ABSTRACT
Public health benefits of childhood vaccinations risk being derailed by low vaccination coverage in low and middle-income countries. One reason for the low coverage is poor parental knowledge of the importance of completing vaccination schedules. We therefore assessed the effects on childhood vaccination coverage, of educating parents and other persons assuming the parental role. We prospectively registered the systematic review, published the protocol, and used standard Cochrane methods to collect and synthesise the evidence. We found six eligible randomised trials with 4248 participants. Three trials assessed health-facility based education of mothers on the importance of completing vaccination schedules; immediately after birth and three months later (one study) or during the first vaccination visit (two studies). The other trials assessed community-based education, including information campaigns on the importance of vaccines using audiotaped presentations and leaflet distributions (one study); structured group discussions on benefits and costs of childhood vaccination and local action plans for improving vaccine uptake (one study); and home-based information sessions using graphic cards showing benefits and costs of childhood vaccinations and location of vaccination centres (one study). Combining the data shows that these interventions lead to substantial improvements in childhood vaccination coverage (relative increase 36%, 95% confidence interval 14% to 62%). There was no difference between the effects of community-based and facility-based education. Therefore, education in communities and health facilities on the importance of childhood vaccinations should be integrated into all vaccination programmes in low and middle-income countries; accompanied by robust monitoring of impacts and use of data for action.
KEYWORDS: Childhood vaccination, demand creation, Education, low and middle-income countries, parents
Introduction
Vaccination is vital not only in averting infections, but also in mitigating the severity of infectious diseases and preventing some cancers.1,2 However, childhood vaccination coverage remains low in many low and middle-income countries, resulting in millions of vaccine-preventable child deaths each year2-4 The low vaccination coverage has been attributed to many reasons, including (but not limited to) parental knowledge and attitudes, and inadequate information and communication.4 In particular, poor understanding of the benefits of vaccines and vaccination schedules among parents is associated with low childhood vaccination coverage.5
Poor understanding of the vaccine benefits may be due to conflicting information parents receive about the importance and safety of vaccines.6 Therefore, it is important that parents and persons with parental roles are provided accurate information on the benefits of childhood vaccinations and adverse events following vaccination, so that they can make informed decisions regarding vaccination of their children.6-9 Active engagement and effective communication between the providers and recipients of vaccination services may be effective in improving vaccination coverage.10,11 We therefore conducted this systematic review to assess the effects of interventions for educating parents, compared to standard vaccination practices, on vaccination coverage in low and middle-income countries.
Methods
We registered this systematic review in the International Prospective Register of Systematic Reviews and published the protocol in a peer-reviewed journal.12,13 Studies eligible for inclusion in the review were randomised trials conducted in low or middle-income countries, as defined by the World Bank.14
We considered any intervention aimed at educating parents about the importance of childhood vaccinations, compared to standard vaccination practices. We defined parents as parents, legal guardians, or other persons assuming the parental role. Our outcome of interest was coverage with three doses of diphtheria-tetanus-pertussis containing vaccines (DTP3) or other vaccination status as reported by the trial authors.
We conducted a comprehensive search of peer-reviewed literature in multiple electronic databases; including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, Cumulative Index of Nursing and Allied Health (CINAHL), and PDQ (Pretty Darn Quick) Evidence. We conducted the initial search in May 2015, with an update in June 2016. Appendix 1 shows the search strategy we used for the various electronic databases. In addition, we searched the WHO International Clinical Trials Registry Platform, Clinicaltrials.gov, and reference lists of relevant reviews.
The output from the May 2015 search was independently screened for potentially eligible studies by LA Lukusa (LAL) and NN Mbeye (NNM). The output from the June 2016 search was independently screened by VN Ndze (VNN) and CS Wiysonge (CSW). Full texts of potentially eligible studies were retrieved and the two researchers independently assessed them for eligibility against the study inclusion criteria. All potentially eligible studies were published in English. At each stage, the two respective researchers compared their results and resolved disagreements by discussion and consensus.
LAL and VNN extracted data from eligible studies in duplicate, using a pre-designed and pilot-tested data collection form); and compared their results and resolved discrepancies by discussion and consensus. The data extracted included study design and methods, country setting, participant characteristics, intervention characteristics, outcome measures, and outcome data. The two authors also independently assessed the risk of bias in each included study using the Cochrane Risk of Bias Tool.15 We assessed the risk of selection bias by considering the adequacy of random sequence generation and allocation concealment, and the risk of performance bias by considering blinding of participants and personnel. For the risk of detection bias we assessed the blinding of outcome assessors. We used completeness of outcome data to assess the risk of attrition bias, and the completeness of outcome reporting for the risk of reporting bias. For each domain, we classified the risk of bias as low if the criterion was adequately addressed, high if the criterion was not adequately addressed, and unclear if the information provided was not sufficient to make an informed judgement. We summarised the assessment and categorised each included study either as having a low or a high risk of bias. Any study that had a high risk of selection, detection or attrition bias was categorised as having a high risk of bias. All other studies were considered to have a low risk of bias.
We conducted data analyses using the Cochrane Review Manager software (http://ims.cochrane.org/RevMan). For each included study, we calculated the natural logarithm of the risk ratio (RR) and its standard error. We then expressed the result of each study as a RR with its 95% confidence interval (CI). We included data from two eligible cluster randomised trials after controlling for the design effect, using the intra-cluster correlation coefficient.15
We assessed statistical heterogeneity using the chi-squared test of homogeneity, with significance defined at the 10% alpha level. When there was significant statistical heterogeneity, we used the random effects model to pool study results and assessed the source of heterogeneity using subgroup analyses. We defined subgroups by type of intervention i.e. community-based versus facility-based education. In addition, we quantified heterogeneity using the Higgins' I-squared statistic.15
We assessed the certainty of the evidence for each outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.16
Results
The two literature searches generated a total of 3568 records, and removing duplicates resulted to 3537 records. After screening summaries of the records, we discarded 3527 clearly irrelevant records. Of the 10 potentially eligible studies, we included six in the review.17-21 The remaining four studies were excluded as they were not randomized trials.22-25 Fig. 1 shows the search and selection of studies for this review. Table 1 summarises the characteristics of the six included studies.
Figure 1.
