Abstract
Purpose
Tumor vessels supported by perivascular cells have been implicated in the failure of some anti-angiogenic agents. The relationship between perivascular cell coverage (PC) and bevacizumab efficacy in metastatic colorectal cancer (mCRC) was analyzed.
Patients and methods
A total of 284 consecutive mCRC patients who received first-line chemotherapy with or without bevacizumab from 2007–2014 in Sun Yat-Sen University Cancer Center were analyzed. Immunohistochemical double-stain for the perivascular cell marker alpha-smooth muscle actin and endothelial cell (cluster of differentiation 31) was performed to characterize the intratumoral microvascular density. Multispectral image capturing and computerized image analyses were used to quantify the microvessels supported by the perivascular cells. The patients were divided into high and low PC group according to a median cutoff value of 0.55.
Results
No significant differences in overall survival (OS) and progression-free survival (PFS) were noted between the high and low PC group. In the low PC group, the patients with bevacizumab treatment had favorable OS (P=0.03), but without PFS benefit. In the high PC group, neither OS nor PFS was significantly different between the B+C and C subgroup. Tumors with perineural invasion had high PC (P=0.03).
Conclusion
The data showed that a low PC value could be a predictor for bevacizumab benefit.
Keywords: perivascular cell coverage, bevacizumab, predictive marker, overall survival, metastatic colorectal cancer
Introduction
Targeting tumor microvascular monoclonal antibody has been a promising therapy in many advanced solid tumors,1 including metastatic colorectal cancer (mCRC).2 As a standard anti-angiogenesis drug inhibiting the vascular endothelial growth factor (VEGF), bevacizumab has been widely used in mCRC.3–6 The combination of bevacizumab plus chemotherapy has substantially improved overall survival (OS) up to 30 months.7 However, the angiogenesis pathway in mCRC is not fully understood, and evidence on selecting predictive biomarkers for treatment outcomes is insufficient.8,9 One possible reason is that most research focuses only on the VEGF signaling pathway,10,11 overlooking the integral microenvironment that is intricately regulated by multiple angiogenic-related molecules.12,13 Hence, it is difficult to distinguish the survival benefit of angiogenesis blockers from VEGF inhibitors.14 Abnormal tumor vasculature typically presents with shattered and/or vanished lumen.15,16 Therefore, the lack of blood supply in the tumor vasculature and the high energy requirement of proliferating cancer cells result in a hypoxic tumor microenvironment and metabolic shift to aerobic glycolysis.17,18 Hypoxia and aerobic glycolysis could induce the production of large amounts of lactic acid by tumor cells, leading to the acidic microenvironment.19,20 Lactate dehydrogenase (LDH), the key enzyme in the production of lactic acid, may reflect the acidity of the tumor microenvironment. Our previous study suggested that the LDH in peripheral blood is a potential predictor of treatment outcomes of anti-angiogenesis therapy.21 However, the LDH in peripheral blood could not accurately reflect the real situation in the tumor microenvironment. The varying cutoff concentrations and several isoforms make LDH an unsatisfactory biomarker candidate. The intricate regulators in the microenvironment will eventually act on tumor vasculature. Therefore, the features of tumor vasculature could be potential predictive markers of anti-angiogenic therapy.
The budding mode of new capillaries through the mutual cooperation of various cells in the tumor microenvironment is the most widely recognized method of angiogenesis.22 With the discovery of other mechanisms of new vessel recruitment,23 the heterogeneity of tumor vasculature had been gaining increasing interest.24 As one of the principal constituents of blood vessels, the pericytes play crucial roles in vessel survival, maturation, stabilization,19 and blood flow regulation.20 The mature blood vessels are characterized by encompassing pericytes.25 The hypothesis of the synergistic effect was that bevacizumab eliminated and normalized the immature tumor vessels, which could enhance blood transfusion to the tumor cells.26,27 Furthermore, pericytes have been reported as a homogeneous cell population in terms of derivation, distribution, phenotype, and function.28
The purpose of our study was to investigate whether there was a particular microvessel characteristic in mCRC and to evaluate the relationship between this type of tumor vessel and bevacizumab efficacy, particularly the immature vessels that lack supporting pericytes.15
Materials and methods
Patients
Patients who met the following inclusion criteria were enrolled: (1) pathologically diagnosed with mCRC and with clinical and/or pathological confirmations of metastasis at the Sun Yat-Sen University Cancer Center from January 2005 to March 2014 and finished the planned course of first-line treatment in this center; (2) with pathological specimens of primary tumor; and (3) an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2. Patients in the chemotherapy plus bevacizumab group received bevacizumab at a dose of 5 mg/kg on day one combined with the standard first-line chemotherapy regimens including oxaliplatin-based and irinotecan-based regimens. Patients who received less than four cycles of bevacizumab were excluded because a therapeutic benefit can only be obtained from at least four cycles of bevacizumab.29 Patients who lack a pathological diagnosis or complete medical history, those lost to followup, and those who have two or more kinds of asynchronous or synchronous tumor were excluded.
