cancer cells. Organelle pH is known to be strictly regulated by transporters and ion exchangers but pH dynamics in pathological conditions remain enigmatic.1 One important challenge in the study of cellular pH dynamics is the lack of methods to accurately monitor organelle pH in living cells without the need for expensive and cutting-edge equipment. Using a recently developed methodology to assess endosomal pH dynamics in living cells, we have observed that exposure of tumor cells to hypoxia triggers rapid acidification (within 2–3h) of the endosomal lumen that was associated with cytosolic alkalinisation of the cells.6,7 Such intracellular pH dysregulation creates a strong cytoplasm to endosome pH gradient leading to an increase in weak-base drug sequestration within acidified endosomes and chemoresistance.7 This work was the first evidence of the role of the tumor physical microenvironment in regulating proton concentrations within the endosomal compartment. Such rapid cellular response to hypoxia suggested that the mechanism involved in its regulation was not related to hypoxia-associated transcriptional activity but more likely to post-translational modifications. V-ATPase shares endosome membrane localization with the sodium/hydrogen exchanger 6 (NHE6) that displays an opposite function allowing protons to leak out from the endosomal lumen. So far, most currently published research on NHE6 has focussed on the link between dysfunctions of the exchanger and the development of various disorders of the brain where NHE6 is abundantly expressed.5 In our study, we emphasized a novel role for NHE6 in cancer drug resistance by demonstrating that the exchanger was relocalized from endosomes to the plasma membrane in hypoxia. This molecular movement was further shown to represent a key event in endosome hyperacidification and weak-base drug sequestration.7 Molecular characterization of NHE6 mobilization revealed the role of the PKC/ receptor of activated protein C kinase 1(RACK1) complex where RACK1 binds NHE6 to prevent endosome relocalization from plasma membrane. In this context, PKC inhibition, RACK1 knockdown or interference with RACK1-NHE6 binding using a competition peptide restored NHE6 localization at endosomes, endosomal pH and weak-base drug sensitivity in hypoxic cells (Fig. 1).
Figure 1.
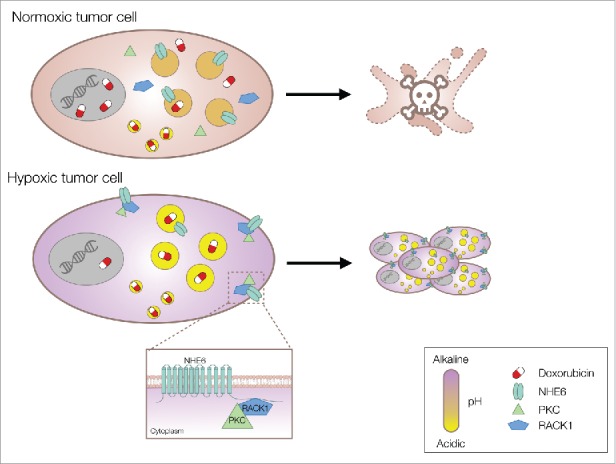
Molecular events involved in hypoxia-induced endosome acidification and chemoresistance. Normoxic cancer cells have organellar pH and a slightly acidic cytophasmic pH. In these cells, weak-base chemotherapeutics, such as doxorubicin, are not efficiently sequestracted and the drugs can efficiently reach their cellular target leading to cell death. In hypoxic cancer cells, the acidification of the endosomal compartments leads to exacerbation of the vesicular pH gradient and drug trapping. Hypoxia-induced acidification of endosomal PH is due to mislocalization of the sodium/ hydrogen exchanger 6 (NHE6) because of its enhanced binding to receptor of activated protenin C kinase 1 (RACK1) throught a PKC-dependent mechanism.
Perspectives for pH-based anti-cancer therapy
Since extracellular acidification and cytosolic alkalinization are two prominent and common drives of cancer progression, targeting pH dysregulation gained a lot of interest in the development of new therapeutic avenues. The approaches tested so far have been centered on exploring the potential therapeutic benefits of buffers, PPI and protein transporter inhibitors (PTI). The use of PTIs in vitro and in animal models of cancer showed positive results in blocking tumor growth, metastasis formation and resistance to various chemotherapeutic agents. Unfortunately, clinical trials were not as encouraging with moderate response and important adverse effects associated to drug toxicity (Granja et al., 2017). The finding of a strong intracellular pH gradient in hypoxic cells suggests that even though drug uptake can be increased by interventions aimed at disrupting the pH gradient at the plasma membrane, hypoxic cancer cells can prevent drug cytotoxicity by pH-dependent drug trapping within acidified endosomes, adding a new physiological barrier to chemotherapy.
