ABSTRACT
Although catch-up campaigns (CCs) at the introduction of pneumococcal conjugate vaccines (PCVs) may accelerate their impact, supply constraints may limit their benefit if the need for additional PCV doses results in introduction delay. We studied the impact of PCV13 introduction with and without CC in Nha Trang, Vietnam – a country that has not yet introduced PCV – through a dynamic transmission model. We modelled the impact on carriage and invasive pneumococcal disease (IPD) of routine vaccination (RV) only and that of RV with CCs targeting <1y olds (CC1), <2y olds (CC2) and <5y olds (CC5). The model was fitted to nasopharyngeal carriage data, and post-PCV predictions were based on best estimates of parameters governing post-PCV dynamics. With RV only, elimination in carriage of vaccine-type (VT) serotypes is predicted to occur across all age groups within 10 years after introduction, with near-complete replacement by non-VT. Most of the benefit of CCs is predicted to occur within the first 3 years with the highest impact at one year, when IPD incidence is predicted to be 11% (95%CrI 9 – 14%) lower than RV with CC1, 25% (21 – 30 %) lower with CC2 and 38% (32 – 46%) lower with CC5. However, CCs would only prevent more cases of IPD insofar as such campaigns do not delay introduction by more than about 6, 12 and 18 months for CC1, CC2 and CC5. Those findings are important to help guide vaccine introduction in countries that have not yet introduced PCV, particularly in Asia.
KEYWORDS: Asia, campaign, catch-up, pneumococcus, viet nam, vaccine
Introduction
Disease due to Streptococcus pneumoniae (the pneumococcus) is a leading cause of morbidity and mortality worldwide, disproportionally so in resource-poor settings.1-3
Ten- and 13-valent pneumococcal conjugate vaccines (PCV10 and PCV13), which cover 10 and 13 of 94 known serotypes,4 are steadily being introduced into the routine immunization programmes of most low and lower-middle income countries with the support from Gavi, the Vaccine Alliance. In 2017, 58 out of 73 countries eligible for Gavi support have introduced the vaccine, and seven additional countries have been approved for introduction and are expected to introduce PCV within the next two years.5 In 2012 Pakistan was the first Asian country to introduce PCV for routine use, followed by Nepal, Cambodia and Lao PDR.6 However, PCV has not yet been introduced into the routine childhood immunisation programme in most of Asia, including Vietnam.
The WHO recommends introducing PCV into childhood immunisation programmes. It states that a catch-up campaign (CC) among older children can be used to accelerate PCV impact.7 The aim of such campaign is to provide direct protection to age groups at particular risk of pneumococcal disease, as well as to accelerate the population impact of the vaccine through enhanced herd protection.8 The WHO recommendation was recently reviewed and is due to be updated but remains unable to guide on the country specific expected impact and efficiency of alternative catch-up strategies.9,10 Moreover, CCs have so far not been conducted in most Gavi-supported countries over concerns that they would lead to vaccine introduction delays given limited vaccine supplies.6 For routine vaccination, WHO currently recommends introducing PCV either as a three primary infant dose schedule (3+0) or as two primary doses with a booster at 12 months of age (2+1 schedule), with the choice between schedules guided by setting-specific epidemiological characteristics.7
In this manuscript we explored the differential impact on carriage and invasive disease of catch-up campaigns targeting various age groups, through a dynamic compartmental model of disease transmission, and we explored the possible impact of vaccine introduction delay. More specifically, we explored four different strategies: (i) routine vaccination only (RV), with two infant doses and a booster dose at 12 months of age (‘2+1’), and routine vaccination with a catch-up campaign in (ii) <1y olds (CC1), (iii) <2y olds (CC2), and (iv) <5y olds (CC5). A 2+1 routine programme was considered here, given the relatively low prevalence of carriage in young children in Nha Trang.11
Results
The carriage prevalence in <5 year olds was 41% (95% CI 38 – 46%) overall, 27% (95% CI 23 – 32%) for VT serotypes and 14% (95% CI 11 – 18%) for NVT serotypes. We estimated the carriage prevalence of VT in 5 – 17 year olds and in adults to be 14% (95% CrI 10 – 18%) and 3% (95% CrI 0 – 7%) respectively, and that of NVT to be 15% (95% CrI 11 – 19%) and 3% (95% CrI 0 – 7%). The model fit to the carriage data is shown in Fig. 1.
Figure 1.