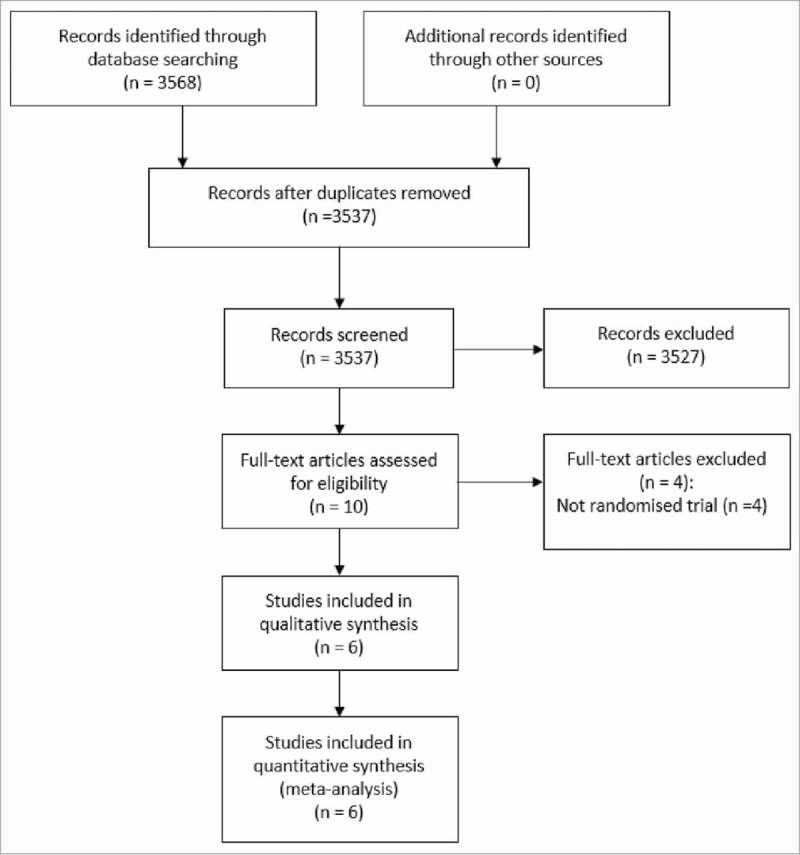
Flow chart showing study selection process.
Table 1.
Characteristics of included studies.
| Study | Design | Location | Interventions and comparisons | Outcome assessment method |
|---|---|---|---|---|
| Bolam 1998 | RCT | Kathmandu, Nepal | Mothers received: Group A: Health education immediately after birth and 3 months later. | DTP3 coverage assessed through self-reports of mothers. |
| Group B: Health education at birth only. | ||||
| Group C: Health education at three months only. | ||||
| Group D: No health education. | ||||
| Pandey 2007 | cRCT | Uttar Pradesh, India | Intervention: Information campaigns among poor rural populations in 2 rounds. Each round comprised 2–3 one-hour meetings using audiotaped presentation, question period, and leaflet distribution. Participants re-interviewed after 12 months. | Children less than one year old receiving one or more vaccine doses |
| Comparison: No intervention | Tetanus toxoid coverage in children assessed through self-reports of mothers. | |||
| Andersson 2009 | cRCT | Lasbela, Pakistan | Intervention: Three structured discussions separately with male and female groups. Sharing of information on local vaccination coverage; costs and benefits of childhood vaccination; & local action plans. Participants spread the information in their communities. | DTP3 coverage assessed through self-reports of mothers. |
| Comparison: No intervention. | ||||
| Usman 2009 | RCT | Pakistan | Group One: Reminder immunisation card. | DTP3 coverage assessed through vaccination cards. |
| Group Two: 2–3 minutes conversation with mother during DTP1 visit to motivate & convey adverse impact of incomplete vaccinations. | ||||
| Group Three: Both interventions. | ||||
| Group Four: No intervention. | ||||
| Owais 2011 | RCT | Karachi, Pakistan | Intervention: 5-minute home-based information sessions on importance of vaccines for low-literacy communities. They used graphic cards with 3 messages: vaccines save children's lives; location of vaccination centres; & importance of retaining cards. | DTP3 coverage assessed through vaccination cards. |
| Comparison: Verbal general health promotion messages delivered by trained CHWs. | ||||
| Usman 2011 | RCT | Karachi, Pakistan | Group One: Reminder immunisation card. | DTP3 coverage assessed through vaccination cards. |
| Group Two: 2–3 minutes discussions with mothers on importance of completing vaccination schedule. | ||||
| Group 3: Both interventions. | ||||
| Group 4: No intervention. |
CHWs: Community health workers; DTP3: Third dose of diphtheria-tetanus-pertussis containing vaccines; RCT: Individually randomised trial; cRCT: cluster randomised trial.
Three studies assessed the effects of educating parents in communities, outside of health facilities.5,17,19 One study was conducted in Karachi, Pakistan, with 179 mothers in the education group and 178 in the “no-education” control group.5 The second study was conducted among 18 education and 14 control clusters in Lasbela district in Pakistan.17 The third study was undertaken in Uttar Pradesh, India, among 11 education and 10 control clusters.19 These studies assessed the vaccination status of children through vaccination cards,5 or by self-reports by parents.17,19
The other three studies assessed the effects of educating parents at health facilities.18,20,21 The first study was conducted in Kathmandu, Nepal, with 205 mothers in the education group and 198 mothers in the control group.18 The second study, undertaken in Karachi, Pakistan, had 375 mothers each in the education and control arms.20 The last study, also conducted in Karachi, Pakistan, randomised 376 mothers to receive education and 378 in the control “no education” arm.21 The studies assessed vaccination coverage through self-reports,18 or vaccination cards.20,21
One study reported including information of adverse events following vaccination in discussions with parents.17
All six studies had adequate randomisation sequence generation. One study had adequate allocation concealment,17 but it was unclear whether the other five had adequate allocation concealment.5,18-21 Outcome assessors were not aware of intervention allocations in four studies, but this was not the case in the remaining two.20,21 One study had a high proportion of participants lost to follow-up (21–29% in each arm),18 but the others had a minimal proportion of participants lost to follow-up. Based on pre-specified criteria, we judged three studies to have a high risk of bias.18,20,21,Table 2 shows a summary of the risk of bias in included studies.
Table 2.
Summary of risk of bias in included studies.
| Bolam 1998 | Pandey 2007 | Andersson 2009 | Usman 2009 | Owais 2011 | Usman 2011 | |
|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | ○+ | ○+ | ○+ | ○+ | ○+ | ○+ |
| Allocation concealment (selection bias) | ○? | ○? | ○+ | ○? | ○? | ○? |
| Blinding of outcome assessment (detection bias) | ○+ | ○+ | ○+ | ○− | ○+ | ○− |
| Incomplete outcome data (attrition bias) | ○− | ○+ | ○+ | ○+ | ○+ | ○+ |
| Selective reporting (reporting bias) | ○? | ○? | ○? | ○? | ○? | ○? |
| Other bias | ○+ | ○− | ○− | ○+ | ○+ | ○+ |
○+ Low risk; ○− High risk; ○? Unclear risk.
Three studies assessed the effects of educating caregivers in communities, outside of health facilities.5,17,19 Two of these studies reported DTP3 coverage5,17 and the third reported coverage with at least one childhood vaccine.19 The latter was a cluster-randomised trial conducted from May 2004 to May 2005 in Uttar Pradesh, India, in which Pandey and colleagues found the vaccination coverage in the intervention and control clusters to be 72% (386/536) and 46% (225/489) respectively.19 In the second cluster randomised trial, conducted in Lasbela district of Balochistan province of Pakistan, from the spring of 2005 to the spring of 2007, Andersson and colleagues reported 53% (283/535) DTP3 coverage in intervention clusters compared to 24% (103/422) in control clusters.17 The third study was an individually randomised trial conducted by Owais and colleagues from August 2008 to March 2009 in Karachi Pakistan.5 The investigators reported DTP3 coverage of 72% (129/179) in the intervention group and 52% (92/178) in the control group. Pooling the data from the studies shows that community-based education improves childhood vaccination coverage (three trials, 2339 participants: RR 1.61, 95% CI 1.19 to 2.18, I2 = 37%) (Fig. 3).
Figure 3.
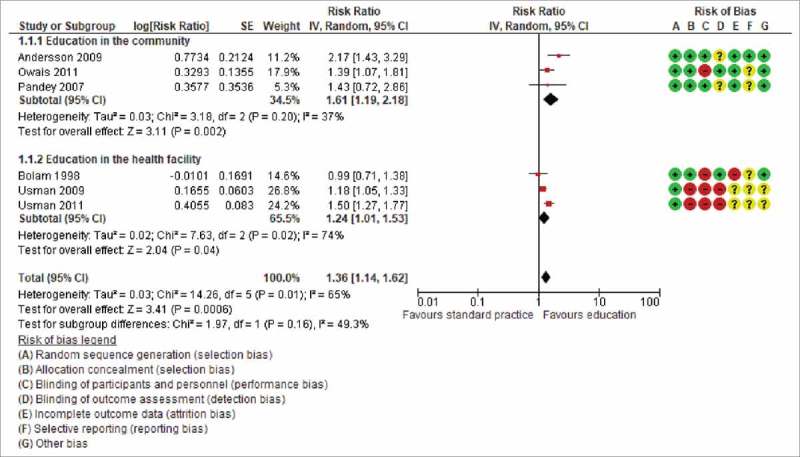
The effect of caregiver education on uptake of any vaccine among children.
The three studies that assessed facility-based education reported DTP3 coverage.18,20,21 The coverage in the education compared to no-education arms was respectively 87% (179/205) and 85% (169/198) in the trial conducted by Bolam and colleagues from November 1994 to May 1996.in Kathmandu, Nepal; 65%(242/375) and 55% (205/375) in the trial conducted by Usman and colleagues from September 2003 to March 2004 in urban Karachi in Pakistan; and 60% (228/378) and 39% (149/378) in the trial conducted by Usman and colleagues from November 2005 to August 2006 in rural Karachi in Pakistan. Combining data from the studies shows that facility-based education improves vaccination coverage (three trials, 1909 participants: RR 1.24, 95% CI 1.01 to 1.53, I2 = 74%) (Figures 2 and 3).
Figure 2.
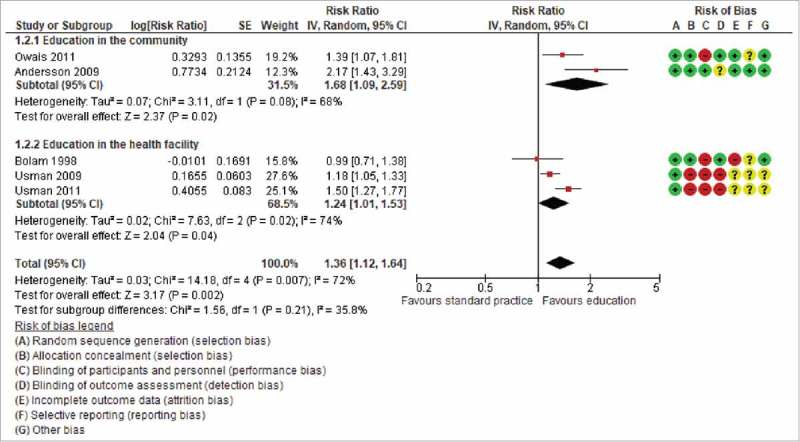
The effect of caregiver education on uptake of three doses of diphtheria-tetanus-pertussis containing vaccines among children.
Overall, the combined data show that education of parents improves vaccination coverage in low and middle-income countries (six trials; 4248 participants: RR 1.36, 95%CI 1.14 to 1.62; I2 = 65%) (Figs. 3). Table 3 shows our confidence in this evidence. Evidence from randomised trials is considered of high certainty in the GRADE framework; but we downgraded this to moderate because three of the six studies had a high risk of bias.
Table 3.
GRADE Summary of findings for the effects of caregiver education on vaccination coverage.
| Population: Parents and other persons assuming the parental role | |||||
| Settings: Low and middle-income countries | |||||
| Intervention: Education on benefits of childhood vaccinations and importance of completing vaccination schedules | |||||
|
Comparison: Standard practice | |||||
|
Illustrative comparative effects (95% CI) |
|||||
|
Outcomes |
Standard practice |
Education |
Relative effect (95% CI) |
No of Participants(studies) |
Quality of the evidence (GRADE) |
| Coverage with any vaccine | 51 per 100 | 69 per 100 (58 to 83) | RR 1.36 (1.14 to 1.62) | 3694 (6 studies) | ⊕⊕⊕⊝ moderate1 |
|
DTP3 coverage |
52 per 100 |
70 per 100 (58 to 85) |
RR 1.36 (1.12 to 1.64) |
3156 (5 studies) |
⊕⊕⊕⊝ moderate1 |
| The effect with education (and its 95% CI) is based on the assumed risk in the comparison group (i.e. standard practice) and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | |||||
| GRADE Working Group grades of evidence | |||||
| High certainty: Further research is very unlikely to change our confidence in the estimate of effect. | |||||
| Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||
| Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | |||||
| Very low certainty: We are very uncertain about the estimate. | |||||
We downgraded the evidence by one level (from high), because three of the studies have a high risk of bias.
Discussion
The target of the Global Vaccine Action Plan was to achieve 90% national coverage with all vaccines on national vaccination schedules in all 194 countries by 201526. However, only 129 (66%) countries achieved this coverage target by 2014.27 The ten countries with the largest numbers of un-immunised children are all low-income or lower-middle income countries.27 There is thus an urgent need for effective interventions that would ensure equitable uptake of existing vaccines by people in all communities around the world.26
In this systematic review, we have shown that caregiver education probably leads to substantial increases in childhood vaccination coverage. Although all six included trials were conducted in only three Asian countries (India, Nepal, and Pakistan), we have no reason to doubt the applicability of the evidence to other low and middle-income countries.
Only one study reported including information of adverse events following vaccination in the education sessions with parents.17 We are, therefore, uncertain of the extent to which the information of adverse events following vaccination impacted the decision making of parents. An adverse event following vaccination is defined as “any untoward medical occurrence which follows vaccination and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom, or disease”.27 Such an adverse event maybe a vaccine product-related reaction, a reaction due to an error in administration, a reaction related to vaccine quality defect, a vaccination anxiety related reaction, or a coincidental event.27
Ours is the most comprehensive and up to date review of randomised trial evidence of the effects of caregiver education on childhood vaccination coverage. However, our findings are consistent with those of related systematic reviews.1,29-31 Community-based evidence based discussions aimed at knowledge translation to community members may prove to be more effective than conventional health education strategies. However, the setting and scale of the targeted population may influence these findings. Kaufman and colleagues previously reviewed face-to-face interventions for informing or educating parents about early childhood vaccination, and reported that these interventions may have little to no impact on vaccination status or knowledge and understanding of vaccination.29 The apparent difference in our conclusions may be because Kaufman and colleagues included studies from all country settings.28 Therefore, provision of accurate vaccine information in communities and health facilities should be integrated into all childhood vaccination programmes in low and middle-income countries; accompanied by robust monitoring of the impact and use of data for action.
Appendix 1. Search strategy for electronic databases
| CENTRAL | |
|---|---|
| ID | Search |
| #1 | MeSH descriptor: [Immunization] this term only |
| #2 | MeSH descriptor: [Immunization Schedule] this term only |
| #3 | MeSH descriptor: [Immunization, Secondary] this term only |
| #4 | MeSH descriptor: [Immunotherapy, Active] this term only |
| #5 | MeSH descriptor: [Mass Vaccination] this term only |
| #6 | MeSH descriptor: [Immunization Programs] this term only |
| #7 | MeSH descriptor: [Vaccination] this term only |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 |
| #9 | MeSH descriptor: [Child] explode all trees |
| #10 | MeSH descriptor: [Infant] explode all trees |
| #11 | MeSH descriptor: [Mothers] this term only |
| #12 | MeSH descriptor: [Women] this term only |
| #13 | MeSH descriptor: [Pregnant Women] this term only |
| #14 | #9 or #10 or #11 or #12 or #13 |
| #15 | #8 and #14 |
| #16 | (immunization or immunisation or vaccination) next (program* or rate* or coverage or adher*):ti |
| #17 | (vaccinat* or revaccinat* or immunization or immunisation) near/3 (child* or infant* or newborn* or neonat* or baby or babies or kid or kids or toddler* or woman or women or mother or mothers):ti,ab,kw |
| #18 | #15 or #16 or #17 |
| #19 | (Africa or Asia or Caribbean or “West Indies” or “South America” or “Latin America” or “Central America”):ti,ab,kw |
| #20 | (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brazil or Brasil or Bulgaria or “Burkina Faso” or “Burkina Fasso” or “Upper Volta” or Burundi or Urundi or Cambodia or “Khmer Republic” or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or “Cape Verde” or “Central African Republic” or Chad or Chile or China or Colombia or Comoros or “Comoro Islands” or Comores or Mayotte or Congo or Zaire or “Costa Rica” or “Cote d'Ivoire” or “Ivory Coast” or Croatia or Cuba or Cyprus or Czechoslovakia or “Czech Republic” or Slovakia or “Slovak Republic”):ti,ab,kw |
| #21 | (Djibouti or “French Somaliland” or Dominica or “Dominican Republic” or “East Timor” or “East Timur” or “Timor Leste” or Ecuador or Egypt or “United Arab Republic” or “El Salvador” or Eritrea or Estonia or Ethiopia or Fiji or Gabon or “Gabonese Republic” or Gambia or Gaza or Georgia or Georgian or Ghana or “Gold Coast” or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or “Isle of Man” or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or “Kyrgyz Republic” or Kirghiz or Kirgizstan or “Lao PDR” or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania):ti,ab,kw |
| #22 | (Macedonia or Madagascar or “Malagasy Republic” or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or “Marshall Islands” or Mauritania or Mauritius or “Agalega Islands” or Mexico or Micronesia or “Middle East” or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or “Netherlands Antilles” or “New Caledonia” or Nicaragua or Niger or Nigeria or “Northern Mariana Islands” or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or “Puerto Rico”):ti,ab,kw |
| #23 | (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or “Saint Kitts” or “St Kitts” or Nevis or “Saint Lucia” or “St Lucia” or “Saint Vincent” or “St Vincent” or Grenadines or Samoa or “Samoan Islands” or “Navigator Island” or “Navigator Islands” or “Sao Tome” or “Saudi Arabia” or Senegal or Serbia or Montenegro or Seychelles or “Sierra Leone” or Slovenia or “Sri Lanka” or Ceylon or “Solomon Islands” or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or “Togolese Republic” or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or “Soviet Union” or “Union of Soviet Socialist Republics” or Uzbekistan or Uzbek or Vanuatu or “New Hebrides” or Venezuela or Vietnam or “Viet Nam” or “West Bank” or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia):ti,ab,kw |
| #24 | (developing or less* next developed or “under developed” or underdeveloped or “middle income” or low* next income or underserved or “under served” or deprived or poor*) next (countr* or nation* or population* or world):ti,ab,kw |
| #25 | (developing or less* next developed or “under developed” or underdeveloped or “middle income” or low* next income) next (economy or economies):ti,ab,kw |
| #26 | low* next (gdp or gnp or “gross domestic” or “gross national”):ti,ab,kw |
| #27 | (low near/3 middle near/3 countr*):ti,ab,kw |
| #28 | (lmic or lmics or “third world” or “lami country” or “lami countries”):ti,ab,kw |
| #29 | (“transitional country” or “transitional countries”):ti,ab,kw |
| #30 | (#19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29) |
| #31 | #18 and #30 in Trials |
MEDLINE
| # | Searches |
|---|---|
| 1 | Immunization/ |
| 2 | Immunization Schedule/ |
| 3 | Immunization, Secondary/ |
| 4 | Immunotherapy, Active/ |
| 5 | Mass Immunization/ |
| 6 | Immunization Programs/ |
| 7 | Vaccination/ |
| 8 | or/1–7 |
| 9 | exp Child/ |
| 10 | exp Infant/ |
| 11 | Mothers/ |
| 12 | Women/ |
| 13 | Pregnant Women/ |
| 14 | or/9–13 |
| 15 | 8 and 14 |
| 16 | ((vaccinat* or revaccinat* or immunization or immunisation) adj3 (child* or infant? or newborn? or neonat* or baby or babies or kid? or toddler? or woman or women or mother?)).ti,ab. |
| 17 | ((immunization or immunisation or vaccination) adj (program* or rate* or coverage or adher*)).ti. |
| 18 | 15 or 16 or 17 |
| 19 | Developing Countries.sh,kf. |
| 20 | (Africa or Asia or Caribbean or West Indies or South America or Latin America or Central America).hw,kf,ti,ab,cp. |
| 21 | (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brazil or Brasil or Bulgaria or Burkina Faso or Burkina Fasso or Upper Volta or Burundi or Urundi or Cambodia or Khmer Republic or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or Cape Verde or Central African Republic or Chad or Chile or China or Colombia or Comoros or Comoro Islands or Comores or Mayotte or Congo or Zaire or Costa Rica or Cote d'Ivoire or Ivory Coast or Croatia or Cuba or Cyprus or Czechoslovakia or Czech Republic or Slovakia or Slovak Republic or Djibouti or French Somaliland or Dominica or Dominican Republic or East Timor or East Timur or Timor Leste or Ecuador or Egypt or United Arab Republic or El Salvador or Eritrea or Estonia or Ethiopia or Fiji or Gabon or Gabonese Republic or Gambia or Gaza or Georgia Republic or Georgian Republic or Ghana or Gold Coast or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or Isle of Man or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or Kyrgyz Republic or Kirghiz or Kirgizstan or Lao PDR or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania or Macedonia or Madagascar or Malagasy Republic or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or Marshall Islands or Mauritania or Mauritius or Agalega Islands or Mexico or Micronesia or Middle East or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or Netherlands Antilles or New Caledonia or Nicaragua or Niger or Nigeria or Northern Mariana Islands or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or Puerto Rico or Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or Saint Kitts or St Kitts or Nevis or Saint Lucia or St Lucia or Saint Vincent or St Vincent or Grenadines or Samoa or Samoan Islands or Navigator Island or Navigator Islands or Sao Tome or Saudi Arabia or Senegal or Serbia or Montenegro or Seychelles or Sierra Leone or Slovenia or Sri Lanka or Ceylon or Solomon Islands or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or Togolese Republic or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or Soviet Union or Union of Soviet Socialist Republics or Uzbekistan or Uzbek or Vanuatu or New Hebrides or Venezuela or Vietnam or Viet Nam or West Bank or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia).hw,kf,ti,ab,cp. |
| 22 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab. |
| 23 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab. |
| 24 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab. |
| 25 | (low adj3 middle adj3 countr*).ti,ab. |
| 26 | (lmic or lmics or third world or lami countr*).ti,ab. |
| 27 | transitional countr*.ti,ab. |
| 28 | or/19–27 |
| 29 | 18 and 28 |
| 30 | randomized controlled trial.pt. |
| 31 | controlled clinical trial.pt. |
| 32 | pragmatic clinical trial.pt. |
| 33 | multicenter study.pt. |
| 34 | non-randomized controlled trials as topic/ |
| 35 | interrupted time series analysis/ |
| 36 | controlled before-after studies/ |
| 37 | (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab. |
| 38 | groups.ab. |
| 39 | (trial or impact or effect or multicenter or multi center or multicentre or multi centre).ti. |
| 40 | (intervention* or controlled or control group? or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. |
| 41 | or/30–40 |
| 42 | exp Animals/ |
| 43 | Humans/ |
| 44 | 42 not (42 and 43) |
| 45 | review.pt. |
| 46 | meta analysis.pt. |
| 47 | news.pt. |
| 48 | comment.pt. |
| 49 | editorial.pt. |
| 50 | cochrane database of systematic reviews.jn. |
| 51 | comment on.cm. |
| 52 | (systematic review or literature review).ti. |
| 53 | or/44–52 |
| 54 | 41 not 53 |
| 55 | 29 and 54 |
CINAHL
| # | Query |
|---|---|
| S54 | S16 AND S34 AND S52 [Exclude MEDLINE records] |
| S53 | S16 AND S34 AND S52 |
| S52 | S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 |
| S51 | TI (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or multicenter or “multi center” or multicentre or “multi centre” or controlled or groups or before N5 after or pre N5 post or ((pretest or “pre test”) and (posttest or “post test”)) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or “time series” or time W0 point* or repeated W0 measur*) OR AB (randomis* or randomiz* or randomly or trial or effect* or impact* or intervention* or multicenter or “multi center” or multicentre or “multi centre” or controlled or groups or before N5 after or pre N5 post or ((pretest or “pre test”) and (posttest or “post test”)) or quasiexperiment* or quasi W0 experiment* or evaluat* or “time series” or time W0 point* or repeated W0 measur*) |
| S50 | (MH “Health Services Research”) |
| S49 | (MH “Experimental Studies+”) |
| S48 | (MH “Time Series”) |
| S47 | (MH “Multiple Time Series”) |
| S46 | (MH “Interrupted Time Series Analysis”) |
| S45 | (MH “Repeated Measures”) |
| S44 | (MH “Multicenter Studies”) |
| S43 | (MH “Quasi-Experimental Studies”) |
| S42 | (MH “Pretest-Posttest Design”) |
| S41 | (MH “Pretest-Posttest Control Group Design”) |
| S40 | (MH “Nonrandomized Trials”) |
| S39 | (MH “Intervention Trials”) |
| S38 | (MH “Clinical Trials”) |
| S37 | (MH “Randomized Controlled Trials”) |
| S36 | PT clinical trial |
| S35 | PT randomized controlled trial |
| S34 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 |
| S33 | TI transitional W0 countr* OR AB transitional W0 countr* |
| S32 | TI ( lmic or lmics or third W0 world or lami W0 countr* ) OR AB ( lmic or lmics or third W0 world or lami W0 countr* ) |
| S31 | TI low N3 middle N3 countr* OR AB low N3 middle N3 countr* |
| S30 | TI ( low* W0 (gdp or gnp or gross W0 domestic or gross W0 national) ) OR AB ( low* W0 (gdp or gnp or gross W0 domestic or gross W0 national) ) |
| S29 | TI ( (developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income) W0 (economy or economies) ) OR AB ( (developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income) W0 (economy or economies) ) |
| S28 | TI ( (developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income or underserved or under W0 served or deprived or poor*) W0 (countr* or nation or nations or population* or world or area or areas) ) OR AB ( (developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income or underserved or under W0 served or deprived or poor*) W0 (countr* or nation or nations or population* or world or area or areas) ) |
| S27 | MW ( Afghanistan or Bangladesh or Benin or “Burkina Faso” or Burundi or Cambodia or “Central African Republic” or Chad or Comoros or Congo or “Cote d'Ivoire” or Eritrea or Ethiopia or Gambia or Ghana or Guinea or Haiti or India or Kenya or Korea or Kyrgyz or Kyrgyzstan or Lao or Laos or Liberia or Madagascar or Malawi or Mali or Mauritania or Melanesia or Mongolia or Mozambique or Burma or Myanmar or Nepal or Niger or Nigeria or Pakistan or Rwanda or “Salomon Islands” or “Sao Tome” or Senegal or “Sierra Leone” or Somalia or Sudan or Tajikistan or Tanzania or Timor or Togo or Uganda or Uzbekistan or Vietnam or “Viet Nam” or Yemen or Zambia or Zimbabwe ) or TI ( Afghanistan or Bangladesh or Benin or “Burkina Faso” or Burundi or Cambodia or “Central African Republic” or Chad or Comoros or Congo or “Cote d'Ivoire” or Eritrea or Ethiopia or Gambia or Ghana or Guinea or Haiti or India or Kenya or Korea or Kyrgyz or Kyrgyzstan or Lao or Laos or Liberia or Madagascar or Malawi or Mali or Mauritania or Melanesia or Mongolia or Mozambique or Burma or Myanmar or Nepal or Niger or Nigeria or Pakistan or Rwanda or “Salomon Islands” or “Sao Tome” or Senegal or “Sierra Leone” or Somalia or Sudan or Tajikistan or Tanzania or Timor or Togo or Uganda or Uzbekistan or Vietnam or “Viet Nam” or Yemen or Zambia or Zimbabwe ) or AB ( Afghanistan or Bangladesh or Benin or “Burkina Faso” or Burundi or Cambodia or “Central African Republic” or Chad or Comoros or Congo or “Cote d'Ivoire” or Eritrea or Ethiopia or Gambia or Ghana or Guinea or Haiti or India or Kenya or Korea or Kyrgyz or Kyrgyzstan or Lao or Laos or Liberia or Madagascar or Malawi or Mali or Mauritania or Melanesia or Mongolia or Mozambique or Burma or Myanmar or Nepal or Niger or Nigeria or Pakistan or Rwanda or “Salomon Islands” or “Sao Tome” or Senegal or “Sierra Leone” or Somalia or Sudan or Tajikistan or Tanzania or Timor or Togo or Uganda or Uzbekistan or Vietnam or “Viet Nam” or Yemen or Zambia or Zimbabwe ) |
| S26 | MW ( Albania or Algeria or Angola or Armenia or Azerbaijan or Belarus or Bhutan or Bolivia or Bosnia or Herzegovina or “Cape Verde” or Cameroon or China or Colombia or Congo or Cuba or Djibouti or “Dominican Republic” or Ecuador or Egypt or “El Salvador” or Fiji or Gaza or Georgia or Guam or Guatemala or Guyana or Honduras or “Indian Ocean Islands” or Indonesia or Iran or Iraq or Jamaica or Jordan or Kiribati or Lesotho or Macedonia or Maldives or “Marshall Islands” or Micronesia or “Middle East” or Moldova or Morocco or Namibia or Nicaragua or Palestin* or Paraguay or Peru or Philippines or Samoa or “Sri Lanka” or Suriname or Swaziland or Syria or “Syrian Arab Republic” or Thailand or Tonga or Tunisia or Turkmenistan or Ukraine or Vanuatu or “West Bank” ) or TI ( Albania or Algeria or Angola or Armenia or Azerbaijan or Belarus or Bhutan or Bolivia or Bosnia or Herzegovina or “Cape Verde” or Cameroon or China or Colombia or Congo or Cuba or Djibouti or “Dominican Republic” or Ecuador or Egypt or “El Salvador” or Fiji or Gaza or Georgia or Guam or Guatemala or Guyana or Honduras or “Indian Ocean Islands” or Indonesia or Iran or Iraq or Jamaica or Jordan or Kiribati or Lesotho or Macedonia or Maldives or “Marshall Islands” or Micronesia or “Middle East” or Moldova or Morocco or Namibia or Nicaragua or Palestin* or Paraguay or Peru or Philippines or Samoa or “Sri Lanka” or Suriname or Swaziland or Syria or “Syrian Arab Republic” or Thailand or Tonga or Tunisia or Turkmenistan or Ukraine or Vanuatu or “West Bank” ) or AB ( Albania or Algeria or Angola or Armenia or Azerbaijan or Belarus or Bhutan or Bolivia or Bosnia or Herzegovina or “Cape Verde” or Cameroon or China or Colombia or Congo or Cuba or Djibouti or “Dominican Republic” or Ecuador or Egypt or “El Salvador” or Fiji or Gaza or Georgia or Guam or Guatemala or Guyana or Honduras or “Indian Ocean Islands” or Indonesia or Iran or Iraq or Jamaica or Jordan or Kiribati or Lesotho or Macedonia or Maldives or “Marshall Islands” or Micronesia or “Middle East” or Moldova or Morocco or Namibia or Nicaragua or Palestin* or Paraguay or Peru or Philippines or Samoa or “Sri Lanka” or Suriname or Swaziland or Syria or “Syrian Arab Republic” or Thailand or Tonga or Tunisia or Turkmenistan or Ukraine or Vanuatu or “West Bank” ) |
| S25 | MW ( “American Samoa” or Argentina or Belize or Botswana or Brazil or Brasil or Bulgaria or Chile or Comoros or “Costa Rica” or Croatia or Dominica or Guinea or Gabon or Grenada or Grenadines or Hungary or Kazakhstan or Latvia or Lebanon or Libia or libyan or Libya or Lithuania or Malaysia or Mauritius or Mayotte or Mexico or Micronesia or Montenegro or Nevis or “Northern Mariana Islands” or Oman or Palau or Panama or Poland or Romania or Russia or “Russian Federation” or Samoa or “Saint Lucia” or “St Lucia” or “Saint Kitts” or “St Kitts” or “Saint Vincent” or “St Vincent” or Serbia or Seychelles or Slovakia or “Slovak Republic” or “South Africa” or Turkey or Uruguay or Venezuela or Yugoslavia ) or TI ( “American Samoa” or Argentina or Belize or Botswana or Brazil or Bulgaria or Chile or Comoros or “Costa Rica” or Croatia or Dominica or Guinea or Gabon or Grenada or Grenadines or Hungary or Kazakhstan or Latvia or Lebanon or Libia or libyan or Libya or Lithuania or Malaysia or Mauritius or Mayotte or Mexico or Micronesia or Montenegro or Nevis or “Northern Mariana Islands” or Oman or Palau or Panama or Poland or Romania or Russia or “Russian Federation” or Samoa or “Saint Lucia” or “St Lucia” or “Saint Kitts” or “St Kitts” or “Saint Vincent” or “St Vincent” or Serbia or Seychelles or Slovakia or “Slovak Republic” or “South Africa” or Turkey or Uruguay or Venezuela or Yugoslavia ) or AB ( “American Samoa” or Argentina or Belize or Botswana or Brazil or Bulgaria or Chile or Comoros or “Costa Rica” or Croatia or Dominica or Guinea or Gabon or Grenada or Grenadines or Hungary or Kazakhstan or Latvia or Lebanon or Libia or libyan or Libya or Lithuania or Malaysia or Mauritius or Mayotte or Mexico or Micronesia or Montenegro or Nevis or “Northern Mariana Islands” or Oman or Palau or Panama or Poland or Romania or Russia or “Russian Federation” or Samoa or “Saint Lucia” or “St Lucia” or “Saint Kitts” or “St Kitts” or “Saint Vincent” or “St Vincent” or Serbia or Seychelles or Slovakia or “Slovak Republic” or “South Africa” or Turkey or Uruguay or Venezuela or Yugoslavia ) |
| S24 | TI ( Africa or Asia or “South America” or “Latin America” or “Central America” ) or AB ( Africa or Asia or “South America” or “Latin America” or “Central America” ) |
| S23 | (MH “Asia+”) |
| S22 | (MH “West Indies+”) |
| S21 | (MH “South America+”) |
| S20 | (MH “Latin America”) |
| S19 | (MH “Central America+”) |
| S18 | (MH “Africa+”) |
| S17 | (MH “Developing Countries”) |
| S16 | S13 OR S14 OR S15 |
| S15 | TI (immunization or immunisation or vaccination) W0 (program* or rate* or coverage or adher*) |
| S14 | TI ( (vaccinat* or revaccinat* or immunization or immunisation) N3 (child* or infant or infants or newborn or neonat* or baby or babies or kid or kids or toddler* or woman or women or mother*) ) OR AB ( (vaccinat* or revaccinat* or immunization or immunisation) N3 (child* or infant or infants or newborn or neonat* or baby or babies or kid or kids or toddler* or woman or women or mother*) ) |
| S13 | S5 AND S12 |
| S12 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 |
| S11 | (MH “Expectant Mothers”) |
| S10 | (MH “Women”) |
| S9 | (MH “Mothers”) |
| S8 | (MH “Infant, Newborn”) |
| S7 | (MH “Infant”) |
| S6 | (MH “Child”) |
| S5 | S1 or S2 or S3 or S4 |
| S4 | (MH “Immunization Programs”) |
| S3 | (MH “Immunotherapy”) |
| S2 | (MH “Immunization Schedule”) |
| S1 | (MH “Immunization”) |
Funding Statement
This article is based on research supported in part by the South African Medical Research Council and the National Research Foundation of South Africa (Grant Numbers: 108571 and 106035).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is sponsored partly by the South African Medical Research Council and the National Research Foundation of South Africa (Grant Numbers: 106035 and 108571).
Contributions of authors
LAL and NVN contributed to screening, data extraction, analysis, and write up. CSW conceived the study, contributed to screening, data extraction, data analysis, and write up. NVN and CSW critically revised successive drafts of the manuscript. All authors reviewed the drafts and approved the final version of the review.
References
- 1.Oyo-Ita A, Wiysonge CS, Oringanje C, Nwachukwu CE, Oduwole O, Meremikwu MM. Interventions for improving coverage of childhood immunisation in low- and middle-income countries. Cochrane Database of Systematic Reviews. 2016;10:CD008145. doi: 10.1002/14651858.CD008145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauke-Moagi BE, Mumba M. New vaccine introduction in the East and Southern African sub-region of the WHO African Region in the context of GIVS and MDGs. Vaccine. 2012;30:C3–C8. doi: 10.1016/j.vaccine.2012.05.086. PMID:22939018. [DOI] [PubMed] [Google Scholar]
- 3.Levine OS, Bloom DE, Cherian T, de Quadros C, Sow S, Wecker J, Duclos P, Greenwood B. The future of immunisation policy, implementation, and financing. Lancet. 2011;378:439–48. doi: 10.1016/S0140-6736(11)60406-6. PMID:21664676. [DOI] [PubMed] [Google Scholar]
- 4.Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. Individual and contextual factors associated with low childhood immunisation coverage in sub-Saharan Africa: A multilevel analysis. PLoS ONE. 2012;7:e37905. doi: 10.1371/journal.pone.0037905. PMID:22662247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owais A, Hanif B, Siddiqui AR, Agha A, Zaidi AK. Does improving maternal knowledge of vaccines impact infant immunization rates? A community-based randomized-controlled trial in Karachi, Pakistan. BMC Public Health. 2011;11:239. doi: 10.1186/1471-2458-11-239. PMID:21496343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis N, Hill S, Kaufman J, Lewin S, Kis-Rigo J, De Castro Freire SB, Bosch-Capblanch X, Glenton C, Lin V, Robinson P, et al.. “Communicate to vaccinate”: the development of a taxonomy of communication interventions to improve routine childhood vaccination. BMC Int Health Hum Rights. 2013;13:23. doi: 10.1186/1472-698X-13-23. PMID:23663327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams SE, Rothman RL, Offit PA, Schaffner W, Sullivan M, Edwards KM. A randomized trial to increase acceptance of childhood vaccines by vaccine-hesitant parents: a pilot study. Acad Pediatr. 2013;13:475–80. doi: 10.1016/j.acap.2013.03.011. PMID:24011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowda C, Schaffer SE, Kopec K, Markel A, Dempsey AF. Pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum Vaccin Immunother. 2013;9:437–45. doi: 10.4161/hv.22821. PMID:23291937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett RJ, Larson HJ, Moloi MH, Tshatsinde EA, Meheus A, Paterson P, François G. Addressing public questioning and concerns about vaccination in South Africa: A guide for healthcare workers. Vaccine. 2012;30:C72–8. doi: 10.1016/j.vaccine.2012.03.037. PMID:22939026. [DOI] [PubMed] [Google Scholar]
- 10.Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335:24–27. doi: 10.1136/bmj.39246.581169.80. PMID:17615222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams N, Woodward H, Majeed A, Saxena S. Primary care strategies to improve childhood immunisation uptake in developed countries: systematic review. JRSM Short Rep. 2011;2:81. doi: 10.1258/shorts.2011.011112. PMID:22046500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukusa LA, Adeniyi F, Wiysonge CS. Effects of interventions to inform or educate parents or caregivers about childhood vaccination in low and middle income countries. PROSPERO2014:CRD42014010141.[http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42014010141]. [DOI] [PMC free article] [PubMed]
- 13.Lukusa LA, Mbeye NN, Adeniyi FB, Wiysonge CS. Protocol for a systematic review of the effects of interventions to inform or educate caregivers about childhood vaccination in low and middle-income countries. BMJ Open. 2015;5:e008113. doi: 10.1136/bmjopen-2015-008113. PMID:26169807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The World Bank Group Data. Countries and Economies. Available from http://data.worldbank.org/country (accessed 16May2015). [Google Scholar]
- 15.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- 16.Wiysonge CS, Ngcobo NJ, Jeena PM, Madhi SA, Schoub BD, Hawkridge A, Shey MS, Hussey GD. Advances in childhood immunisation in South Africa where to now? Programme managers' views and evidence from systematic reviews. BMC Public Health. 2012;12:578. doi: 10.1186/1471-2458-12-578. PMID:22849711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson N, Cockcroft A, Ansari NM, Omer K, Baloch M, Ho Foster A, Shea B, Wells GA, Soberanis JL. Evidence-based discussion increases childhood vaccination uptake: a randomised cluster controlled trial of knowledge translation in Pakistan. BMC International Health and Human Rights. 2009;9(Suppl 1):S8. doi: 10.1186/1472-698X-9-S1-S8. PMID:19828066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolam A, Manandhar DS, Shrestha P, Ellis M, Costello AM. The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: a randomised controlled trial. BMJ. 1998;316:805–11. doi: 10.1136/bmj.316.7134.805. PMID:9549449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey P, Sehgal RA, Riboud M, Levine D, Goyal M. Informing Resource-Poor Populations and the Delivery of Entitled Health and Social Services in Rural India A Cluster Randomized Controlled Trial. JAMA. 2007;298:1867–75. doi: 10.1001/jama.298.16.1867. PMID:17954538. [DOI] [PubMed] [Google Scholar]
- 20.Usman RH, Akhtarb S, Habibc F, Jehana I. Redesigned immunization card and center-based education to reduce childhood immunization dropouts in urban Pakistan: A randomized controlled trial. Vaccine. 2009;27:467–72. doi: 10.1016/j.vaccine.2008.10.048. PMID:18996423. [DOI] [PubMed] [Google Scholar]
- 21.Usman RH, Rahbar HM, Kristensen S, Vermund SH, Kirby RS, Habib F, Chamot E. Randomized controlled trial to improve childhood immunization adherence in rural Pakistan: redesigned immunization card and maternal education. Trop Med Int Health. 2011;16:334–42. doi: 10.1111/j.1365-3156.2010.02698.x. PMID:21159080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul Rahman MA, Al-Dabbagh SA, Al-Habeeb QS. Health education and peer leaders' role in improving low vaccination coverage in Akre district, Kurdistan region, Iraq. Eastern Mediterranean Health Journal. 2013;19:125–9. doi: 10.26719/2013.19.2.125. PMID:23516821. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Luo S, Tang X, Lou L, Chen Y, Guo J, Zhang B. Does introducing an immunization package of services for migrant children improve the coverage, service quality and understanding? An evidence from an intervention study among 1548 migrant children in eastern China. BMC Public Health. 2015;15:664. doi: 10.1186/s12889-015-1998-5. PMID:26173803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johri M, Chandra D, Kone GK, Dudeja S, Sylvestre MP, Sharma JK, et al.. Interventions to increase immunisation coverage among children 12–23 months of age in India through participatory learning and community engagement: pilot study for a cluster randomised trial. BMJ Open. 2015;5:e007972. doi: 10.1136/bmjopen-2015-007972. PMID:26384721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keoprasith B, Kizuki M, Watanabe M, Takano T. The impact of community-based, workshop activities in multiple local dialects on the vaccination coverage, sanitary living and the health status of multiethnic populations in Lao PDR. Health Promotion International. 2013;28:453–65. doi: 10.1093/heapro/das030. PMID:22773609. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization Global Vaccine Action Plan 2011 – 2020. http://www.who.int/immunization/global_vaccine_action_plan/en/ (accessed 07 December 2015). [Google Scholar]
- 27.Strategic Advisory Group of Experts on Immunisation 2015. Assessment report of the Global Vaccine Action Plan. http://www.who.int/immunization/global_vaccine_action_plan/SAGE_GVAP_Assessment_Report_2015_EN.pdf (accessed, 07December2015).
- 28.World Health Organization Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification (Second edition). Geneva: WHO; 2018. http://www.who.int/vaccine_safety/publications/gvs_aefi/en/ (accessed, 07March2018) [Google Scholar]
- 29.Kaufman J, Synnot A, Ryan R, Hill S, Horey D, Willis N, Lin V, Robinson P. Face to face interventions for informing or educating parents about early childhood vaccination. Cochrane Database syst Rev. 2013;31(5):CD010038. [DOI] [PubMed] [Google Scholar]
- 30.Saeterdal I, Lewin S, Austvoll-Dahlgren A, Glenton C, Munabi-Babigumira S. Interventions aimed at communities to inform and/or educate about early childhood vaccination. Cochrane Database syst Rev. 2014;19(11):CD010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey H, Reissland N, Mason J. Parental reminder, recall and educational interventions to improve early childhood immunisation uptake: A systematic review and meta-analysis. Vaccine. 2015;33:2862–80. doi: 10.1016/j.vaccine.2015.04.085. [DOI] [PubMed] [Google Scholar]