An important question for future studies is how can we exploit this knowledge for the benefit of chemotherapy-treated patients. Since membrane transporters are highly “druggable” targets with proven success in the clinic, a search for selective inhibitors or potentiators of NHE6 activity would likely be an immediate priority. Inhibitors of the better-characterized plasma membrane NHE1 isoform could also be screened for efficacy against NHE6 using in silico, in vitro, and in vivo approaches.2 Also, the complex PKC/RACK1/NHE6 could be used as target for the development of first-in-class drugs that potentiate the efficacy of existing chemotherapeutics. A large panel of weak-base drugs potentially affected by this ion-trapping mechanism are currently used in treatment regimens for several types of metastatic cancers. Despite patient's initial response to treatment, they tend to experience treatment failure leading to cancer recurrence.
Intrinsic resistance of tumor cell subpopulations represents a profound challenge in cancer treatment and ion-trapping mechanisms have to be further studied not only to design new treatment options but also to assess the potential of molecular regulators as predictive biomarkers of tumor response to weak-base drugs.
Funding Statement
This work was supported by the Canadian Institute of Health research, (MOP-126173) and an internal (UdeS/Merck Sharp & Dohme) operating grant.
Conflict of interest
The authors declare no potential conflicts of interest.
Financial support
This work was supported by the Canadian Institutes of Health Research (CIHR) Grant MOP-126173 (to CMD) and an internal UdeS/Merck Sharp & Dohme operating grant. CMD is a member of the Fonds de la Recherche en Santé du Québec-funded Centre de Recherche Clinique du Centre Hospitalier Universitaire de Sherbrooke.
References
- 1.Casey J. R., Grinstein S., & Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. PMID:19997129. [DOI] [PubMed] [Google Scholar]
- 2.Faraone S. V, & Zhang-James Y. Can sodium/hydrogen exchange inhibitors be repositioned for treating attention deficit hyperactivity disorder? An in silico approach. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:711–7. doi: 10.1002/ajmg.b.32155. PMID:24132903. [DOI] [PubMed] [Google Scholar]
- 3.Gillies R. J., Verduzco D., & Gatenby R. A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–93. doi: 10.1038/nrc3298. PMID:22695393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granja S., Tavares-Valente D., Queiros O., & Baltazar F. Value of pH regulators in the diagnosis, prognosis and treatment of cancer. Semin Cancer Biol. 2016;43:17–34. doi: 10.1016/j.semcancer. PMID:28065864. [DOI] [PubMed] [Google Scholar]
- 5.Kondapalli K. C., Prasad H., & Rao R.. 2014. An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front Cell Neurosci. 2017;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucien F., Harper K., Pelletier P. P., Volkov L., & Dubois C. M. Simultaneous pH measurement in endocytic and cytosolic compartments in living cells using confocal microscopy. J Vis Exp. 2014;28:1–7. doi: 10.3791/51395. PMID:24798000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucien F., Pelletier P. P., Lavoie R. R., Lacroix J. M., Roy S., Parent J. L., Arsenault D., Harper K., & Dubois C. M. Hypoxia-induced mobilization of NHE6 to the plasma membrane triggers endosome hyperacidification and chemoresistance. Nat Commun. 2017;8:15884. doi: 10.1038/ncomms15884. PMID:28635961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Numico G., Fusco V., Franco P., & Roila F. 2017. Proton Pump Inhibitors in cancer patients: How useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol. 111:144–51. doi: 10.1016/j.critrevonc.2017.01.014. PMID:28259289. [DOI] [PubMed] [Google Scholar]
- 9.Taylor S., Spugnini E. P., Assaraf Y. G., Azzarito T., Rauch C., & Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat. 2015;23:69–78. doi: 10.1016/j.drup.2015.08.004. PMID:26341193. [DOI] [PubMed] [Google Scholar]
- 10.Wojtkowiak J. W., Verduzco D., Schramm K. J., & Gillies R. J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–8. doi: 10.1021/mp200292c. PMID:21981633. [DOI] [PMC free article] [PubMed] [Google Scholar]


