Abstract
Cyclic peptides are cyclic compounds formed mainly by the amide bonds between either proteinogenic or non-proteinogenic amino acids. This review highlights the occurrence, structures and biological activities of fungal cyclic peptides (excluding cyclodipeptides, and peptides containing ester bonds in the core ring) reported until August 2017. About 293 cyclic peptides belonging to the groups of cyclic tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca-, undeca-, dodeca-, tetradeca-, and octadecapeptides as well as cyclic peptides containing ether bonds in the core ring have been isolated from fungi. They were mainly isolated from the genera Aspergillus, Penicillium, Fusarium, Acremonium and Amanita. Some of them were screened to have antimicrobial, antiviral, cytotoxic, phytotoxic, insecticidal, nematicidal, immunosuppressive and enzyme-inhibitory activities to show their potential applications. Some fungal cyclic peptides such as the echinocandins, pneumocandins and cyclosporin A have been developed as pharmaceuticals.
Keywords: cyclopeptides, fungi, biological activities, occurrence, applications
1. Introduction
Cyclic peptides (also called cyclopeptides) are cyclic compounds formed mainly by proteinogenic or non-proteinogenic amino acids joined together by amide bonds (or peptide bonds). Cyclic peptides have been isolated from plants [1], fungi [2], bacteria (including actinomycetes) [3], sponges [4], algae [5,6], and mammals [7]. Among these organisms, fungi are well-known producers of a diversity of cyclic peptides with interesting structures and biological activities [8]. Here, we focus on the cyclic peptides derived from fungi, including insect pathogenic fungi, plant pathogenic fungi, soil-derived fungi, marine-derived fungi, plant or insect endophytic fungi, and the macroscopic fungi which we usually call mushrooms. Some of the fungal cyclic peptides are mycotoxins which pose a hazard to animals and plants [2]. Interest in cyclic peptides is due to their significant biological activities, such as antimicrobial, insecticidal, cytotoxic, and anticancer activities, in addition to their important physiological and ecological functions. Therefore, they have the potential to be developed as pharmaceuticals and agrochemicals [9].
Fungal cyclic peptides mainly include cyclic di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and decapeptides. Recent special reviews covering the chemical synthesis [10], biosynthesis [11,12], as well as developments and applications (i.e., echinocandins, pneumocandins and cyclosporin A) [13,14] of fungal cyclic peptides are available. In this review, we describe the occurrence, biological activities, structures, and potential applications of the fungal cyclic peptides and their analogs, with the exception of cyclodipeptides (called 2,5-diketopiperazines), which were previously reviewed [15]. Cyclic depsipeptides from organisms including fungi, which are a big group of cyclic peptides in which one or more amino acid is replaced by a hydroxy acid, resulting in the formation of at least one ester bond in the core ring structure, have also been reviewed [16]. The cyclic peptides containing at least one ether bond in the core ring were considered as a special group and included in this review.
2. Cyclic Tripeptides
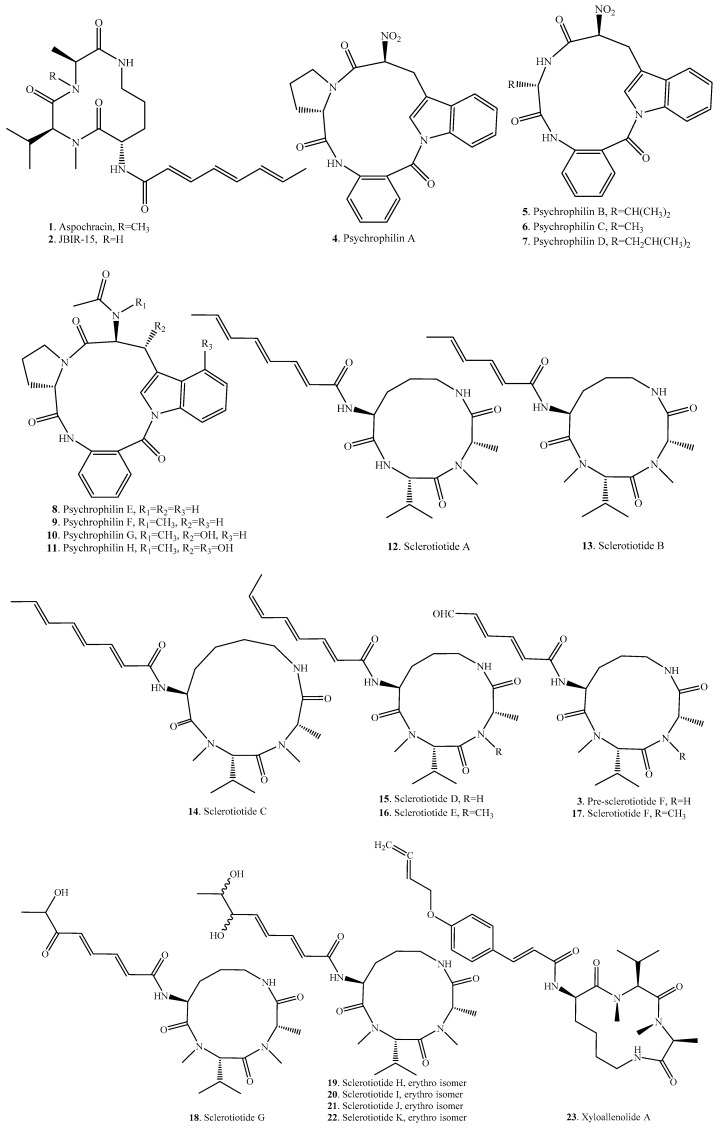
Cyclic tripeptides are composed of three amino acid residues in the core ring. They are described in the genera Aspergillus, Penicillium and Xylaria. Cyclic tripeptides exhibit insecticidal [17], cytotoxic [18] and antifungal activities [19]. Their origins, and biological activities are listed in Table 1, and the structures are provided in Figure 1.
Table 1.
Fungal cyclic tripeptides and their biological activities.
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Aspochracin (1) | Aspergillus kumbius | - | [24] |
| Aspergillus ochraceus | Insecticidal activity | [17] | |
| Aspergillus sclerotiorum PT06-1 | - | [19] | |
| JBIR-15 (2) | Aspergillus sclerotiorum | Antifungal activity | [19,25] |
| Pre-sclerotiotide F (3) | Aspergillus insulicola | - | [26] |
| Psychrophilin A (4) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] |
| Psychrophilin B (5) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [21] |
| Psychrophilin C (6) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [21] |
| Psychrophilin D (7) | Penicillium algidum | Cytotoxic activity | [18] |
| Psychrophilin E (8) | Aspergillus versicolor ZLN-60 | Antiproliferative activity | [22,23] |
| Psychrophilin F (9) | Aspergillus versicolor ZLN-60 | - | [23] |
| Psychrophilin G (10) | Aspergillus versicolor ZLN-60 | Lipid-lowering effect | [23] |
| Psychrophilin H (11) | Aspergillus versicolor ZLN-60 | - | [23] |
| Sclerotiotide A (12) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide B (13) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide C (14) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide D (15) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide E (16) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide F (17) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Aspergillus insulicola | - | [26] | |
| Sclerotiotide G (18) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide H (19) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide I (20) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide J (21) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide K (22) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Xyloallenolide A (23) | Xylaria sp. No. 2508 | - | [27] |
Figure 1.
Structures of the cyclic tripeptides isolated from fungi.
Aspochracin (1) was the first cyclic tripeptide isolated from fungi in 1969. It was obtained from the culture broth of Aspergillus ochraceus, a pathogenic fungus causing muscardine on insects. This compound shows contact toxicity on the first instar larvae as well as the eggs of silkworm [17].
Aspochracin (1), JBIR-15 (2) and sclerotiotides A–K (12–22) were obtained from the halotolerant fungus Aspergillus sclerotiorum PT06-1. Only sclerotiotides A (12), B (13), F (17) and I (20), and JBIR-15 (2) show selective antifungal activity against Candida albicans, with minimum inhibitory concentration (MIC) values of 7.5, 3.8, 30, 6.7, and 30 μM, respectively [19].
Psychrophilins A–H (compounds 4–11) were isolated from Aspergillus and Penicillium species [18,20,21,22,23]. Among them, psychrophilin D (7) from Penicillium algidum show cytotoxic activity [18], psychrophilin E (8) from Aspergillus sp. shows antiproliferative activity [22], and psychrophilin G (10) from Aspergillus versicolor ZLN-60 shows lipid-lowering effects [23].
3. Cyclic Tetrapeptides
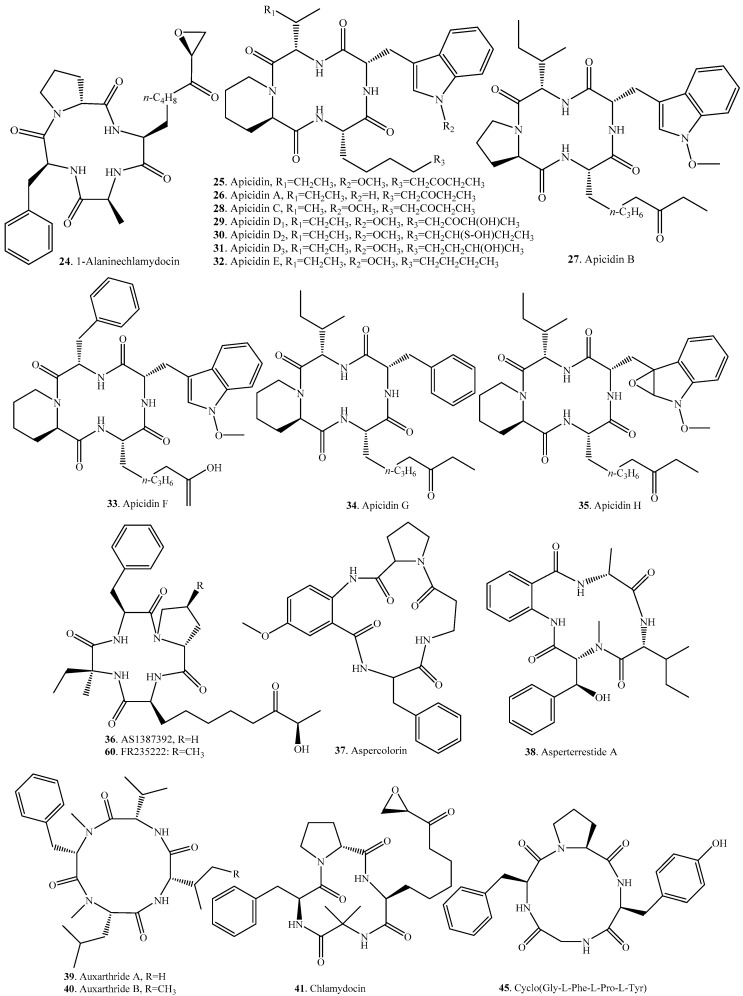
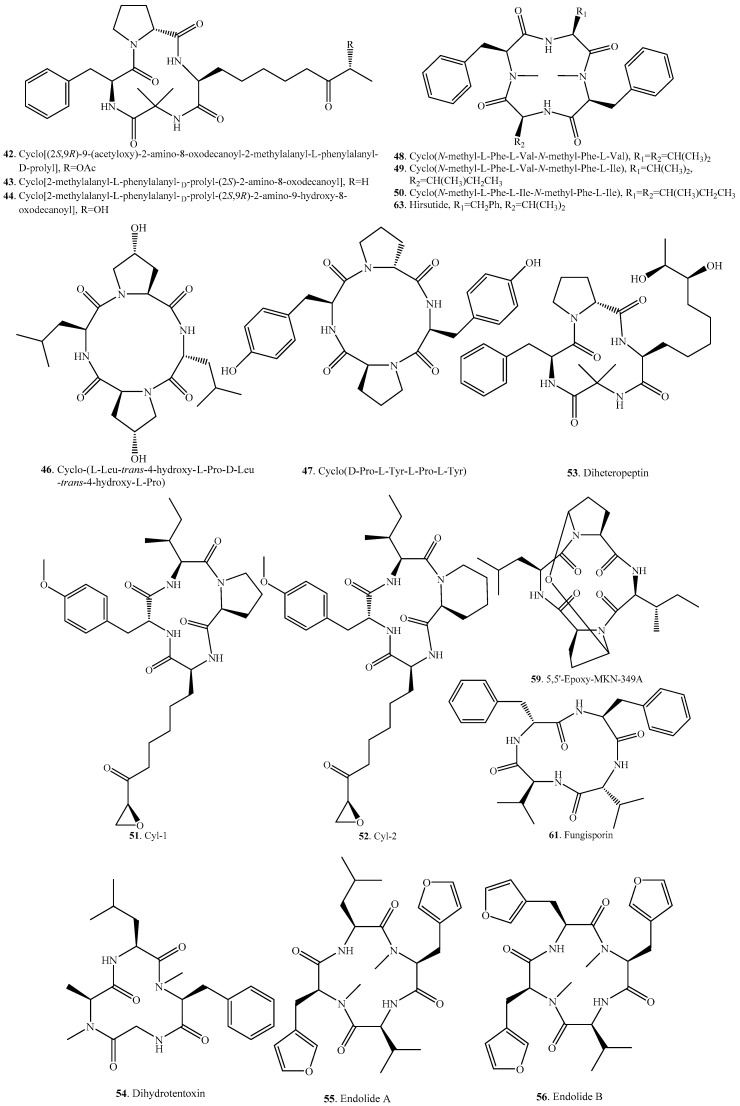
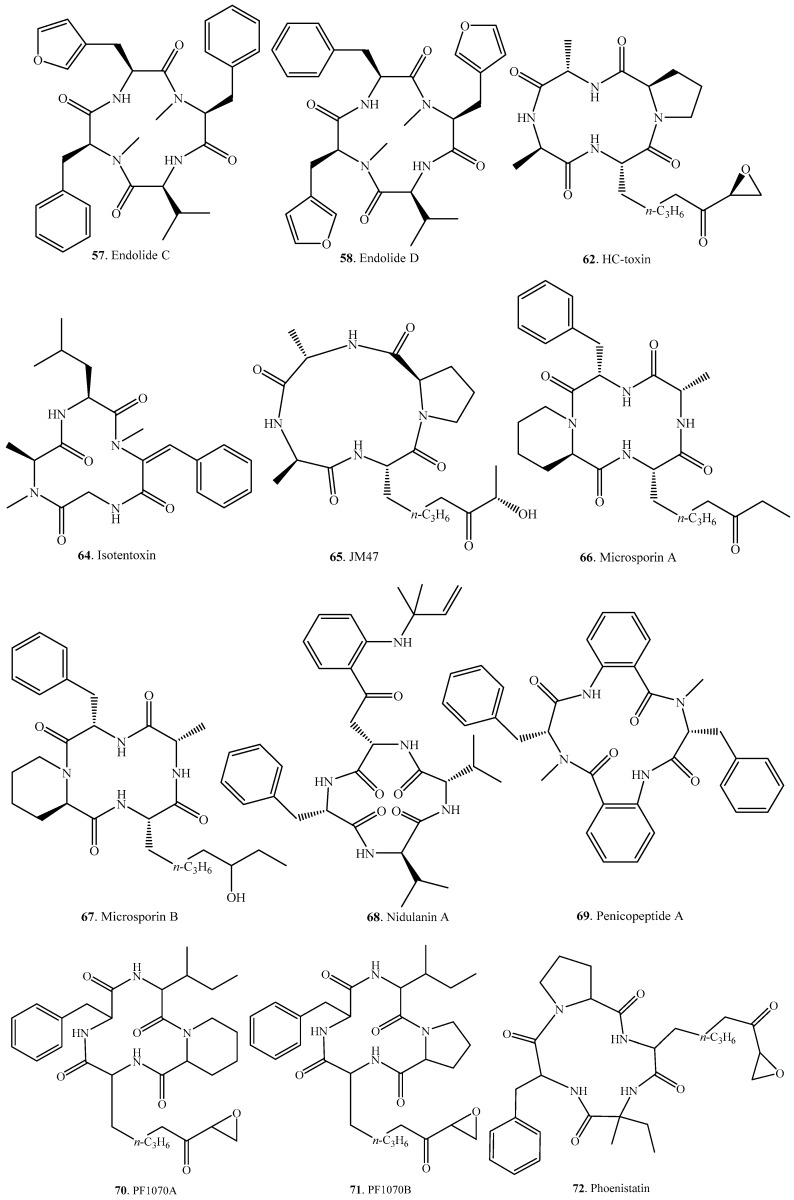
Cyclic tetrapeptides have been found in many genera such as Acremonium, Alternaria, Cylindrocladium, Fusarium, Helicoma, Penicillium, Peniophora, Phoma, Phomopsis, Pseudoxylaria, Stachylideium, and Verticillium. Their origins and biological activities are listed in Table 2, and the corresponding structures are shown in Figure 2.
Table 2.
