Abstract
Background
Randomized controlled trials of the first-line combination of bevacizumab and chemotherapy in patients with metastatic breast cancer (MBC) have shown improvements in tumor response and progression-free survival (PFS).
Objective
The aim of this ambispective, observational study (LORENA) was to describe the clinical characteristics of long-term responders to bevacizumab-based therapy.
Patients and methods
This study consisted of a retrospective and a prospective phase. During the retrospective phase, patients with HER2-negative MBC who were treated with bevacizumab-based first-line therapy were included. During the prospective phase, patients with PFS of ≥12 months were treated according to routine clinical practice procedures. Overall survival (OS) and PFS were estimated using the Kaplan–Meier method. Univariate and multivariate analyses of prognostic factors were performed.
Results
In total, 148 women were included (median age: 50 years; range: 29–81 years). The mean duration of exposure to bevacizumab was 18 months. The majority of patients experienced objective response (complete: 23%; partial: 57%). Median PFS was 22.7 months and median OS was 58.2 months. In multivariate analyses, patients receiving maintenance hormonal therapy (MHT) had longer PFS (P=0.002; hazard ratio [HR] =1.8) and OS (P=0.009; HR=2.0), while patients not previously treated with taxanes had longer OS (P<0.0001; HR =3.3). No unexpected adverse events were observed.
Conclusion
The results of this study suggest, that among long-term responders, first-line bevacizumab-based therapy is more effective in patients who had not been previously treated with taxanes, and that MHT provides additional therapeutic benefits by extending PFS and OS.
Keywords: bevacizumab, metastatic breast cancer, real-world, maintenance hormonal therapy, taxane
Plain language summary
While first-line combination therapy with bevacizumab and chemotherapy has shown some efficacy in the treatment of patients with MBC in Phase III clinical trials, it has failed to significantly improve OS. In addition, the use of bevacizumab in this indication has been considered controversial due to the withdrawal of marketing approval in the USA. Therefore, identification of factors that predict long-term response to bevacizumab would provide patients with a valuable therapeutic option. This ambispective, observational study (LORENA) was conducted to describe the clinical characteristics of patients with MBC who have experienced long-term response to bevacizumab. The results suggest that long-term responders are relatively young (<50 years of age), more often premenopausal, have stage IV disease at diagnosis, and have <3 sites of metastases. The benefit of bevacizumab may be greater in patients who have not received prior treatment with taxanes and in those who received MHT.
Introduction
Breast cancer is the most common type of cancer in women,1 and in 2015, it was responsible for approximately 571,000 deaths worldwide.2 At present, the median duration of survival of patients with metastatic breast cancer (MBC) is estimated to be 2–3 years,3 while the 5-year survival rate is estimated at 26.9%.4 In recent decades, outcomes in patients with MBC have improved; however, the greatest improvements in outcomes have been seen in women with human epidermal growth factor receptor 2 (HER2)-positive tumors, while outcomes in patients with HER2-negative tumors have lagged behind.5
Members of the vascular endothelial growth factor (VEGF) family of proteins are key players in the process of neoangiogenesis, which is crucial for the development and progression of breast cancer.6 The VEGF family of glycoproteins are powerful mitogens that, once bound to a VEGF receptor (principally VEGF-receptor 2), activate intracellular signaling cascades that lead to endothelial cell proliferation and migration.7 VEGF expression is upregulated in the hypoxic conditions that predominate in tumors, and its transcription is activated by hypoxia-inducible factor-1 (HIF-1).7 A number of factors may contribute to VEGF expression in breast cancer, including hypoxia and estrogen,7 and high levels of VEGF and VEGF receptor expression are adverse prognostic marker in breast cancers.8,9
It has been demonstrated that some chemotherapies, in particular metronomic chemotherapy, have anti-angiogenic effects.10,11 HIF-1 is activated in response, and the tumor mounts a reactive response by upregulating VEGF.12 This feedback loop can be blocked by combining chemotherapy with an anti-angiogenic drug,12 which is the rationale for using chemotherapy in combination with an anti-VEGF drug to treat MBC. The combination can help to restore the balance between pro- and anti-angiogenic stimuli in the tumor, “normalizing” perfusion, and vascular permeability in the tumor vasculature, and allowing more efficient delivery of chemotherapies.12