Double immunohistochemical staining
All specimens were fixed in formalin and embedded in paraffin blocks; the blocks were then cut into 4-µm sections. All tissue sections were double stained to assess the blood vessel endothelial cell marker cluster of differentiation 31 (CD31) and the perivascular cell marker alpha smooth muscle actin (α-SMA).
For immunohistochemical (IHC) staining, we used a Polymer Double-stain System (Mo/HRP Rb/AP, Zhong Shan Gold Bridge Biotechnology Co., Ltd, Beijing, China) according to the manufacturer’s instructions. Tissue sections were incubated simultaneously with two primary antibodies: rabbit anti-human CD31 polyclonal antibody (working dilution 1:200, Abcam, Cambridge, UK) and mouse anti-human α-SMA monoclonal antibody (working dilution 1:12,000, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). Sections were incubated with anti-rabbit multimer labeled with horseradish peroxidase (brown staining) for CD31 and with anti-mouse multimer labeled with alkaline phosphatase substrate (red staining) for α-SMA. The nuclear-specific hematoxylin was used for counterstaining.
Tumor area and multispectral imaging
We selected the five most vascularized spots (×20 objective) in all sections. Images were spectrally analyzed to quantify horseradish peroxidase-stained, alkaline phosphatase substrate-stained, and hematoxylin-stained regions using the Nuance multispectral imaging system (Cambridge Research, Woburn, MA, USA). The resulting image cubes were converted to optical density (OD) units and were mathematically unmixed into separated CD31 substrate, SMA substrate, and hematoxylin components using spectra deduced from control specimens. The component images were pseudo-colored for further analyses.
Images were analyzed on Image Pro Plus 6.0 software. The mean values of perivascular cell coverage (PC, percentage) for the five selected spots in each area were considered the final values.
Follow-up and statistical analysis
The latest follow-up was performed on June 30, 2016 through telephone interview or medical records review. OS was measured from the date of diagnosis with mCRC to the date of death. Progression-free survival (PFS) was measured from the initiation of first-line therapy to the progression. Both OS and PFS were estimated via the Kaplan–Meier method, and survival differences were analyzed via the log-rank test.
The distributions of the baseline characteristics of the patients were assessed via the Chi-squared test. The correlation between PC and clinicopathologic characteristics was assessed using Spearman rank correlations for categorical data and Wilcoxon rank-sum test and Kruskal-Wallis test for measured data. All statistical was analyses were conducted using SPSS version 22 software. Significance was set at a P-value of <0.05.
Results
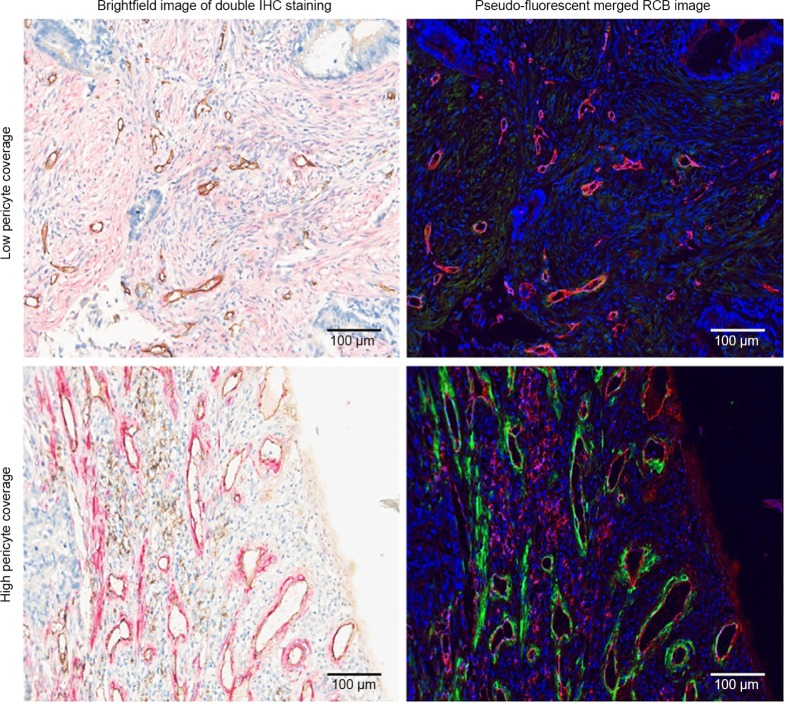
A total of 284 patients with available pathological sections were recruited in this retrospective study. Of them, 59 patients received chemotherapy combined with bevacizumab (B+C group), while 225 patients received chemotherapy alone (C group). The association between endothelial cells and perivascular cells was assessed by analyzing the co-staining of CD31 and α-SMA via Nuance multispectral imaging.30 Figure 1 revealed the correlations between perivascular cells and endothelial cells. The blood vessels in mCRC were covered with different proportions of perivascular cells. The median value30 of PC was 0.55 and was used as the cutoff point to divide the patients into high and low PC groups. High and low PC were defined as a median PC of >0.55 and ≤0.55, respectively.