Fungal cyclic tetrapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 1-Alaninechlamydocin (24) | Tolypocladium sp. | Inhibitory activity on histone deacetyl (HDAC) activity, antiproliferation, cytotoxicity, cell cycle arrest, and apoptosis induction | [30] |
| Apicidin (25) | Fusarium pallidoroseum | Antimalarial activity | [32] |
| Fusarium semitectum | Phytotoxic activity | [53] | |
| - | Antiprotozoal activity by inhibiting parasite HDAC | [35] | |
| Apicidin A (26) | Fusarium pallidoroseum | Antimalarial activity | [32] |
| Fusarium semitectum | - | [53] | |
| Apicidin B (27) | Fusarium pallidoroseum | Antiprotozoal activity | [33] |
| Apicidin C (28) | Fusarium pallidoroseum | Antiprotozoal activity | [33] |
| Apicidin D1 (29) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Apicidin D2 (30) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Fusarium semitectum | Phytotoxic activity | [53] | |
| Apicidin D3 (31) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Apicidin E (32) | Fusarium semitectum mutant | - | [54] |
| Apicidin F (33) | Fusarium fujikuroi | Antimalarial activity | [55] |
| Apicidin G (34) | Fusarium semitectum | - | [56] |
| Apicidin H (35) | Fusarium semitectum | - | [56] |
| AS1387392 (36) | Acremonium sp. No. 27082 | Immunosuppressive activity | [37] |
| Aspercolorin (37) | Aspergillus versicolor | - | [57] |
| Asperterrestide A (38) | Aspergillus terreus SCSGAF0162 | Cytotoxic and antiviral activity | [38] |
| Auxarthride A (39) | Coprophilous fungus Auxarthron pseudauxarthron | - | [58] |
| Auxarthride B (40) | Coprophilous fungus Auxarthron pseudauxarthron | - | [58] |
| Chlamydocin (41) | Diheterospora chlantydosporia | Cytostatic activity | [39] |
| Penicillium sp. BCC18034 | Antimalarial and cytotoxic activity | [40] | |
| Cyclo[(2S,9R)-9-(acetyloxy)-2-amino-8-oxodecanoyl-2-methylalanyl-l-phenylalanyl-d-prolyl] (42) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S)-2-amino-8-oxodecanoyl] (43) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S,9R)-2-amino-9-hydroxy-8-oxodecanoyl] (44) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Verticillium coccosporum | Phytotoxic activity | [59] | |
| Cyclo(Gly-l-Phe-l-Pro-l-Tyr) (45) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [42] |
| Cyclo(l-Leu-trans-4-hydroxy-l-Pro-d-Leu-trans-4-hydroxy-l-Pro) (46) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [43] |
| Cyclo(d-Pro-l-Tyr-l-Pro-l-Tyr) (47) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [42] |
| Cyclo(N-methyl-l-Phe-l-Val-N-methyl-Phe-l-Val) (48) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyclo(N-methyl-l-Phe-l-Val-N-methyl-l-Phe-l-Ile) (49) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyclo(N-methyl-l-Phe-l-Ile-N-methyl-l-Phe-l-Ile) (50) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyl-1 (51) | Cylindrocladium scoparium | Phytotoxic activity | [45,46] |
| Cyl-2 (52) | Cylindrocladium scoparium | Phytotoxic activity | [46,60] |
| Diheteropeptin (53) | Diheterospora sp. | TGF-β-like activity | [61] |
| Diheterospora chlamydosporia | TGF-β-like activity | [62] | |
| Dihydrotentoxin (54) | Alternaria porri | - | [63] |
| Phoma sp. | - | [51] | |
| Endolide A (55) | Stachylideium sp. from the sponge Callyspongia sp. | Affinity to the vaspopressin receptor 1A with a Ki of 7.04 μM | [64] |
| Endolide B (56) | Stachylideium sp. from the sponge Callyspongia sp. | Be selevtive toward the serotonin receptor 5HT2b with a Ki of 0.77 μM | [64] |
| Endolide C (57) | Marine-sponge-derived Stachylidium sp. 293 K04 | - | [65] |
| Endolide D (58) | Marine-sponge-derived Stachylidium sp. 293 K04 | - | [65] |
| 5,5’-Epoxy-MKN-349A (59) | Endophytic fungus Penicillium sp. GD6 from the mangrove Bruguiera gymnorrhiza | - | [66] |
| FR235222 (60) | Acremonium sp. No. 27082 | Immunosuppressant that inhibits mammalian histone deacetylase | [67,68,69] |
| Fungisporin (61) | Penicillium chrysogenum | - | [70] |
| HC-toxin (62) | Helminthosporium carbonum = Cochliobolus carbonum | Phytotoxic activity | [71,72] |
| Hirsutide (63) | Spider-derived fungus Hirsutella sp. | - | [73] |
| Isotentoxin (64) | Alternaria porri | - | [63] |
| JM47 (65) | Marine-derived Fusarium sp. from the alga Codium fragile | - | [29] |
| Microsporin A (66) | Microsporum cf. gypseum CNL-692 | Inhibitory activity on histone deacetylase; cytotoxic activity | [47] |
| Microsporin B (67) | Microsporum cf. gypseum CNL-692 | Inhibitory activity on histone deacetylase; cytotoxic activity | [47] |
| Nidulanin A (68) | Aspergillus nidulans | - | [74] |
| Penicopeptide A (69) | Endophytic Penicillium commune from Vitis vinifera | Inhibitrory activity on 11β-hydroxysteroid dehydrogense | [48] |
| PF1070A (70) | Calonectria ilicicola | Phytotoxic activity | [75] |
| Humicola sp. | Antitumour activity | [76] | |
| PF1070B (71) | Humicola sp. | Antitumour activity | [76] |
| Phoenistatin (72) | Acremonium fusigerum | Enhancing activity on gene expression | [77] |
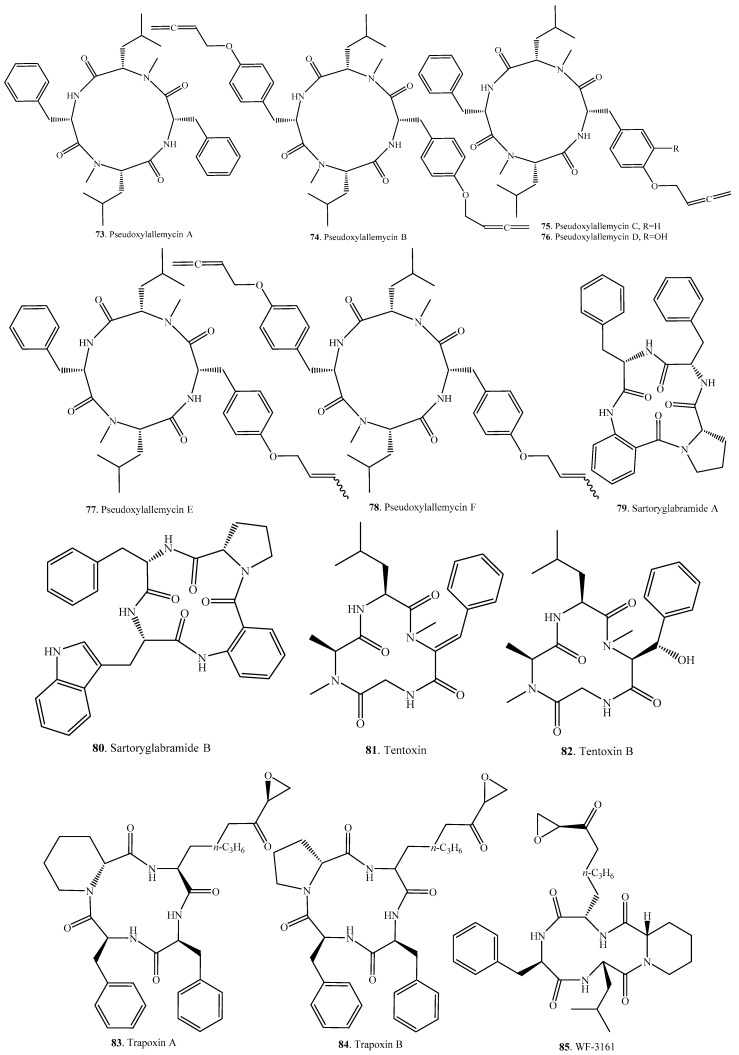
| Pseudoxylallemycin A (73) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin B (74) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin C (75) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin D (76) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin E (77) | Termite-associated fungus Pseudoxylaria sp. X802 | - | [49] |
| Pseudoxylallemycin F (78) | Termite-associated fungus Pseudoxylaria sp. X802 | - | [49] |
| Sartoryglabramide A (79) | Marine-derived Neosartorya glabra KUFA 0702 from the sponge Mycale sp. | - | [78] |
| Sartoryglabramide B (80) | Marine-derived Neosartorya glabra KUFA 0702 from the sponge Mycale sp. | - | [78] |
| Tentoxin (81) | Alternaria linicola | Phytotoxic activity | [79] |
| Alternaria porri | - | [63] | |
| Phoma sp. | - | [51] | |
| Tentoxin B (82) | Marine-derived fungus Phoma sp. from the giant jellyfish Nemopilema nomurai | Weak suppressive effect on the production of nitric oxide in murine macrophage cells without notable cytotoxicity | [51] |
| Trapoxin A (83) | Helicoma ambiens RF-1023 | Detransformation activity against v-sis oncogene-transformed NIH3T3 cells | [52] |
| - | HDAC inhibitory activity | [31] | |
| Trapoxin B (84) | Helicoma ambiens RF-1023 | Detransformation activity against v-sis oncogene-transformed NIH3T3 cells | [52] |
| WF-3161 (85) | Petriella guttlata | Antitumor activity | [80] |
Figure 2.
Structures of the cyclic tetrapeptides isolated from fungi.
Histone deacetylases (HDACs) are important regulators of gene expression and have been implicated as key participants in a variety of diseases. HC-toxins are host-selective toxins and act as inhibitors of HDAC [28]. JM47 (65) from a marine Fusarium species belongs to the HC-toxin family, and contains a 2-amino-8-oxo-9,10-epoxydecanoic acid residue [29]. Other cyclic tetrapeptides such as 1-alaninechlamydocin (24), apicidin (25), chlamydocin (41), FR235222 (60), microsporins A (66) and B (67), and trapoxins A (83) and B (84) also show inhibitory activity on HDAC [30,31]. Apicidin analogs (25–35) from Fusarium species show antiprotozoal activity by inhibiting parasite HDAC [32,33,34,35]. These HDAC inhibitors were considered as the potential therapeutics for spinal muscular atrophy [36].
AS1387392 (36) from Acremonium sp. No. 27082 showed a strong inhibitory effect against mammalian HDAC and T-cell proliferation which suggested its potential as immunosuppressant [37].
Asperterrestide A (38) from the fermentation broth of the marine-derived fungus Aspergillus terreus SCSGAF0162 showed cytotoxicity against U937 and MOLT4 hunman carcinoma cell lines and inhibitory effects on influenza virus strains H1N1 and H3N2 [38].
Chlamydocin (41) was isolated from Diheterospora chlantydosporia [39] and Penicillium sp. BCC18034 [40], respectively. This compound showed antimalarial and cytotoxic activities. Three chlamydocin analogs cyclo[(2S,9R)-9-(acetyloxy)-2-amino-8-oxodecanoyl-2-methylalanyl-l-phenylvalanyl-d-prolyl] (42), cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S)-2-amino-8-oxo-decanoyl] (43), and cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S,9R)-2-amino-9-hydroxy-8-oxodecanoyl] (44) were isolated from the culture filtrate of the soil fungus Peniophora sp. They all showed plant growth-retarding activity by reducing the height of rice seedlings without blotch and wilting [41].
Three cyclic tetrapeptides cyclo(Gly-l-Phe-l-Pro-l-Tyr) (45), cyclo(l-Leu-trans-4-hydroxy-l-Pro-l-Leu-trans-4-hydroxy-l-Pro) (46) and cyclo(d-Pro-l-Tyr-l- Pro- l -Tyr) (47) were isolated from the co-culture broth of two mangrove fungi, Phomopsis sp. K38 and Alternaria sp. E33. These compounds all had moderate antifungal activity against Candida albicans, Gaeumannomyces graminis, Rhzioctonia cerealis, Helminthosporium sativum and Fusarium graminearum [42,43].
Three cyclic tetrapeptides cyclo(N-methyl-l-Phe-l-Val-N-methyl-Phe-l-Val) (48), cyclo (N-methyl-l-Phe-l-Val-N-methyl-l-Phe-l-Ile) (49), and cyclo(N-methyl-l-Phe-l-Ile-N-methyl-l-Phe-l-Ile) (50) were isolated from the crude fermentation extract of Onychocola sclerotica. They all display inhibitory activity as cardiac calcium channel blockers on wild-type HEK or C1-6-37-3 cells with IC50 values of 5.0–7.1 μM [44].
Cyl-1 (51) and Cyl-2 (52) from the plant fungal pathogen Cylindrocladium scoparium exhibited marked inhibition on the growth of rice and lettuce seedlings, especially on their root growth, which suggested that they could be used to inhibit plant growth [45,46].
Microsporins A (66) and B (67) from the marine-derived fungus Microsporum cf. gypseum CNL-692 obtained from a sample of the bryozoan Bugula sp. collected in the U.S. Virgin Islands. Both compounds showed inhibitory activity on HDAC and demonstrated cytotoxic activity against human colon adenocarcinoma (HCT-116) cells [47].
Penicopeptide A (69) was isolated from the endophytic fungus Penicillium commune from Vitis vinifera. It exhibited significant inhibitory activity against 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in vitro and showed strong binding affinity to recombinant human 11β-HSD1 by microscale thermophoresis assay. Moreover, penicopeptide A (69) decreased the lipid droplet accumulation associate with the inhibiton of 11β-HSD1 activity of 3T3-L1 cells in vivo [48].
Pseudoxylallemycins A–F (73–78) were isolated from the termite-associated fungus Pseudoxylaria sp. X802. Pseudoxylallemycins A–D (73–76) showed antibacterial activity against Gram-negative human-pathogenic Pseudomonas aeruginosa and antiproliferative activity against human umbilical vein endothelial cells and K-562 cell lines [49].
Tentoxin (81) and its derivatives dihydrotentoxin (54) and isotentoxin (64) were important mycotoxins from Alternaria spp. [50]. Tentoxin B (82) was isolated from the marine-derived fungus Phoma sp. from the giant jellyfish Nemopilema nomurai. This compound showed weak suppressive effect on the production of nitric oxide in murine macrophage cells [51].
Trapoxins A (83) and B (84) from Helicoma ambiens RF-1023 exhibited detransformation activity against v-sis oncogene-transformed NIH3T3 cells (sis/NIH3T3) [52]. In addition, trapoxin A (83) showed HDAC inhibitory activity [31]. Both compounds displayed their potential as antitumor agents.
4. Cyclic Pentapeptides
Cyclic pentapeptides have been mainly isolated from the genera of Aspergillus, Fusarium, Hamigera, Penicillum, Pseudallescheria and Xylaria. Their distributions in fungi, and biological activities are listed in Table 3, and the structures are provided in Figure 3.
Table 3.
Fungal cyclic pentapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
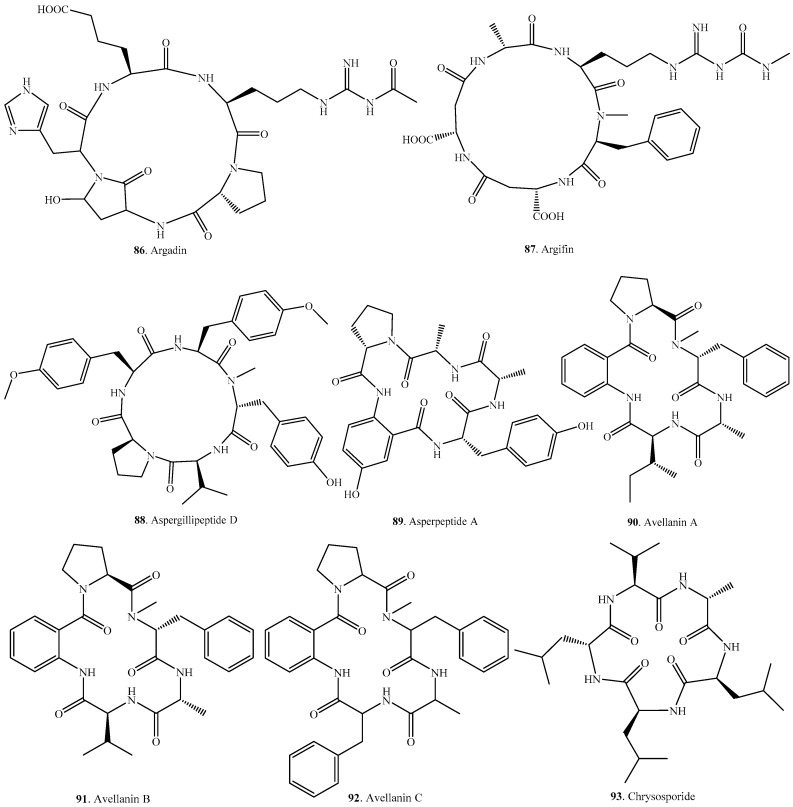
| Argadin (86) | Clonostachys sp. FO-7314 | Chitinase inhibitor | [81] |
| Argifin (87) | Gliocladium sp. FTD-0668 | Chitinase inhibitor | [82,97] |
| Aspergillipeptide D (88) | Marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501 | Antiviral activity | [84] |
| Asperpeptide A (89) | Marine gorgonian-derived Aspergillus sp. XS-20090B15 | Antibacterial activity | [98] |
