Abstract
The effective production and usage of ginsenosides, given their distinct pharmacological effects, are receiving increasing amounts of attention. As the ginsenosides content differs in different parts of Panax ginseng, we wanted to assess and compare the ginsenosides content in the ginseng roots, leave, stems, and berries. To extract the ginsenosides, 70% (v/v) methanol was used. The optimal ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (UPLC-QTOF/MS) method was used to profile various ginsenosides from the different parts of P. ginseng. The datasets were then subjected to multivariate analysis including principal component analysis (PCA) and hierarchical clustering analysis (HCA). A UPLC-QTOF/MS method with an in-house library was constructed to profile 58 ginsenosides. With this method, a total of 39 ginsenosides were successfully identified and quantified in the ginseng roots, leave, stem, and berries. PCA and HCA characterized the different ginsenosides compositions from the different parts. The quantitative ginsenoside contents were also characterized from each plant part. The results of this study indicate that the UPLC-QTOF/MS method can be an effective tool to characterize various ginsenosides from the different parts of P. ginseng.
Keywords: Panax ginseng, ginsenosides, UPLC-QTOF/MS
1. Introduction
In the herbal markets and food industries of Korea and other East Asian countries, Panax ginseng C.A. Meyer is one of the most important herbal plants. Its roots have been used as a main constituent in oriental medicine [1,2]. P. ginseng root contains many bioactive compounds including ginsenosides, peptides, polyacetylenic alcohols, fatty acids, and essential oils [3]. Among these compounds, ginsenosides are considered critical ingredients, having various pharmacological effects, such as enhancing the immune system, and anti-tumor and anti-diabetes activities [4,5,6,7]. Each ginsenoside has specific activities, and the efficacy of ginseng is determined by the ginsenoside contents. Due to its biological utility, the production and use of ginsenosides has been receiving increased attention. More than 150 ginsenosides have been isolated and identified from the P. ginseng plant [8,9]. In particular, its distinct parts including the roots, leaves, stems, and berries are considered as good sources of ginsenosides [10,11,12]. The qualitative and quantitative ginsenoside contents may differ depending on the plant part of P. ginseng [13,14]. Hence, to evaluate the efficacy of different ginseng parts and their effective production of ginsenosides, profiling the various ginsenosides from the distinct parts of P. ginseng is required.
To assess the levels of various ginsenosides from a complex P. ginseng extract, a sensitive and reliable analytical method is necessary. To analyze ginsenosides, high performance liquid chromatography (HPLC) coupled with a UV detector [15,16], or an evaporative light scattering detector (ELSD) [17,18] are typically used. However, due to the limited availability of standard samples, only a few ginsenosides have been quantified. Liquid chromatography (LC) coupled with electrospray ionization/mass spectrometry (ESI/MS) has emerged as a robust tool for the comprehensive profiling of various ginsenosides [19,20,21]. Within MS, a full mass scan based on quadrupole time of flight (QTOF)/MS is a sensitive detector used to measure the exact mass values of target and non-target compounds [22,23]. The ultra-performance LC (UPLC) system provides high resolution separation using a small particle size column, and is effective for analyzing various compounds in a complex mixture [24].
In this study, UPLC-QTOF/MS with an in-house library was used to perform a comprehensive profiling of ginsenosides from the roots, leave, stems, and berries of P. ginseng. To construct an in-house library and the quantification of ginsenosides, we used a total of 58 standard samples, including malonyl-ginsenosides (MGRs), isolated in our laboratory. The qualitative and quantitative contents of ginsenosides were characterized in the distinct parts of P. ginseng.
2. Materials and Methods
2.1. Standard Constituents and Reagents
HPLC-grade methanol (MeOH), water, and ACN were obtained from Fisher Scientific Korea (Seoul, Korea). HPLC-grade formic acid was purchased from Fluka Analytical, Sigma Aldrich Chemie GmbH (Steinheim, Germany). The ginsenoside standards used in this study were isolated and purified from P. ginseng roots and red ginseng by a series of chromatography procedures in our laboratory, and the information for 30 ginsenosides was reported in our previous study [25]. The structures of other ginsenosides were determined based on the spectroscopic data (MS, hydrogen nuclear magnetic resonance (1H-NMR), and carbon-13 (13C)-NMR) in the previous literatures: fKa [26], nR4 [27], nR2-R [28], Rh1-R [29], Ra3 [26], M-Rb1 [30], M-Rc [31], M-Rb2 [30], M-Rd [31], CO [32], GyL [33], GyLI [33], nFt1 [34], Rg3-R [29], Mc [35], and CY [35]. Rf, Rk1, Ro, Rg2-R, PPD, and PPT were purchased from Sigma Aldrich (St. Louis, MO, USA). Rk3, Rh4, GyXVII, and XLIX were purchased from Chemfaces (Wuhan, China). Rk2 and GyA were purchased from ALB technology (Mongkok Kowloon, Hong Kong, China).
2.2. Panax ginseng Samples
Panax ginseng was cultivated in the experimental field of the National Institute of Horticultural and Herbal Science, Rural Development Administration (RDA) at Chungbuk, Eumseong, Korea (36° N, 127° E), according to the “ginseng GAP standard cultivation guide” protocol developed by RDA, Republic of Korea [36]. A voucher specimen (NIHHS-14S01) was deposited at the Herbarium of the Department of Herbal Crop Research, National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong, Republic of Korea. Six years into its growing season, in the month of July, the roots, stems and leaves, and berries were harvested, and stored in a deep freezer at −80 °C until further processing.
2.3. Extraction of Ginsenosides
The samples of ginseng roots, stems and leaves, and berries were dried at 40 °C in a forced-air, convection-drying oven for 48 h after washing, and then weighed. For the ginseng root, the main roots were used for experiments after removing the lateral and fine roots. Each sample was ground (<0.5 mm) using a mixer (Hanil, Seoul, Korea) and thoroughly mixed, and subsamples were homogenized further using a Retsch MM400 mixer mill (Retsch GmbH, Haan, Germany) for analyses. Fine powder was weighed (200 mg), suspended in 2 mL of 70% (v/v) methanol, and ultrasonically extracted for 30 min at 50 °C. Each sample was centrifuged at 13,500 rpm for 5 min. The solution was filtered through a syringe filter (0.22 µm) and injected directly into the UPLC system.
2.4. UPLC-QTOF/MS Conditions for Ginsenoside Profiling
UPLC was performed using a Waters ACQUITY H-Class UPLC (Waters Corp., Milford, MA, USA) with an ACQUITY BEH C18 column (2.1 mm × 100 mm; 1.7 µm). The column oven was maintained at 40 °C, and the mobile phases included solvent A [water and 0.1% formic acid (v/v)] and solvent B [acetonitrile and 0.1% formic acid (v/v)]. The elution gradient was as follows: 0–0.5 min, B 15%; 0.5–1 min, B 15–20%; 1–6 min, B 20%; 6–13 min, B 20–30%; 13–23 min, B 30–35%; 23–24 min, B 35–38%; 24–27 min, B 38–60%; 27–31 min, B 60–90%; 31–32 min, B 90–15%; and 32–35 min, B 15%. The flow rate was 450 μL/min, and the injection volume was 2 μL for each run.
Next, MS analysis was performed using a Waters Xevo G2-S QTOF MS (Waters Corp., Milford, MA, USA) operating in negative ion mode. The mass spectrometers performed alternating high- and low-energy scans, known as the MSE acquisition mode. The operating parameters were set as follows: cone voltage 40 V; capillary 3.0 kV; source temperature 120 °C; desolvation temperature 550 °C; cone gas flow 30 L/h; and desolvation gas flow at 800 L/h. Accurate mass measurements were obtained by means of an automated calibration delivery system that contained an internal reference [Leucine-enkephalin, m/z 554.262 (ESI-)]. Data were collected between 100 and 2000 m/z.
2.5. Data Processing and Multivariate Analysis
All MSE data were collected and processed using UNIFI 1.8 (Waters Corp., Milford, MA, USA). Data within UNIFI 1.8 were passed through the apex peak detection and alignment processing algorithms. The intensity of each ion was normalized with respect to the total ion count to generate a data matrix having RT, m/z value, and the normalized peak area. Charged species, salt adducts, and fragments were all automatically aligned and grouped. The three-dimensional data, including peak number (RT-m/z pair), sample name, and normalized peak areas, were exported to the EZinfo software 3.0.3 (UMETRICS) for PCA. The processed data were also entered into the MetaboAnalyst website (http://www.metaboanalyst.ca) to perform HCA.
3. Results and Discussion
3.1. UPLC-QTOF/MS with an In-House Library to Profile Various Ginsenosides
The sensitivity of MS-based analytical methods has increased to enable the detection of compounds with limited abundance in biological samples. Thus, LC-MS methods have been widely used to analyze major compounds in various herbal medicines. For the detailed characterization of P. ginseng, assessing the levels of both the high- and low-abundance ginsenosides is important. In our previous study [25], UPLC-QTOF/MS was used to analyze 30 ginsenosides. The optimal UPLC method was effective for separating various ginsenosides. In the identification of ginsenosides, QTOF/MS was also sensitive and reliable for measuring the exact mass values of the compounds. In this study, the LC-MS method was simultaneously applied to profile 58 ginsenosides. Figure S1 represented the molecular structures of these ginsenosides. First, these ginsenoside standards were purchased or obtained from the experiments by isolating and purifying them from P. ginseng. In our laboratory, we collected four MGRs that contained a malonyl residue, M-Rb1, M-Rb2, M-Rc, and M-Rd, which is linked to the glucose in the compounds. Isolating and purifying the MGRs from the extracted mixture of P. ginseng is difficult in practice. Due to their instability when exposed to heat, care must be taken when handling and preserving MGR compounds.
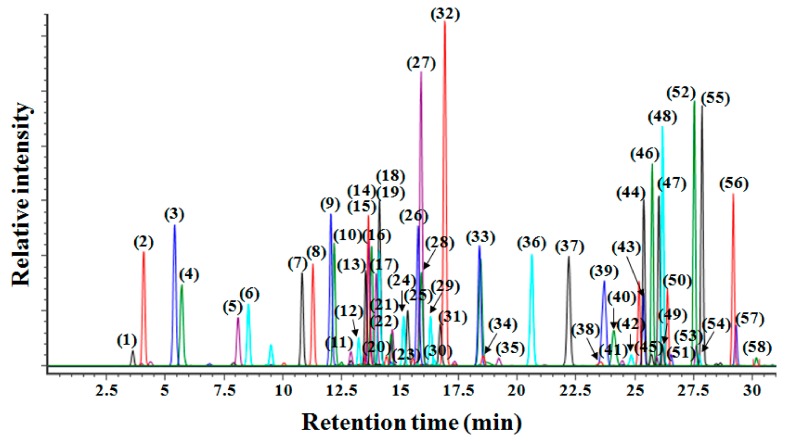
Next, for the comprehensive profiling of various ginsenosides, 58 ginsenoside standards were used to optimize the UPLC-QTOF/MS conditions. By using the UPLC system with an ACQUITY BEH C18 column, these standards were well separated after 30 min at a flow rate of 450 µL/min. The base peak intensity (BPI) chromatograms of the 58 ginsenosides are shown in Figure 1. Among these ginsenosides, 16 isomeric well-separated pairs were found: C36H60O7 (Rk2 or Rh3), C36H60O8 (Rk3, Rh4, fKa, or Rh6), C36H62O8 (Rh2 or CK), C36H62O9 (Rh1, Rh1-R, or F1), C41H70O12 (Mc or CY), C41H70O13 (nR2, F5, nR2-R, or F3), C42H70O12 (Rg4, F4, Rk1, or Rg5), C42H72O13 (Rg2, Rg2-R, F2, Rg3, or Rg3-R), C42H72O14 (Rg1, pF11, Rf, GyL, or GyLI), C47H80O17 (nFe, CO, or nFt1), C48H82O18 (Re, Rd, or GyXVII), C48H82O19 (gRf or vR4), C53H90O22 (Rc, Rb2, or Rb3), C56H92O25 (M-Rc or M-Rb2), C58H98O26 (Ra1 or Ra2), and C59H100O27 (nR4 or Ra3). Although several peaks overlapped with each other, identifying each peak was possible, based on its exact mass values obtained by QTOF/MS. In the negative ion mode, ginsenosides were detected as [M + COOH]− ions and [M − H]− ions with high mass accuracy (<2.5 ppm). For the exact identification of ginsenosides, we constructed an in-house library using the UNIFI software. Information about the compound’s name, molecular formula, retention time (RT), mass accuracy, and adduct was listed in the library for the 58 ginsenosides (Table 1). Next, a validation study was performed to prove the performance of the UPLC-QTOF/MS method for profiling the ginsenosides. For this, the standard curves of the 58 ginsenosides were constructed, and their linearity range and correlation (R2) were calculated. The limits of detection (LODs) of each standard are provided in Table S1. The R2 of each ginsenoside analysis was, at minimum, above 0.9908, proving the high reliability of the quantitative analysis of the ginsenosides. LODs also showed the high sensitivity of this method for detecting ginsenosides. For example, in the case of Ra3, Ro, M-Rb2, Rd, GyL, GyLI, Rg3, Mc, and Cy, the two picogram (pg) of these compounds was detected by UPLC-QTOF/MS.
Figure 1.
Base peak intensity (BPI) chromatogram of 58 ginsenoside standards. The peaks of (1)–(58) are aligned with the 58 ginsenosides listed in Table 1.
Table 1.
In-house library for the analysis of 58 ginsenosides using ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (UPLC-QTOF/MS).
| No. | RT (min) | Ginsenosides | Molecular Formula | Expected Neutral Mass (Da) | Observed Neutral Mass (Da) | QTOF/MS (ESI-) (m/z) | Mass Accuracy (ppm) | Adducts |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.58 | 20-O-Glucoginsenoside Rf (gRf) | C48H82O19 | 962.545 | 962.5453 | 1007.5435 | 0.26 | +HCOO |
| 2 | 4.07 | Notoginsenoside R1 (nR1) | C47H80O18 | 932.5345 | 932.5343 | 977.5325 | 0.2 | +HCOO |
| 3 | 5.36 | Ginsenoisde Rg1 (Rg1) | C42H72O14 | 800.4922 | 800.4908 | 845.489 | 1.62 | +HCOO |
| 4 | 5.66 | Ginsenoside Re (Re) | C48H82O18 | 946.5501 | 946.5503 | 991.5485 | 0.16 | +HCOO |
| 5 | 8.05 | Floralginsenoside Ka (fKa) | C36H62O11 | 670.4292 | 670.4295 | 715.4277 | 0.34 | +HCOO |
| 6 | 8.46 | Ginsenoside Rh6 (Rh6) | C36H62O11 | 670.4292 | 670.4291 | 715.4273 | 0.19 | +HCOO |
| 7 | 10.72 | Ginsenoside Rh23 (Rh23) | C37H64O10 | 668.4499 | 668.4507 | 713.4489 | 1.06 | +HCOO |
| 8 | 11.2 | Vinaginsenoside R4 (vR4) | C48H82O19 | 962.545 | 962.5452 | 1007.5434 | 0.19 | +HCOO |
| 9 | 11.94 | Pseudo-ginsenoside F11 (pF11) | C42H72O14 | 800.4922 | 800.4932 | 845.4914 | 1.22 | +HCOO |
| 10 | 12.11 | Ginsenoside Rf (Rf) | C42H72O14 | 800.4922 | 800.4922 | 845.4904 | 0.01 | +HCOO |
| 11 | 12.81 | Notoginsenoside R2 (nR2) | C41H70O13 | 770.4816 | 770.4823 | 815.4805 | 0.83 | +HCOO |
| 12 | 13.15 | Notoginsenoside R4 (nR4) | C59H100O27 | 1240.6452 | 1240.6455 | 1285.6437 | 0.2 | +HCOO |
| 13 | 13.43 | Ginsenoside F5 (F5) | C41H70O13 | 770.4816 | 770.4819 | 815.4801 | 0.33 | +HCOO |
| 14 | 13.56 | Ginsenoside Rh1 (Rh1) | C36H62O9 | 638.4394 | 638.4392 | 683.4374 | 0.31 | +HCOO |
| 15 | 13.58 | 20(R)-Notoginsenoside R2 (nR2-R) | C41H70O13 | 770.4816 | 770.4815 | 815.4797 | 0.18 | +HCOO |
| 16 | 13.7 | Ginsenoside Rg2 (Rg2) | C42H72O13 | 784.4973 | 784.4984 | 829.4966 | 1.38 | +HCOO |
| 17 | 13.9 | Ginsenoside F3 (F3) | C41H70O13 | 770.4816 | 770.4811 | 815.4793 | 0.63 | +HCOO |
| 18 | 14.03 | 20(R)-Ginsenoside Rg2 (Rg2-R) | C42H72O13 | 784.4973 | 784.496 | 829.4942 | 1.58 | +HCOO |
| 19 | 14.03 | 20(R)-Ginsenoside Rh1 (Rh1-R) | C36H62O9 | 638.4394 | 638.4397 | 683.4379 | 0.47 | +HCOO |
| 20 | 14.35 | Ginsenoside Ra2 (Ra2) | C58H98O26 | 1210.6346 | 1210.6346 | 1255.6328 | 0.01 | +HCOO |
| 21 | 14.51 | Ginsenoside Ra3 (Ra3) | C59H100O27 | 1240.6452 | 1240.6447 | 1285.6429 | 0.36 | +HCOO |
| 22 | 14.53 | Ginsenoside Rb1 (Rb1) | C54H92O23 | 1108.6029 | 1108.6006 | 1153.5988 | 2.02 | +HCOO |
| 23 | 14.58 | Gypenoside XLIX (XLIX) | C52H86O21 | 1046.5662 | 1046.5658 | 1091.564 | 0.31 | +HCOO |
| 24 | 15.04 | Malonyl ginsenoside Rb1 (M-Rb1) | C57H94O26 | 1194.6033 | 1194.6057 | 1193.5984 | 2 | –H |
| 25 | 15.25 | Ginsenoside Rc (Rc) | C53H90O22 | 1078.5924 | 1078.5925 | 1123.5907 | 0.1 | +HCOO |
| 26 | 15.47 | Ginsenoside Ra1 (Ra1) | C58H98O26 | 1210.6346 | 1210.633 | 1255.6312 | 1.27 | +HCOO |
| 27 | 15.69 | Ginsenoside Ro (Ro) | C48H76O19 | 956.4981 | 956.4978 | 955.4905 | 0.28 | –H |
| 28 | 15.8 | Ginsenoside F1 (F1) | C36H62O9 | 638.4394 | 638.4395 | 683.4377 | 0.11 | +HCOO |
| 29 | 15.9 | Malonyl ginsenoside Rc (M-Rc) | C56H92O25 | 1164.5928 | 1164.5947 | 1163.5874 | 1.6 | –H |
| 30 | 16.25 | Ginsenoside Rb2 (Rb2) | C53H90O22 | 1078.5924 | 1078.5913 | 1123.5895 | 0.99 | +HCOO |
| 31 | 16.6 | Ginsenoside Rb3 (Rb3) | C53H90O22 | 1078.5924 | 1078.5915 | 1123.5897 | 0.81 | +HCOO |
| 32 | 16.9 | Malonyl ginsenoside Rb2 (M-Rb2) | C56H92O25 | 1164.5928 | 1164.5923 | 1163.585 | 0.4 | –H |
| 33 | 18.28 | Gypenoside A (GyA) | C46H74O17 | 898.4926 | 898.4915 | 943.4897 | 1.21 | +HCOO |