Figure 1.
Double IHC staining and image analyses of mCRC tissue.
Notes: Tissue sections were stained with anti-CD31 antibody (brown in the brightfield IHC images, red in the pseudo-fluorescent merged RGB images) and anti-α-SMA antibody (red in the brightfield IHC images, green in the pseudo-fluorescent merged RGB images). Brightfield and pseudo-fluorescent RGB images are shown to highlight the intensity of the staining. Scale bars, 100 µm.
Abbreviations: IHC, immunohistochemical; mCRC, metastatic colorectal cancer; CD31, cluster of differentiation 31; RGB, red-green-blue; α-SMA, alpha-smooth muscle actin.
PC and patients’ survival
The median OS in the high PC group was lower than that in the PC group at 26.5 (95% CI=21.8–31.1) months vs 30.0 (95% CI=25.0–35.0) months (P=0.48, Figure 2), respectively, but the difference was not significant. The difference in PFS between the two groups also showed no significance (9.3 months in the high PC group and 9.8 months in the low PC group, P=0.29, Figure 2). The patient characteristics are shown in Table 1. All of the factors were balanced in statistical analysis.
Figure 2.
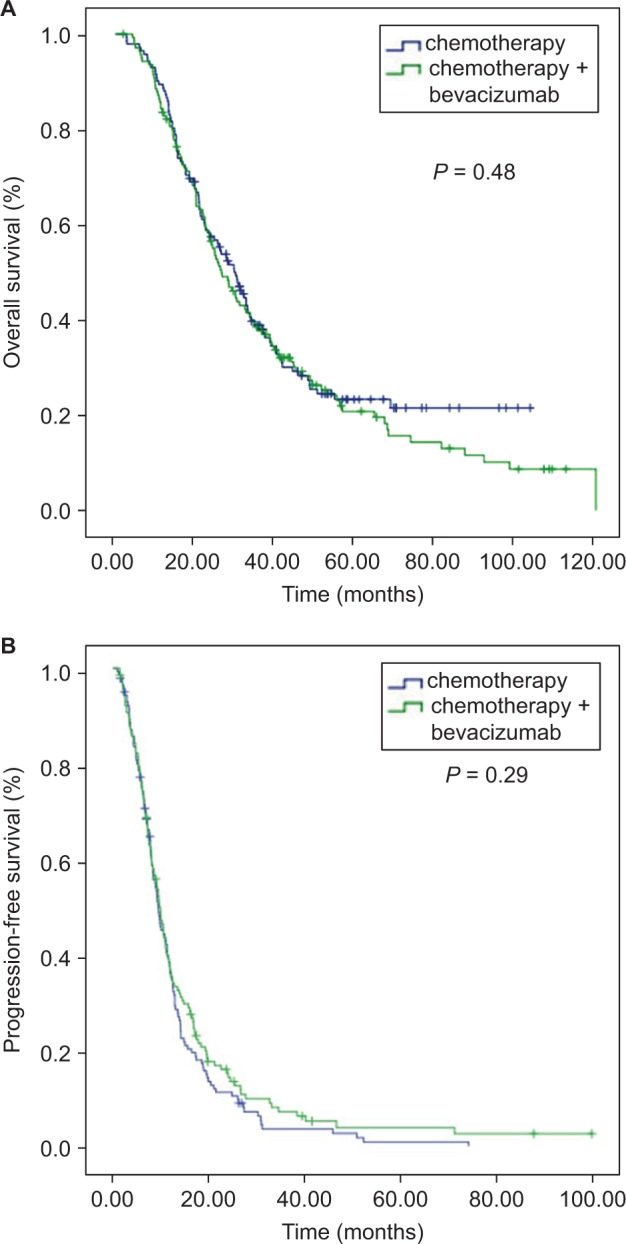
Kaplan–Meier curve analyses in the entire cohort. There was no significant difference in OS (A) and PFS (B) between patients who had high PC and low PC.