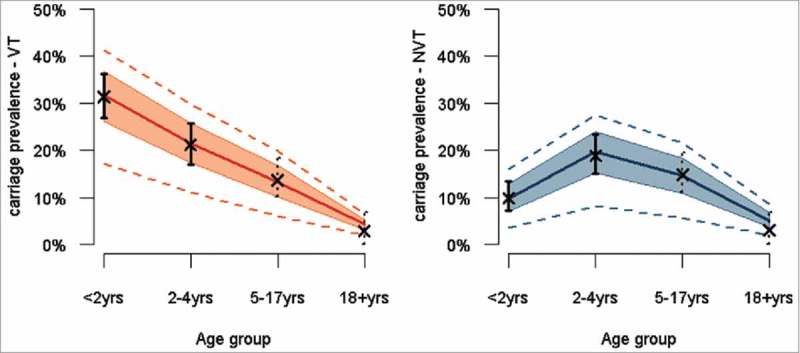
Pre-PCV carriage estimates across age groups for VT (left panel) and NVT (right panel), based on survey (plain vertical lines) and meta-regression model (dotted vertical lines) estimates, and estimates from the corresponding transmission model. Legend: Dots and bars correspond to the point and 95% confidence interval for the carriage estimates from the survey data (plain dot and plain lines) and the meta-regression model (cross and dotted lines). The dark red and dark blue plain lines represent the median transmission model estimate for VT and NVT respectively, the shaded areas the 50% credible interval (CrI) around the median and the dotted red and blue lines the 95% CrI.
Nasopharyngeal carriage
Our model predicts elimination of VT serotypes across all age groups within 10 years of PCV introduction in RV, with near-complete replacement by NVT serotypes, resulting in little or no change in the overall carriage prevalence (Fig. 2 A & B), particularly in children aged >5 years and in adults. CCs are predicted to reduce VT carriage more quickly through combined direct and indirect (i.e. herd) effects (Fig. 2 C & D).
Figure 2.
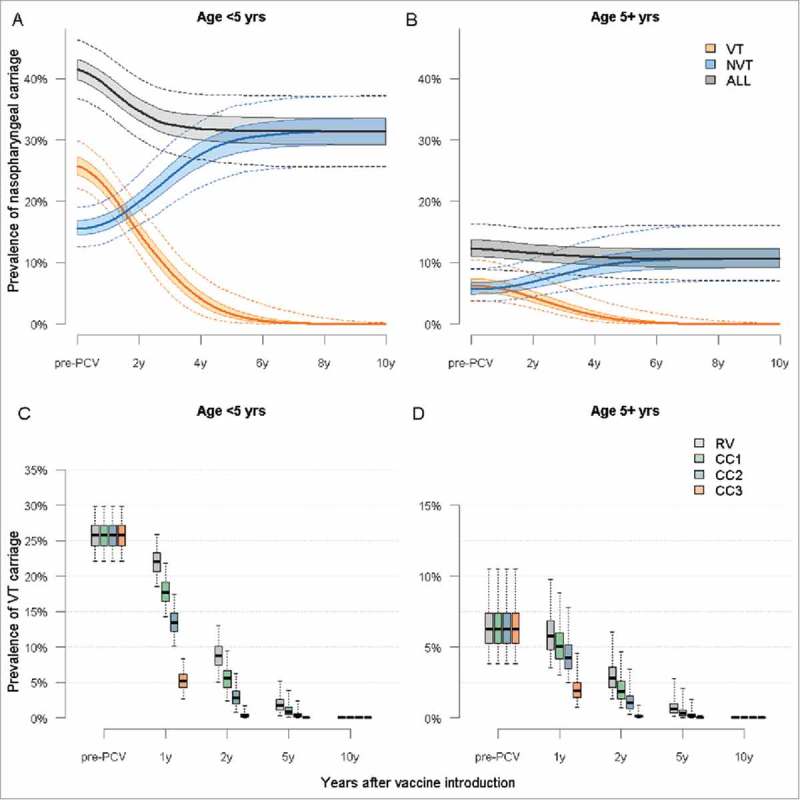
Predicted trends in nasopharyngeal carriage following PCV13 introduction in Nha Trang. Legend: Predicted trends in VT, NVT and overall carriage in <5 year olds without a catch-up campaign. B: Predicted trends in VT, NVT, and overall carriage in ≥5 year olds in RV. C: Predicted prevalence of VT carriage in <5 year olds for each vaccination strategy. D: Predicted prevalence of VT carriage in ≥5 year olds. In all four panels: plain line = median, shaded areas = 50% CrI and dotted line or whiskers = 95% CrI.
In children under five years the VT carriage in children is predicted to decrease by >99% within 6 years and 7 months (95% CI 5y2 m – 9 y6 m) in RV, 5 years and 11 months (4y6 m– 8 y10 m) in CC1, 5 years and 1 month (3y9m – 7y10m) in CC2 and 3 yrs (2y2 m–4y10 m) in CC5. Similar trends are predicted in older children and adults (Fig. 3C and 3D). And in all scenarios NVT would increase proportionally to the decrease in VT.
Figure 3.