| Avellanin A (90) | Hamigera sp. | Vasoconstriction and pressor effect | [99] |
| Avellanin B (91) | Hamigera sp. | Vasoconstriction and pressor effect | [99] |
| Avellanin C (92) | Hamigera paravellanea | Vasoconstriction and pressor effect | [99] |
| Chrysosporide (93) | Sepedonium chrysospermum | Weak cytotoxic to murine leukemia cell line | [100] |
| Clavatustide C (94) | Aspergillus clavatus C2WU | - | [101] |
| Cotteslosin A (95) | Aspergillus versicolor | Weak cytotoxic to human cancer cell lines | [102] |
| Cotteslosin B (96) | Aspergillus versicolor | Weak cytotoxic to human cancer cell lines | [102] |
| Cyclo(l-Ile-l-Leu-l-Leu-l-Leu-l-Leu) (97) | Endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii | Motility inhibitory and lytic activities against zoospores; Antifungal activity; weak cytotoxic activity on brine shrimp larvae | [103] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Ile) (98) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Leu) (99) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Val) (100) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Phe-l-Leu-l-Leu-l-Leu-l-Ile) (101) | Unidentified endophytic fungus No. 2524 from the mangrove Avicennia marina | - | [105] |
| Cyclo(l-Phe-l-Leu-l-Leu-l-Leu-l-Leu) (102) | Endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii | Motility inhibitory and lytic activities against zoospores; Antifungal activity; Weak cytotoxic activity on brine shrimp larvae | [103] |
| Cycloaspeptide A (103) | Aspergillus sp. NE-45 | - | [85] |
| Penicillium algidum | Antiplasmodial activity | [18] | |
| Penicillium jamesonlandense | - | [106] | |
| Penicillium janczewskii | - | [107] | |
| Penicillium lanosum | - | [106] | |
| Penicillium ribium | - | [106] | |
| Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] | |
| Penicillium soppii | - | [106] | |
| Cycloaspeptide B (104) | Aspergillus sp. NE-45 | - | [85] |
| Cycloaspeptide C (105) | Aspergillus sp. NE-45 | - | [85] |
| Cycloaspeptide D (106) | Penicillium algidum | Antiplasmodial activity | [18] |
| Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] | |
| Cycloaspeptide E (107) | Penicillia sp. | Insecticidal activity | [86] |
| Tricothecium sp. | - | [86] | |
| Cycloaspeptide F (108) | Isaria farinosa | Cytotoxic activity | [87] |
| Cycloaspeptide G (109) | Isaria farinosa | Cytotoxic activity | [87] |
| Cyclochlorotine (110) | Penicillium islandicum | Liver fibrosis, liver injuries | [108,109,110] |
| Hydroxycyclochlorotine (111) | Penicillium islandicum | - | [108] |
| Islanditoxin (112) | Penicillium islandicum | - | [111] |
| Lajollamide A (113) | Marine-derived fungus Asteromyces cruciatusis | Weak antibacterial on Gram-positive bacteria | [112] |
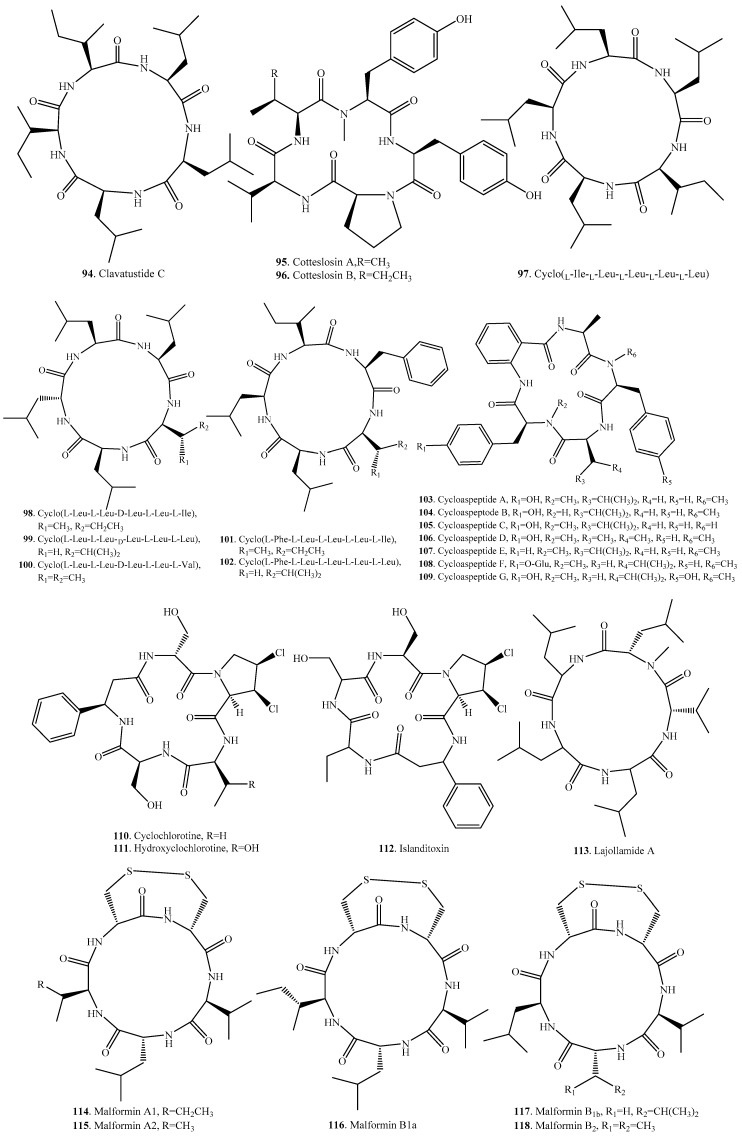
| Malformin A1 (114) | Aspergillus niger | Malformation activity in the corn root; Cytotoxic activity | [92,93] |
| Aspergillus tubingensis | Cytotoxic activity; Anti-TMV activity | [89,90] | |
| Malformin A2 (115) | Aspergillus niger | - | [92] |
| Malformin B1a (116) | Aspergillus niger | Curvature activity in the corn root test | [88] |
| Malformin B1b (117) | Aspergillus niger | - | [88] |
| Malformin B2 (118) | Aspergillus niger | - | [88] |
| Malformin C (119) | Aspergillus niger | Anticancer activity | [94,95] |
| Malformin E (120) | Endophytic fungus Aspergillus tamari from Ficus carica | Cytotoxic and antibiotic activity | [91] |
| MBJ-0174 (121) | Mortierella alpine f28740 from a soil smaple collected in Ise, Japan | Stimulators of cellular fibrinolytic activity | [113] |
| PF1171B (122) | Hamigera sp. | - | [99] |
| PF1171D (123) | Hamigera sp. | - | [99] |
| PF1171E (124) | Hamigera sp. | - | [99] |
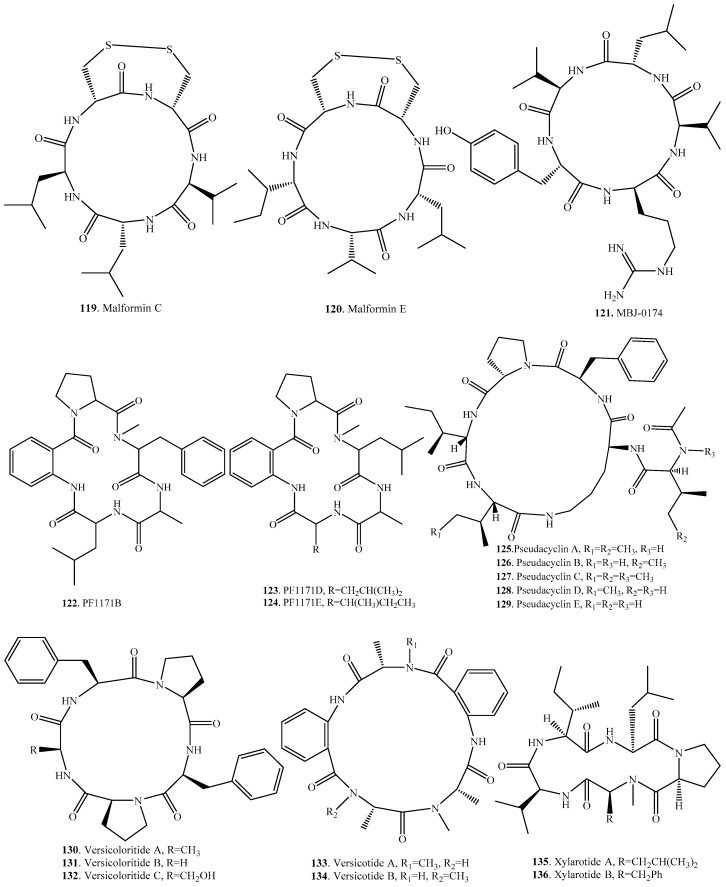
| Pseudacyclin A (125) | Animal fungal pathogen Pseudallescheria boydii | Immunosuppressive activity | [96] |
| Pseudacyclin B (126) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin C (127) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin D (128) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin E (129) | Anmal fungal pathogen Pseudallescheria boydii | - | [96] |
| Versicoloritide A (130) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicoloritide B (131) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicoloritide C (132) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicotide A (133) | Aspergillus versicolor ZLN-60 from the sediment of the Yellow Sea | - | [115] |
| Versicotide B (134) | Aspergillus versicolor ZLN-60 from the sediment of the Yellow Sea | - | [115] |
| Xylarotide A (135) | Xylaria sp. 101 | - | [116] |
| Xylarotide B (136) | Xylaria sp. 101 | - | [116] |
Figure 3.
Structures of the cyclic pentapeptides isolated from fungi.
Argadin (86) and argifin (87) produced by Clonostachys sp. FO-7314 and Gliocladium sp. FTD-0668, respectively, were identified in screening as chitinase inhibitors [81,82]. Chitinases play key roles in organisms, ranging from bacteria to humans. There is a need for specific, potent inhibitors to probe the function of these chitinases in different organisms. Such molecules could also provide leads for the development of chemotherapeuticals with fungicidal, insecticidal, or anti-inflammatory potential. Argadin (86) and argifin (87) could be used as the leads for the development of novel chitinase inhibitors [83].
Aspergillipeptide D (88) was isolated from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. It showed evident antiviral activity against herpes simplex virus type 1 (HSV-1) with a median inhibitory concentration (IC50) value of 9.5 μM under their non-cytotoxic concentration against a Vero cell line, and also had antiviral activity against acyclovir-resistant clinical isolates of HSV-1 [84].
Cycloaspeptides A–F (103–108) are anthranilic acid (ABA)-containing cyclic pentapeptides. Among them, cycloaspeptides A–C (103–105) were isolated from Aspergillus sp. NE-45 [85], cycloaspeptide D (106) with antiplasmodial activity from Penicillium algidum [18] and the psychrotolerant fungus Penicillium ribeum [20], cycloaspeptide E (107) with insecticidal activity from several Penicillia species and a Tricothecium strain [86], and cycloaspeptides F (108) and G (109) from Isaria farinosa [87]. The presence of a glucose unit in cycloaspeptide F (108) was very rare in the isolated cyclic peptides.
Seven malformins (114–120) were isolated from a few Aspergillus species [88,89,90,91]. Malformin A1 (114) from Aspergillus niger and Aspergillus tubingensis showed malformation activity in the corn root, and cytotoxic activity, and anti-TMV activity [89,90,92,93]. Malformin C (119) from Aspergillus niger had potent cell growth inhibition activity [94], but its therapeutic index was too low to be an anti-cancer drug [95].
Pseudacyclins A–E (125–129) were isolated from Pseudallescheria boydii which is an emerging fungal pathogen causing fatal infections in both immunocompromised and immunocompetent hosts. Among them, pseudacyclin A (125) exhibited immunosuppressive activity [96].
5. Cyclic Hexapeptides
About 40 cyclic hexapeptides have been isolated from fungi. Their distributions in fungi, and biological activities are shown in Table 4, and the structures are provided in Figure 4.
Table 4.
Fungal cyclic hexapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
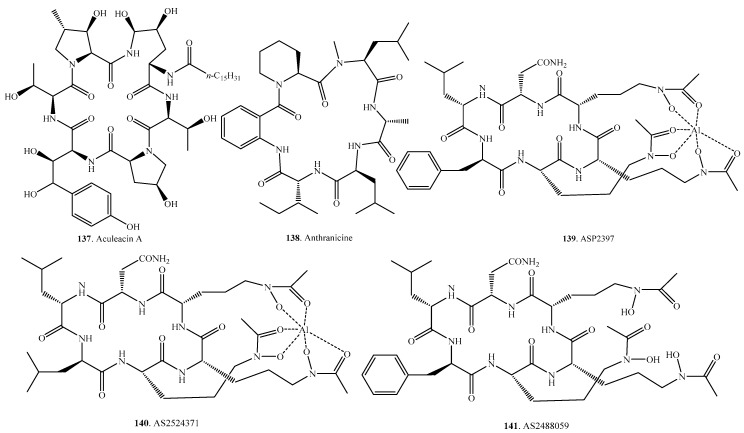
| Aculeacin A (137) | Aspergillus aculeatus M-4214 | Antifungal activity by inhibiting synthesis of β-1,3-glucan | [117,118] |
| Anthranicine (138) | Acremonium sp. A29-2004 | - | [130] |
| ASP2397 (139) | Acremonium persicinum MF-347833 from Malaysian leaf litter | Antifungal activity | [131] |
| AS2524371 (140) | Acremonium persicinum MF-347833 from Malaysian leaf litter | - | [131] |
| AS2488059 (141) | Acremonium persicinum MF-347833 from Malaysian leaf litter | - | [131] |
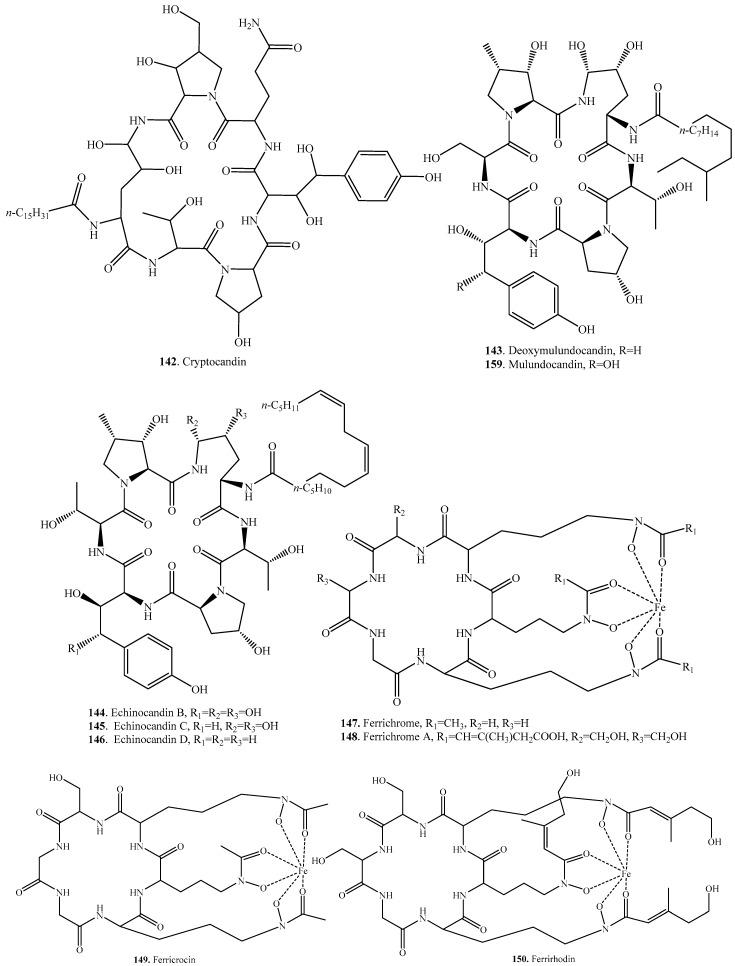
| Cryptocandin (142) | Cryptosporiopsis cf. quercina | Antifungal activity | [132] |
| Deoxymulundocandin (143) | Aspergillus sydowii | Antifungal activity | [133] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin B (144) | Aspergillus nidulans var. roseus ATCC 58397 | Antifungal activity | [134] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin C (145) | Aspergillus sp. | Antifungal activity | [135] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin D (146) | Aspergillus sp. | Antifungal activity | [135] |
| Ferrichrome (147) | Ustilago maydis | - | [136] |
| Ferrichrome A (148) | Ustilago maydis | - | [136] |
| Ferricrocin (149) | Colletotrichum gloeosporioides | Phytotoxic activity | [137] |
| Ferrirhodin (150) | Botrytis cinerea | - | [138] |
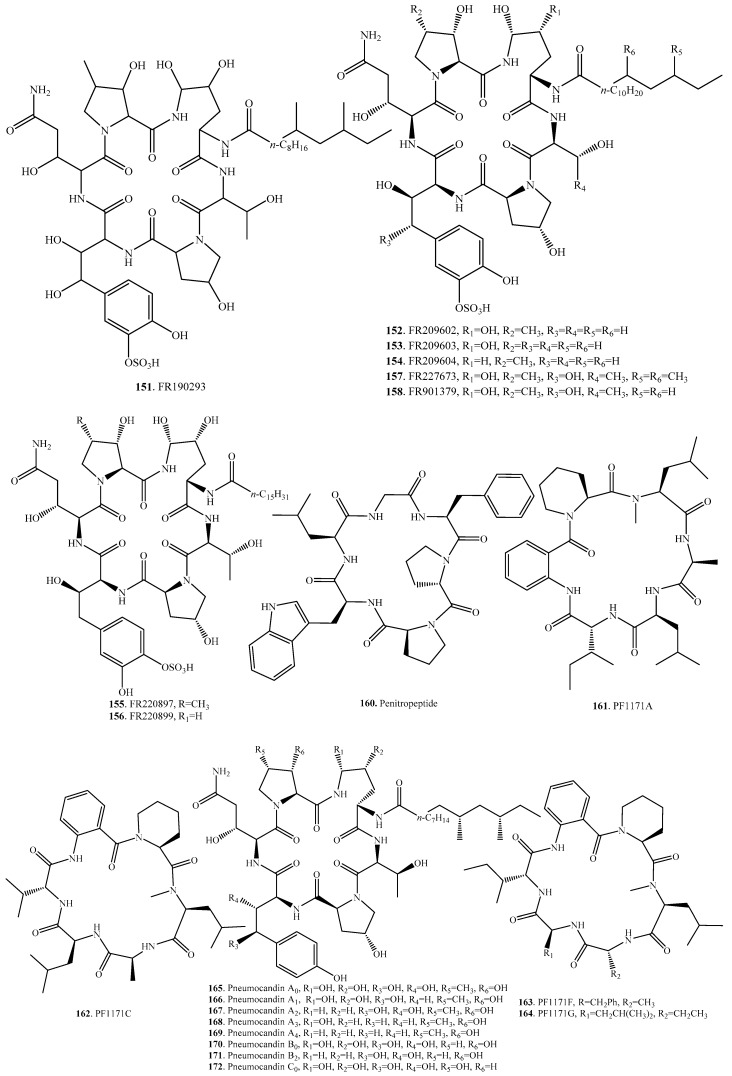
| FR190293 (151) | Tolypocladium parasiticum No. 16616 | Antifungal activity | [139] |
| FR209602 (152) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR209603 (153) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR209604 (154) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR220897 (155) | Coleophoma empetri No. 14573 | Antifungal activity | [141] |
| FR220899 (156) | Coleophoma empetri No. 14573 | Antifungal activity | [141] |
| FR227673 (157) | Chalara sp. No. 22210 | Antifungal activity | [139] |
| FR901379 (158) | Coleophoma empetri F-11899 | Antifungal activity | [142] |
| Mulundocandin (159) | Aspergillus sydowii | Antifungal activity | [122] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Penitropeptide (160) | Endophytic fungus Penicillium tropicum isolated from leaves of Sapium ellipticum | - | [143] |
| PF1171A (161) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [124] |
| PF1171C = Similanamide (162) | Marine sponge-associated fungus Aspergillus similanensis KUFA 0013 | Weak cytotoxic activity against the cancer cell lines | [125,126] |
| Hamigera sp. | - | [99] | |
| Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [124] | |
| PF1171F (163) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [144] |
| PF1171G (164) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [144] |
| Pneumocandin A0 = L-671,329 (165) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Zalerion arboricola | Antifungal activity | [145] | |
| Pneumocandin A1 (166) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A2 (167) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A3 (168) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A4 (169) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin B0 (170) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin B2 (171) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3- β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin C0 (172) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3- β-d-glucan synthesis inhibition activity | [127,128] |
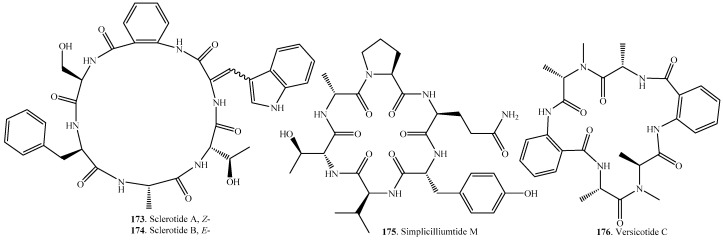
| Sclerotide A (173) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [129] |
| Sclerotide B (174) | Aspergillus sclerotiorum PT06-1 | Antifungal, antibacterial and cytotoxic activity | [129] |
| Simplicilliumtide M (175) | Deep-sea derived Simplicillium obclavatum EIODSF 020 | - | [146] |
| Versicotide C (176) | Aspergillus versicolor ZLN-60 | - | [23] |
Figure 4.
Structures of the cyclic hexapeptides isolated from fungi.
Aculeacin A (137) was isolated from Aspergillus aculeatus M-4214. This compound showed marked antifungal activity by inhibiting synthesis of β-1,3-glucan [117,118].
The echinocandins have emerged as first-line antifungal agents for many Candida and Aspergillus infections. They have a unique mechanism of action by inhibiting the synthesis of β-1,3-d-glucan, a key component of the fungal cell wall. Caspofungin was the first echinocandin antifungal agent to become licensed for treatment of invasive fungal infecitons [119]. Caspofungin has been used in immunocompromised children and neonates with invasive fungal infections [120]. The second commercial antifungal agent in the echinocandin series was micafungin which has been used worldwide in chemotherapy for life-threatening fungal infections [121].