Abbreviations: OS; overall survival; PFS, progression-free survival; PC, perivascular cell coverage.
Table 1.
Patient characteristics
| Characteristics | Low PC group (%)
|
High PC group (%)
|
P |
|---|---|---|---|
| (n=142) | (n=142) | ||
| Sex | 0.62 | ||
| Male | 96 (67.6) | 91 (64.1) | |
| Female | 46 (32.4) | 51 (35.9) | |
| Age (median, range) years | 56 (23–87) | 53 (19–80) | 0.13 |
| ≤60 | 89 (62.7) | 102 (71.8) | |
| >60 | 53 (37.3) | 40 (28.2) | |
| Primary tumor location | 0.32 | ||
| Right colon | 43 (30.3) | 44 (31.0) | |
| Left colon | 53 (37.3) | 42 (29.6) | |
| Rectum | 46 (32.4) | 56 (39.4) | |
| Pathological type | 0.24 | ||
| Well differentiated | 3 (2.1) | 0 (0) | |
| Moderately differentiated | 93 (65.5) | 84 (59.2) | |
| Poorly differentiated | 29 (20.4) | 35 (24.6) | |
| Mucinous adenocarcinoma | 14 (9.9) | 21 (14.8) | |
| Unknowna | 3 (2.1) | 2 (1.4) | |
| Pathologic tumor classification | 0.37 | ||
| T1 | 2 (1.4) | 2 (1.4) | |
| T2 | 2 (1.4) | 7 (5.0) | |
| T3 | 24 (16.9) | 26 (18.3) | |
| T4 | 114 (80.3) | 105 (73.9) | |
| Unknownb | 0 (0) | 2 (1.4) | |
| Lymphatic invasion | 101 | 100 | 0.9 |
| Vascular invasion | 10 (7.0) | 16 (11.3) | 0.3 |
| Perineural invasion | 2 (1.4) | 6 (4.3) | 0.17 |
| First-line bevacizumab therapy | 0.38 | ||
| With bevacizumab | 26 (18.3) | 33 (23.2) | |
| Without bevacizumab | 116 (81.7) | 109 (76.8) | |
| First-line chemotherapy regimen | 0.31 | ||
| Oxaliplatin-based | 110 (77.5) | 103 (72.5) | |
| Irinotecan-based | 23 (16.2) | 30 (21.1) | |
| Fluorouracil alone | 1 (0.7) | 4 (2.8) | |
| Oxaliplatin plus irinotecan | 8 (5.6) | 5 (3.5) |
Notes:
Five patients pathologically diagnosed with colorectal adenocarcinoma without the differentiation degree by the biopsy specimen or pathology consultation of specimen from other hospitals.
There was no surgical primary tumor specimen from these patients.
Abbreviation: PC, perivascular cell coverage.
PC and bevacizumab effect
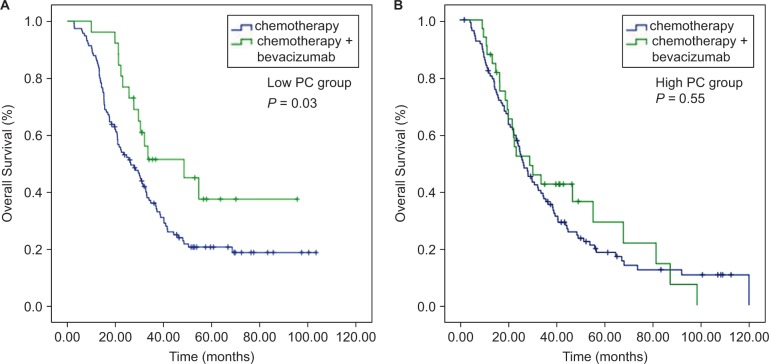
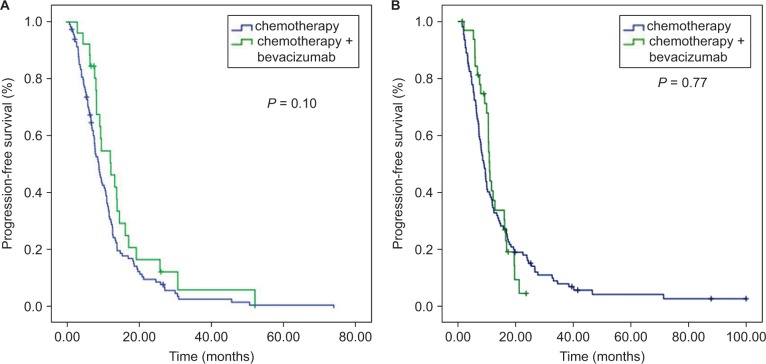
In the low PC cohort, the median OS of the B+C group was 48.5 (95% CI=22.9–74.1) months, while it was 26.4 (95% CI=19.0–33.7) months (P=0.03) in the C group. Meanwhile, no significant difference in median OS was noted between the B+C and C subgroups in the high PC group (26.1 months in the C group and 29.0 months in the B+C group, P=0.55, Figure 3). The patients who received bevacizumab therapy did not obtain a PFS benefit in both the low and high PC groups (9.1 and 12.4 months in the C and B+C subgroups, respectively, in the low PC group, P=0.10; 9.00 and 10.87 months in the C and B+C subgroups in the high PC group, P=0.77, Figure 4).
Figure 3.
Kaplan–Meier curve analyses of OS. (A) In the low PC group, patients who received bevacizumab had better OS. (B) In the high PC group, there was no significant difference in OS in terms of bevacizumab treatment.
Abbreviations: OS; overall survival; PC, perivascular cell coverage.
Figure 4.
Kaplan–Meier curve analyses of PFS. There was no significant difference in PFS in terms of bevacizumab treatment in both the low PC group (A) and the high PC group (B).
Abbreviations: PFS, progression-free survival; PC, perivascular cell coverage.
The results suggested that patients with low PC could benefit more from bevacizumab than those with high PC. Analyses of patient characteristics according to the B+C subgroup and C subgroup in the low PC cohort showed that they were equally distributed (Table 2). Meanwhile, in the high PC cohort, a higher percentage of patients in the B+C subgroup was female (P=0.01), and the number of patients with vascular (P=0.003) and perineural invasion (P=0.03) was also higher than that in the C subgroup.
Table 2.
Patient characteristics in PC subgroup analyses
| Characteristics | Low PC group
|
P | High PC group
|
P | ||
|---|---|---|---|---|---|---|
| B+C (%) (n=26) | C (%) (n=116) | B+C (%) (n=33) | C (%) (n=109) | |||
| Sex | 0.36 | 0.01 | ||||
| Male | 20 (76.9) | 76 (65.5) | 15 (45.5) | 76 (69.7) | ||
| Female | 6 (23.1) | 40 (34.5) | 18 (54.5) | 33 (30.3) | ||
| Age (median, range) years | 54 (32–75) | 57 (23–87) | 0.12 | 49 (24–75) | 55 (19–80) | 0.19 |
| ≤60 | 20 (76.9) | 69 (59.5) | 27 (81.8) | 75 (68.8) | ||
| >60 | 6 (23.1) | 47 (40.5) | 6 (18.2) | 34 (31.2) | ||
| Primary tumor location | 0.66 | 0.91 | ||||
| Right colon | 6 (23) | 37 (31.9) | 11 (33.3) | 33 (30.2) | ||
| Left colon | 10 (38.5) | 43 (37.1) | 10 (30.3) | 32 (29.4) | ||
| Rectum | 10 (38.5) | 36 (31.0) | 12 (36.4) | 44 (40.4) | ||
| Pathological type | 0.07 | 0.32 | ||||
| Well differentiated | 1 (3.8) | 2 (1.7) | 0 (0) | 0 (0) | ||
| Moderately differentiated | 19 (73.0) | 74 (63.8) | 22 (66.7) | 62 (56.9) | ||
| Poorly differentiated | 1 (3.8) | 28 (24.1) | 9 (27.3) | 26 (23.9) | ||
| Mucinous adenocarcinoma | 5 (19.2) | 9 (7.8) | 2 (6.0) | 19 (17.4) | ||
| Unknowna | 0 (0) | 3 (2.6) | 0 (0) | 2 (1.8) | ||
| Pathologic tumor classification | 0.71 | 0.61 | ||||
| T1 | 1 (3.8) | 1 (0.9) | 1 (3.0) | 1 (0.9) | ||
| T2 | 0 (0) | 2 (1.7) | 2 (6.1) | 5 (4.6) | ||
| T3 | 9 (34.6) | 15 (12.9) | 8 (24.2) | 18 (16.5) | ||
| T4 | 16 (61.5) | 98 (84.5) | 22 (66.7) | 83 (76.2) | ||
| Unknownb | 0 (0) | 0 (0) | 0 (0) | 2 (1.8) | ||
| Lymphatic invasion | 16 (61.5) | 85 (73.3) | 0.34 | 24 (72.7) | 76 (69.7) | 0.83 |
| Vascular invasion | 2 (7.7) | 8 (6.9) | 0.89 | 9 (27.3) | 7 (6.4) | 0.003 |
| Perineural invasion | 1 (3.8) | 1 (0.9) | 0.33 | 4 (12.1) | 2 (1.8) | 0.03 |
| First-line chemotherapy regimen | 0.18 | 0.06 | ||||
| Oxaliplatin-based | 17 (65.4) | 93 (80.2) | 18 (54.5) | 85 (78.0) | ||
| Irinotecan-based | 8 (30.8) | 15 (12.9) | 12 (36.4) | 18 (16.4) | ||
| Fluorouracil alone | 0 (0) | 1 (0.9) | 1 (3.0) | 3 (2.8) | ||
| Oxaliplatin plus irinotecan | 1 (3.8) | 7 (6.0) | 2 (6.1) | 3 (2.8) | ||
Notes:
These patients were pathologically diagnosed with colorectal adenocarcinoma without the differentiation degree using the biopsy specimen or pathology consultation of specimen from other hospitals.;
These patients had no primary tumor specimen.
Abbreviations: PC, perivascular cell coverage; B+C, bevacizumab plus chemotherapy group; C, chemotherapy group.
Correlation between PC and clinicopathologic characteristics
We analyzed the relationship between PC and clinicopathologic characteristics in mCRC and found that high PC was correlated with the occurrence of perineural invasion (P=0.03). However, there was no correlation between PC and sex, age, tumor size, primary tumor location, pathological type, T-classification, lymphatic invasion, stage, and vascular invasion in mCRC (Table 3).
Table 3.
Correlation between clinicopathologic characteristics and PC
| A
| |||
|---|---|---|---|
| Variables | No. of patients | PC
|
|
| Median (range) | P | ||
| Sex | 0.9a | ||
| Male | 187 | 0.53 (0–5.40) | |
| Female | 97 | 0.60 (0–3.77) | |
| Primary tumor | 0.79b | ||
| Right colon | 87 | 0.56 (0–5.4) | |
| Left colon | 95 | 0.52 (0–2.98) | |
| Rectum | 102 | 0.62 (0–3.7) | |
| Pathological type | 0.12b | ||
| Well differentiated | 3 | 0.16 (0.03–0.27) | |
| Moderately differentiated | 177 | 0.49 (0–3.77) | |
| Poorly differentiated | 64 | 0.61 (0–3.14) | |
| Mucinous adenocarcinoma | 35 | 0.69 (0–5.4) | |
| Unknown | 5 | 0.47 (0.20–1.04) | |
| Pathologic tumor classification | 0.22b | ||
| T1 | 4 | 0.60 (0.45–1.05) | |
| T2 | 9 | 0.81 (0.05–2.57) | |
| T3 | 50 | 0.49 (0–3.77) | |
| T4 | 219 | 2.23 (0–3.77) | |
| Unknown | 2 | 2.23 (0.76–3.7) | |
| Lymphatic invasion | 0.91a | ||
| Yes | 201 | 0.55 (0–5.40) | |
| No | 83 | 0.56 (0–3.14) | |
| Vascular invasion | 0.51a | ||
| Yes | 26 | 0.60 (0.03–3.14) | |
| No | 258 | 0.53 (0–5.40) | |
| Perineural invasion | 0.03a | ||
| Yes | 8 | 0.99 (0.34–2.76) | |
| No | 276 | 0.53 (0–5.40) | |
|
| |||
| B | |||
|
| |||
| Spearman rank correlation | r | P | |
| Age at diagnosis | −0.11 | 0.08 | |
| Tumor size | 0.04 | 0.55 | |
Notes:
These P-values were determined using the Wilcoxon rank-sum test.
These P-values were determined using the Kruskal-Wallis test.
Abbreviations: PC, perivascular cell coverage; r, Spearman correlation coefficient.
Discussion
Blockage of tumor angiogenesis is a promising approach in cancer treatment. However, due to the complexity of tumor angiogenesis, the survival benefit from bevacizumab administration is still limited in mCRC patients.31,32 Bevacizumab, as well as other anti-angiogenic agents targeting VEGF-A or its receptors, perhaps only inhibit the particular endothelial cells that rely on exogenous VEGF-A.24 At least six well-defined tumor vessel types develop from angiogenesis and arterio-venogenesis with respect to structure and function, including glomeruloid microvascular proliferations, mother vessels, vascular malformations, capillaries, draining veins, and feeder arteries.24 Although all six types of blood vessels could be induced by VEGF-A in mouse models, only the mother vessel and glomeruloid microvascular proliferations remained sensitive to anti-VEGF-A therapy.33
During tumor angiogenesis, the recruitment and coverage of perivascular cells is an essential condition for vessel maturation.14 Bergers and Hanahan34 found that maturation of the vasculature with increased PC might be responsible for the failure of anti-VEGF therapy. Suppressing pericyte migration has been reported to be better than anti-angiogenic therapy alone,35 implying that suppressing pericyte migration could result in production of microvessels with low PC, which are sensitive to anti-angiogenic therapy.
Aside from inducing angiogenesis, tumors exploit multiple mechanisms to recruit blood vessels,23 including vessel co-option, which is a process of hijacking the normal vessel counterpart similar to tumor invasion.36 The co-opted vessels are usually supported by pericytes surrounding the endothelial cells.18 Pericytes not only stabilize blood vessels,37 but also induce the autocrine VEGF-A signaling that could promote endothelial cell survival.38 Weisshardt et al39 have reported that bevacizumab-resistant vessels in mCRC are covered by pericytes that have much longer diameters than capillary vessels, suggesting the formation of co-option vessels in the tumor. The vessel co-option has been reported as an essential mechanism for anti-angiogenic resistance in mouse neuroblastoma40 and in patients with colorectal cancer liver metastases.41 In addition, the pericytes have been found to be involved in vascular immunosurveillance,42 and promote tumor growth via immunosuppression.43 Hence, the low proportion of perivascular cells could serve as a predictive marker of anti-angiogenic therapy. In our study, we discovered a novel finding that low PC is a potential predictive marker for bevacizumab therapy.
Our study found that PC was not a prognostic factor in mCRC. In contrast, some reports have demonstrated that poor PC indicated unfavorable prognoses in patients with colorectal cancer44 and breast cancer.45 However, the value of PC as a prognostic marker is controversial. In clear-cell renal cell carcinoma, high PC was associated with more aggressive characteristics such as high tumor grades, high tumor stages, high necrosis rates, and poor outcome.30 In the current study, we found that high PC was associated with more perineural invasion. To the best of our knowledge, the significance of the association between PC and patient prognosis in mCRC is unclear.
This study has some limitations, as follows. First, it is a retrospective and non-randomized study with a small number of patients. Second, in the low PC group, the addition of bevacizumab significantly improved OS, but not PFS. The results are similar to those of the FLEX study of non-small-cell lung cancer.46 The small sample size may have been inadequate to show a statistically significant PFS difference, because bevacizumab seemed to prolong the PFS for 3.3 months in the low PC group. Third, in the high PC cohort, the proportion of patients with vascular and perineural invasion was higher in the B+C subgroup than that in the C subgroup. Both vascular and perineural invasion are poor prognostic factors that may offset the bevacizumab benefit in the high PC group, although the number of patients with such factors was small in this cohort.
Conclusion
PC is a potential predictive marker of bevacizumab therapy. Patients with low PC value could benefit more from bevacizumab treatment than those with high PC. Our findings need to be validated in large-scale prospective studies. The clinical value of PC for choosing the optimal therapeutic modality should be assessed.
Ethics approval
This study was approved by the ethics committee of the Sun Yat-Sen University Cancer Center (Approval no: GZR2015-034). Written informed consent was obtained from each patient.
Data availability
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDB2018000380.
Acknowledgments
We thank the participants for their cooperation in this study. This study was funded by the Natural Science Foundation of Guangdong, China (2015A030313010); Science and Technology Program of Guangzhou, China (1563000305); and National Natural Science Foundation of China (81272641 and 81572409).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60(4):222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23(15):3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Yin C, Liao F, et al. Bevacizumab plus chemotherapy as third- or later-line therapy in patients with heavily treated metastatic colorectal cancer. Onco Targets Ther. 2015;8:2407–2413. doi: 10.2147/OTT.S88679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 7.Elez E, Argilés G, Tabernero J. First-Line Treatment of Metastatic Colorectal Cancer: Interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16(11):52. doi: 10.1007/s11864-015-0369-x. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11(12):1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 9.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31(9):1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y. VEGF-targeted cancer therapeutics-paradoxical effects in endocrine organs. Nat Rev Endocrinol. 2014;10(9):530–539. doi: 10.1038/nrendo.2014.114. [DOI] [PubMed] [Google Scholar]
- 11.Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res. 2013;19(11):2824–2827. doi: 10.1158/1078-0432.CCR-12-3409. [DOI] [PubMed] [Google Scholar]
- 12.Stacker SA, Achen MG. The VEGF signaling pathway in cancer: the road ahead. Chin J Cancer. 2013;32(6):297–302. doi: 10.5732/cjc.012.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L. Molecular targeted agents–where we are and where we are going. Chin J Cancer. 2013;32(5):225–232. doi: 10.5732/cjc.013.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013;338(2):219–228. doi: 10.1016/j.canlet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Yao X, Qian CN, Zhang ZF, et al. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res. 2007;13(1):161–169. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 16.Qian CN, Huang D, Wondergem B, Teh BT. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer. 2009;115(10 Suppl):2282–2289. doi: 10.1002/cncr.24238. [DOI] [PubMed] [Google Scholar]
- 17.Adighibe O, Leek RD, Fernandez-Mercado M, et al. Why some tumours trigger neovascularisation and others don’t: the story thus far. Chin J Cancer. 2016;35:18. doi: 10.1186/s40880-016-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian CN, Tan MH, Yang JP, Cao Y. Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin J Cancer. 2016;35:10. doi: 10.1186/s40880-015-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonavicius N, Ashenden M, van Weverwijk A, et al. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120(7):1516–1527. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]
- 20.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin C, Jiang C, Liao F, et al. Initial LDH level can predict the survival benefit from bevacizumab in the first-line setting in Chinese patients with metastatic colorectal cancer. Onco Targets Ther. 2014;7:1415–1422. doi: 10.2147/OTT.S64559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36(3):321–331. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao YY, Xue C, Jiang W, et al. Predictive value of intratumoral microvascular density in patients with advanced non-small cell lung cancer receiving chemotherapy plus bevacizumab. J Thorac Oncol. 2012;7(1):71–75. doi: 10.1097/JTO.0b013e31823085f4. [DOI] [PubMed] [Google Scholar]
- 26.Sabo E, Boltenko A, Sova Y, Stein A, Kleinhaus S, Resnick MB. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res. 2001;7(3):533–537. [PubMed] [Google Scholar]
- 27.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 28.Paiva AE, Lousado L, Guerra DAP, et al. Pericytes in the Premetastatic Niche. Cancer Res. 2018;78(11):2779–2786. doi: 10.1158/0008-5472.CAN-17-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bei Zhang WH, Zhang WHB. A Potential Administration-time Dependent Effect of Bevacizumab in Improving Overall Survival and Increasing Metastasis in Metastatic Colorectal Cancer. Chemotherapy. 2013;02(01) [Google Scholar]
- 30.Cao Y, Zhang ZL, Zhou M, et al. Pericyte coverage of differentiated vessels inside tumor vasculature is an independent unfavorable prognostic factor for patients with clear cell renal cell carcinoma. Cancer. 2013;119(2):313–324. doi: 10.1002/cncr.27746. [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 32.Hayes DF. Bevacizumab treatment for solid tumors: boon or bust? JAMA. 2011;305(5):506–508. doi: 10.1001/jama.2011.57. [DOI] [PubMed] [Google Scholar]
- 33.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72(8):1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ peri-vascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7(9):870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian CN. Hijacking the vasculature in ccRCC–co-option, remodelling and angiogenesis. Nat Rev Urol. 2013;10(5):300–304. doi: 10.1038/nrurol.2013.26. [DOI] [PubMed] [Google Scholar]
- 37.Schrimpf C, Xin C, Campanholle G, et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23(5):868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118(10):2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisshardt P, Trarbach T, Dürig J, et al. Tumor vessel stabilization and remodeling by anti-angiogenic therapy with bevacizumab. Histochem Cell Biol. 2012;137(3):391–401. doi: 10.1007/s00418-011-0898-8. [DOI] [PubMed] [Google Scholar]
- 40.Kim ES, Serur A, Huang J, et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc Natl Acad Sci U S A. 2002;99(17):11399–11404. doi: 10.1073/pnas.172398399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark K, Pekayvaz K, Massberg S. Role of pericytes in vascular immunosurveillance. Front Biosci. 2018;23:767–781. doi: 10.2741/4615. [DOI] [PubMed] [Google Scholar]
- 43.Sena IFG, Paiva AE, Prazeres P, et al. Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. 2018;7(4):1232–1239. doi: 10.1002/cam4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mezheyeuski A, Bradic Lindh M, Guren TK, et al. Survival-associated heterogeneity of marker-defined perivascular cells in colorectal cancer. Oncotarget. 2016;7(27):41948–41958. doi: 10.18632/oncotarget.9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooke VG, Lebleu VS, Keskin D, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21(1):66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDB2018000380.