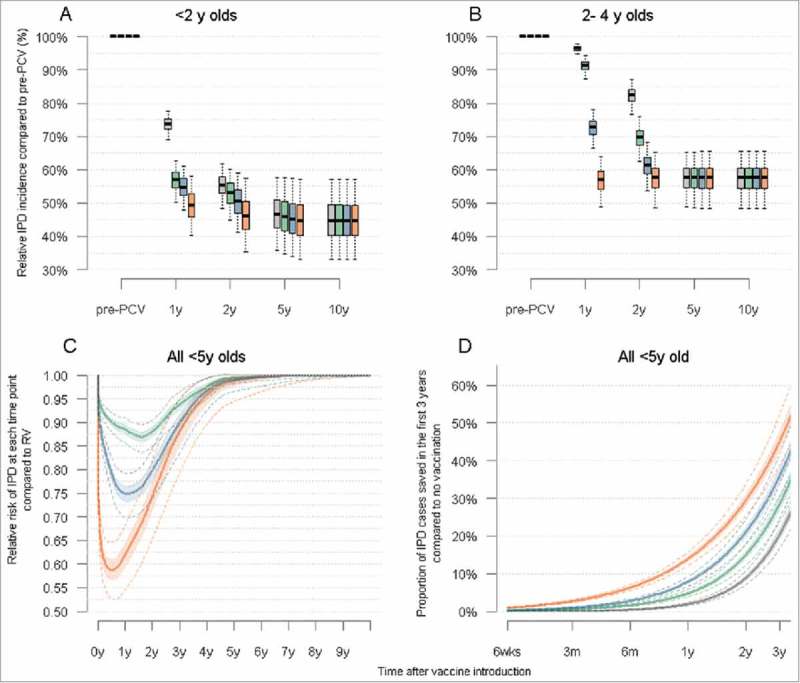
Trends in IPD following PCV introduction in children under five years of age in Nha Trang Legend: A: Predicted trends the cumulative annual incidence of IPD in children <2 years for each vaccination strategy considered, at a 90% vaccination coverage B: Predicted trends the cumulative annual incidence of IPD in children aged 2–4 years for each vaccination strategy considered, at a 90% vaccination coverage C: Cumulative number of IPD cases saved for each catch up strategy compared to RV, in children <5 years of age. D: Overall cumulative number of cases saved for each vaccination strategy, compared to no vaccination, in the first 3 years post PCV introduction. In all four panels: plain line = median, shaded areas = 50% credible intervals and dotted line or whiskers = 95% credible interval.
Invasive pneumococcal disease (IPD)
Our model predicts that the decline of IPD incidence will be proportionally highest among <2 year olds, falling to about 45% (95%CrI 33% – 57%) of its pre-PCV level and would be almost halved (57% (48 – 66%)) in children aged 2 – 4 years respectively (Fig. 2A and 2B).
Most of the benefit of catch-up campaigns over routine vaccination in children <5 years is predicted to occur within the first three years after PCV introduction, with no noticeable difference at 5 years (Fig. 2C). Our model predicts that the relative risk of IPD after CCs compared to RV would be lowest about 1 year after PCV introduction, with differences between strategies then steadily reducing thereafter until the new equilibrium is reached. Compared to RV, one year after vaccine introduction the number of cases of IPD is predicted to be 11% (95%CrI 9 – 14%) lower with CC1, 25% (21–30%) lower with CC2 and 38% (32 – 46%) lower with CC5 (Fig 2C).
The impact of each strategy on the cumulative proportion of cases averted in the first 3 years post PCV introduction, compared to no vaccination, is illustrated in Fig. 3.
Based on an average annual incidence risk of 49 cases per 100,000 children <5 years before PCV introduction,11 a routine introduction of PCV would result in a total of 74 cases (95%CrI 62 – 86) per 100,000 children <5 years averted over the first five years of programme implementation, considering all IPD (both VT and NVT), and catch-up campaigns would lead to the prevention of an additional 13 (95%CrI 11 – 16) cases with CC1, 25 (95%CrI 21 – 30) cases with CC2 and 39 (95%CrI 31 – 49) cases with CC5.
Delayed PCV introduction
We estimated the relative impact on IPD of catch-up campaigns for increasing delays. Our results suggest that, compared to RV, more IPD cases would be prevented in children <5 years insofar as PCV introduction is not delayed by more than 31 weeks (95%CI 30 – 32 weeks) for CC1, 58 weeks (53 – 63 weeks) for CC2 and 89 weeks (78 – 101 weeks) for CC5. Vaccination delays would negatively impact under 2 year olds more rapidly than 2 – 4 year old (Fig. 4).
Figure 4.

Impact of delayed PCV introduction with a catch-up campaign on VT carriage (panel A) and on IPD cases saved (panel B) in children under five years of age. Legend: A: <2 year olds. B: 2 – 4 year olds C: <5 years olds. The middle plain trend line corresponds to the median estimate (Green = CC1, Blue = CC2, Red = CC5), the dark shaded areas the 50% CrI and the light shaded areas the 95%CrI. The plain horizontal line at 1.00 represents the point below which interventions will be more favourable than a timely RV.
Sensitivity analyses
Vaccination coverage
We explored model outputs with lower vaccination coverage in both cohort and catch-up immunization. Our model predicts a lengthening of the time to near-elimination of VT serotypes (and hence, the time reach the new post-PCV disease equilibrium) as vaccination coverage lowers but a similar differential impact of catch-up campaigns compared to routine vaccination. Full details are provided in the Supporting Information Part Three.
Duration of protection
A duration of protection of 3 years would increase the time to elimination of VT carriage, and thus prevent fewer IPD cases overall, while any average duration of protection longer than 6 years would not change model outcomes. With a duration of 3 years the median prevalence of VT in <5 year olds is predicted to reach near elimination about 2 years later in RV and CC1, 1.5 years later with CC2 and about 1 year later with CC5. Similar differences were predicted for the ≥5 year olds (Figure S5 in the Supporting Information Part Three). The relative impact of one vaccination strategy over another was predicted to be similar than with a duration of 6 years.