Mulundocandin (159) and deoxymulundocandin (143) were isolated from Aspergillus sydowii [122] and Aspergillus mulundensis [123], respectively. Both compounds showed antifungal activity against yeasts and filamentous fungi.
PF1171A (161) and PF1171C (162) were isolated from an unidentified ascomycete OK-128. Both compounds showed paralytic activity against silkworms [124]. PF1171C (162) was reisolated as similanamide (162) from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. It showed weak cytotoxic activity against the cancer cell lines [125,126].
Pneumocandin anlogues (165–172) were isolated from Zalerion arboricola. They all showed anticandial activity by inhibiting 1,3-β-d-glucan synthesis [127,128].
Sclerotides A (173) and B (174) were isolated from Aspergillus sclerotiorum PT06-1. They all showed moderate antifungal activity against Candida albicans. In addition, sclerotide B (174) exhibited antibacterial and cytotoxic activities [129].
6. Cyclic Heptapeptides
There are 37 cyclic heptapeptides isolated from fungi. Their distributions in fungi, and biological activities are listed in Table 5, and the structures are shown in Figure 5.
Table 5.
Fungal cyclic heptapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
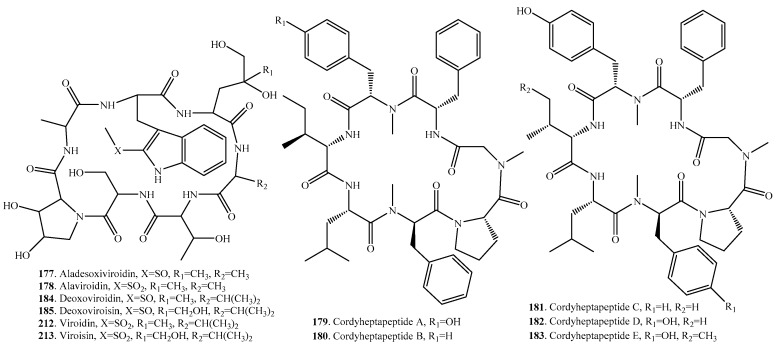
| Aladesoxiviroidin (177) | Amanita phalloides | Toxic to some animal species | [150] |
| Alaviroidin (178) | Amanita phalloides | Toxic to some animal species | [150] |
| Cordyheptapeptide A (179) | Cordyceps sp. BCC1788 | Antimalarial activity against Plasmodium falciparum K1 | [147] |
| Cordyceps sp. BCC 16176 | Cytotoxic activity | [148] | |
| Cordyheptapeptide B (180) | Cordyceps sp. BCC 16176 | Cytotoxic activity | [148] |
| Cordyheptapeptide C (181) | Acremonium persicinum SCSIO 115 | Cytotoxic activity | [149] |
| Cordyheptapeptide D (182) | Acremonium persicinum SCSIO 115 | - | [149] |
| Cordyheptapeptide E (183) | Acremonium persicinum SCSIO 115 | Cytotoxic activity | [149] |
| Deoxoviroidin (184) | Mushroom Amanita virosa | Hepatotoxicity | [159] |
| Deoxoviroisin (185) | Mushroom Amanita virosa | Hepatotoxicity | [159] |
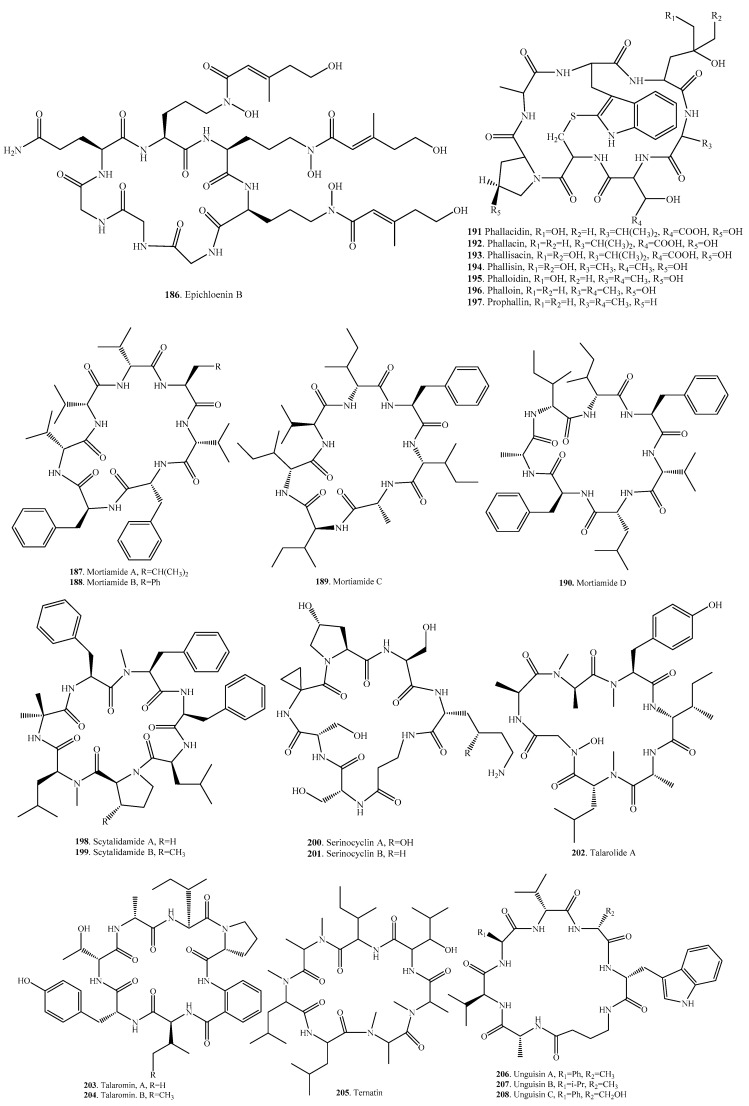
| Epichloenin B (186) | Epichloe festucae | - | [160] |
| Mortiamide A (187) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide B (188) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide C (189) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide D (190) | Marine-derived Mortierella sp. | - | [161] |
| Phallacidin (191) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallacin (192) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallisacin (193) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallisin (194) | Amanita phalloides | Toxic to some animal species | [150] |
| Phalloidin (195) | Amanita phalloides | Toxic to some animal species | [150] |
| Phalloin (196) | Amanita phalloides | Toxic to some animal species | [150] |
| Prophallin (197) | Amanita phalloides | Toxic to some animal species | [150] |
| Scytalidamide A (198) | Acremonium sp. | - | [152] |
| Scytalidiu sp. | Cytotoxic activity on HCT-116 | [153] | |
| Scytalidamide B (199) | Acremonium sp. | - | [152] |
| Scytalidiu sp. | Cytotoxic activity on HCT-116 | [153] | |
| Serinocyclin A (200) | Metarhizium anisopliae | Insecticidal activity on mosquito larvae | [162] |
| Serinocyclin B (201) | Metarhizium anisopliae | - | [162] |
| Talarolide A (202) | Marine tunicte-associated fungus Talaromyces sp. CMB-TU011 | - | [163] |
| Talaromin A (203) | Talaromyces wortmannii | - | [164] |
| Talaromin B (204) | Talaromyces wortmannii | - | [164] |
| Ternatin (205) | Cladobotryum sp. | Cytotoxic activity; antifungal activity | [165] |
| Unguisin A (206) | Marine-derived Emericella unguis | Moderate antibacterial activity | [154,155] |
| Unguisin B (207) | Marine-derived Emericella unguis | Moderate antibacterial activity | [154,155] |
| Unguisin C (208) | Marine-derived Emericella unguis | - | [155] |
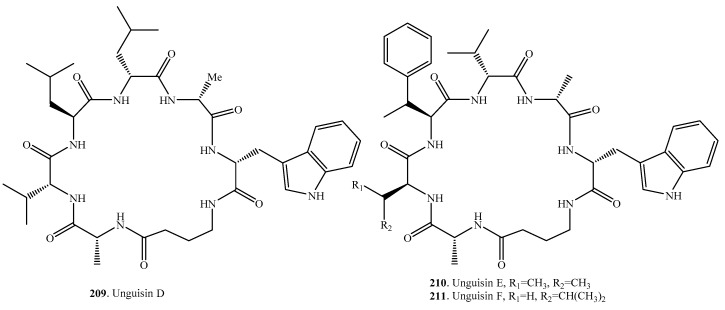
| Unguisin D (209) | Marine-derived Emericella unguis | - | [155] |
| Unguisin E (210) | Aspergillus sp. AF119 | - | [156] |
| Endophytic fungus Mucor irregularis from the medicinal plant Moringa stenopetala | - | [157] | |
| Unguisin F (211) | Endophytic fungus Mucor irregularis from the medicinal plant Moringa stenopetala | - | [157] |
| Viroidin (212) | Mushroom Amanita virosa | - | [159] |
| Amanita phalloides | Toxic to some animal species | [150] | |
| Viroisin (213) | Mushroom Amanita virosa | - | [159] |
Figure 5.
Structures of the cyclic heptapeptides isolated from fungi.
Cordyheptapeptides A (179) and B (180) were isolated from Cordyceps sp. which is an insect fungal pathogen [147,148]. Cordyheptapeptides C (181), D (182) and E (183) were isolated from the marine-derived fungus Acremonium persicinum SCSIO 115 [149]. Cordyheptapeptides A (179), B (180), C (181) and E (183) all showed cytotoxic activity [148,149].
Phallotoxins (191–197) were bicyclic heptapeptides cross-linked by the 2’-bound sulfur group from the poisonous mushroom Amanita phalloides. Seven phallotoxins: phallacidin (191), phallacin (192), phallisacin (193), phallisin (194), phalloidin (195), phalloin (196), and prophallin (197), have been identified so far [150]. Phallotoxins were shown to be ribosomally synthesized and posttranslationally modified peptides (RiPPs) [151].
Scytalidamides A (198) and B (199) were isolated from Acremonium sp. [152] and Scytalidiu sp. [153], respectively. Both compounds showed cytotoxic activity toward HCT-116 human colon adenocarcinoma with IC50 values of 2.7 and 11.0 μM, respectively [153].
Unguisins A–D (206–209) were isolated from Emericella unguis [154,155], unguisin E (210) from Aspergillus sp. AF119 [156] and Mucor irregularis [157], and unguisin F (211) from Mucor irregularis [157]. Only unguisins A (206) and B (207) were screened to display moderate antibacterial activity [154,155]. Further investigation showed that unguisin A (206) was an anion receptor with high affinity for phosphate and pyrophosphate [158].
Virotoxins (177,178,184,185,212,213) are mono-cyclic heptapeptides containg a tryptophan residue substituted at position 2 of the indol ring by a sulfur atom. A series of virotoxins such as aladesoxiviroidin (177), alaviroidin (178), deoxoviroidin (184), deoxoviroisin (185), viroidin (212), and viroisin (213) have been identified from the mushrooms Amanita phalloides and Amanita virosa so far. They were toxic to animals [150,159].
7. Cyclic Octapeptides
The distributions and biological activities of fungal cyclic octapeptides are listed in Table 6, and the structures are shown in Figure 6.
Table 6.
Fungal cyclic octapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
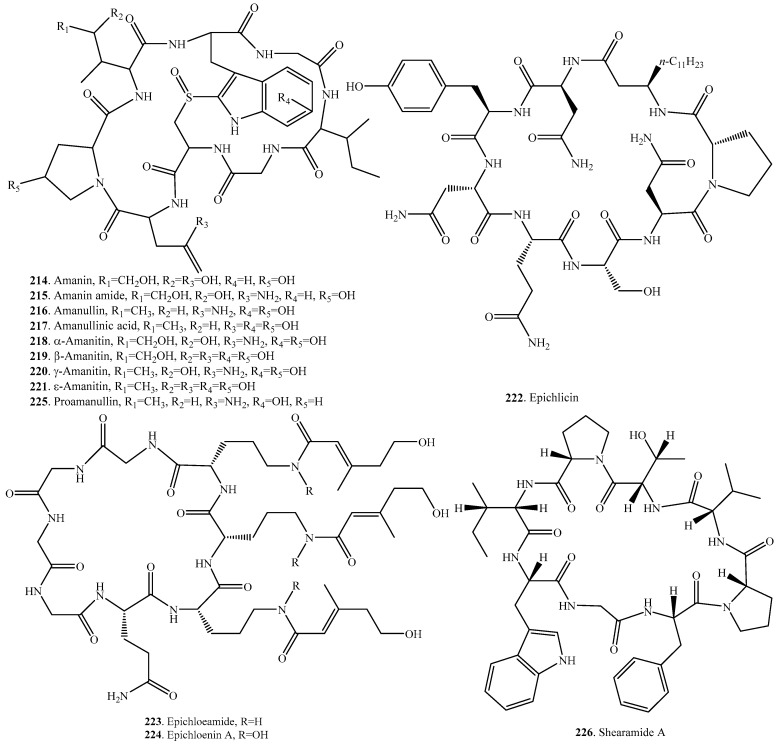
| Amanin (214) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanin amide (215) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanullin (216) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanullinic acid (217) | Amanita phalloides | Toxic to some animal species | [150] |
| α-Amanitin (218) | Amanita exitialis | - | [168] |
| Amanita subjunquillea | Cytotoxic activity | [169] | |
| Amanita phalloides | Toxic to some animal species | [150] | |
| β-Amanitin (219) | Amanita exitialis | - | [168] |
| Amanita subjunquillea | Cytotoxic activity | [169] | |
| Amanita phalloides | Toxic to some animal species | [150] | |
| γ-Amanitin (220) | Amanita phalloides | Toxic to some animal species | [150] |
| ε-Amanitin (221) | Amanita phalloides | Toxic to some animal species | [150] |
| Epichlicin (222) | Endophytc Epichloe typhina from Phleum pratense | Inhibitory activity on the spore germination of Cladosporium phlei | [166] |
| Epichloeamide (223) | Epichloe festucae | - | [160] |
| Epichloenin A (224) | Epichloe festucae | - | [160] |
| Proamanullin (225) | Amanita phalloides | Toxic to some animal species | [150] |
| Shearamide A (226) | Eupenicillium shearii | Insecticidal effect | [167] |
Figure 6.
Structures of the cyclic octapeptides isolated from fungi.
Fungal cyclic octapeptides mainly include the amatoxins, which are bicyclic octapeptides distributed in the poisonous mushroom Amanita phalloides. Nine amatoxin analogs amanin (214), amanin amide (215), amanullin (216), amanullinic acid (217), α-amanitin (218), β-amanitin (219), γ-amanitin (220), ε-amanitin (221), and proamanullin (225) have been identified [150]. The most important biochemical effect of amatoxins is the inhibition of RNA polymerases (especially polymerase II). This interaction leads to a tight complex, and the inhibition is of a non-competitive type. Non-mammalian polymerases show little sensitivity to amanitins. The amatoxins cause necrosis of the liver, also partly in the kidney, with the cellular changes causing the fragmentation and segregation of all nuclear components. Various groups of somatic cells of emanation resistance have been isolated, including from a mutant of Drosophila melanogaster [150].
Amatoxins are synthesized as proproteins on ribosomes, and are considered as ribosomally synthesized and post-translationally modified peptides (RiPPs). The proproteins are 34–35 amino acids in length and do not have predicted signal peptides [151].
Epichlicin (222) was isolated from the endophytic fungus Epichloe typhina from the timothy plant (Pheum pretense). This compound exhibited inhibitory activity toward the spore germination of Cladosporium phlei, a fungal pathogen of the timothy plant at an IC50 value of 22 nM [166].
Both epichloeamide (223) and epichloenin A (224) were identified from the endophytic fungus Epichloe festucae and endophyte-infected Lolium perenne [160].
Shearamide A (226) was isolated from the ascostromata of Eupenicillium shearii. This compound showed insecticidal effect against Helicoverpa zea larvae [167].
8. Cyclic Nonapeptides
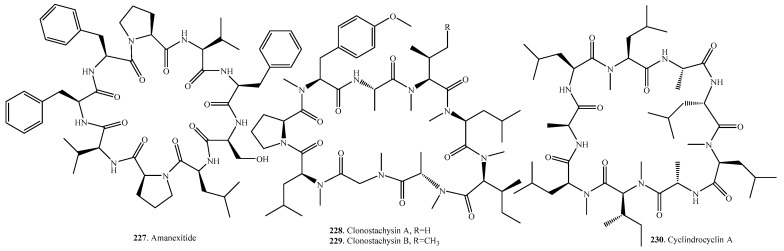
Only four cyclic nonapeptides have been identified in fungi, with their occurrence, biological activities and structures shown in Table 7 and Figure 7, respectively. Amanexitide (227) was isolated from the fruiting bodies of Amanita exitialis, a poisonous mushroom endemic in China [168]. Both clonostachysins A (228) and B (229) were obtained from a marine sponge-derived fungus Clonostachys rogersoniana strain HJK9. They exhibited a selectively inhibitory activity on the dinoflagellate Prorocentrum micans at 30 μM [170]. Cylindrocyclin A (230) from Cylindrocarpon sp. exhibited cytotoxic activity against six cell lines with IC50 values ranging from 11 to 53 μM [171].
Table 7.
Fungal cyclic nonapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amanexitide (227) | Amanita exitialis | - | [168] |
| Clonostachysin A (228) | Marine-derived fungus Clonostachys rogersoniana HJK9 from sponge | Anti-dinoflagellate activity | [170] |
| Clonostachysin B (229) | Marine-derived fungus Clonostachys rogersoniana HJK9 from sponge | Anti-dinoflagellate activity | [170] |
| Cylindrocyclin A (230) | Cylindrocarpon sp. | Cytotoxic activity | [171] |
Figure 7.
Structures of the cyclic nonapeptides isolated from fungi.
9. Cyclic Decapeptides
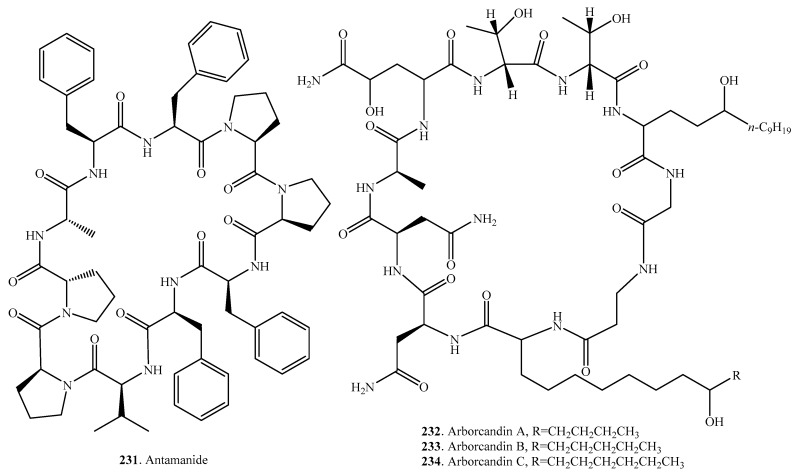
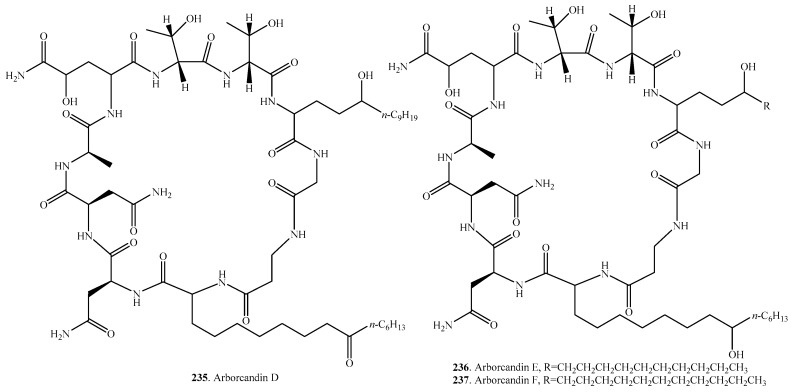
A total of seven cyclic decapeptides were identified in fungi. Their distributions, biological activities and structures are shown in Table 8 and Figure 8.
Table 8.
Fungal cyclic decapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Antamanide (231) | Amanita phalloides | Inhibition of the mitochondrial permeability transition pore, antidote activity against phallotoxins and amatoxins, inhibition of tumor cell growth in vitro | [172,175] |
| Arborcandin A (232) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin B (233) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin C (234) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin D (235) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin E (236) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin F (237) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
Figure 8.
Structures of the cyclic decapeptides isolated from fungi.
Antamanide (231) derived from the fungus Amanita phalloides inhibited the mitochondrial permeability transition pore, a central effector of apoptosis induction, by targeting the pore regulator cyclophillin D [172].
Arborcandins A–F (232–237) were isolated from the culture broth of an unidentified filamentous fungus SANK 17397. They possessed potent 1,3-β-glucan synthase inhibitory activity [173,174].
10. Cyclic Undecapeptides
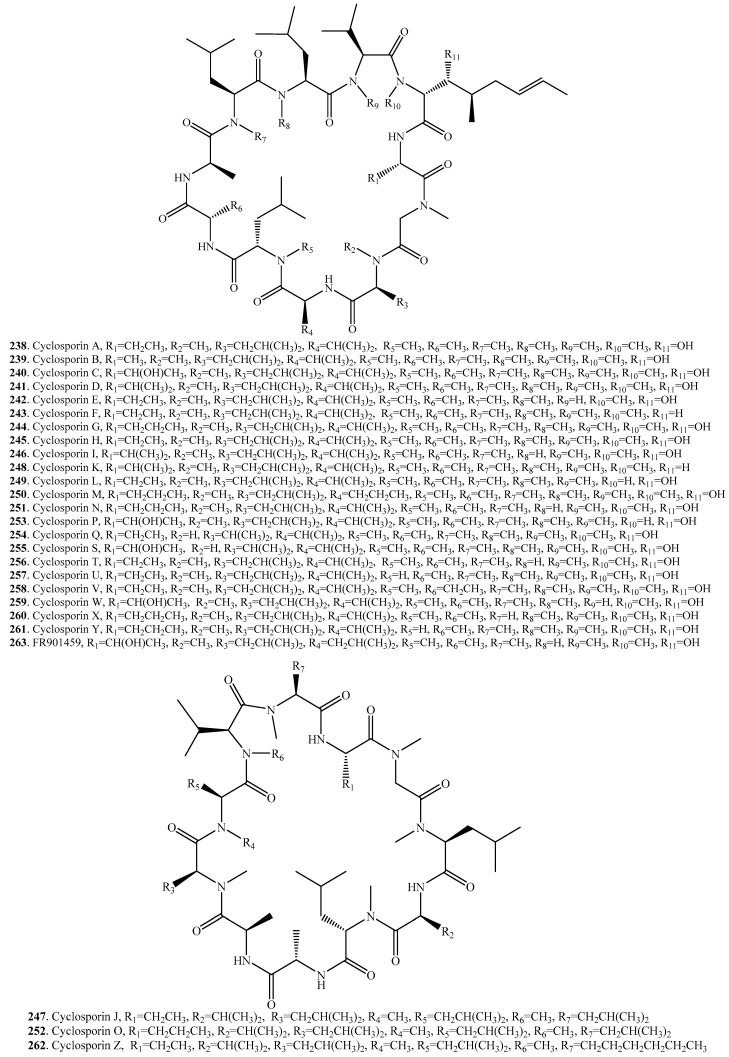
Twenty-six cyclosporin analogs were identified in fungi. They belong to the cyclic undecapeptide group, and their distributions, biological activities and structures are shown in Table 9 and Figure 9, respectively.
Table 9.
Fungal cyclic undecapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Cyclosporin A (238) | Aspergillus fumigatus | - | [177] |
| Aspergillus terreus NRC FA 200 | - | [178] | |
| Beauveria nivea | - | [179] | |
| Fusarium oxysporum | Immunosuppressive, antifungal, anti-inflammatory, anti-parasitic and anticancer activities | [180] | |
| Tolypocladium inflatum | - | [182] | |
| Tolypocladium sp. | - | [183] | |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin B (239) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin C (240) | Acremonium luzulae | Antifungal activity | [187] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] | |
| Cyclosporin D (241) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin E (242) | Trichoderma polysporum | - | [181] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] | |
| Cyclosporin F (243) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] |
| Cyclosporin G (244) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186,188] |
| Cyclosportin H (245) | Tolypocladium inflatum | - | [188] |
| Cyclosportin I (246) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] |
| Cyclosportin J (247) | Tolypocladium terricola | Increased cell proliferation | [189] |
| Cyclosporin K (248) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin L (249) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin M (250) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin N (251) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin O (252) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin P (253) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Q (254) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin S (255) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin T (256) | Epichloe bromicola | Antifungal activity | [190] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] | |
| Cyclosporin U (257) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin V (258) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin W (259) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin X (260) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Y (261) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Z (262) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| FR901459 (263) | Stachybotrys chartarum No. 19392 from a soil collected in Tokyo, Japan | Immunosuppressive activity | [184] |
Figure 9.
Structures of the cyclic undecapeptides isolated from fungi.
Among them, cyclosporin A (238) has received the most meticulous attention owing to its immunosuppressive and antifungal activity [176]. Cyclosporin A (238) can be produced by a series of fungi such as Aspergillus fumigatus [177], Aspergillus terreus [178], Beauveria nivea [179], Fusarium oxysporum [180], Trichoderma polysporum [181], Tolypocladium inflatum [182], and Tolypocladium sp. [183]. It was mainly produced by various types of fermentation techniques using Tolypocladium inflatum. It has a variety of biological activities including immunosuppressive, anti-inflammatory, antifungal and antiparasitic properties. The mechanism of action has been considered as the phosphatase activity inhibition of calcineurin, and production of IL-2 and other cytokines [176].
FR901459 (263), a derivative of cyclosporin A (238), was discovered in the fermentation broth of Stachybotrys chartarum No. 19392 (isolated from soil collected in Tokyo Prefecture, Japan). This compound showed antifungal activity, and was capable of prolonging the survival time of skin allografts in rats with one-third the potency of cyclosporin A [184]. It also prevented mitochondrial swelling and protected against delayed neuronal cell death [185].
11. Cyclic Dodecapeptides
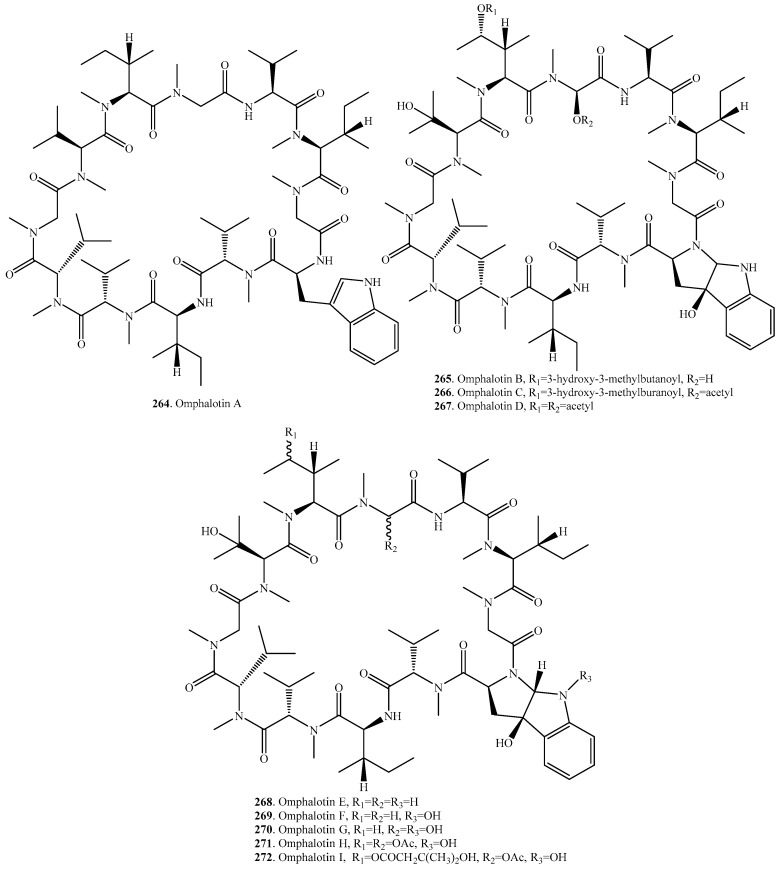
Omphalotin analogs are the only cyclic dodecapeptides idolated from fungi so far. Omphalotins (264–272) were isolated from the basidiomycetes mushroom Omphalotus olearius. These compounds are toxic to humans and animals. Their distributions, biological activities and structures are shown in Table 10 and Figure 10, respectively. Nine cyclic dodecapeptides omphalotins A–I (264–272) with nematicidal activity were identified in the mushroom O. olearius [191,192]. They have been considered as the potent nematicidal agents that seemed to be highly selective for Meloidogyne incognita [193]. Omphalotins were recently identified as the ribosomally synthesized and posttranslationally modified peptides (RiPPs). A self-sacrificing N-methyltransferase was shown to be the precursor of omphalotin A (264) [194,195,196].
Table 10.
Fungal cyclic dodecapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Omphalotin A = Omphalotin (264) | Mushroom Omphalotus olearius | Nematicidal activity | [191,197,198,199] |
| Omphalotin B (265) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin C (266) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin D (267) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin E (268) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin F (269) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin G (270) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin H (271) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin I (272) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
Figure 10.
Structures of the cyclic dodecapeptides isolated from fungi.
12. Cyclic Tetradecapeptides
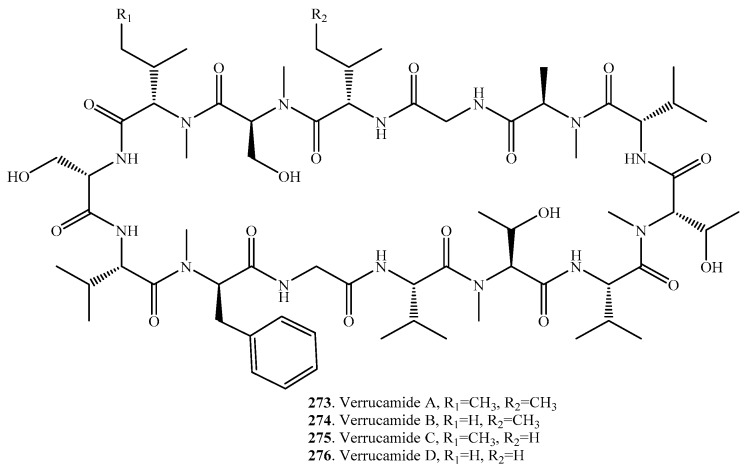
Only four cyclic tetradecapeptides, verrucamides A–D (273–276), were identified from the plant pathogenic fungus Myrothecium verrucaria that attacked important crop plants and weeds. Their distributions, biological activities and structures are shown in Table 11 and Figure 11, respectively. They were screened to show antibacterial activity against Staphylococcus aureus [200].
Table 11.
Fungal cyclic tetradecapeptides and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Verrucamide A (273) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide B (274) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide C (275) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide D (276) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
Figure 11.
Structures of the cyclic tetradecapeptides isolated from fungi.
13. Cyclic Octadecapeptides
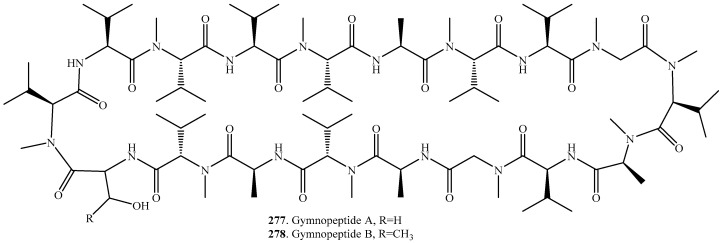
Only two highly N-methylated cyclic octadecapeptides namely gymnopeptides A (277) and B (278) were isolated from the mushroom Gymnopus fusipes (Table 12 and Figure 12). They exhibited striking antiproliferative activity on several human cancer cell lines [201]. So far, gymnopeptides A (277) and B (278), constituted by 18 monomers, are the largest cyclic peptides to be isolated from fungi. Fortunately, the total synthesis of gymnopeptides A (277) and B (278) has been achieved [202]. Further biological studies are needed to clarify the aspects of absorption and metabolism of gymnopeptides A (277) and B (278) after consumption of the fruiting bodies by humans, as well as to explore the potential role of these metabolites in the parasitic lifestyle of the mushroom G. fusipes.
Table 12.
Fungal cyclic octadecapeptides and their biological activities.
Figure 12.
Structures of the cyclic octadecapeptides isolated from fungi.
14. Cyclic Peptides Containing Ether Bonds in the Core Ring
This group of cyclic peptides contained at least one ether linkage except the normal amide and carbon-carbon bonds in the core ring of each molecule. Their distributions, biological activities and structures are shown in Table 13 and Figure 13, respectively.
Table 13.
Fungal cyclic peptides containing ether bonds in the core ring and their biological activities.
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
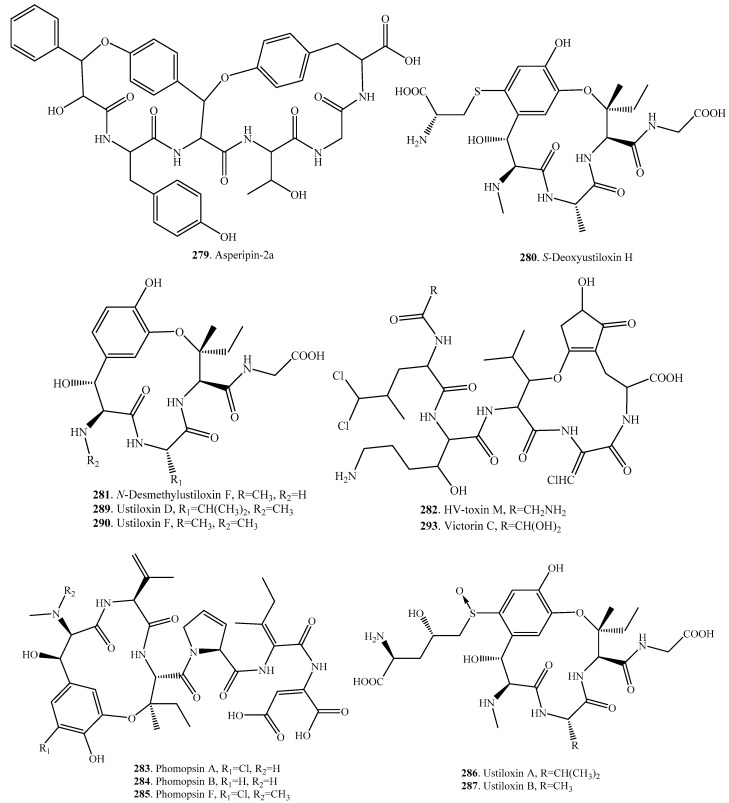
| Asperipin-2a (279) | Aspergillus flavus | - | [203] |
| S-Deoxyustiloxin H (280) | Aspergillus flavus | - | [214] |
| N-Desmethylustiloxin F (281) | Aspergillus flavus | - | [214] |
| HV-toxin M (282) | Plant pathogenic fungus Cochliobus victoriae = Helminthosporium victoria from oat | Phytotoxic activity | [205] |
| Phomopsin A (283) | Plant pathogenic fungus Phomopsis leptostromiformis from lupinis (Lupinus spp.) | Phytotoxic activity, toxic to animals | [206] |
| Phomopsin B (284) | Plant pathogenic fungus Phomopsis leptostromiformis from lupinis (Lupinus spp.) | Toxic to animals | [207] |
| Phomopsin F (285) | Plant pathogenic fungus Diaporthe toxica | Cytotoxic activity against Hepg2 cells | [208] |
| Ustiloxin A (286) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209,211] |
| Ustiloxin B (287) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209,211] |
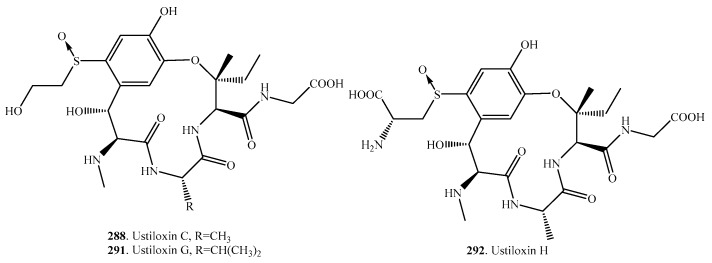
| Aspergillus flavus | - | [213] | |
| Ustiloxin C (288) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [208] |
| Ustiloxin D (289) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [208] |
| Ustiloxin F (290) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209] |
| Ustiloxin G (291) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [212] |
| Ustiloxin H (292) | Aspergillus flavus | - | [214] |
| Victorin C (293) | Plant pathogenic fungus Cochliobus victoriae = Helminthosporium victoria | Phytotoxic activity | [215] |
Figure 13.
Structures of the fungal cyclic peptides containing ether bonds in the core ring.
Asperipin-2a (279) containing five amino acids was isolated from Aspergillus flavus. It was a bicycle peptide containing two ether linkages that was converted from the repeated sequence containing aromatic residues [203].
HV-toxin M (282), with phytotoxic activity, was isolated from the plant pathogenic fungus Cochliobus victoriae (previously as Helminthosporium victoria) from oat (Avena sativa). This compound had three amino acids in the core rings, and two amino acids linked and attached as the side chains [204].
Phomopsins A (283), B (284) and F (285) were isolated from Phomopsis leptostromiformis [205,206] and Diaporthe toxica [207]. They have a structure similar to HV-toxin M (282). Phomopsins are mycotoxins known to be responsible for fatal liver disease of lupin-fed sheep, and displayed phytotoxic and cytotoxic activities [206,207].
Ustiloxins (286–291) have a 13-membered ring including a phenol ether linkage. They were isolated from the water extract of the false smut balls caused by Ustilaginoidea virens on the panicles of rice plants [208,209,210,211,212]. There are three amino acids in the core ring for each ustiloxin. For ustiloxins C (288), D (289), F (290) and G (291), there is a glycine in the side chain. For ustiloxins A (286) and B (287), there are two amino acids in the side chains, whose tyrosine was modified with norvaline, a non-proteinogenic amino acid. They are biosynthesized by the ribosome, and are ribosomally synthesized and post-translationally modified peptides (RiPPs). The cluster possessed a gene, termed ustA, whose translated product, UstA, contained a 16-fold repeated peptide embedding a tetrapeptide, Tyr-Ala-Ile-Gly, that was converted into the cyclic moiety of ustiloxin B (287) [213]. Through gene inactivation, heterologous expression, and in vitro functional analyses the entire biosynthetic pathway of the ustiloxins was unveiled to involve at least nine enzymes. The oxidative cyclization of the core peptide is not clear yet though [214].
15. Conclusions and Future Perspectives
In this review, we describe the progress on the chemistry and biological activities of the cyclic peptides (excluding cyclic dipeptides and peptides containing ester bonds in the core ring) discovered from fungi during the past 50 years, especially the recent 20 years.
It seems that the fungi in genus Aspergillus can produce various types of cyclic peptides, especially cyclic tripeptides to pentapeptides, while the mushrooms, which we call macroscopic fungi, produce relatively big cyclic peptides such as amanin (an octapeptide), amanexitide (a nonapeptide), antamanide (decapeptide), omphalotins (dodecapeptide), and gymnopeptides (octadecapeptides). Most of cyclosporins, which belong to the cyclic undecapeptides, show immunosuppressive and antifungal activities. The isolated cyclic dodecapeptides have nematicidal activity, tetradecapeptides possess antibacterial activity, and octadecapeptides show antiproliferative activity on several human cancer cell lines. The cyclic peptides with a big ring seem to display more biological activities than the small-ring peptides.
Based on the biosynthetic machineries of fungal cyclic peptides, most of them belong are nonribosomal peptides (NRPs) which are synthesized by multimodular nonribosomal peptide synthetases (NRPSs). Just a few of them belong to the ribosomally synthesized and post-translationally modified peptides (RiPPs) such as α-amanitin (218) from Amanita exitialis [150], asperipin-2a (279) from Aspergillus flavus [203], phallacidin (191) from Amanita bisporigera [216], phomopsin A (283) from Phomopsis leptostromiformis [206], and ustiloxins (286-291) from Ustilaginoidea virens (teleomorph: Villosiclva virens) [208,209,212] and Apsergillus flavus [214], omphalotins (260–268) from Omphalotus olearius [194,195,196].
Some fungal cyclic peptides have potential to be developed into pharmaceuticals and agrochemicals. Some cyclic tetrapeptides such as 1-alaninechlamydocin (24) [30], apicidin (25) [35], AS1387392 (36) [37] and trapoxin A (83) [31] show inhibitory activity on histone deacetylase (HDAC) and have potential applications as therapeutics for spinal muscular atrophy [36], anti-cancer agents [30], antiprotozoal agents [35], and immunosuppressants [37]. Some cyclic peptides with phytotoxic activity show potential applications such as herbicides against weeds [217]. Biological analysis revealed a pro-apoptotic effect for most of the cyclic peptides that indicated that the cyclic peptides could be developed as the pro-apoptotic agents [218].
Some cyclic peptides have been developed as drugs, which are listed in Table 13. One noteworthy example is caspofungin which has strong antifungal activity. Caspofungin is a semisynthetic derivative of pneumocandin B0 (170), a lipophilic cyclic hexapeptide from the fungus Glarea lozoyensis. It was commercialized as the antifungal drug caspofungin acetate (CANCIDAS®) which has subsequently saved thousands of lives [14]. Micafungin was the second approved antifungal agent in the echinocandin series, and has been used worldwide in chemotherapy for life-threatening fungal infections. Micafungin is an inhibitor of 1,3-β-glucan synthase, an enzyme necessary for cell-wall synthesis of several fungal pathogens [121]. Other application examples included cyclosporin A (238), an immunosuppressant isolated from Tolypocladium inflatum [176]; omphalotin A (264), a potent nematicidal agent from Omphalotus olearius [197]; and the echinocandins, antifungal lipopeptides isolated from Glarea lozoyensis, Coleophoma empetri, and Aspergillus nidulans var. echinulatus [219]. Commercial cyclic peptide-derived drugs are listed in Table 14.
Table 14.
Some examples of the commercial drugs developed from fungal cyclic peptides.
| Product Name | Original Cyclic Peptide | Development Way | Application | Ref. |
|---|---|---|---|---|
| Anidulafungin | Echinocandin B (144) | Semisynthesis | Antifungal agent | [220] |
| CANCIDAS® | Pneumocandin B0 (170) | Semisynthesis | Antifungal agent | [14] |
| Caspofungin | Echinocandins | Direct application | Antifungal agent for many Candida infections | [120] |
| Cyclosporin A | Cyclosporin A (238) | Direct application | Immunosuppressive and antifungal drug | [176] |
| Micafungin | Echinocandins | Semisynthesis | Antifugal agent | [221] |
It is worth mentioning that many fungal cyclic peptides have been isolated from plant endophytic and marine-derived fungi, which indicates that plant endophytic and marine-drived fungi are mines of biologically active natural products [222,223,224,225,226]. Although the number of fungal cyclic peptides is gradually increasing, it remains a group of natural products with relatively little skeletal diversity. On many occasions, the relative or absolute configurations of the chiral centers have not been determined yet. This remains a challenge for future chemical and spectroscopic work. Many isolated cyclic peptides have not been screened for their biological activities as the quantities obtained were very limited. Even fewer compounds have been the subject of systematic investigations to establish structure-activity relationships. Further high throughput screening of biological activities as well as detailed studies on the action mechanisms of fungal cyclic peptides are needed.
Acknowledgments
This work was co-financed by the grants from the National Key R&D Program of China (2017YFD0201105), and the National Natural Science Foundation of China (31271996).
Author Contributions
Xiaohan Wang performed bibliographic research, drafted and corrected the manuscript. Minyi Lin and Dan Xu retrieved literature, participated in the discussions and supported manuscript corrections. Daowan Lai reviewed the manuscript and helped to revise it. Ligang Zhou conceived the idea, designed the review structure, supervised manuscript drafting, and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tan N.-H., Zhou J. Plant cyclopeptides. Chem. Rev. 2006;106:840–895. doi: 10.1021/cr040699h. [DOI] [PubMed] [Google Scholar]
- 2.Taevernier L., Wynendaele E., De Vreese L., Burvenich C., De Spiegeleer B. The mycotoxin definition reconsidered towards fungal cyclic depsipeptides. J. Environ. Sci. Health Part C. 2016;34:114–135. doi: 10.1080/10590501.2016.1164561. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Zhu X., Kim S.J., Zhang W. Antimycin-type depsipeptides: Discovery, biosynthesis, chemical synthesis, and bioactivities. Nat. Prod. Rep. 2016;33:1146–1165. doi: 10.1039/C6NP00004E. [DOI] [PubMed] [Google Scholar]
- 4.Andavan G.S.B., Lemmens-Gruber R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs. 2010;8:810–834. doi: 10.3390/md8030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2016;33:382–431. doi: 10.1039/C5NP00156K. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P., Li X., Wang B.-G. Secondary metabolites from the marine algal-derived endophtic fungi: Chemical diversity and biological activity. Planta Med. 2016;82:832–842. doi: 10.1055/s-0042-103496. [DOI] [PubMed] [Google Scholar]
- 7.Falanga A., Nigro E., De biasi M.G., Daniele A., Morelli G., Galdiero S., Scudiero O. Cyclic peptides as novel therapeutic microbicides engineering of human defensing mimetics. Molecules. 2017;22:1217. doi: 10.3390/molecules22071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brase S., Encinas A., Keck J., Nising C. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009;109:3903–3990. doi: 10.1021/cr050001f. [DOI] [PubMed] [Google Scholar]
- 9.Zorzi A., Deyle K., Heinis C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017;38:24–29. doi: 10.1016/j.cbpa.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 10.De Leon Rodriguez L.M., Weidkamp A.J., Brimble M.A. An update on new methods to synthesize cyclotetrapeptides. Org. Biomol. Chem. 2015;13:6906–6921. doi: 10.1039/C5OB00880H. [DOI] [PubMed] [Google Scholar]
- 11.Sussmuth R., Muller J., von Dohren H., Molnar I. Fungal cyclooligomer depsipeptides; from classical biochemistry to combinatorial biosynthesis. Nat. Prod. Rep. 2011;28:99–124. doi: 10.1039/C001463J. [DOI] [PubMed] [Google Scholar]
- 12.Kries H. Biosynthetic engineering of nonribosomal peptide synthases. J. Pept. Sci. 2016;22:564–570. doi: 10.1002/psc.2907. [DOI] [PubMed] [Google Scholar]
- 13.Thorstholm L., Craik D.J. Discovery and applications of naturally occurring cyclic peptides. Drug Discov. Today Technol. 2012;9:e13–e21. doi: 10.1016/j.ddtec.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Balkovec J.M., Hughes D.L., Masurekar P.S., Sable C.A., Schwartz R.E., Singh S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014;31:15–34. doi: 10.1039/C3NP70070D. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Li Y., Zhang X., Lai D., Zhou L. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules. 2017;22:2026. doi: 10.3390/molecules22122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taevernier L., Wynendaele E., Gevaert B., De Spiegeleer B. Chemical classification of cyclic depsipeptides. Curr. Protein Pept. Sci. 2017;18:425–452. doi: 10.2174/1389203717666161128141438. [DOI] [PubMed] [Google Scholar]
- 17.Myokei R., Sakurai A., Chang C.-F., Kodaira Y., Takahashi N., Tamura S. Structure of aspochracin, an insecticidal metabolite of Aspergillus ochraceus. Tetrahedron Lett. 1969;10:695–698. doi: 10.1016/S0040-4039(01)87785-6. [DOI] [PubMed] [Google Scholar]
- 18.Dalsgaard P.W., Larsen T.O., Christophersen C. Bioactive cyclic peptides from the psychrotolerant fungus Penicillium algidum. J. Antibiot. 2005;58:141–144. doi: 10.1038/ja.2005.16. [DOI] [PubMed] [Google Scholar]
- 19.Zheng J., Xu Z., Wang Y., Hong K., Liu P., Zhu W. Cyclic tripeptides from the halotolerant fungus Aspergillus sclerotiorum PT06-1. J. Nat. Prod. 2010;73:1133–1137. doi: 10.1021/np100198h. [DOI] [PubMed] [Google Scholar]
- 20.Dalsgaard P.W., Larsen T.O., Frydenvang K., Christophersen C. Psychrophilin A and cycloaspeptide D, novel cyclic peptides from the psychrotolerant fungus Penicillium ribeum. J. Nat. Prod. 2004;67:878–881. doi: 10.1021/np0303714. [DOI] [PubMed] [Google Scholar]
- 21.Dalsgaard P.W., Blunt J.W., Munro M.H.G., Larsen T.O., Christophersen C. Psychrophilin B and C: Cyclic nitropeptides from the psychrotolerant fungus Penicillium rivulum. J. Nat. Prod. 2004;67:1950–1952. doi: 10.1021/np0497954. [DOI] [PubMed] [Google Scholar]
- 22.Ebada S.S., Fischer T., Hamacher A., Du F.-Y., Roth Y.O., Kassack M.U., Wang B.-G., Roth E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Apsergillus. Nat. Prod. Res. 2014;28:776–781. doi: 10.1080/14786419.2014.880911. [DOI] [PubMed] [Google Scholar]
- 23.Peng J., Gao H., Zhang X., Wang S., Wu C., Gu Q., Guo P., Zhu T., Li D. Psychrophilins E–H and versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014;77:2218–2223. doi: 10.1021/np500469b. [DOI] [PubMed] [Google Scholar]
- 24.Lacey H.J., Vuong D., Pitt J.I., Lacey E., Piggott A.M. Kumbicins A–D: Bis-indolyl benzenoids and benzoquinones form an Australian soil fungus, Aspergillus kumbius. Aust. J. Chem. 2016;69:152–160. doi: 10.1071/CH15488. [DOI] [Google Scholar]
- 25.Motohashi K., Inaba S., Takagi M., Shin-ya K. JBIR-15, a new aspochracin derivative, isolated from a sponge-derived fungus, Aspergillus sclerotiorum Huber Sp080903f04. Biosci. Biotechnol. Biochem. 2009;73:1898–1900. doi: 10.1271/bbb.90228. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q.-X., Jin X.-J., Draskovic M., Crews M.S., Tenney K., Valeriote F.A., Yao X.-J., Crews P. Unraveling the numerous biosynthetic products of the marine sediment-derived fungus, Aspergillus insulicola. Phytochem. Lett. 2012;5:114–117. doi: 10.1016/j.phytol.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y., Wu X., Feng S., Jiang G., Zhou S., Vrijmoed L.L.P., Jones E.B.G. A novel N-cinnamoylcyclopeptide containing an allenic ether from the fungus Xylaria sp. (strain #2508) from the South China Sea. Tetrahedron Lett. 2001;42:449–451. [Google Scholar]
- 28.Taunton J., Hassig C.A., Schreiber S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z., Barret M.-O., Boyd K.G., Adams D.R., Boyd A.S.F., Burgess J.G. JM47, a cyclic tetrapeptide HC-toxin analogue from a marine Fusarium species. Phytochemistry. 2002;60:33–38. doi: 10.1016/S0031-9422(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 30.Du L., Risinger A.L., King J.B., Powell D.R., Cichewicz R.H. A potent HDAC inhibitor, 1-alaninechlamydocin, from a Tolypocladium sp. induces G2/M cell cycle arrest and apoptosis in MIA PaCa-2 cells. J. Nat. Prod. 2014;77:1753–1757. doi: 10.1021/np500387h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter N.J., Christianson D.W. Binding of the microbial cyclic tetrapeptide trapoxin A to the class I histone deacetylase HDAC8. ACS Chem. Biol. 2017;12:2281–2286. doi: 10.1021/acschembio.7b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S.B., Zink D.L., Polishook J.D., Dombrowski A.W., Darkin-Rattray S.J., Schmatz D.M., Goetz M.A. Apicidins: Novel cyclic tetrapeptides as coccidiostats and antimalarial agents from Fusarium pallidoroseum. Tetrahedron Lett. 1996;37:8077–8080. doi: 10.1016/0040-4039(96)01844-8. [DOI] [Google Scholar]
- 33.Singh S.B., Zink D.L., Liesch J.M., Dombrowski A.W., Darkin-Rattray S.J., Schmatz D.M., Goetz M.A. Structure, histone deacetylase, and antiprotozoal activities of apicidins B and C, congeners of apicidin with proline and valine substitutions. Org. Lett. 2001;3:2815–2818. doi: 10.1021/ol016240g. [DOI] [PubMed] [Google Scholar]
- 34.Singh S.B., Zink D.L., Liesch J.M., Mosley R.T., Dombrowski A.W., Bills G.F., Darkin-Rattray S.J., Schmatz D.M., Goetz M.A. Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J. Org. Chem. 2002;67:815–825. doi: 10.1021/jo016088w. [DOI] [PubMed] [Google Scholar]
- 35.Darkin-Rattray S.J., Gurnett A.M., Myers R.W., Dulski P.M., Crumley T.M., Allocco J.J., Cannova C., Meinke P.T., Colletti S.L., Bendnarek M.A., et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA. 1996;93:13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai J., Leman L.J., Ku S., Vickers C.J., Olsen C.A., Montero A., Ghadiri M.R., Gottesfeld J.M. Cyclic tetrapeptide HDAC inhibitors as potential therapeutics for spinal muscular atrophy: Screending with iPSC-derived neuronal cells. Bioorg. Med. Chem. Lett. 2017;27:3289–3293. doi: 10.1016/j.bmcl.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Sasamura S., Sakamoto K., Takagaki S., Yamada T., Takase S., Mori H., Fujii T., Hino M., Hashimoto M. AS1387392, a novel immunosuppressive cyclic tetrapeptide compound with inhibitory activity against mammalian histone deacetylase. J. Antibiot. 2010;63:633–636. doi: 10.1038/ja.2010.51. [DOI] [PubMed] [Google Scholar]
- 38.He F., Bao J., Zhang X.-Y., Tu Z.-C., Shi Y.-M., Qi S.-H. Asperterrestide A, a cyctotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013;76:1182–1186. doi: 10.1021/np300897v. [DOI] [PubMed] [Google Scholar]
- 39.Close A., Huguenin R. Isolation and structure elucidation of chlamydocin. Helv. Chim. Acta. 1974;57:533–545. doi: 10.1002/hlca.19740570306. [DOI] [PubMed] [Google Scholar]
- 40.Bunbamrung N., Intaraudom C., Boonyuen N., Rachtawee P., Laksanacharoen P., Pittayakhajonwut P. Penicisochromans from the endophytic fungus Penicillium sp. BCC18034. Phytochem. Lett. 2014;10:13–18. doi: 10.1016/j.phytol.2014.06.018. [DOI] [Google Scholar]
- 41.Tani H., Fujii Y., Nakajima H. Chlamydocin analogues from the soil fungus Peniophora sp.: Structures and plant growth-retardant activity. Phytochemistry. 2001;58:305–310. doi: 10.1016/S0031-9422(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 42.Huang S., Ding W., Li C., Cox D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014;10:410–414. doi: 10.4103/0973-1296.141781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C., Wang J., Luo C., Ding W., Cox D.G. A new cyclopepitde with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014;28:616–621. doi: 10.1080/14786419.2014.887074. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Victoria I., Martin J., Gonzalez-Menendez V., De Pedro N., Aouad N.E., Ortiz-Lopez F.J., Tormo J.R., Platas G., Vicente F., Bills G.F., et al. Isolation and structural eluciation of cyclic tetrapeptides from Onychocola sclerotica. J. Nat. Prod. 2012;75:1210–1214. doi: 10.1021/np3000987. [DOI] [PubMed] [Google Scholar]
- 45.Takayama S., Lsogai A., Nakata M., Suzuki H., Susuki A. Structure of Cyl-1, a novel cyclic tetrapetide from Cylindrocladium scoparium. Agric. Biol. Chem. 1984;48:839–842. [Google Scholar]
- 46.Isogai A., Takayama S., Hirota A., Suzuki A. Determination of the absolute stereochemistry of the epoxide of aoe (2-amino-8-oxa-9,10-epoxy-decanoic acid) in Cyl-1 and Cyl-2 by CD spectra. Agric. Biol. Chem. 1986;50:517–518. [Google Scholar]
- 47.Gu W., Cueto M., Jensen P.R., Fenical W., Silverman R.B. Microsporins A and B: New histone deacetylase inhibitors from the marine-derived fungus Microsporum cf. gypseum and the solid-phase synthesis of microsporin A. Tetrahedron. 2007;63:6535–6541. doi: 10.1016/j.tet.2007.04.025. [DOI] [Google Scholar]
- 48.Sun W., Chen X., Tong Q., Zhu H., He Y., Lei L., Xue Y., Yao G., Luo Z., Wang J., et al. Novel small molecule 11β-HSD1 inhibitor from the endophytic fungus Penicillium commune. Sci. Rep. 2016;6:26418. doi: 10.1038/srep26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo H., Kreuzenbeck N.B., Otani S., Garcia-Altares M., Dahse H.-M., Christiane W., Aanen D.K., Hertweck C., Poulsen M., Beemelmanns C. Pseudoxylallemycins A–F, cyclic tetrapeptides with rare allenyl modifications isolated from Pseudoxylaria sp. X802: A competor of fungus-growing termite cultivars. Org. Lett. 2016;18:3338–3341. doi: 10.1021/acs.orglett.6b01437. [DOI] [PubMed] [Google Scholar]
- 50.Lou J., Fu L., Peng Y., Zhou L. Metabolites form Alternaria fungi and their bioactivities. Molecules. 2013;18:5891–5935. doi: 10.3390/molecules18055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim E.L., Li J.L., Xiao B., Hong J., Yoo E.S., Yoon W.D., Jung J.H. A new tetrapeptide from the jellyfish-derived fungus Phoma sp. Chem. Pharm. Bull. 2012;60:1590–1593. [PubMed] [Google Scholar]
- 52.Itazaki H., Nagashima K., Sugita K., Yoshida H., Kawamura Y., Yasuda Y., Matsumoto K., Ishii K., Uotani N., Nakai H., et al. Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation activities as antitumor agents. J. Antibiot. 1990;43:1524–1532. doi: 10.7164/antibiotics.43.1524. [DOI] [PubMed] [Google Scholar]
- 53.Jin J., Baek S.-R., Lee K.-R., Lee J., Yun S.-H., Kang S., Lee Y.-W. Purification and phytotoxicity of apicidins produced by the Fusarium semitectum KCTC16676. Plant Pathol. J. 2008;24:417–422. doi: 10.5423/PPJ.2008.24.4.417. [DOI] [Google Scholar]
- 54.Jin J.-M., Lee S., Lee J., Baek S.-R., Kim J.-C., Yun S.-H., Park S.-Y., Kang S., Lee Y.-W. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol. Microbiol. 2010;76:456–466. doi: 10.1111/j.1365-2958.2010.07109.x. [DOI] [PubMed] [Google Scholar]
- 55.Von Bargen K.W., Niehaus E.-M., Bergander K., Brun R., Tudzynski B., Humpf H.-U. Structure elucidation and antimalarial activity of apicidin F: An apicidin-like compound produced by Fusarium fujikuroi. J. Nat. Prod. 2013;76:2136–2140. doi: 10.1021/np4006053. [DOI] [PubMed] [Google Scholar]
- 56.Garson M.J. Isolation of the tetrapeptide apcidins G, H and I from the fungus Fusarium semitectum. Nat. Prod. Commun. 2014;9:233–236. [PubMed] [Google Scholar]
- 57.Aucamp P.J., Holzapfel C.W. Toxic substances from Aspergillus versicolor. J. S. Afr. Chem. Inst. 1969;22:35–40. [Google Scholar]
- 58.Li Y., Yue Q., Jayanetti D.R., Swenson D.C., Bartholomeusz G.A., An Z., Gloer J.B., Bills G.F. Anti-Cryptococcus phenalenones and cyclic tetrapeptides from Auxarthron pseudauxarthron. J. Nat. Prod. 2017;80:2101–2109. doi: 10.1021/acs.jnatprod.7b00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S., Peiser G., Nkajima T., Hwang Y.-S. Characterization of a phytotoxic cyclotetrapeptide, a novel chlamydocin analogue, from Verticillium coccosporum. Tetrahedron Lett. 1994;35:6009–6012. doi: 10.1016/0040-4039(94)88061-1. [DOI] [Google Scholar]
- 60.Hirota A., Suzuki A., Aizawa K., Tamura S. Structure of Cyl-2, a novel cyclopeptide from Cylindrocladium scoparium. Agric. Biol. Chem. 1973;37:955–956. doi: 10.1080/00021369.1973.10860775. [DOI] [Google Scholar]
- 61.Masuoka Y., Shin-ya K., Furihata K., Hayakawa Y., Seto H. A novel substance with TGF-β like activity, diheteropeptin, produced by a fungus, Diheterospora sp. J. Antibiot. 1997;50:1058–1060. doi: 10.7164/antibiotics.50.1058. [DOI] [PubMed] [Google Scholar]
- 62.Masuoka Y., Shin-ya K., Kim Y., Yoshida M., Nagai K., Suzuki K. Diheteropeptin, a new substance with TGF-β-like activity, produced by a fungus, Diheterospora chlamydosporia. J. Antibiot. 2000;53:788–792. doi: 10.7164/antibiotics.53.788. [DOI] [PubMed] [Google Scholar]
- 63.Horiuchi M., Akimoto N., Ohnishi K., Yamashita M., Maoka T. Rapid and simultaneous determination of tetra cyclic peptide phytotoxins, tentoxin, isotentoxin and dihydrotentoxin, from Alternaria porri by LC/MS. Chromatography. 2003;24:109–116. [Google Scholar]
- 64.Almeida C., Maddah F.E., Kehraus S., Schnakenburg G., Konig G.M. Endolides A and B, vasopressin and serotonin-receptor interacting N-methylated peptides from the sponge-derived fungus Stachylidium sp. Org. Lett. 2016;18:528–531. doi: 10.1021/acs.orglett.5b03553. [DOI] [PubMed] [Google Scholar]
- 65.Maddah F.E., Kehraus S., Nazir M., Almeida C., Konig G.M. Insights into the biosynthetic origin of 3-(3-furyl)alanine in Stachylidium sp. 293 K04 tetrapeptides. J. Nat. Prod. 2016;79:2838–2845. doi: 10.1021/acs.jnatprod.6b00601. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z.-F., Yang X.-H., Liu H.-L., Gu Y.-C., Ye B.-P., Guo Y.-W. A new cyclic peptide and a new steroid from the fungus Penicillium sp. GD6 isolated from the mangrove Bruguiera gymnorrhiza. Helv. Chim. Acta. 2014;97:1564–1570. doi: 10.1002/hlca.201400062. [DOI] [Google Scholar]
- 67.Mori H., Urano Y., Abe F., Furukawa S., Furukawa S., Tsurumi Y., Sakamoto K., Hashimoto M., Takase S., Hino M., et al. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC). I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2003;56:72–79. doi: 10.7164/antibiotics.56.72. [DOI] [PubMed] [Google Scholar]
- 68.Mori H., Abe F., Furukawa S., Furukawa S., Sakai F., Hino M., Fujii T. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC). II. Biological activities in animal models. J. Antibiot. 2003;56:80–86. doi: 10.7164/antibiotics.56.80. [DOI] [PubMed] [Google Scholar]
- 69.Mori H., Urano Y., Kinoshita T., Yoshimura S., Takase S., Hino M. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase III. Structure determination. J. Antibiot. 2003;56:181–185. doi: 10.7164/antibiotics.56.181. [DOI] [PubMed] [Google Scholar]
- 70.Ali H., Ries M.I., Lankhorst P.P., Hoeven R.A.M., Schouten O.L., Noga M., Hankemeier T., Peij N.N.M.E., Bovenberg R.A.L., Vreeken R.J., et al. A non-canonical NPRS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum. PLoS ONE. 2014;9:e98212. doi: 10.1371/journal.pone.0098212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liesch J.M., Sweeley C.C., Staffeld G.D., Anderson M.S., Weber D.J., Scheffer R.P. Structure of HC-toxin, a cyclic tetrapeptide from Helminthosporium carbonum. Tetrahedron. 1982;38:45–48. doi: 10.1016/0040-4020(82)85043-6. [DOI] [Google Scholar]
- 72.Kawai M., Jasensky R.D., Rich D.H. Conformational analysis by NMR spectrometry of the highly substituted cyclic tetrapeptides, chlamydocin and Ala-chlamydocin. Evidence for a unique amide bond sequence in dimethyl-d sulfoxidy. J. Am. Chem. Soc. 1983;105:4456–4462. doi: 10.1021/ja00351a052. [DOI] [Google Scholar]
- 73.Lang G., Blunt J.W., Cummings N.J., Cole A.L.J., Munro M.H.G. Hirsutide, a cyclic tetrapeptide from a spider-derived entomopathogenic fungus, Hirsutella sp. J. Nat. Prod. 2005;68:1303–1305. doi: 10.1021/np0501536. [DOI] [PubMed] [Google Scholar]
- 74.Klitgaard A., Nielsen J.B., Frandsen R.J.N., Andersen M.R., Nielsen K.F. Combining stable isotope labeling and molecular networking for biosynthetic pathway characterization. Anal. Chem. 2015;87:6520–6526. doi: 10.1021/acs.analchem.5b01934. [DOI] [PubMed] [Google Scholar]
- 75.Ochi S., Yoshida M., Nakagawa A., Natsume M. Identification and activity of a phytotoxin produced by Calonectria ilicicola, the causal agent of soybean red crown rot. Can. J. Plant Pathol. 2011;33:347–354. doi: 10.1080/07060661.2011.593558. [DOI] [Google Scholar]
- 76.Yoshida S., Yoshioka M., Yaguchi T., Nagasawa M., Koyama M. PF1070A and B, new antitumour antibiotics produced by Humicola sp. Sci. Rep. Meiji Seika Kaisha. 1993;32:58–63. [Google Scholar]
- 77.Masuoka Y., Shin-Ya K., Furihata K., Nagai K., Suzuki K.-I., Hayakawa Y., Seto H. Phoenistatin, a new gene expression-enhancing substance produced by Acremonium fusigerum. J. Antibiot. 2001;54:187–190. doi: 10.7164/antibiotics.54.187. [DOI] [PubMed] [Google Scholar]
- 78.Zin W.W.M., Buttachon S., Dethoup T., Fernandes C., Cravo S., Pinoto M.M.M., Gales L., Pereira J.A., Silva A.M.S., Sekeroglu N., et al. New cyclotetrapeptides and a new diketopiperazine derivative from the marine sponge-associated fngus Neosartorya glabra KUFA 0702. Mar. Drugs. 2016;14:136. doi: 10.3390/md14070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evans N., Mcroberts N., Hill R.A., Marshall G. Phytotoxin production by Alternaria linicola and phytoalexin production by the linseed host. Ann. Appl. Biol. 1996;129:415–431. doi: 10.1111/j.1744-7348.1996.tb05765.x. [DOI] [Google Scholar]
- 80.Umehara K., Nakahara K., Kiyoto S., Iwami M., Okamoto M., Tanaka H., Hohsaka M., Aoki H., Imanaka H. Studies on WF-3161, a new antitumor antibiotic. J. Antibiot. 1983;36:478–483. doi: 10.7164/antibiotics.36.478. [DOI] [PubMed] [Google Scholar]
- 81.Arai N., Shiomi K., Yamaguchi Y., Masuma R., Iwai Y., Turberg A., Kolbl H., Omura S. Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem. Pharm. Bull. 2000;48:1442–1446. doi: 10.1248/cpb.48.1442. [DOI] [PubMed] [Google Scholar]
- 82.Arai N., Shiomi K., Iwai Y., Omura S. Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668 II. Isolation, physicochemical properties, and structure elucidation. J. Antibiot. 2000;53:609–614. doi: 10.7164/antibiotics.53.609. [DOI] [PubMed] [Google Scholar]
- 83.Rao F.V., Houston D.R., Boot R.G., Aerts J.M.F.G., Hodkinson M., Adam D.J., Shimoi K., Omura D.J., Shiomi K., Omura S., et al. Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem. Biol. 2005;12:65–76. doi: 10.1016/j.chembiol.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Ma X., Nong X.-H., Ren Z., Wang J., liang X., Wang L., Qi S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017;58:1151–1155. doi: 10.1016/j.tetlet.2017.02.005. [DOI] [Google Scholar]
- 85.Kobayashi R., Samejima Y., Nakajima S., Kawai K., Udagawa S. Studies on fungal products. XI. Isolation and structures of novel cyclic pentapeptides from Aspergillus sp. NE-45. Chem. Pharm. Bull. 1987;35:1347–1352. doi: 10.1248/cpb.35.1347. [DOI] [PubMed] [Google Scholar]
- 86.Lewer P., Graupner P.R., Hahn D.R., Karr L.L., Duebelbeis D.O., Lira J.M., Anzeveno P.B., Fields S.C., Gilbert J.R., Pearce C. Discovery, synthesis, and insecticidal activity of cycloaspeptide E. J. Nat. Prod. 2006;69:1506–1510. doi: 10.1021/np060219c. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y., Liu S., Liu H., Liu X., Che Y. Cycloaspeptides F and G, cyclic pentapeptides from a cordyceps-colonizing isolate of Isaria farinosa. J. Nat. Prod. 2009;72:1364–1367. doi: 10.1021/np900205m. [DOI] [PubMed] [Google Scholar]
- 88.Kim K.-W., Sugawara F., Uzawa J., Yoshida S., Murofushi N., Takahashi N., Curtis R.W., Kanai M. Structure of malformin B, phytotoxic metabolites produced by Aspergillus niger. Tetrahedron Lett. 1991;32:6715–6718. doi: 10.1016/S0040-4039(00)93584-6. [DOI] [Google Scholar]
- 89.Zhan J., Gunaherath G.M.K.B., Wijeratne E.M.K., Gunatilaka A.A.L. Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. Phytochemistry. 2007;68:368–372. doi: 10.1016/j.phytochem.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan Q.-W., Gao F.-L., Wang F.-R., Chen Q.-J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015;16:5750–5761. doi: 10.3390/ijms16035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma Y.-M., Liang X.-A., Zhang H.-C., Liu R. Cytotoxic and antibiotic cyclic pentapeptide from an endiphytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 2016;64:3789–3793. doi: 10.1021/acs.jafc.6b01051. [DOI] [PubMed] [Google Scholar]
- 92.Sugawara F., Kim K.-W., Uzawa J., Yoshida S., Takahashi N., Curtis R.W. Structure of malformin A2, reinvestigation of phytotoxic metabolites produced by Aspergillus niger. Tetrahedron Lett. 1990;31:4337–4340. doi: 10.1016/S0040-4039(00)97615-9. [DOI] [Google Scholar]
- 93.Li X.-B., Xie F., Liu S.-S., Li Y., Zhou J.-C., Liu Y.-Q., Yuan H.-Q., Lou H.-X. Naphtho-γ-pyrones from endophyte Aspergillus niger occurring in the liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2013;10:1193–1201. doi: 10.1002/cbdv.201300042. [DOI] [PubMed] [Google Scholar]
- 94.Anderegg R.J., Biemann K., Buechi G., Cushman M. Malformin C, a new metabolite of Aspergillus niger. J. Am. Chem. Soc. 1976;98:3365–3370. doi: 10.1021/ja00427a051. [DOI] [PubMed] [Google Scholar]
- 95.Wang J., Jiang Z., Lam W., Gullen E.A., Yu Z., Wei Y., Wang L., Zeiss C., Beck A., Cheng E.-C., et al. Study of malformin C, a fungal source cyclic pentapeptide, as an anti-cancer drug. PLoS ONE. 2015;10:e0140069. doi: 10.1371/journal.pone.0140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavlaskova K., Nedved J., Kuzma M., Zabka M., Sulc M., Sklenar J., Novak P., Benada O., Kofronova O., Hajduch M., et al. Characterization of pseudacyclins A–E, a suite of cyclic peptides prduced by Pseudallescheria boydii. J. Nat. Prod. 2010;73:1027–1032. doi: 10.1021/np900472c. [DOI] [PubMed] [Google Scholar]
- 97.Shiomi K., Arai N., Iwai Y., Turberg A., Kolbl H., Omura S. Structure of argifin, a new chitinase inhibitor produced by Gliocladium sp. Tetrahedron Lett. 2000;41:2141–2143. doi: 10.1016/S0040-4039(00)00099-X. [DOI] [Google Scholar]
- 98.Chen M., Shao C.-L., Fu X.-M., Kong C.-J., She Z.-G., Wang C.-Y. Lumazine peptides penilumandes B–D and the cyclic pentapeptide asperpeptide A from a gorgonian-derived Aspergillus sp. fungus. J. Nat. Prod. 2014;77:1601–1606. doi: 10.1021/np5001686. [DOI] [PubMed] [Google Scholar]
- 99.Igarashi Y., Hanafusa T., Gohda F., Peterson S., Bills G. Species-level assessment of secondary metabolite diversity among Hamigera species and a taxonomic note on the genus. Mycology. 2014;5:102–109. doi: 10.1080/21501203.2014.917736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitova M.I., Stuart B.G., Cao G.H., Blunt J.W., Cole A.L.J., Munro M.H.G. Chrysosporide, a cyclic pentapeptide from a New Zealand sample of the fungus Sepedonium chrysospermum. J. Nat. Prod. 2006;69:1481–1484. doi: 10.1021/np060137o. [DOI] [PubMed] [Google Scholar]
- 101.Ye P., Shen L., Jiang W., Ye Y., Chen C.-T.A., Wu X., Wang K., Wu B. Zn-driven discovery of a hydrothermal vent fungal metabolite clavatustide C, and an experimental study of the anti-cancer mechanism of clavatustide B. Mar. Drugs. 2014;12:3203–3217. doi: 10.3390/md12063203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fremlin L.J., Piggott A.M., Lacey E., Capon R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- 103.Talontsi F.M., Facey P., Tagong M.D.K., Islam M.T., Frauendorf H., Draeger S., von Tiedemann A., Laatsch H. Zoosporicidal metabolties from an endophytic fungus Cryptosporiospsis sp. of Zanthoxylum leprieurii. Phytochemistry. 2012;83:87–94. doi: 10.1016/j.phytochem.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Li G., Kusari S., Golz C., Strohmann C., Spiteller M. Three cyclic petapeptides and a cyclic lipopeptide produced by endophytic Fusarium decemcellulare LG53. RSC Adv. 2016;6:54092–54098. doi: 10.1039/C6RA10905E. [DOI] [Google Scholar]
- 105.Li H.-J., Lin Y.-C., Yao J.-H., Vrijmoed L.L.P., Jones E.B.G. Two new metabolites from the mangrove endophytic fungus No. 2524. J. Asian Nat. Prod. Res. 2004;6:185–191. doi: 10.1080/102860201653237. [DOI] [PubMed] [Google Scholar]
- 106.Frisvad J.C., Larsen T.O., Dalsgaard P.W., Seifert K.A., Louis-Seize G., Lyhne E.K., Jarvis B.B., Fettinger J.C., Overy D.P. Four psychrotolerant species with high chemical diversity consistently producing cycloaspeptide A, Penicillium jamesonlandense sp. nov., Penicillium ribium sp. nov., Penicillium soppii and Penicillium lanosum. Int. J. Syst. Evol. Microbiol. 2006;56:1427–1437. doi: 10.1099/ijs.0.64160-0. [DOI] [PubMed] [Google Scholar]
- 107.Schmeda-Hirschmann G., Hormazabal E., Rodriguez J.A., Theoduloz C. Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. Z. Naturforsch. C. 2008;63c:383–388. doi: 10.1515/znc-2008-5-612. [DOI] [PubMed] [Google Scholar]
- 108.Mizutani K., Hirasawa Y., Sugita-Konishi Y., Mochizuki N., Morita H. Structural and conformational analysis of hydroxycyclochlorotine and cyclochlorotine, chlorinated cyclic peptides from Penicillium islandicum. J. Nat. Prod. 2008;71:1297–1300. doi: 10.1021/np800150m. [DOI] [PubMed] [Google Scholar]
- 109.Terao K., Ito E. Liver fibrosis induced in mice by cyclochlorotine. Mycotoxins. 1983;17:59–61. doi: 10.2520/myco1975.1983.59. [DOI] [Google Scholar]
- 110.Terao K., Ito E., Tatsuno T. Liver injuries induced by cyclochlorotine isolated from Penicillium islandicum. Arch. Toxicol. 1984;55:39–46. doi: 10.1007/BF00316584. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh A.C., Ramgopal M. Cyclic peptides from Penicillium isolandicum. A review and a reevaluation of the structure of islanditoxin. J. Heterocycl. Chem. 1980;17:1809–1812. doi: 10.1002/jhet.5570170838. [DOI] [Google Scholar]
- 112.Gulder T., Hong H., Correa J., Egereva E., Wiese J., Imhoff J., Gross H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs. 2012;10:2912–2935. doi: 10.3390/md10122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawahara T., Itoh M., Izumikawa M., Sakata N., Tsuchida T., Shin-ya K. Novel arginine-containing peptides MBJ-0173 and MBJ-0174 from Mortierella alpine f28740. J. Antibiot. 2017;70:226–229. doi: 10.1038/ja.2016.116. [DOI] [PubMed] [Google Scholar]
- 114.Zhuang Y., Teng X., Wang Y., Liu P., Wang H., Li J., Li G., Zhu W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron. 2011;67:7085–7089. doi: 10.1016/j.tet.2011.07.003. [DOI] [Google Scholar]
- 115.Zhou L.-N., Gao H.-Q., Cai S.-X., Zhu T.-J., Gu Q.-Q., Li D.-H. Two new cyclic pentapeptides from the marine-derived fungus Aspergillus versicolor. Helv. Chim. Acta. 2011;94:1065–1070. doi: 10.1002/hlca.201000408. [DOI] [Google Scholar]
- 116.Li Y.-Y., Hu Z.-Y., Shen Y.-M. Two new cyclopeptides and one new nonenolide from Xylaria sp. 101. Nat. Prod. Commun. 2011;6:1843–1846. [PubMed] [Google Scholar]
- 117.Mizuno K., Yagi A., Satoi S., Takada M., Hayashi M., Asano K., Matsuda T. Studies on aculeacin. I. Isolation and characeterazation of aculeacin A. J. Antibiot. 1977;30:297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- 118.Yamaguchi H., Hiratani T., Iwata K., Yamamoto Y. Studies on the mechanism of antifungal action of aculeacin A. J. Antibiot. 1982;35:210–219. doi: 10.7164/antibiotics.35.210. [DOI] [PubMed] [Google Scholar]
- 119.Song J.C., Stevens D.A. Caspofungin: Pharmacodynamics, pharmacokinetics, clinical uses and treatment outcomes. Crit. Rev. Microbiol. 2016;42:813–846. doi: 10.3109/1040841X.2015.1068271. [DOI] [PubMed] [Google Scholar]
- 120.Somer A., Torun S.H., Salman N. Caspofungin therapy in immunocompromised children and neonates. Expert Rev. Anti-Infect. Ther. 2011;9:347–355. doi: 10.1586/eri.11.4. [DOI] [PubMed] [Google Scholar]
- 121.Hashimoto S. Micafungin: A sulfated echinocandin. J. Antibiot. 2009;62:27–35. doi: 10.1038/ja.2008.3. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay T., Ganguli B.N., Fehlhaber H.W., Kogler H., Vertesy L. Mulundocandin, a new lipopeptide antibiotic. II. Structure elucidation. J. Antibiot. 1987;40:281–289. doi: 10.7164/antibiotics.40.281. [DOI] [PubMed] [Google Scholar]
- 123.Bills G., Yue Q., Chen L., Li Y., An Z., Frisvad J. Aspergillus mulundensis sp. nov., a new species for the fungus producing the antifungal echinocandin lipopeptides, mulundocandins. J. Antibiot. 2016;69:141–148. doi: 10.1038/ja.2015.105. [DOI] [PubMed] [Google Scholar]
- 124.Kai K., Yoshikawa H., Kuo Y.-H., Akiyama K., Hayashi H. Determination of absolute structures of cyclic peptides, PF1171A and PF1171C, from unidentified ascomycete OK-128. Biosci. Biotechnol. Biochem. 2010;74:1309–1311. doi: 10.1271/bbb.100098. [DOI] [PubMed] [Google Scholar]
- 125.Prompanya C., Fernandes C., Cravo S., Pinoto M.M.M., Dethoup T., Silva A.M.S., Kijjoa A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanenesis KUFA 0013. Mar. Drugs. 2015;13:1432–1450. doi: 10.3390/md13031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masuda Y., Tanaka R., Ganesan A., Doi T. Structure revision of similanamide to PF1171C by total synthesis. J. Nat. Prod. 2015;78:2286–2291. doi: 10.1021/acs.jnatprod.5b00643. [DOI] [PubMed] [Google Scholar]
- 127.Hensens O.D., Liesch J.M., Zink D.L., Smith J.L., Wichmann C.F., Schwartz R.E. Pneumocandins from Zalearion arboricola. III. Structure elucidation. J. Anibiot. 1992;45:1875–1885. doi: 10.7164/antibiotics.45.1875. [DOI] [PubMed] [Google Scholar]
- 128.Schmatz D.M., Abruzzo G., Powles M.A., Mcfadden D.C., Balkovec J.M., Black R.M., Nollstadt K., Bartizal K. Pneumocandins from Zalearion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumoystis carinii and Candida species. J. Anibiot. 1992;45:1886–1891. doi: 10.7164/antibiotics.45.1886. [DOI] [PubMed] [Google Scholar]
- 129.Zheng J., Zhu H., Hong K., Wang Y., Liu P., Wang X., Peng X., Zhu W. Novel cyclic hexapeptides from marine-derived fungus, Aspergillus sclerotiorum PT06-1. Org. Lett. 2009;11:5262–5265. doi: 10.1021/ol902197z. [DOI] [PubMed] [Google Scholar]
- 130.Liermann J.C., Thines E., Anke H., Opatz T. Anthranicine, an unusual cyclic hexapeptide from Acremonium sp. A29-2004. Z. Naturforsch. B. 2009;64:727–730. [Google Scholar]
- 131.Nakamura I., Yoshimura S., Masaki T., Takase S., Ohsumi K., Hashimoto M., Furukawa S., Fujie A. ASP2397: A novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. 2017;70:45–51. doi: 10.1038/ja.2016.107. [DOI] [PubMed] [Google Scholar]
- 132.Strobel G.A., Miller R.V., Martinez-Miller C., Condron M.M., Teplow D.B., Hess W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis c.f. quercina. Microbiology. 1999;145:1919–1926. doi: 10.1099/13500872-145-8-1919. [DOI] [PubMed] [Google Scholar]
- 133.Mukhopadhyay T., Roy K., Bhat R.G., Sawant S.N., Blumbach J., Ganguli B.N., Fehlhaver H.W., Kogler H. Deoxymulundocandin-a new echinocandin type antifungal antibiotic. J. Antibiot. 1992;45:618–623. doi: 10.7164/antibiotics.45.618. [DOI] [PubMed] [Google Scholar]
- 134.Toth V., Nagy C.T., Pocsi I., Emri T. The echinocandin B producer fungus Aspergillus nidulans var. roseus ATCC 58397 does not possess innate resistance against its lipopeptide antimycotic. Appl. Microbiol. Biotechnol. 2012;95:113–122. doi: 10.1007/s00253-012-4027-y. [DOI] [PubMed] [Google Scholar]
- 135.Traber R., Keller-Juslen C., Loosli H.R., Kuhn M., Von Wartburg A. Cyclopeptide antibiotics from Aspergillus species. Structure of echinocandins C and D. Helv. Chim. Acta. 1979;62:1252–1267. doi: 10.1002/hlca.19790620436. [DOI] [Google Scholar]
- 136.Bolker M., Basse C.W., Schirawski J. Ustilago maydis secondary metabolism-from genomics to chemistry. Fungal Genet. Biol. 2008;45:588–598. doi: 10.1016/j.fgb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 137.Ohra J., Morita K., Tsujino Y., Tazaki H., Fujimori T., Goering M., Evans S., Zorner P. Production of the phytotoxic metabolite, ferricrocin, by the fungus Colletotrichum gloeosporioides. Biosci. Biotechnol. Biochem. 1995;59:113–114. doi: 10.1271/bbb.59.113. [DOI] [PubMed] [Google Scholar]
- 138.Konetschny-Rapp S., Jung G., Huschka H.G., Winkelmann G. Isolation and identification of the principal siderophore of the plant pathogenic fungus Botrytis cinerea. Biol. Met. 1988;1:90–98. doi: 10.1007/BF01138066. [DOI] [Google Scholar]
- 139.Kanasaki R., Kobyashi M., Fujine K., Sato I., Hashimoto M., Takase S., Tsurumi Y., Fujie A., Hino M., Hashimoto S., et al. FR227673 and FR190293, novel antifungal lipopeptides from Chalara sp. No. 22210 and Tolypocladium parasiticum No. 16616. J. Antibiot. 2006;59:158–167. doi: 10.1038/ja.2006.23. [DOI] [PubMed] [Google Scholar]
- 140.Kanasaki R., Sakamoto K., Hashimoto M., Takase S., Tsurumi Y., Fujie A., Hino M., Hashimoto S., Hori Y. FR209602 and related compounds, novel antifungal lipopeptides from Coleophoma craterifomis No. 738. J. Antibiot. 2006;59:137–144. doi: 10.1038/ja.2006.20. [DOI] [PubMed] [Google Scholar]
- 141.Kanasaki R., Abe F., Kobayashi M., Katsuoka M., Hashimoto M., Takase S., Tsurumi Y., Fujie A., Hino M., Hashimoto S., et al. FR220897 and FR220899, novel antifungal lipopeptides from Coleophoma empetri No. 14573. J. Antibiot. 2006;59:149–157. doi: 10.1038/ja.2006.22. [DOI] [PubMed] [Google Scholar]
- 142.Fujie A. Discovery of micafungin (FK463): A novel antifungal drug derived from a natural product lead. Pure Appl. Chem. 2007;79:603–614. doi: 10.1351/pac200779040603. [DOI] [Google Scholar]
- 143.Zeng Y., Wang H., Kamdem R.S.T., Orfali R.S., Dai H., Makhloufi G., Janiak C., Liu Z., Proksch P. A new cyclohexapeptide, penitropeptide and a new polyketide, penitropone from the endophytic fungus Penicillium tropicum. Tetrahedron Lett. 2016;57:2998–3001. doi: 10.1016/j.tetlet.2016.05.095. [DOI] [Google Scholar]
- 144.Kuo Y.-H., Kai K., Akiyama K., Hayashi H. Novel bioactive peptides, PF1171F and PF1171G, from unidentified ascomycete OK-128. Tetrahedron Lett. 2012;53:429–431. doi: 10.1016/j.tetlet.2011.11.068. [DOI] [Google Scholar]
- 145.Wichmann C.F., Liesch J.M., Schwartz R.E. L-671,329, a new antifungal agent. II. Structure determination. J. Antibiot. 1989;42:168–173. doi: 10.7164/antibiotics.42.168. [DOI] [PubMed] [Google Scholar]
- 146.Liang X., Nong X.-H., Huang Z.-H., Qi S.-H. Antifungal and antiviral cyclic peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. J. Agric. Food Chem. 2017;65:5114–5121. doi: 10.1021/acs.jafc.7b01238. [DOI] [PubMed] [Google Scholar]
- 147.Rukachaisirikul V., Chantaruk S., Tansakul C., Saithong S., Chaicharemwimonkoon L., Pakawatchai C., Isaka M., Intereya K. A cyclopeptide from the insect pathogenic fungus Cordyceps sp. BCC 1788. J. Nat. Prod. 2006;69:305–307. doi: 10.1021/np050433l. [DOI] [PubMed] [Google Scholar]
- 148.Isaka M., Srisanoh U., Lartpornmatulee N., Boonruangprapa T. ES-242 derivatives and cycloheptapeptides from Cordyceps sp. strains BCC 16173 and BCC 16176. J. Nat. Prod. 2007;70:1601–1604. doi: 10.1021/np070357h. [DOI] [PubMed] [Google Scholar]
- 149.Chen Z., Song Y., Chen Y., Huang H., Zhang W., Ju J. Cyclic heptapeptides, cordyheptapeptides C–E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J. Nat. Prod. 2012;75:1215–1219. doi: 10.1021/np300152d. [DOI] [PubMed] [Google Scholar]
- 150.Vetter J. Toxins of Amanita phalloides. Toxicon. 1998;36:13–24. doi: 10.1016/S0041-0101(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 151.Walton J.D., Hallen-Adams H.E., Luo H. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Biopolymers. 2010;94:659–664. doi: 10.1002/bip.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boot C.M., Amagata T., Tenney K., Compton J.E., Pietraszkiewicz H., Valeriote F.A., Crews P. Four classes of structurally unusual peptides from two marine-derived fungi: Structures and bioactivities. Tetrahedron. 2007;63:9903–9914. doi: 10.1016/j.tet.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan L.T., Cheng X.C., Jensen P.R., Fenical W. Scytalidamides A and B, new cytotoxic cyclic heptapeptides from a marine fungus of the genus Scytalidium. J. Org. Chem. 2003;68:8767–8773. doi: 10.1021/jo030191z. [DOI] [PubMed] [Google Scholar]
- 154.Malmstrom J. Uguisins A and B. New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999;62:787–789. doi: 10.1021/np980539z. [DOI] [PubMed] [Google Scholar]
- 155.Malmstrom J., Ryager A., Anthoni U., Nielsen P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry. 2002;60:869–872. doi: 10.1016/S0031-9422(02)00150-4. [DOI] [PubMed] [Google Scholar]
- 156.Liu S., Shen Y. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chem. Nat. Compd. 2011;47:786–788. doi: 10.1007/s10600-011-0059-2. [DOI] [Google Scholar]
- 157.Akone S.H., Daletos G., Lin W., Proksch P. Unguisin F, a new cyclic peptide from the endophytic fungus Mucor irregularis. Z. Natufors. C. 2016;71c:15–19. doi: 10.1515/znc-2015-0137. [DOI] [PubMed] [Google Scholar]
- 158.Ariawan A.D., Webb J.E.A., Howe E.N.W., Gale P.A., Thordarson P., Hunter L. Cyclic peptide unguisin A is an anion receptor with high affinity for phosphate and pyrophosphate. Org. Biomol. Chem. 2017;15:2962–2967. doi: 10.1039/C7OB00316A. [DOI] [PubMed] [Google Scholar]
- 159.Loranger A., Tuchweber B., Gicquaud C., St-Pierre S., Cote M.G. Toxicity of peptides of Amanita virosa mushrooms in mice. Fundam. Appl. Toxicol. 1985;5:1144–1152. doi: 10.1016/0272-0590(85)90151-4. [DOI] [PubMed] [Google Scholar]
- 160.Koulman A., Lee T.V., Fraser K., Johnson L., Arcus V., Lott J.S., Rasmussen S., Lane G. Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloe festucae in culture and endophyte-infected Lolium perenne. Phytochemistry. 2012;75:128–139. doi: 10.1016/j.phytochem.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grunwald A.L., Berrue F., Robertson A.W., Overy D.P., Kerr R.G. Mortiamides A–D, cyclic heptapeptides from a novel Mortierella sp. obtained from Frobisher Bay. J. Nat. Prod. 2017;80:2677–2683. doi: 10.1021/acs.jnatprod.7b00383. [DOI] [PubMed] [Google Scholar]
- 162.Krasnoff S.B., Keresztes I., Gillilan R.E., Szebenyi D.M.E., Donzelli B.G.G., Churchill A.C.L., Gibson D.M. Serinocyclins A and B, cyclic heptapeptides from Metarhizium anisopliae. J. Nat. Prod. 2007;70:1919–1924. doi: 10.1021/np070407i. [DOI] [PubMed] [Google Scholar]
- 163.Dewapriya P., Prasad P., Damodar R., Salim A.A., Capon R.J. Talarolide A, a cyclic heptapeptide hydroxamate from an Australian marine tunicate-associated fungus, Talaromyces sp. (CMB-TU011) Org. Lett. 2017;19:2046–2049. doi: 10.1021/acs.orglett.7b00638. [DOI] [PubMed] [Google Scholar]
- 164.Bara R., Aly A.H., Wray V., Lin W.H., Proksch P., Debbab A. Talaromins A and B, new cyclic peptides from the endophytic fungus Talaromyces wortmannii. Tetrahedron Lett. 2013;54:1686–1689. doi: 10.1016/j.tetlet.2013.01.064. [DOI] [Google Scholar]
- 165.Feng Y., Blunt J.W., Cole A.L.J., Cannon J.F., Robinson W.T., Munro M.H.G. Two novel cytotoxic cyclodepsipeptides from a mycoparasitic Cladobotryum sp. J. Org. Chem. 2003;68:2002–2005. doi: 10.1021/jo0263059. [DOI] [PubMed] [Google Scholar]
- 166.Seto Y., Takahashi K., Matsuura H., Kogami Y., Yada H., Yoshihara T., Nabeta K. Novel cyclic peptide, epichlicin, from the endophytic fungus Epichloe typhina. Biosci. Biotechnol. Biochem. 2007;71:1470–1475. doi: 10.1271/bbb.60700. [DOI] [PubMed] [Google Scholar]
- 167.Belofsky G.N., Gloer J.B., Wicklow D.T., Dowd P.F. Shearamide A: A new cyclic peptide from the ascostromata of Eupenicillium shearii. Tetrahedron Lett. 1998;39:5497–5500. doi: 10.1016/S0040-4039(98)01161-7. [DOI] [Google Scholar]
- 168.Xue J.-H., Wu P., Chi Y.-L., