ABSTRACT
The mitotic spindle checkpoint delays anaphase onset until all chromosomes have achieved stable kinetochore-microtubule attachments. Here, we discuss recent findings showing that CHMP4C, a component of the endosomal sorting complex required for transport (ESCRT) machinery, protects human cells against chromosome missegregation by promoting localisation of the ROD–ZW10–ZWILCH (RZZ) spindle checkpoint complex to unattached kinetochores.
KEYWORDS: CHMP4C, spindle checkpoint, mitosis, RZZ, ESCRT, ZW10, Biology of malignant cells
The mitotic spindle checkpoint protects against chromosome missegregation and aneuploidy by delaying sister chromatid separation in the presence of unattached or improperly-attached kinetochores.1 Conserved components of the spindle checkpoint pathway include the mitotic arrest deficient (MAD; MAD1, MAD2, and BUBR1) and the budding uninhibited by benzimidazole (BUB; BUB1 and BUB3) proteins. Accumulation of MAD and BUB proteins on unattached kinetochores is essential to prevent activation of the anaphase-promoting complex/cyclosome (APC/C) and delay mitotic exit.1 In metazoans, recruitment of the MAD1-MAD2 heterodimer to unattached kinetochores requires the activity of the ROD–ZW10–ZWILCH (RZZ) complex and cells lacking RZZ proteins are spindle checkpoint deficient.2,3 However, how RZZ is targeted to kinetochores is a matter of active investigation.
Charged multivesicular body protein 4C (CHMP4C), a component of the endosomal sorting complex required for transport-III (ESCRT-III) machinery, is a human orthologue of the yeast protein Snf7 that is involved in multivesicular body sorting.4 CHMP4C is required for the abscission checkpoint that delays the final cut of the narrow cytoplasmic canal between daughter cells in the presence of trapped chromatin and functions as an abscission timer in normally segregating cells.5 During the abscission checkpoint, the AURORA B kinase phosphorylates CHMP4C and this phosphorylation targets CHMP4C to the midbody centre to prevent downstream ESCRT components including the ATPase VPS4 from relocalizing to the abscission site to deliver the final cut.5
Recent evidence from our lab has shown an unexpected role for CHMP4C in the mitotic spindle checkpoint, independently from its membrane-directed activities.6 CHMP4C localises to prometaphase kinetochores in the absence of spindle poisons or after treatment of cells with a concentration of nocodazole that completely depolymerizes spindle microtubules. However, CHMP4C is reduced from kinetochores in chromosomes aligned at the metaphase plate or in prometaphase cells treated with taxol, a spindle drug that stabilizes microtubules. CHMP4C is required for optimal chromosome alignment and segregation in the absence of spindle poisons and for mitotic arrest when kinetochores are unattached by nocodazole-treatment. Furthermore, CHMP4C binds to ZW10 in cell extracts and in vitro and promotes localization of BUBR1, RZZ and MAD1-MAD2 complexes to prometaphase kinetochores. These results show that CHMP4C acts as a loading factor for the RZZ complex to unattached kinetochores (Figure 1). However, CHMP4C is dispensable for RZZ kinetochore localization and mitotic arrest when spindle function is disrupted by taxol, suggesting that additional proteins are required for RZZ localisation to improperly-attached kinetochores (Figure 1). One possibility is that RZZ makes physical contacts with KNL1-BUB1 and/ or KNL1-ZWINT1 kinetochore complexes independently of CHMP4C.7,8 Also, the mitotic kinase AURORA B that is required for mitotic arrest in the presence of taxol, could promote localisation of RZZ to misattached kinetochores by phosphorylating ZWINT1.9 Such alternative mechanisms may allow the spindle checkpoint to respond to more than one type of defect ensuring a higher level of chromosome segregation fidelity.
Figure 1.
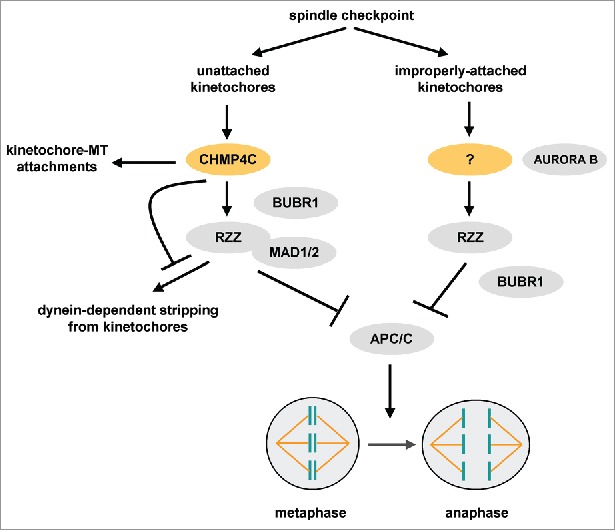
Newly-identified functions of CHMP4C in the spindle checkpoint. CHMP4C promotes localisation of the RZZ and MAD1-MAD2 (MAD1/2) complexes to unattached kinetochores. CHMP4C also prevents dynein-dependent stripping of RZZ from attached kinetochores and promotes stable kinetochore-microtubule (MT) interactions. In the presence of improper kinetochore-microtubule attachments, additional proteins (indicated by a question mark) mediate RZZ-kinetochore targeting. See text for details.
In addition, CHMP4C-deficient cells in prometaphase exhibit reduced cold-stable microtubule polymers, suggesting that CHMP4C is required for robust kinetochore–microtubule attachments (Figure 1).6 These results indicate that CHMP4C has the additional task of regulating kinetochore-microtubule interactions, as previously shown for other spindle checkpoint proteins.10 After chromosomes bi-orient, stable kinetochore-microtubule attachments may lead to dissociation of CHMP4C from metaphase kinetochores.
Why would cells employ CHMP4C, an abscission checkpoint protein, in spindle checkpoint signalling? By recycling proteins, such as CHMP4C and AURORA B, in the spindle checkpoint and cytokinesis/abscission, cells may coordinate chromosome alignment and segregation with the late stages of cell division to ensure the ordered coupling of these mitotic processes. Perhaps in agreement with this hypothesis, constitutive CHMP4C kinetochore targeting induces a checkpoint metaphase arrest that is dependent on ZW10 and MAD1-MAD2 kinetochore binding, suggesting that removal of CHMP4C from kinetochores is a prerequisite for spindle checkpoint silencing and onset of anaphase.6
While this work by Petsalaki et al clearly establishes a role for CHMP4C in the spindle checkpoint,6 it also raises many interesting questions. For example, how does RZZ localise to improperly-attached kinetochores? How does CHMP4C itself localise to unattached kinetochores? How does CHMP4C prevent dynein-dependent removal of RZZ from attached kinetochores? And what is the mechanism by which CHMP4C promotes stable kinetochore-microtubule attachments? Future studies aimed at answering these questions may reveal additional, unexpected mechanisms by which CHMP4C promotes accurate chromosome segregation in human cells and facilitate evaluation of CHMP4C-targeting as a possible means to restrain cancer cell proliferation.
Funding Statement
Worldwide Cancer Research, Fondation Sante.
Acknowledgments/ disclosure statement
Work in our lab was supported by Worldwide Cancer Research and by Fondation Santé. The authors declare no potential conflicts of interest.
References
- 1.Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol. 2015;25:3017. doi: 10.1016/j.cub.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Caldas GV, Lynch TR, Anderson R, Afreen S, Varma D, DeLuca JG. The RZZ complex requires the N-terminus of KNL1 to mediate optimal Mad1 kinetochore localization in human cells. Open Biol. 2015;5:150160. doi: 10.1098/rsob.150160. PMID:26581576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silio V, McAinsh AD, Millar JB. KNL1-Bubs and RZZ Provide two separable pathways for checkpoint activation at human kinetochores. Dev Cell. 2015;35:600–13. doi: 10.1016/j.devcel.2015.11.012. PMID:26651294 [DOI] [PubMed] [Google Scholar]
- 4.Campsteijn C, Vietri M, Stenmark H. Novel ESCRT functions in cell biology: Spiraling out of control? Curr Opin Cell Biol. 2016;41:1–8. doi: 10.1016/j.ceb.2016.03.008. PMID:27031044 [DOI] [PubMed] [Google Scholar]
- 5.Nahse V, Christ L, Stenmark H, Campsteijn C. The abscission checkpoint: Making It to the Final Cut. Trends Cell Biol. 2017;27:1–11. doi: 10.1016/j.tcb.2016.10.001. PMID:27810282 [DOI] [PubMed] [Google Scholar]
- 6.Petsalaki E, Dandoulaki M, Zachos G. The ESCRT protein Chmp4c regulates mitotic spindle checkpoint signaling. J Cell Biol. 2018;217:861–76. doi: 10.1083/jcb.201709005. PMID:29362225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Lischetti T, Hayward DG, Nilsson J. Distinct domains in Bub1 localize RZZ and BubR1 to kinetochores to regulate the checkpoint. Nat Commun. 2015;6:7162. doi: 10.1038/ncomms8162. PMID:26031201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HM, Hu XY, Ding X, Dou Z, Yang ZH, Shaw AW, Teng MK, Cleveland DW, Goldberg ML, Niu LW, et al.. Human Zwint-1 specifies localization of zeste white 10 to kinetochores and is essential for mitotic checkpoint signaling. J Biol Chem. 2004;279:54590–8. doi: 10.1074/jbc.M407588200. PMID:15485811 [DOI] [PubMed] [Google Scholar]
- 9.Kasuboski JM, Bader JR, Vaughan PS, Tauhata SB, Winding M, Morrissey MA, Joyce MV, Boggess W, Vos L, Chan GK, et al.. Zwint-1 is a novel Aurora B substrate required for the assembly of a dynein-binding platform on kinetochores. Mol Biol Cell. 2011;22:3318–30. doi: 10.1091/mbc.E11-03-0213. PMID:21775627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elowe S. Bub1 and BubR1: At the interface between chromosome attachment and the spindle checkpoint. Mol Cell Biol. 2011;31:3085–93. doi: 10.1128/MCB.05326-11. PMID:21628528 [DOI] [PMC free article] [PubMed] [Google Scholar]