Bevacizumab is a recombinant humanized monoclonal antibody against VEGF.13 In Phase III clinical studies, the first-line combination of bevacizumab and chemotherapy significantly improved progression-free survival (PFS), without affecting overall survival (OS), in patients with metastatic or locally recurrent breast cancer.14–17 These findings were later confirmed in a large, international, open-label study of >2,200 patients.18 Furthermore, in a real-world, non-randomized study of 3,426 patients with HER2-negative MBC, the first-line combination of bevacizumab and paclitaxel was associated with significant improvement in PFS, as well as OS, compared with paclitaxel alone.19 The efficacy of bevacizumab has also been evaluated in combination with hormonal therapy (letrozole or fulvestrant). However, in patients with advanced breast cancer, these treatment regimens failed to produce significant improvements in PFS or OS compared with hormonal therapy alone in a Phase III clinical study.20
The European Medicines Agency (EMA) and the Food and Drug Administration (FDA) of the USA approved bevacizumab in combination with chemotherapy for first-line treatment of HER2-negative MBC. The FDA later withdrew its approval due to the risk of adverse events (AEs) and lack of evidence supporting long-term clinical benefit. While the EMA’s approval remained in place, the clinical use of bevacizumab to treat breast cancer was considered controversial by some experts. The third European School of Oncology – European Society of Medical Oncology International Consensus Guidelines for Advanced Breast Cancer21 state that, due to the absence of improvement in OS and because no factors predicting effective bevacizumab treatment have been identified, its use can only be recommended in selected cases.
The aim of this ambispective, observational, real-world study was to describe the clinical characteristics of patients with HER2-negative MBC who experienced long-term benefits with first-line bevacizumab-based therapy.
Patients and methods
Design and objectives
This ambispective, observational, multicenter study was conducted at 38 hospitals in Spain. Patients were eligible to participate if they were aged ≥18 years, had a histologically confirmed, metastatic or locally-advanced, HER2-negative breast cancer, received first-line treatment with a combination of bevacizumab and chemotherapy, and had a PFS ≥12 months from the beginning of bevacizumab treatment.
Data on patient characteristics, tumor description, duration of bevacizumab treatment, and prior neo/adjuvant chemotherapy or other therapies, were collected retrospectively at the time of enrollment. Grade ≥3 AEs related to bevacizumab and AEs related to bevacizumab that led to discontinuation were recorded in accordance with the Medical Dictionary for Regulatory Activities 17.1 and their intensity was assessed using the Common Terminology Criteria for Adverse Events v4.0.22 For patients who were alive at the time of inclusion, data were prospectively updated over the next 18 months. During the intervening period, patients continued to attend routine follow-up visits.
The main objective of the study was to describe the clinical characteristics of patients with HER2-negative MBC who were treated with the first-line combination of bevacizumab and chemotherapy and had a PFS ≥12 months. Secondary objectives included assessment of patients who experienced objective response (overall response rate, ORR), PFS, OS, and factors predictive of clinical benefit, as well as assessment of safety and tolerability. Clinical response was evaluated using the Response Evaluation Criteria in Solid Tumors 1.0. PFS was defined as the time from treatment initiation to progression or death due to any cause, and was determined by an investigator using routine clinical practice criteria. OS was defined as the time from the date of treatment initiation to the date of death due to any cause.
The study was approved by the ethics committee from Centro Oncológico de Galicia (A Coruña, Spain), and patients participating in the prospective phase provided written informed consent.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Qualitative variables were described using frequency and percentage per modality. When relevant, the 95% CI was calculated. OS and PFS were estimated using the Kaplan–Meier method. A post hoc investigation of factors predictive of long-term survival was performed using a Cox multivariate model for PFS and OS. Factors analyzed included age, tumor characteristics, and antineoplastic treatments. Factors entered into the regression were first tested using univariate analysis. The final model was constructed with all variables presenting log-rank P-value ≤0.05. Hazard ratios (HR) and 95% CIs were calculated for factors predictive of long-term survival. SPSS software version 21.0 was used for all statistical calculations.
Results
Patient characteristics
Between March 2012 and October 2013, 166 patients were screened for inclusion, and 148 met the eligibility criteria and were included in the final analysis (median age: 50 years; range: 29–81 years) (Table 1). At the time of inclusion, 110 patients were alive and included in the 18-month prospective phase of the study.
Table 1.
Baseline patient demographics and tumor characteristics (n=148)
| Characteristics | Value |
|---|---|
| Median age (range), years | 50 (29, 81) |
| ECOG performance status, n (%) | |
| 0–1 | 132 (89.19) |
| ≥2 | 8 (5.41) |
| ND | 8 (5.41) |
| Menopausal status, n (%) | |
| Pre-menopausal | 64 (43.24) |
| Post-menopausal | 73 (49.32) |
| ND | 11 (7.43) |
| TNM stage of disease at initial diagnosis, n (%) | |
| I | 12 (8.11) |
| II | 53 (35.81) |
| III | 38 (25.67) |
| IV | 42 (28.38) |
| ND | 3 (2.03) |
| Number of metastasis organ sites, n (%) | |
| ≤3 | 140 (94.59) |
| >3 | 8 (5.41) |
| Site of metastasis, n (%) | |
| Non-visceral | 56 (37.84) |
| Visceral | 39 (26.35) |
| Both | 53 (35.81) |
| Histology, n (%) | |
| Invasive ductal carcinoma | 126 (85.14) |
| Invasive lobular carcinoma | 12 (8.11) |
| Other | 10 (6.76) |
| Histological grade, n (%) | |
| I | 10 (6.76) |
| II | 49 (33.11) |
| III | 44 (29.73) |
| ND | 45 (30.41) |
| Estrogen receptors, n (%) | |
| Positive | 110 (74.32) |
| Negative | 32 (21.62) |
| ND | 6 (4.06) |
| Progesterone receptors, n (%) | |
| Positive | 91 (61.49) |
| Negative | 52 (35.14) |
| ND | 5 (3.38) |
| Ki67, n (%) | |
| <10% | 17 (11.49) |
| 10%–20% | 32 (21.62) |
| >20% | 45 (30.41) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ND, no data.
At initial diagnosis, 42 (28%) patients had stage IV cancer and 28 (19%) had triple-negative tumors. The majority (n=140, 95%) had ≤3 sites of metastases and in 92 patients (62%), at least 1 visceral site was affected (Table 1). The most common sites of metastasis were the bones (n=82, 55%), lungs (n=57, 39%), liver (n=46, 31%), lymph nodes (n=30, 20%), and soft tissues (n=15, 10%). At baseline, 110 patients had received prior treatment for non-MBC, including surgery (n=110, 74%), radiotherapy (n=83, 58%), chemotherapy (n=99, 67%), and hormonal therapy (n=76, 51%). Among those who had received chemotherapy, 36 (24% of total) were treated only with anthracyclines and 4 (3%) only with taxanes, while 48 (32%) were treated with both. A total of 52 patients (35%) received prior treatment with taxanes.
At baseline, 30 patients (20%) had concomitant cardiovascular conditions, while 10 (7%) had neurological, 24 (16%) had hormonal, 11 (7%) had gastrointestinal, 1 (<1%) had hemostatic, and 6 (4%) had infectious conditions; 66 patients (45%) had other concomitant conditions.
Bevacizumab treatment
The mean duration of first-line bevacizumab treatment was 19.1 months (SD 11.5 months). In 108 patients (73%), the duration of treatment was ≥12 months, while in 84 (57%), it was >15 months. Bevacizumab 10 mg/kg every 2 weeks was used in 100 patients (68%), while 15 mg/kg every 3 weeks was used in 46 (31%; data not available for 2 patients). A total of 138 patients discontinued first-line bevacizumab treatment for reasons that included disease progression in 67 patients (48.5%), AEs in 25 (18%), and death in 1 (<1%); 45 (33%) withdrew for other reasons, primarily the oncologist’s or patient’s decision.
First-line bevacizumab treatment was given in combination with chemotherapy in 138 patients (93%) and the mean (SD) number of cycles was 10.7 (6.9). Chemotherapy consisted primarily of taxanes (n=128; 93%), most commonly paclitaxel (n=105; 82%).
Following first-line chemotherapy, 89 patients (60%) received maintenance hormonal therapy (MHT), and 19 of these 89 patients (21%) continued to receive MHT until the last visit. Aromatase inhibitors were used in 55 patients (62%), while tamoxifen or fulvestrant was used in 34 patients (38%). The mean (SD) duration of MHT was 17.4 (12.9) months.
Second-line therapy was prescribed to 91 patients (61%); of these, 43 patients (47%) received capecitabine (alone or in combination) and 29 (32%) continued to receive bevacizumab. Overall, 73 patients (49%) received ≥3 lines of therapy at the end of the study.
Effectiveness
Objective response data were available for 120 patients. Overall, 85 patients had a partial response (71%) and 35 had a complete response (29%), yielding an ORR of 81% (response data were not available for 28 patients).
Median PFS was 22.7 months (95% CI: 20.2–25.2 months; Figure 1). Factors associated with increased PFS on univariate analysis were the absence of adjuvant treatment with taxanes (HR: 1.83, 95% CI: 1.26–2.65, P=0.001) and the use of MHT (HR: 1.96, 95% CI: 1.34–2.86, P=0.0004). A multivariate analysis that included these 2 factors demonstrated that MHT was the only factor independently associated with increased PFS (HR: 1.86, 95% CI: 1.25–2.77, P=0.002; Figure 2). Median PFS was 25.3 months (95% CI: 21.5–32.6) in patients who received MHT and 19.1 months (95% CI: 16.0–21.0) in those who did not.
Figure 1.
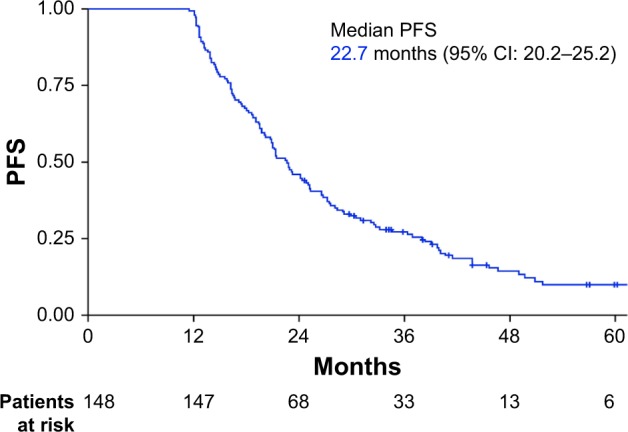
PFS in the entire patient population (n=148).
Abbreviation: PFS, progression-free survival.
Figure 2.
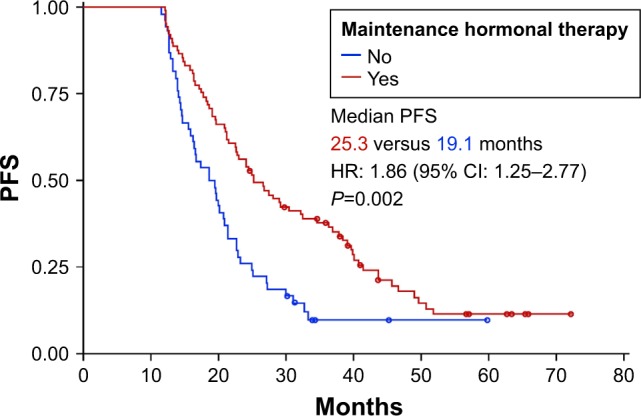
Multivariate analysis. PFS in patients with and without maintenance hormonal therapy.
Abbreviations: HR, hazard ratio; PFS, progression-free survival.
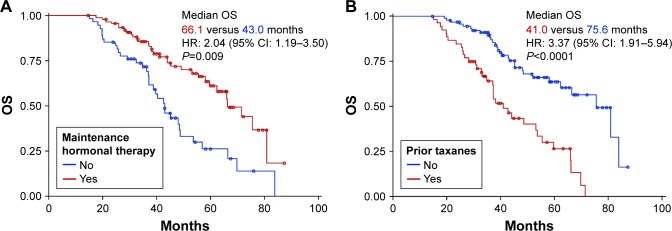
The median OS was 58.2 months (95% CI: 48.4–66.4; Figure 3). Univariate analysis revealed that advanced age, prior surgery, chemotherapy, treatment with taxanes, and the absence of MHT had a statistically significant negative influence on OS (Table 2). Two variables showed near-significant negative influence: diagnosis of triple-negative breast cancer (P=0.055) and prior radiotherapy (P=0.055). Multivariate analysis of prognostic factors for OS included all variables that demonstrated statistical significance or near significance in the univariate analysis. Only 2 of these were independently associated with improved OS: MHT (HR: 2.04, 95% CI: 1.19–3.50, P=0.009) and the absence of prior treatment with taxanes (HR: 3.37, 95% CI: 1.91–5.94, P<0.0001; Figure 4). Median OS in patients who received MHT was 66.1 months (95% CI: 59.8–80.9) compared with 43.0 months in those who did not (95% CI: 37.2–48.8).
Figure 3.
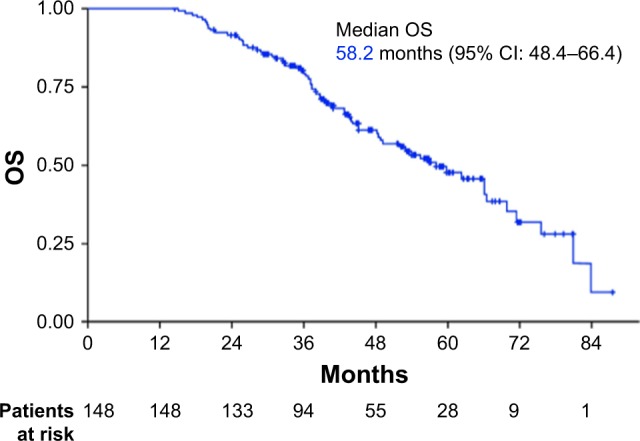
OS in the entire patient population (n=148).
Abbreviation: OS, overall survival.
Table 2.
Univariate analysis of predictive factors for overall survival (Cox proportional hazards analysis)
| Variables | HR (95% CI) | P-value (log-rank) |
|---|---|---|
| Age (<50 vs ≥50 years old) | 0.58 (0.36–0.94) | 0.02 |
| Prior surgery (no vs yes) | 2.13 (1.01–4.48) | 0.04 |
| Prior chemotherapy (no vs yes) | 2.19 (1.17–4.12) | 0.01 |
| Prior taxanes (no vs yes) | 3.34 (2.01–5.56) | <0.0001 |
| Hormonal maintenance therapy (yes vs no) | 2.45 (1.51–4.00) | 0.0002 |
Abbreviation: HR, hazard ratio.
Figure 4.
Multivariate analysis.
Note: (A) Overall survival in patients with and without maintenance hormonal therapy and (B) overall survival in patients with and without prior taxane therapy.
Abbreviations: HR, hazard ratio; OS, overall survival.
Safety
All patients who received at least 1 dose of bevacizumab were included in the safety analysis (n=148). Sixty-nine patients died during the course of the study, but none of these deaths occurred as a result of AEs. There were 57 AEs of grades 1–4 that led to discontinuation of bevacizumab therapy, of which 26 AEs were of grade ≥3. A total of 44 AEs of grade ≥3 related to bevacizumab were reported in 37 patients (25%; Table 3).
Table 3.
Adverse events of grade ≥3 related to bevacizumab
| Adverse events | Grade 3 n (%) | Grade 4 n (%) | Total n (%) |
|---|---|---|---|
| Hypertension | 19 (12.83) | 0 | 19 (12.83) |
| Hypertensive crisis | 2 (1.35) | 1 (0.68) | 3 (2.03) |
| Proteinuria | 5 (3.38) | 0 | 5 (3.38) |
| Kidney failure | 0 | 1 (0.68) | 1 (0.68) |
| Fatigue | 4 (2.70) | 0 | 4 (2.70) |
| Epistaxis | 2 (1.35) | 0 | 2 (1.35) |
| Pulmonary embolism | 1 (0.68) | 0 | 1 (0.68) |
| Neutropenia | 2 (1.35) | 0 | 2 (1.35) |
| Periodontal osteonecrosis | 1 (0.68) | 0 | 1 (0.68) |
| Mandibular osteonecrosis | 0 | 1 (0.68) | 1 (0.68) |
| Hemorrhagic diarrhea | 1 (0.68) | 0 | 1 (0.68) |
| Oral bleeding | 1 (0.68) | 0 | 1 (0.68) |
| Paresthesia | 1 (0.68) | 0 | 1 (0.68) |
| Skin toxicity | 1 (0.68) | 0 | 1 (0.68) |
| Anorexia | 1 (0.68) | 0 | 1 (0.68) |
Discussion
This ambispective, observational study in Spain showed that, during routine clinical practice, patients with metastatic or locally advanced HER2-negative breast cancer who have a PFS of ≥12 months after first-line bevacizumab + chemotherapy have a high ORR (≥81%), a PFS of almost 2 years (median 22.7 months), and survival for almost 5 years (median 58.2 months). The only independent predictor of prolonged PFS in this cohort was MHT, but predictive factors of improved OS were MHT and the absence of prior treatment with taxanes. Bevacizumab demonstrated a favorable safety profile, with no new safety signals identified.
In the present study, bevacizumab-based treatment regimens showed a greater effectiveness compared with previous randomized controlled and real-world studies. This was likely due to the recruitment criteria that restricted entry to patients who were long-term responders. To the best of our knowledge, the median PFS and OS observed in the present study are the highest described to date, exceeding those of the subgroup of patients from the ATHENA study who were treated with bevacizumab for >12 months (PFS: 19.9 months; OS: 29.6 months).23 In real-world studies, the median PFS and OS ranged from 8 to 14 months and from 21 to 40 months, respectively.19,24–27 Furthermore, the ORR was also higher in the present study than in other real-world studies (60%–70%),26,27 as well as in the long-term treatment subgroup in the ATHENA study (68%).23
It should be noted that in the present study, some of the patients’ baseline and clinical characteristics were different from those of other real-world studies of bevacizumab in the treatment of MBC. Patients in the present study were younger (median age: 50 vs 53–58 years), and a greater proportion of them were premenopausal (43% vs 16%) and had stage IV cancer at diagnosis (28% vs 16%–19%).19,26 The younger median age of the patients in our study, and the consequent higher proportion of premenopausal women, relative to other published series could be related to the tolerability of bevacizumab in older women, and the impact this has on maintaining long-term bevacizumab treatment. Data show that older women receiving bevacizumab may be more susceptible to certain AEs, particularly hypertension, than their younger counterparts.28
The proportion of patients who had >3 metastatic sites was lower in the present study (5%) than in the ATHENA study (69%), even when only the subgroup of patients who were treated for ≥12 months was considered (63%).23 Nevertheless, the proportion of patients in our study who had visceral metastases, and liver metastases specifically, was similar to that observed in other studies. In regard to hormone receptor status, there were few differences between our patient cohort and those of other similar studies: the proportion of patients with triple-negative tumors was similar to that reported in a real-world study conducted in Germany and in the subgroup of patients from the ATHENA study who received long-term treatment (19%, 18%, and 16%, respectively), while it was lower than in the entire patient population of the ATHENA study (26%) and in a real-world study conducted in France (31%).19,23,26 While in the ATHENA study (both the entire patient population and the subgroup of patients who were treated for ≥12 months) and the real-world study conducted in France, the majority of patients received neo/adjuvant chemotherapy (88%–95%); in the present study, only 67% did, a proportion similar to that reported in a real-world study conducted in Germany.19,23,26 Compared with the latter, the proportion of patients who received prior treatment with taxanes was higher in the present study (35% vs 24%), a surprising finding considering the fact that the use of taxanes was identified as a potential negative prognostic factor.26
What cannot be determined from our data is whether these patient characteristics could, in any way, be considered predictive factors of a longer benefit/response with the weekly paclitaxel–bevacizumab combination, or if they are simply prognostic factors that would be present in any series of patients with MBC and affect their long-term response to any form of therapy.
The subgroup analysis from the E2100 study showed that the benefit of combining bevacizumab with weekly paclitaxel was somewhat greater in patients who had previously received chemotherapy (taxanes or anthracyclines), in younger patients, and in patients with <3 metastatic sites.14 In contrast, among those who received paclitaxel without bevacizumab, PFS was worse in younger vs older patients and those who had been pretreated with taxanes vs those without taxane pretreatment.14 Therefore, some of the characteristics of our long-term responders may not be specific for bevacizumab–paclitaxel treatment, and include factors generally associated with better PFS and OS in MBC, such as stage IV at diagnosis or <3 metastatic sites. Other characteristics, such as younger age and prior treatment with taxanes, may be specific indicators of a favorable response to bevacizumab + paclitaxel, because they are generally associated with worse prognosis.29,30
The absence of prior treatment with taxanes and the use of MHT were identified in the present study as factors independently predictive of improved OS, while the latter was also a predictor of improved PFS. The results of the E2100 and AVADO randomized trials showed that patients who had received adjuvant treatment with taxanes experienced greater clinical benefits with the addition of bevacizumab.15,16 However, in the E2100 trial, the improvement in the median PFS in patients previously treated with taxanes, with chemotherapy without taxanes, and without prior chemotherapy were relatively small (12.0, 10.8, and 13.6 months, respectively).14,16 Nevertheless, similar to the findings of the present study, absence of prior taxane therapy was a predictor of increased PFS in a real-world study of 78 patients treated with bevacizumab at La Paz University Hospital (13.7 vs 8.3 months, HR: 0.63, P=0.062).31 Therefore, it is likely that patients with a history of taxane treatment can benefit from adding bevacizumab to first-line therapy, but they would be expected to have a shorter PFS than those without such history.
In routine clinical practice, the use of MHT is common in patients with hormone receptor-positive tumors after completion of chemotherapy. The benefit of MHT after chemotherapy is not well established in the literature. A systematic review of studies demonstrated that MHT is associated with improvements in PFS, control of symptoms, and quality of life in patients with MBC.32 Furthermore, several real-world studies have shown that MHT in combination with maintenance bevacizumab is associated with either improved PFS,33 or improved PFS and OS.27,31 These findings, in conjunction with the findings of the present study, suggest that the addition of hormonal therapy to maintenance bevacizumab after first-line bevacizumab-based therapy is a promising treatment option for patients with hormone receptor-positive MBC.
Limitations
The limitations of the present study include its observational design, which also included a retrospective phase. These features increase the risk of selection bias and reduce the possibility of inferring causality in comparison with a purely prospective, interventional study. Nevertheless, by examining outcomes in real-world patients representative of those that physicians encounter in day-to-day practice, observational studies provide a valuable adjunct to data from randomized controlled studies.
Conclusion
The results of this study suggest that patients with metastatic or locally advanced HER2-negative breast cancer who experience long-term response to first-line bevacizumab-based therapy are relatively young (<50 years of age), more often premenopausal, have stage IV disease at diagnosis, and have <3 sites of metastases. In this patient population, the benefit of bevacizumab-based therapy is increased if there is no prior treatment with taxanes and when MHT is added to maintenance bevacizumab after stopping chemotherapy.
Acknowledgments
The authors would like to thank Ana Moreno Cerro on behalf of Springer Healthcare Communications, and Georgii Filatov of Springer Healthcare Communications for medical writing assistance. This assistance was funded by Roche Farma, Spain. This study was funded by the Fundación Centro Oncológico Regional de Galicia.
Footnotes
Author contributions
MRV and AR planned and designed the study, prepared the first draft of the paper, tables, and figures, and contributed to the discussion. AR critically edited the final manuscript. LM, MJGG, IGL, EGG, CAR, JIC, and GLV contributed to the design of the study, recruitment and follow-up of patients, and to the discussion of the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Andrés Redondo served as an advisor for, and received honoraria and research funding from Roche. Miguel J Gil Gil served as advisors and received honoraria from Roche. Elisa García-Garre has received honoraria from Roche for lectures. The other authors report no conflicts of interest in this work.
References
- 1.Cardoso F, Fallowfield L, Costa A, Castiglione M, Senkus E, ESMO Guidelines Working Group Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi25–vi30. doi: 10.1093/annonc/mdr372. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation Cancer. 2017. [Accessed August 22, 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 3.Pfizer Inc Global Status of Advanced/Metastatic Breast Cancer: 2005–2015 Decade Report. 2016. [Accessed August 22, 2017]. Available from: http://www.breastcancervi-sion.com/sites/default/files/Decade%20Report_Full%20Report_Final.pdf.
- 4.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer. 2017. [Accessed August 22, 2017]. Available from: https://seer.cancer.gov/statfacts/html/breast.html.
- 5.Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985–2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 6.DeVita VT, Hellman S, Rosenberg SA. Cancer, Principles and Practice of Oncology. Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 7.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13(16):1845–1857. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 8.Eppenberger U, Kueng W, Schlaeppi JM, et al. Markers of tumor angiogenesis and proteolysis independently define high- and low-risk subsets of node-negative breast cancer patients. J Clin Oncol. 1998;16(9):3129–3136. doi: 10.1200/JCO.1998.16.9.3129. [DOI] [PubMed] [Google Scholar]
- 9.Mylona E, Alexandrou P, Giannopoulou I, et al. The prognostic value of vascular endothelial growth factors (VEGFs)-A and -B and their receptor, VEGFR-1, in invasive breast carcinoma. Gynecol Oncol. 2007;104(3):557–563. doi: 10.1016/j.ygyno.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 11.Drevs J, Fakler J, Eisele S, et al. Antiangiogenic potency of various chemotherapeutic drugs for metronomic chemotherapy. Anticancer Res. 2004;24(3a):1759–1763. [PubMed] [Google Scholar]
- 12.Blagosklonny MV. How Avastin potentiates chemotherapeutic drugs: action and reaction in antiangiogenic therapy. Cancer Biol Ther. 2005;4(12):1307–1310. doi: 10.4161/cbt.4.12.2315. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency . Avastin: EPAR – Product Information. London: European Medicines Agency; 2009. [Google Scholar]
- 14.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27(30):4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 17.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 18.Smith IE, Pierga JY, Biganzoli L, et al. First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2,251 patients. Ann Oncol. 2011;22(3):595–602. doi: 10.1093/annonc/mdq430. [DOI] [PubMed] [Google Scholar]
- 19.Delaloge S, Pérol D, Courtinard C, et al. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol. 2016;27(9):1725–1732. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
- 20.Martín M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol. 2015;33(9):1045–1052. doi: 10.1200/JCO.2014.57.2388. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017;28(1):16–33. doi: 10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2016. [Accessed August 10, 2017]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 23.Smith I, Pierga JY, Biganzoli L, et al. Final overall survival results and effect of prolonged (≥1 year) first-line bevacizumab-containing therapy for metastatic breast cancer in the ATHENA trial. Breast Cancer Res Treat. 2011;130(1):133–143. doi: 10.1007/s10549-011-1695-8. [DOI] [PubMed] [Google Scholar]
- 24.Cannita K, Paradisi S, Cocciolone V, et al. New schedule of bevacizumab/paclitaxel as first-line therapy for metastatic HER2-negative breast cancer in a real-life setting. Cancer Med. 2016;5(9):2232–2239. doi: 10.1002/cam4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller V, Dank M, de Ducla S, Mitchell L, Schneeweiss A. Real-world effectiveness and safety of first-line bevacizumab (BEV) + paclitaxel (PAC) in >2000 patients (pts) with HER2-negative metastatic breast cancer (mBC) Ann Oncol. 2016;27(suppl_6):237P. [Google Scholar]
- 26.Schneeweiss A, Förster F, Tesch H, et al. First-line Bevacizumab-containing Therapy for HER2-negative Metastatic Breast Cancer: Final Results from a Prospective German Study. Anticancer Res. 2016;36(3):967–974. [PubMed] [Google Scholar]
- 27.Gamucci T, Mentuccia L, Natoli C, et al. A Real-World Multicentre Retrospective Study of Paclitaxel-Bevacizumab and Maintenance Therapy as First-Line for HER2-Negative Metastatic Breast Cancer. J Cell Physiol. 2017;232(6):1571–1578. doi: 10.1002/jcp.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biganzoli L, di Vincenzo E, Jiang Z, et al. First-line bevacizumab-containing therapy for breast cancer: results in patients aged ≥70 years treated in the ATHENA study. Ann Oncol. 2012;23(1):111–118. doi: 10.1093/annonc/mdr043. [DOI] [PubMed] [Google Scholar]
- 29.Puente J, López-Tarruella S, Ruiz A, et al. Practical prognostic index for patients with metastatic recurrent breast cancer: retrospective analysis of 2,322 patients from the GEICAM Spanish El Alamo Register. Breast Cancer Res Treat. 2010;122(2):591–600. doi: 10.1007/s10549-009-0687-4. [DOI] [PubMed] [Google Scholar]
- 30.Shen T, Gao C, Zhang K, Siegal GP, Wei S. Prognostic outcomes in advanced breast cancer: the metastasis-free interval is important. Hum Pathol. 2017;70:70–76. doi: 10.1016/j.humpath.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Redondo A, Martínez V, Zamora P, et al. Continuation of bevacizumab and addition of hormone therapy following weekly paclitaxel therapy in HER2-negative metastatic breast cancer. Onco Targets Ther. 2014;7:2175–2181. doi: 10.2147/OTT.S70654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi S, Schinzari G, Basso M, et al. Maintenance hormonal and chemotherapy treatment in metastatic breast cancer: a systematic review. Future Oncol. 2016;12(10):1299–1307. doi: 10.2217/fon-2015-0065. [DOI] [PubMed] [Google Scholar]
- 33.Fabi A, Russillo M, Ferretti G, et al. Maintenance bevacizumab beyond first-line paclitaxel plus bevacizumab in patients with Her2-negative hormone receptor-positive metastatic breast cancer: efficacy in combination with hormonal therapy. BMC Cancer. 2012;12:482. doi: 10.1186/1471-2407-12-482. [DOI] [PMC free article] [PubMed] [Google Scholar]