Discussion
We explored the possible impact of introducing PCV13 with and without a catch-up campaign in Vietnam through a dynamic transmission model. Our results feed into current debates about introduction strategies, particularly in South-East Asia where pneumococcal disease burden is high,12 where many countries have not yet introduced PCV,6 and where epidemiological data to guide decision making remain scarce.12,13 Although Vietnam is Gavi-eligible and is expected to introduce PCV in the coming years, it has not yet been approved for introduction.6 Our results provide estimates about how much catch-up campaigns would decrease disease burden compared to routine vaccine introduction without a campaign, for different scenarios. Our study also shows that, although catch-up campaigns would decrease disease burden more rapidly across age groups, their impact would only be beneficial insofar as the additional supply and operational constraints of their implementation does not delay PCV introduction by more than about 6 months to 2 years, depending on the age cohorts targeted by those campaigns.
The availability of data on both social mixing patterns – which are central to transmission models.14-16 – and carriage in the same population, allowed for thorough parameterisation of a transmission model.
Nevertheless, our study has a number of limitations. In the absence of post-PCV data, predictions were based on the best available estimates of parameters governing vaccine effects that were observed elsewhere,17-19 which may not fully capture the local characteristics. Given that serotypes differ in their pathogenicity, fitness and transmissibility,20,21 and that vaccine efficacy and duration of protection differs by serotype, 22 our predictions based on homogeneous characteristics for the group of VT and NVT serotypes may overlook local epidemiological characteristics. This uncertainty was nonetheless captured to some extent by sampling from the known uncertainty around those parameters.19 Moreover, we were not able to assess the impact of PCV on pneumococcal pneumonia, the burden of which is much higher than that of IPD,23 given the lack of robust data and the challenges in the aetiological assessment of clinical pneumonia. Results from several large pneumonia aetiological studies.24 might help modelling work on the impact of PCV on pneumonia in the future.
Our predictions are in line with the experience of PCV7 in Europe and North America,25-32 as well with post-PCV trends observed in the few studies from resource-poor settings.33,34
The implementation of PCV in various settings has consistently shown little or no change in overall carriage prevalence, due to replacement effects by NVT serotypes colonising the space left vacant by VT in the nasopharynx, but a reduction in severe disease given the lower pathogenicity of the latter, in accordance with our model output. Our predictions were also robust to estimates of duration of protection of vaccination coverage, and thus provide useful estimates of the impact of introducing PCV in a semi-urban Southeast Asian setting.
The generalisability to other settings of the differential impact of CCs needs to be considered in light of the epidemiological and socio-demographic characteristics of Nha Trang. In particular, the low prevalence of carriage and an ageing population – with only 5% of children under the age of five years – likely contribute to the rapid predicted reduction of disease due to PCV under all scenarios. Although similar epidemiological and demographic characteristics are observed in many other South(east) Asian settings,35,36 the differential impact of CCs and the establishment of herd effects in settings with a younger population and a higher carriage prevalence is likely to differ, and should be addressed with models applied to such settings.
Results from our study are important to inform current debates around PCV introduction strategies in GAVI-eligible countries. Where the number of vaccine doses available is limited, and ignoring any other supply side, staffing and outreach challenges that could potentially delay the implementation of CCs, our study can inform whether delaying the introduction of the vaccine to allow for CCs would potentially be beneficial, compared to a routine-only strategy. Moreover, this model also provides a framework that could feed into economic evaluations to further guide decisions about vaccine introduction with and without campaigns, and evaluate strategies in the short and medium term.
In conclusion, our study offers insights into the current debate about PCV introduction strategies when particularly for South-East Asia. Our model suggests that catch-up campaigns have the potential to rapidly establish control of vaccine serotypes, potentially also aiding accelerated implementation of cost saving reduced dose schedules,37 but are only offering added reduction in disease burden insofar their implementation results in little to no implementation delay.
Materials and methods
The model was applied to the population of Nha Trang (∼360,000 inhabitants), an urban and semi-rural area in south-central Vietnam.
Data
Nasopharyngeal carriage
Two surveys were conducted among children aged 0 – 59 months randomly drawn from the population census, with 350 children included in January 2008.38 and another 350 children in July 2008.39
Samples were processed and cultured as per WHO recommendations.40 Serotyping was done using polymerase chain reaction with 29 specific primer pairs, and did not differentiate between 6A and 6B serotypes.38 Given that both antigens are included in PCV13 but that PCV10 does not include 6A, we decided to implement our model for PCV13.
The carriage prevalence (and its uncertainty) of VT and NVT among 5–17 year olds and adults (≥18 years) was estimated based on the prevalence and serotype distribution in children <5 years of age, using a random effect meta-regression model.22
Social mixing patterns
We derived the age-specific contact patterns from a survey conducted in the same area in 2010.
Study participants were interviewed about their contact on a given day, similarly to previous studies.41 Participants were first asked to fill in some background information, and were then asked to remember their social encounter occurring in the following 24 hours, before being interviewed about those shortly after. As for other social contact studies, the questionnaire recorded information on the location and type of contacts, including whether or not contacts included physical (i.e. skin-to-skin touch).
In the study, household were randomly selected from all non-touristic urban and semi-rural areas of Nha Trang (i.e. matching the areas where the nasopharyngeal carriage was undertaken)., and one person per household was included in the study. The sample size was based on POLYMOD calculations.41), but was higher to account for urban/rural differences.
The corresponding mixing matrix used in the model was derived as in Melegaro et al.42 We parameterised our model based on physical (skin-to-skin) contacts only, given that S.pneumoniae is generally assumed to be transmitted through close interpersonal contact.43
A total of 2002 individuals were included in the study. The mean number of physical contacts per age group is shown in Fig. 5. The manuscript is currently being written up for publication, and further results can be obtained on request.
Figure 5.

Mean number for physical contacts between age groups of study participants.
Model structure
We built an age-structured deterministic Susceptible-Infected-Susceptible transmission model of carriage acquisition and clearance, in which we modelled VT jointly and separately from NVT, but allowed for co-colonisation, as in previous models.19 Details about the model structure and model equations can be found in the Supporting Information (Part 1).
The model comprised of three levels of vaccine-induced immunity; (1) no protection, (2) partial protection and (3) full protection. The latter refers to the efficacy and duration of protection conferred after completion of the infant schedule (i.e. 2 infant doses and a booster at 12 months (‘2+1’ schedule)) or the completion of a catch-up programme in older children (2 doses in <18 months and 1 dose in ≥18 months). Partial protection was gained form two primary infant doses, or after the first catch-up dose in children aged 12 – 17 months. The difference between full and partial protection relied in the magnitude of vaccine efficacy against carriage and in the duration of protection.
We applied the model to a population of 81 annual age cohorts (0 to 80 years) divided into 52 weekly age bands of 100 individuals. In the calculation of the force of infection the population figure was adjusted to represent the actual population, based on census data.
Model fitting
We fitted the model to pre-vaccination nasopharyngeal carriage data using a Markov Chain Monte-Carlo (MCMC) algorithm, and estimating the age-specific probability of effective transmission in the absence of PCV. For each unique posterior sample we simulated up to 15 years after vaccine introduction.
The model was fitted the model to pre-vaccination nasopharyngeal carriage data using an MCMC algorithm. Estimates of the age-specific probability of effective transmission, which were obtained from the contact and carriage data were updated in the MCMC. Fitting was performed using a Poisson log likelihood, as follows:
where is the modelled number of VT carriers in age group a, is the observed number of VT carriers in age group a, is the modelled number of NVT carriers in age group a, is the observed number of NVT in age group a. The constant here is independent of the modelled number of cases.
The MCMC was run with 100,000 iterations of the chain and for each accepted set of parameters the post-vaccination simulation up to 15 years after vaccine introduction was computed, after a burn-in of 20,000 iterations.
Convergence of the log likelihood was first assessed visually and then with the Geweke test of convergence.49
In the calculation of the log-likelihood for carriage, model estimates of VT carriage prevalence included VT carriers and VT-NVT co-colonization given the higher likelihood of the PCR assay to detect VT than NVT colonies in case of multiple colonization.38
Model parameters and input
Table 1 displays the value assigned to the different parameters governing transmission and vaccination. Uncertainty around parameters was taken into account by sampling from their posterior distribution in the MCMC process.
Table 1.
Parameters used in the model.
| Parameter | Value | Source* |
|---|---|---|
| Competition for carriage acquisition (CN and CV)$ | 0.1 (SD 0.01) | [18,19] |
| Duration of carriage (δi) | ||
| 0–11 months | 47.1 days | [49] |
| 12–23 months | 39.4 days | [49] |
| 24–35 months | 31.6 days | [49] |
| 36–47 months | 21.5 days | [49] |
| 48–59 months | 21.3 days | [49] |
| 5–17 years | 17.0 days | [48,50] |
| 18 years and over | 18.0 days | [48,50] |
| Vaccine efficacy against carriage | ||
| Full protection (VECf) | 63% (95%CrI 53 −73 %) | [17] |
| Partial protection (VECp) | 0.78 (95%CI 0.64 – 0.92) * VECf | Meta-analysis of [44–47] (Supporting Information Part Two) |
| Mean duration of protection against carriage | ||
| In fully protected (DF) | 6.0 years | [17](Supporting Information Part Two) |
| In partially protected (DP) | 0.78 (95%CI 0.64 – 0.92) * DF | Based on same assumptions as for Vaccine efficacy |
| Vaccine efficacy against IPD (VEIPD) | 0.80 (0.61 – 0.90) | [51] |
| Vaccine efficacy against invasiveness (VEINV) | 0.48 (0.34 – 0.65) | Derived from estimates of [51] and [17] (Supporting Information Part Two) |
| Mixing matrix | Social contact study (Nha trang) | Calculated as in Melegaro et al. [42] |
Where 0.1 means a force of infection which is 10% that of a situation with no competition.
We obtained the vaccine efficacy against carriage conferring full protection () and its uncertainty from a meta-regression model.17 We estimated the partial efficacy against carriage () as 0.78 (95% CI 0.64 – 0.92) that of through a meta-analysis of the relative risk of VT carriage in 2+0 schedules compared to 3+0 or 2+1 schedules, which included four trials and eight entry points.44-47 Further details are provided in the Supporting Information Part Two.
We assumed an exponential decay function for the waning of vaccine efficacy, as in previous models.19,48 We fixed the average duration of protection to 6 years, which best matched the output of an asymptomatic meta-regression model of waning efficacy,17 and analysed the impact of shorter (3 years) and longer (20 years) average protection in sensitivity analyses, based on the 95% credible intervals (CrI) of a model of waning efficacy.17
The average duration of protection following particular vaccination was assumed to be 0.78 (95%CI 0.64 – 0.92) that of a complete schedule, as for vaccine efficacy. Details are provided in the Supporting Information (Part Two).
In our base case model we set the vaccination coverage of both routine and catch-up strategies at 90%, in accordance with coverage data from Vietnam for routine immunization programmes and supplementary immunization activities.49
Sensitivity analyses
We ran the model for coverage levels of 50% and 70% in both routine and catch-up programmes, and compared the results with that of the main model (coverage 90%). We also explored the impact of duration of protection on our model outputs, based on lower values of 3 years and 20 years, which span across the range of likely values (Supporting Information Part Three).
Supplementary Material
Funding Statement
For this work Olivier le Polain was supported by a doctoral research fellowship from the AXA Research Fund. The baseline pneumococcal carriage and population data in Nha Trang was obtained from Nha Trang population based cohort study which was supported by Japan Global Research Network for Infectious Diseases (JGRID). For parts of this work Stefan Flasche was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 208812/Z/17/Z).
Ethics
Ethical approval for both the contact and nasopharyngeal surveys was granted by the institutional review board of the London School of Hygiene and Tropical Medicine and the Ethics Commission of National Institute of Hygiene and Epidemiology, Hanoi and the Nagasaki University.
Disclosure of potential conflicts of interest
OLP: none
WJE: WJE's partner works for GSK, who manufacture PCV10.
KT: none
KA: none
EKM: Kim Mulholland has consulted for GSK on PCV vaccine use and nutritional strategies to improve vaccine effectiveness.
DG: has served on ad-hoc advisory boards for Pfizer, GlaxoSmithKline and Merck, and the University College London Institute of Child Health Laboratory receives contract research funding from Pfizer, GlaxoSmithKline and Merck
YHC: none
DDA: none
LMY: none
SF: none
Acknowledgments
We would like to thank Ana Maria Henao Restrepo, Hope Johnson and Kate O'Brien for helpful discussions around the study objectives. We are grateful to the participants of this study and their parents as well as to the staff from Khanh-Hoa Health Service and the medical staff from Khanh Hoa General Hospital for their support. We also thank the staff from the Japan-Vietnam Friendship Laboratory at National Institute of Hygiene and Epidemiology, Hanoi, and Institute of Tropical Medicine, Nagasaki University.
References
- 1.Global Action Plan for prevention and control of pneumonia (GAPP). World Health Organization/The United Nations Children's Fund (UNICEF). 2009. [accessed 15 May 2018]. [http://whqlibdoc.who.int/hq/2009/WHO_FCH_CAH_NCH_09.04_eng.pdf].-
- 2.Cohen AL, Hyde TB, Verani J, Watkins M. Integrating pneumonia prevention and treatment interventions with immunization services in resource-poor countries. Bulletin of the World Health Organization. 2012;90(4):289–94. doi: 10.2471/BLT.11.094029. PMID:22511825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R et al.. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. PMID:20466419. [DOI] [PubMed] [Google Scholar]
- 4.Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem. 2012;287(33):27885–94. doi: 10.1074/jbc.M112.380451. PMID:22736767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global PCV introduction status. [Accessed 15 May 2018]. [http://view-hub.org/viz/].
- 6.Gavi, The Vaccine Alliance Advance Market Commitment for Pneumococcal Vaccines. Annual Report 1 January–31 December 2017. [accessed 15 May 2018]. [https://www.gavi.org/library/gavi.../amc/2017-pneumococcal-amc-annual-report/]. [Google Scholar]
- 7.Publication WHO Pneumococcal vaccines WHO position paper – 2012 – recommendations. Vaccine. 2012;30(32):4717–8. doi: 10.1016/j.vaccine.2012.04.093. PMID:22621828. [DOI] [PubMed] [Google Scholar]
- 8.Whitney CG, Goldblatt D, O'Brien KL. Dosing schedules for pneumococcal conjugate vaccine: considerations for policy makers. Pediatr Infect Dis J. 2014;33(Suppl 2):S172–181. doi: 10.1097/INF.0000000000000076. PMID:24336059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meeting of the Strategic Advisory Group of Experts on immunization October 2017 – conclusions and recommendations. Wkly Epidemiol Rec. 2017;92(48):729–47. PMID:29192459. [PubMed] [Google Scholar]
- 10.Flasche S, Ojal J, Le Polain de Waroux O, Otiende M, O'Brien KL, Kiti M, Nokes DJ, Edmunds WJ, Scott JAG. Assessing the efficiency of catch-up campaigns for the introduction of pneumococcal conjugate vaccine: a modelling study based on data from PCV10 introduction in Kilifi, Kenya. BMC Med. 2017;15(1):113. doi: 10.1186/s12916-017-0882-9. PMID:28592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anh DD, Kilgore PE, Slack MP, Nyambat B, Tho LH, Yoshida LM, Nguyen HA, Nguyen CD, Chong CY, Nguyen D et al.. Surveillance of pneumococcal-associated disease among hospitalized children in Khanh Hoa Province, Vietnam. Clin Infect Dis: Official publication Infect Dis Soc Am. 2009;48(Suppl 2):S57–64. doi: 10.1086/596483. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T et al.. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. PMID:19748398. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal N, Singh M, Das RR, Jindal I, Agarwal A, Thumburu KK, Kumar A, Chauhan A. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: a systematic review. PloS one. 2014;9(9):e108617. doi: 10.1371/journal.pone.0108617. PMID:25268974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmunds WJ, O'Callaghan CJ, Nokes DJ. Who mixes with whom? A method to determine the contact patterns of adults that may lead to the spread of airborne infections. Proc Biol Sci R Soc. 1997;264(1384):949–57. doi: 10.1098/rspb.1997.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J et al.. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoSMed. 2008;5(3):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. AmJEpidemiol. 2006;164(10):936–44. [DOI] [PubMed] [Google Scholar]
- 17.Le Polain De Waroux O, Flasche S, Prieto-Merino D, Goldblatt D, Edmunds WJ. The efficacy and duration of protection of pneumococcal conjugate vaccines against nasopharyngeal carriage: A meta-regression model. Pediatr Infect Dis J. 2015;34(8):858–64. doi: 10.1097/INF.0000000000000717. PMID:26075814. [DOI] [PubMed] [Google Scholar]
- 18.Auranen K, Mehtala J, Tanskanen A, Kaltoft S. Between-strain competition in acquisition and clearance of pneumococcal carriage–epidemiologic evidence from a longitudinal study of day-care children. AmJEpidemiol. 2010;171(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi YH, Jit M, Gay N, Andrews N, Waight PA, Melegaro A, George R, Miller E. 7-Valent pneumococcal conjugate vaccination in England and Wales: is it still beneficial despite high levels of serotype replacement? PLoSOne. 2011;6(10):e26190. doi: 10.1371/journal.pone.0026190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsitch M, Abdullahi O, D'Amour A, Xie W, Weinberger DM, Tchetgen Tchetgen E, Scott JA. Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology. 2012;23(4):510–9. doi: 10.1097/EDE.0b013e31824f2f32. PMID:22441543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J Clin Microbiol. 2007;45(3):948–52. doi: 10.1128/JCM.01913-06. PMID:17202280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Polain de Waroux O, Flasche S, Prieto-Merino D, Edmunds WJ. Age-dependent prevalence of nasopharyngeal carriage of streptococcus pneumoniae before conjugate vaccine introduction: a prediction model based on a meta-analysis. PloS one. 2014;9(1):e86136. doi: 10.1371/journal.pone.0086136. PMID:24465920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudan I, El Arifeen S, Bhutta ZA, Black RE, Brooks A, Chan KY, Chopra M, Duke T, Marsh D, Pio A et al.. Setting research priorities to reduce global mortality from childhood pneumonia by 2015. PLoS med. 2011;8(9):e1001099. doi: 10.1371/journal.pmed.1001099. PMID:21980266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine OS, Bhat N, Crawley J, Deloria-Knoll M, DeLuca AN, Driscoll AJ, Feikin DR, Karron RA, Murdoch DR, O'Brien KL et al.. Pneumonia etiology research for child health. Introduction. Clin Infect Dis Official publication Infect Dis Soc Am. 2012;54(Suppl 2):S87–88. doi: 10.1093/cid/cir1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flasche S, van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, George R, Miller E. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoSMed. 2011;8(4):e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124(1):e1–11. doi: 10.1542/peds.2008-3099. PMID:19564254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spijkerman J, van Gils EJ, Veenhoven RH, Hak E, Yzerman EP, van der Ende A, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerg Infect Dis. 2011;17(4):584–91. doi: 10.3201/eid1704.101115. PMID:21470445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghaffar F, Barton T, Lozano J, Muniz LS, Hicks P, Gan V, Ahmad N, McCracken GH Jr.. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis Official publication Infect Dis Soc Am. 2004;39(7):930–8. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 29.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004;23(11):1015–22. doi: 10.1097/01.inf.0000143645.58215.f0. PMID:15545856. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy TW, Singleton RJ, Bulkow LR, Bruden DL, Hurlburt DA, Parks D, Moore M, Parkinson AJ, Schuchat A, Butler JC. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23(48–49):5464–73. doi: 10.1016/j.vaccine.2005.08.100. PMID:16188350. [DOI] [PubMed] [Google Scholar]
- 31.Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, Hennessy TW. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006;193(11):1487–94. doi: 10.1086/503805. PMID:16652275. [DOI] [PubMed] [Google Scholar]
- 32.Nzenze SA, Shiri T, Nunes MC, Klugman KP, Kahn K, Twine R, de Gouveia L, von Gottberg A, Madhi SA. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J. 2013;32(11):1270–8. doi: 10.1097/01.inf.0000435805.25366.64. PMID:24061271. [DOI] [PubMed] [Google Scholar]
- 33.Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, Akisanya A, Litchfield T, Nsekpong DE, Oluwalana C et al.. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoSMed. 2011;8(10):e1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, Bwanaali T, Mumbo E, Kamau T, Sharif SK et al.. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2(7):e397–405. doi: 10.1016/S2214-109X(14)70224-4. PMID:25103393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adegbola RA, DeAntonio R, Hill PC, Roca A, Usuf E, Hoet B, Greenwood BM. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PloS one. 2014;9(8):e103293. doi: 10.1371/journal.pone.0103293. PMID:25084351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UNFPA. State of the World Population 2017. Worlds Apart, Reproductive Health and Rights in an Age of Inequality. Report. 2017 [https://www.unfpa.org/sites/default/files/sowp/downloads/UNFPA_PUB_2017_EN_SWOP.pdf].
- 37.Flasche S, Van Hoek AJ, Goldblatt D, Edmunds WJ, O'Brien KL, Scott JA, Miller E. The potential for reducing the number of pneumococcal conjugate vaccine doses while sustaining herd immunity in high-income countries. PLoS Med. 2015;12(6):e1001839. doi: 10.1371/journal.pmed.1001839. PMID:26057994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vu HT, Yoshida LM, Suzuki M, Nguyen HA, Nguyen CD, Nguyen AT, Oishi K, Yamamoto T, Watanabe K, Vu TD. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30(1):11–18. doi: 10.1097/INF.0b013e3181f111a2. PMID:20686433. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida LM, Nguyen HA, Watanabe K, Le MN, Nguyen AT, Vu HT, Yoshino H, Suzuki M, Takahashi K, Le T et al.. Incidence of radiologically-confirmed pneumonia and Haemophilus influenzae type b carriage before Haemophilus influenzae type b conjugate vaccine introduction in Central Vietnam. J Pediatr. 2013;163(1 Suppl):S38–43. doi: 10.1016/j.jpeds.2013.03.029. PMID:23773592. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien KL, Nohynek H. World Health Organization Pneumococcal Vaccine Trials Carriage Working G: Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22(2):e1–11. doi: 10.1097/01.inf.0000049347.42983.77. PMID:12586987. [DOI] [PubMed] [Google Scholar]
- 41.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J et al.. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. PMID:18366252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melegaro A, Jit M, Gay N, Zagheni E, Edmunds WJ. What types of contacts are important for the spread of infections?: using contact survey data to explore European mixing patterns. Epidemics. 2011;3(3–4):143–51. doi: 10.1016/j.epidem.2011.04.001. PMID:22094337. [DOI] [PubMed] [Google Scholar]
- 43.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301. doi: 10.1038/nrmicro1871. PMID:18340341. [DOI] [PubMed] [Google Scholar]
- 44.Dagan R, Givon-Lavi N, Porat N, Greenberg D. The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine. 2012;30(34):5132–40. doi: 10.1016/j.vaccine.2012.05.059. PMID:22683519. [DOI] [PubMed] [Google Scholar]
- 45.Russell FM, Carapetis JR, Satzke C, Tikoduadua L, Waqatakirewa L, Chandra R, Seduadua A, Oftadeh S, Cheung YB, Gilbert GL et al.. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clin Vaccine Immunol. 2010;17(12):1970–6. doi: 10.1128/CVI.00117-10. PMID:20943882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Gils EJ, Veenhoven RH, Hak E, Rodenburg GD, Bogaert D, Ijzerman EP, Bruin JP, van AL, Sanders EA. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA. 2009;302(2):159–67. doi: 10.1001/jama.2009.975. PMID:19584345. [DOI] [PubMed] [Google Scholar]
- 47.Ferraro CF, Trotter CL, Nascimento MC, Jusot JF, Omotara BA, Hodgson A, Ali O, Alavo S, Sow S, Daugla DM et al.. Household crowding, social mixing patterns and respiratory symptoms in seven countries of the African meningitis belt. PloS one. 2014;9(7):e101129. doi: 10.1371/journal.pone.0101129. PMID:24988195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melegaro A, Choi Y, Pebody R, Gay N. Pneumococcal carriage in United Kingdom families: estimating serotype-specific transmission parameters from longitudinal data. Am J Epidemiol. 2007;166(2):228–35. doi: 10.1093/aje/kwm076. PMID:17517684. [DOI] [PubMed] [Google Scholar]
- 49.Ali M, Canh GD, Clemens JD, Park JK, von Seidlein L, Minh TT, Thiem DV, Tho HL, Trach DD, Vaccine Safety Datalink G. The use of a computerized database to monitor vaccine safety in Viet Nam. Bull World Health Organ. 2005;83(8):604–10. PMID:16193545. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