| 34 | 18.29 | Ginsenoside Rd (Rd) | C48H82O18 | 946.5501 | 946.5495 | 991.5477 | 0.59 | +HCOO |
| 35 | 18.97 | Malonyl ginsenoside Rd (M-Rd) | C51H84O21 | 1032.5505 | 1032.5498 | 1031.5425 | 0.7 | –H |
| 36 | 20.4 | Gypenoside XVII (GyXVII) | C48H82O18 | 946.5501 | 946.5497 | 991.5479 | 0.47 | +HCOO |
| 37 | 21.96 | Notoginsenoisde Fe (nFe) | C47H80O17 | 916.5396 | 916.5393 | 961.5375 | 0.22 | +HCOO |
| 38 | 23.29 | Compound O (CO) | C47H80O17 | 916.5396 | 916.5396 | 961.5378 | 0.1 | +HCOO |
| 39 | 23.46 | Ginsenoside Rg4 (Rg4) | C42H70O12 | 766.4867 | 766.4875 | 811.4857 | 0.91 | +HCOO |
| 40 | 23.84 | Ginsenoside Rk3 (Rk3) | C36H60O8 | 620.4288 | 620.4292 | 665.4274 | 0.51 | +HCOO |
| 41 | 24.28 | Ginsenoside F4 (F4) | C42H70O12 | 766.4867 | 766.4865 | 811.4847 | 0.27 | +HCOO |
| 42 | 24.68 | Ginsenoside Rh4 (Rh4) | C36H60O8 | 620.4288 | 620.4275 | 665.4257 | 2.01 | +HCOO |
| 43 | 25.15 | Gypenoside L (GyL) | C42H72O14 | 800.4922 | 800.4905 | 845.4887 | 2.1 | +HCOO |
| 44 | 25.19 | Ginsenoside F2 (F2) | C42H72O13 | 784.4973 | 784.4968 | 829.495 | 0.6 | +HCOO |
| 45 | 25.34 | Gypenoside LI (GyLI) | C42H72O14 | 800.4922 | 800.4909 | 845.4891 | 1.6 | +HCOO |
| 46 | 25.57 | Notoginsenoside Ft1 (nFt1) | C47H80O17 | 916.5396 | 916.5417 | 961.5399 | 2.26 | +HCOO |
| 47 | 25.78 | Protopanaxatiol (PPT) | C30H52O4 | 476.3866 | 476.3862 | 521.3844 | 0.71 | +HCOO |
| 48 | 26.01 | 20(S)-Ginsenoside Rg3 (Rg3) | C42H72O13 | 784.4973 | 784.498 | 829.4962 | 0.9 | +HCOO |
| 49 | 26.2 | 20(R)-Ginsenoside Rg3 (Rg3-R) | C42H72O13 | 784.4973 | 784.4989 | 829.4971 | 1.97 | +HCOO |
| 50 | 26.37 | Ginsenoisde Mc (Mc) | C41H70O12 | 754.4867 | 754.4867 | 799.4849 | 0 | +HCOO |
| 51 | 26.52 | Compound Y (CY) | C41H70O12 | 754.4867 | 754.4849 | 799.4831 | 2.3 | +HCOO |
| 52 | 27.35 | Compound K (CK) | C36H62O8 | 622.4445 | 622.4433 | 667.4415 | 1.8 | +HCOO |
| 53 | 27.43 | Ginsenoside Rk1 (Rk1) | C42H70O12 | 766.4867 | 766.4861 | 811.4843 | 0.81 | +HCOO |
| 54 | 27.55 | Ginsenoside Rg5 (Rg5) | C42H70O12 | 766.4867 | 766.4873 | 811.4855 | 0.73 | +HCOO |
| 55 | 27.7 | Ginsenoside Rh2 (Rh2) | C36H62O8 | 622.4445 | 622.4441 | 667.4423 | 0.52 | +HCOO |
| 56 | 29.04 | Ginsenoside Rk2 (Rk2) | C36H60O7 | 604.4339 | 604.4341 | 649.4323 | 0.35 | +HCOO |
| 57 | 29.15 | Ginsenoside Rh3 (Rh3) | C36H60O7 | 604.4339 | 604.434 | 649.4322 | 0.17 | +HCOO |
| 58 | 30.04 | Protopanaxadiol (PPD) | C30H52O3 | 460.3916 | 460.3917 | 505.3899 | 0.19 | +HCOO |
3.2. Profiling of Various Ginsenosides from the Roots, Stems, Leaves, and Berries of Panax ginseng
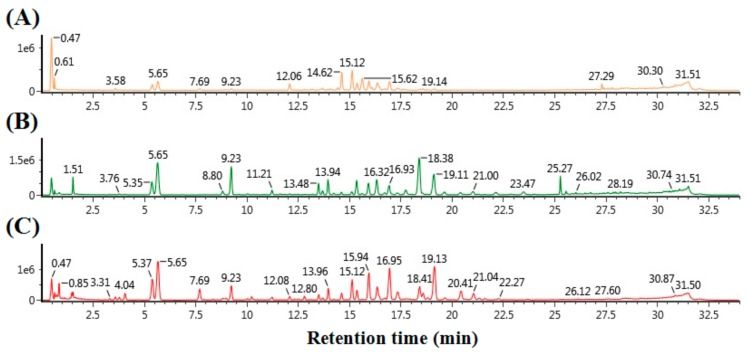
In this study, the constructed UPLC-QTOF/MS with an in-house library was applied to profile various ginsenosides from the roots, stems, leaves, and berries of P. ginseng. The numbers of individual samples were as follows: ginseng root (GR) (n = 10), stem and leaf (GS) (n = 10), and berry (GB) (n = 10). To extract various ginsenosides from individual samples, 70% (v/v) methanol was used. The extracts were analyzed using UPLC-QTOF/MS; each piece of data was then processed using the UNIFI software. Figure 2 shows the representative BPI chromatograms of ginsenoside extracts from GR, GS, and GB. UPLC was able to separate the ginsenosides and other metabolites in 30 min. The intensities of several peaks were different dependent on the sample. For example, overall, the GR sample had lower intensity peaks compared to the other samples. The GS sample had higher intensity peaks than the GB sample, eluted for 5–10 min and 18–26 min. However, the GB sample had higher intensity peaks than the GS sample, eluted for 5–10 min. These demonstrated that GR, GS, and GB have distinct metabolite compositions, including ginsenosides.
Figure 2.
BPI chromatogram of ginsenoside extracts from three samples of (A) ginseng roots (GR), (B) stems and leaves (GS), and (C) berries (GB).
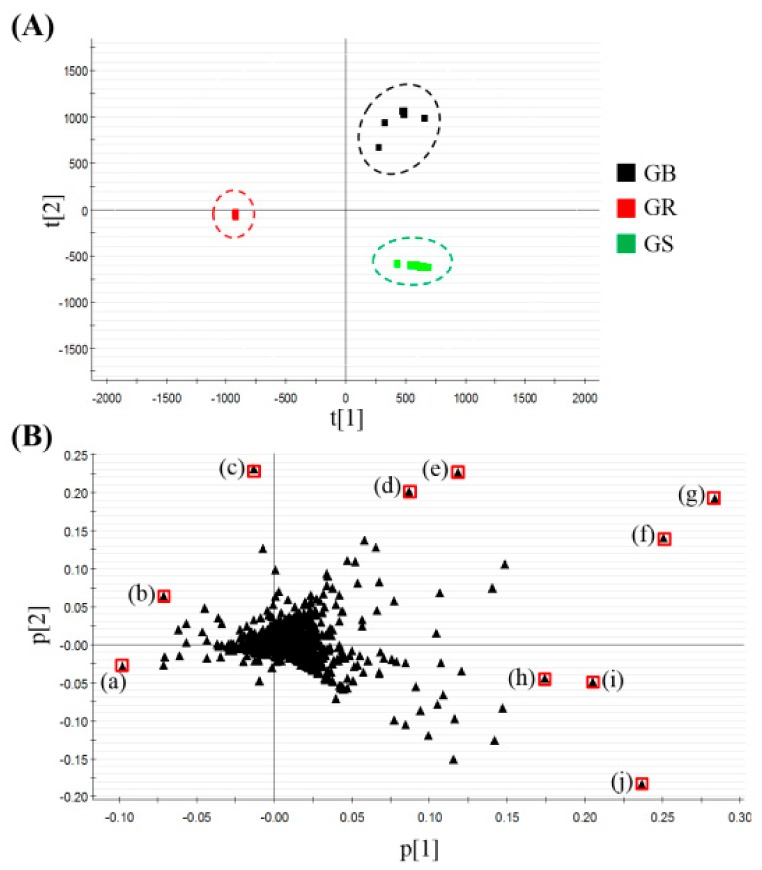
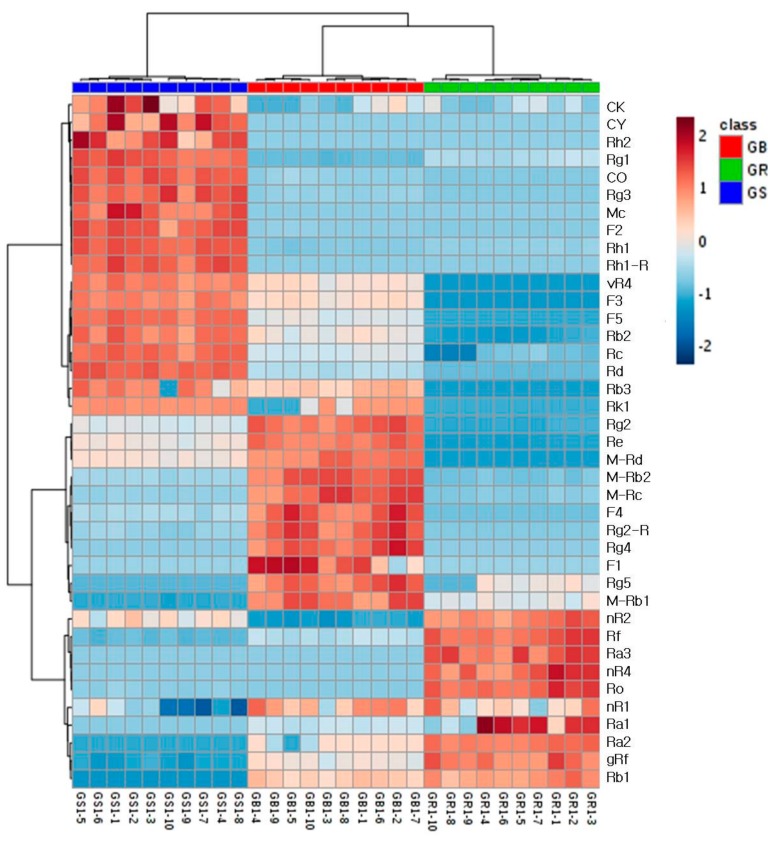
First, all the processed data were exported to the EZinfo software for principal component analysis (PCA). PCA helps visualize the general clustering trends among individual samples, and the scatter of the points indicates the similarities or differences between the metabolite data of the samples. In the score plot, the GR, GS, and GB were well-differentiated (Figure 3A). Moreover, in the loading plot, we identified the main variables that differentiate the three groups in the score plot. Each point represented the m/z-RT pairs of molecules. Based on the in-house library, the nine variables of the loading plot were identified as several ginsenosides (Figure 3B). Next, in the data processing, a total of 39 ginsenosides were identified from the three samples. Using the in-house library, the numbers of identified ginsenosides from three samples were as follows: 27 in GR, 32 in GS, and 29 in GB. The ginsenosides datasets were then subjected to hierarchical clustering analysis (HCA). As a result, the three groups were clearly differentiated. The different levels of the 39 ginsenosides in the ginseng roots, stems and leave, and berries are described in the heatmap in Figure 4. This demonstrated the major ginsenosides that were found in each ginseng part.
Figure 3.
(A) Principal component analysis (PCA) score plot and (B) loading plot of ginsenoside extracts from ginseng roots (GR) (n = 10), stems and leaves (GS) (n = 10), and berries (GB) (n = 10). In the PCA loading plot, each point was identified as follows: ginsenoside (a) Ro (b) Rb1, (c) M-Rb1, (d) M-Rc, (e) M-Rb2, (f) M-Rd, (g) Re, (h) F3, (i) unknown, and (j) Rd.
Figure 4.
Hierarchical clustering analysis of the 39 ginsenoside datasets from the ginseng root (GR) (n = 10), stems and leaves (GS) (n = 10), and berries (GB) (n =10).
3.3. Quantification of Ginsenosides from the Roots, Stems and Leaves, and Berries of Panax ginseng
Next, we quantified the 39 ginsenosides in the individual samples. The standard curve of each ginsenoside was applied to determine its actual amount in the ginseng samples. The quantification was based on 10 biological replicate analysis of each ginseng sample (n = 10), and the amounts are described as mean ± SD in Table 2. This displays the qualitative and quantitative ginsenoside contents in the different P. ginseng parts. Previously, Wang et al. has reported the HPLC-UV–based quantification of 12 ginsenosides from ginseng root, rootlet, leaf, and berry [14]. From the five parts such as root, root-hair, rhizome, stem, and leaf, Shi et al. quantified seven ginsenosides by HPLC-UV [13], and Qu et al. quantified 12 ginsenosides by HPLC-ELSD [36]. Compared to these reports, this study detail characterized a total of 39 ginsenosides with high- or low-abundance from ginseng root, stem and leaf, and berry. As our results, ginsenosides, such as nR4, Ra2, Ra3, and Ra1, were uniquely found in GR. Furthermore, GS uniquely contained Rb3, Mc, CY, Rh2, and Rh1-R. F1 and Rg4 were only detected in GB. Although most of unique ginsenosides are low-abundant, their profiles are valuable to characterize the ginsenoside contents of each ginseng part. In the quantification, GR had highest amounts of Rf, Rb1, and Ro, compared to the other samples. GS had highest amounts of Rg1, vR4, F5, Rh1, F3, Rc, Rb2, Rd, CO, F2, Rg3, CK, and Rk1. GB had highest amounts of gRf, nR1, Re, nR2, Rg2, Rg2-R, M-Rb1, M-Rc, M-Rb2, M-Rd, F4, and Rg5. Previously, two papers have reported that GR had the highest amounts of Rb1, GB had the highest amounts of Rg2, and GS had the highest amounts of Rg1, Rh1, Rd, and Rg3 [14,36]. Interestingly, these results have been matched to ours. Rb3 and Rh1-R are also equally analyzed from GS [13,14]. On the other hand, Wang et al. has reported that Re and Rc are mainly consisted in GS and GR, respectively [14]. As our results, GB had highest amounts of Re, and this is matched to another previous paper [37]. The quantification of several ginsenosides can differ depending on ginseng’s cultivars and growing conditions.
Table 2.
Quantification of 39 ginsenosides from the roots, stems and leaves, and berries of P. ginseng. Values are expressed as mg/g.
| No. | Ginsenosides | Roots | Stems and Leaves | Berries | No. | Ginsenosides | Root | Stems and Leaves | Berry |
|---|---|---|---|---|---|---|---|---|---|
| 1 | gRf | 1.8 ± 0.1 | 0.3 ± 0.1 | 3.6 ± 1.1 | 21 | Ro | 0.15 ± 0.01 | 0.014 ± 0.002 | 0.014 ± 0.002 |
| 2 | nR1 | 0.06 ± 0.01 | 0.03 ± 0.01 | 1.2 ± 0.2 | 22 | M-Rc | 0.9 ± 0.1 | 1.08 ± 0.04 | 4.7 ± 0.5 |
| 3 | Rg1 | 3.1 ± 0.2 | 9.6 ± 0.5 | 8.9 ± 4.3 | 23 | Rb2 | 0.6 ± 0.1 | 2.8 ± 0.1 | 2.3 ± 0.6 |
| 4 | Re | 3.0 ± 0.3 | 17 ± 1 | 26 ± 8 | 24 | Rb3 | not detected | 0.5 ± 0.1 | not detected |
| 5 | vR4 | 0.007 ± 0.001 | 1.2 ± 0.1 | 0.9 ± 0.1 | 25 | M-Rb2 | 0.9 ± 0.1 | 1.6 ± 0.1 | 6.8 ± 0.5 |
| 6 | Rf | 4.6 ± 0.2 | 0.30 ± 0.01 | 3.4 ± 1.1 | 26 | Rd | 0.15 ± 0.01 | 7.1 ± 0.2 | 2.3 ± 0.5 |
| 7 | nR2 | 1.1 ± 0.1 | 0.7 ± 0.1 | 4.5 ± 1.3 | 27 | M-Rd | 0.014 ± 0.001 | 3.1 ± 0.1 | 5.3 ± 0.4 |
| 8 | nR4 | 0.14 ± 0.02 | not detected | not detected | 28 | CO | 0.06 ± 0.01 | 12.6 ± 0.1 | 0.4 ± 0.1 |
| 9 | F5 | not detected | 5.8 ± 0.2 | 2.8 ± 0.3 | 29 | Rg4 | not detected | not detected | 0.06 ± 0.03 |
| 10 | Rh1 | 0.021 ± 0.002 | 0.24 ± 0.01 | 0.04 ± 0.02 | 30 | F4 | not detected | 0.4 ± 0.1 | 2.1 ± 0.8 |
| 11 | Rg2 | 0.17 ± 0.01 | 0.61 ± 0.03 | 0.6 ± 0.5 | 31 | F2 | 0.0019 ± 0.0006 | 2.7 ± 0.3 | 0.03 ± 0.01 |
| 12 | F3 | not detected | 5.3 ± 0.2 | 4.1 ± 0.4 | 32 | Rg3 | 0.0004 ± 0.0002 | 0.09 ± 0.01 | 0.007 ± 0.001 |
| 13 | Rg2-R | not detected | 0.008 ± 0.001 | 0.06 ± 0.03 | 33 | Mc | not detected | 0.24 ± 0.04 | not detected |
| 14 | F1 | not detected | not detected | 0.005 ± 0.001 | 34 | CY | not detected | 0.08 ± 0.02 | not detected |
| 15 | Ra2 | 3.2 ± 0.1 | not detected | not detected | 35 | CK | 0.0010±0.0004 | 0.003±0.001 | 0.002 ± 0.001 |
| 16 | Ra3 | 0.9 ± 0.1 | not detected | not detected | 36 | Rk1 | not detected | 1.18±0.02 | 1.153 ± 0.004 |
| 17 | Rb1 | 3.8 ± 0.3 | 0.08 ± 0.01 | 2.8 ± 0.6 | 37 | Rg5 | 0.031 ± 0.004 | not detected | 0.08 ± 0.01 |
| 18 | M-Rb1 | 4.7 ± 0.5 | 0.7 ± 0.1 | 9.9 ± 1.2 | 38 | Rh2 | not detected | 0.011 ± 0.002 | not detected |
| 19 | Rc | 0.9 ± 0.1 | 3.5 ± 0.1 | not detected | 39 | Rh1-R | not detected | 0.0028 ± 0.0004 | not detected |
| 20 | Ra1 | 0.6 ± 0.2 | not detected | not detected |
Each ginsenoside has specific activities, and the efficacies of GR, GS, and GB are determined by the ginsenoside contents. Here, we focused on the pharmacological activities of ginsenosides which are mainly or uniquely consisted in each ginseng part. By searching previous literatures [5,6,15,38,39,40,41,42,43,44,45,46], we found six kinds of activities in 17 ginsenosides (Table S2). In summary, three ginsenosides (Rf, Rb1, Ro) consisted in GR shows anti-inflammation effect and lymphocyte proliferative capacity. Moreover, 10 ginsenosides (Rg1, Rc, Rb2, Rd, F2, Rg3, CK, Rb3, Rh2, Rh1-R) consisted in GS shows six activities (anti-cancer, anti-diabetes, anti-inflammation, lymphocyte proliferative capacity, immunomodulating activity, chemopreventive effect). Lastly, four ginsenosides (Re, Rg2, Rg2-R, F1) consisted in GB shows four activities (anti-cancer, anti-diabetes, anti-inflammation, lymphocyte proliferative capacity). These results demonstrated that not only GR, but also GS and GB are good sources to obtain ginsenosides having pharmacological activities. By detail profiling of various ginsenosides, it was possible to find the efficacies of ginseng root, stems, leaves, and berries.
4. Conclusions
In conclusion, this study optimized an analytical method based on UPLC-QTOF/MS with an in-house library to profile 58 ginsenosides. Furthermore, using this method, we determined the quantitative contents of 39 ginsenosides from GR, GS, and GB. These results demonstrated that not only the ginseng root, but also the stems, leaves, and berries are good sources of ginsenosides. Each ginsenoside has specific activities. Thus, in the basis of ginsenosides profiling and quantification, we can assess the pharmacological utility of different ginseng parts. Moreover, ginsenoside contents may differ depending on ginseng’s cultivars, growing years, growing regions, etc. Finally, the proposed UPLC-QTOF/MS method for ginsenoside profiling is a good tool for evaluating various ginseng samples as sources for ginsenosides.
Acknowledgments
This work was supported by the Rural Development Administration Grant (PJ01136203), Republic of Korea.
Supplementary Materials
Supplementary materials are available online. Figure S1. Molecular structures of 58 ginsenosides listed in Table 1. Table S1. Validation study for the analysis of the 58 ginsenosides using UPLC-QTOF/MS. Table S1. Pharmacological activities of 17 ginsenosides which are mainly or uniquely consisted in ginseng root (GR), stem and leaf (GS), and berry (GB).
Author Contributions
J.W.L. and D.Y.L. conceived and designed the experiments; B.-R.C. and D.J.C. performed the NMRs, and TOF-mass spectrometry of the samples; Y.-S.L. and G.-S.K. isolated the standard compounds and elucidated the structures; Y.-C.K. and S.-Y.K. contributed to the plant materials preparation; J.W.L. and D.Y.L. wrote the paper and N.-I.B. managed the research project.
Conflicts of Interest
The authors declare no conflicts of interest. The founding sponsors had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; or decision to publish the results.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Wang Y., Pan J.Y., Xiao X.Y., Lin R.C., Cheng Y.Y. Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography-mass spectrometry. Phytochem. Anal. 2006;17:424–430. doi: 10.1002/pca.944. [DOI] [PubMed] [Google Scholar]
- 2.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng CA Meyer. Phytochem. Rev. 2005;4:159–175. doi: 10.1007/s11101-005-2835-8. [DOI] [Google Scholar]
- 3.Wang A., Wang C.Z., Wu J.A., Osinski J., Yuan C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem. Anal. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 4.Xie J.-T., Mehendale S.R., Li X., Quigg R., Wang X., Wang C.-Z., Wu J.A., Aung H.H., Rue P.A., Bell G.I. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005;1740:319–325. doi: 10.1016/j.bbadis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Kenarova B., Neychev H., Hadjiivanova C., Petkov V.D. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn. J. Pharmacol. 1990;54:447–454. doi: 10.1254/jjp.54.447. [DOI] [PubMed] [Google Scholar]
- 6.Yue P.Y.K., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Fan D.T.P., Yeung H.W., Wong R.N.S. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007;2:6. doi: 10.1186/1749-8546-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang W., Zhang Y., Gao J., Ding X., Gao S. The anti-fatigue effect of 20 (R)-ginsenoside Rg3 in mice by intranasally administration. Biol. Pharm. Bull. 2008;31:2024–2027. doi: 10.1248/bpb.31.2024. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.Y., Kim Y.K., Park N.I., Kim C.S., Lee C.Y., Park S.U. Chemical constituents and biological activities of the berry of Panax ginseng. J. Med. Plants Res. 2010;4:349–353. [Google Scholar]
- 9.Qiu S., Yang W.-Z., Yao C.-L., Qiu Z.-D., Shi X.-J., Zhang J.-X., Hou J.-J., Wang Q.-R., Wu W.-Y., Guo D.-A. Nontargeted metabolomic analysis and “commercial-homophyletic” comparison-induced biomarkers verification for the systematic chemical differentiation of five different parts of Panax ginseng. J. Chromatogr. A. 2016;1453:78–87. doi: 10.1016/j.chroma.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 10.Li X.-G., Yan Y.Z., Jin X.-J., Kim Y.K., Uddin M.R., Kim Y.B., Bae H., Kim Y.C., Lee S.W., Park S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012;9:670–683. [Google Scholar]
- 11.Zhao J.-M., Li N., Zhang H., Wu C.-F., Piao H.-R., Zhao Y.-Q. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bioorg. Med. Chem. Lett. 2011;21:1027–1031. doi: 10.1016/j.bmcl.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Sritularak B., Morinaga O., Yuan C.-S., Shoyama Y., Tanaka H. Quantitative analysis of ginsenosides Rb1, Rg1, and Re in American ginseng berry and flower samples by ELISA using monoclonal antibodies. J. Nat. Med. 2009;63:360–363. doi: 10.1007/s11418-009-0332-x. [DOI] [PubMed] [Google Scholar]
- 13.Shi W., Wang Y., Li J., Zhang H., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. doi: 10.1016/j.foodchem.2006.05.053. [DOI] [Google Scholar]
- 14.Wang C.-Z., Wu J.A., McEntee E., Yuan C.-S. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J. Agric. Food Chem. 2006;54:2261–2266. doi: 10.1021/jf052993w. [DOI] [PubMed] [Google Scholar]
- 15.Li X.-L., Wang C.-Z., Sun S., Mehendale S.R., Du W., He T.-C., Yuan C.-S. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol. Rep. 2009;22:943–952. [PubMed] [Google Scholar]
- 16.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J. Agric. Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.N., Ha Y.W., Shin H., Son S.H., Wu S.J., Kim Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng CA Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharm. Biomed. Anal. 2007;45:164–170. doi: 10.1016/j.jpba.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Sun B.-S., Gu L.-J., Fang Z.-M., Wang C.-Y., Wang Z., Lee M.-R., Li Z., Li J.-J., Sung C.-K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC–ELSD. J. Pharm. Biomed. Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Joo K.-M., Lee J.-H., Jeon H.-Y., Park C.-W., Hong D.-K., Jeong H.-J., Lee S.J., Lee S.-Y., Lim K.-M. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J. Pharm. Biomed. Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Xie G.X., Ni Y., Su M.M., Zhang Y.Y., Zhao A.H., Gao X.F., Liu Z., Xiao P.G., Jia W. Application of ultra-performance LC-TOF MS metabolite profiling techniques to the analysis of medicinal Panax herbs. Metabolomics. 2008;4:248–260. doi: 10.1007/s11306-008-0115-5. [DOI] [Google Scholar]
- 21.Ji Q.C., Harkey M.R., Henderson G.L., Eric Gershwin M., Stern J.S., Hackman R.M. Quantitative determination of ginsenosides by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 2001;12:320–326. doi: 10.1002/pca.593. [DOI] [PubMed] [Google Scholar]
- 22.Wan J.-Y., Liu P., Wang H.-Y., Qi L.-W., Wang C.-Z., Li P., Yuan C.-S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Mao Q., Yang J., Cui X.-M., Li J.-J., Qi Y.-T., Zhang P.-H., Wang Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of Panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H.-M., Li S.-L., Zhang H., Wang Y., Zhao Z.-L., Chen S.-L., Xu H.-X. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J. Pharm. Biomed. Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.W., Ji S.-H., Lee Y.-S., Choi D.J., Choi B.-R., Kim G.-S., Baek N.-I., Lee D.Y. Mass Spectrometry Based Profiling and Imaging of Various Ginsenosides from Panax ginseng Roots at Different Ages. Int. J. Mol. Sci. 2017;18:1114. doi: 10.3390/ijms18061114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai H., Wang S., Liu J., Gao D., Jiang Y., Liu H., Cai Z. Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B. 2016;1026:263–271. doi: 10.1016/j.jchromb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Woo S.-S., Song J.-S., Lee J.-Y., In D.S., Chung H.-J., Liu J.R., Choi D.-W. Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochemistry. 2004;65:2751–2761. doi: 10.1016/j.phytochem.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 28.Yang W., Zhang J., Yao C., Qiu S., Chen M., Pan H., Shi X., Wu W., Guo D. Method development and application of offline two-dimensional liquid chromatography/quadrupole time-of-flight mass spectrometry-fast data directed analysis for comprehensive characterization of the saponins from Xueshuantong Injection. J. Pharm. Biomed. Anal. 2016;128:322–332. doi: 10.1016/j.jpba.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Qi L.-W., Wang H.-Y., Zhang H., Wang C.-Z., Li P., Yuan C.-S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 30.Qiu S., Yang W.-Z., Shi X.-J., Yao C.-L., Yang M., Liu X., Jiang B.-H., Wu W.-Y., Guo D.-A. A green protocol for efficient discovery of novel natural compounds: Characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal. Chim. Acta. 2015;893:65–76. doi: 10.1016/j.aca.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 31.Xu X.-F., Cheng X.-L., Lin Q.-H., Li S.-S., Jia Z., Han T., Lin R.-C., Wang D., Wei F., Li X.-R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng Res. 2016;40:344–350. doi: 10.1016/j.jgr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun M., Che Y., Liu Z. A tractable method for the preparation of the ginsenoside compounds O and Mc1. Anal. Methods. 2015;7:4757–4762. doi: 10.1039/C5AY00117J. [DOI] [Google Scholar]
- 33.Wu Q., Jang M., Piao X.-L. Determination by UPLC-MS of four dammarane-type saponins from heat-processed Gynostemma pentaphyllum. Biosci. Biotechnol. Biochem. 2014;78:311–316. doi: 10.1080/09168451.2014.882751. [DOI] [PubMed] [Google Scholar]
- 34.Wang R.-F., Li J., Hu H.-J., Li J., Yang Y.-B., Yang L., Wang Z.-T. Chemical transformation and target preparation of saponins in stems and leaves of Panax notoginseng. J. Ginseng Res. 2016 doi: 10.1016/j.jgr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee G.-W., Kim K.-R., Oh D.-K. Production of rare ginsenosides (compound Mc, compound Y and aglycon protopanaxadiol) by β-glucosidase from Dictyoglomus turgidum that hydrolyzes β-linked, but not α-linked, sugars in ginsenosides. Biotechnol. Lett. 2012;34:1679–1686. doi: 10.1007/s10529-012-0949-9. [DOI] [PubMed] [Google Scholar]
- 36.Qu C., Bai Y., Jin X., Wang Y., Zhang K., You J., Zhang H. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009;115:340–346. doi: 10.1016/j.foodchem.2008.11.079. [DOI] [Google Scholar]
- 37.Ko S.K., Bae H.M., Cho O.S., Im B.O., Chung S.H., Lee B.Y. Analysis of ginsenoside composition of ginseng berry and seed. Food Sci. Biotechnol. 2008;17:1379–1382. [Google Scholar]
- 38.Mai T.T., Moon J., Song Y., Viet P.Q., Van Phuc P., Lee J.M., Yi T.-H., Cho M., Cho S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012;321:144–153. doi: 10.1016/j.canlet.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 39.Xie J.-T., Wang C.-Z., Zhang B., Mehendale S.R., Li X.-L., Sun S., Han A.H., Du W., He T.-C., Yuan C.-S. In vitro and in vivo anticancer effects of American ginseng berry: Exploring representative compounds. Biol. Pharm. Bull. 2009;32:1552–1558. doi: 10.1248/bpb.32.1552. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y., Jin Y., Lim W., Ji S., Choi S., Jang S., Lee S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2003;84:463–468. doi: 10.1016/S0960-0760(03)00067-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Choi K.-D., Yu H., Jin F., Im W.-T. Production of ginsenoside F1 using commercial enzyme Cellulase KN. J. Ginseng Res. 2016;40:121–126. doi: 10.1016/j.jgr.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attele A.S., Zhou Y.-P., Xie J.-T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.K., Kang H., Baek C.W., Jung Y.H., Woo Y.C., Choi G.J., Shin H.Y., Kim K.S. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J. Ginseng Res. 2017 doi: 10.1016/j.jgr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C.-K., Cho D.H., Lee K.-S., Lee D.-K., Park C.-W., Kim W.G., Lee S.J., Ha K.-S., Goo Taeg O., Kwon Y.-G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid.-Based Complement. Altern. Med. 2012 doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D.-H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan S.-M., Luo J.-G., Huang F., Kong L.-Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng CA Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J. Pharm. Biomed. Anal. 2014;89:76–82. doi: 10.1016/j.jpba.2013.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.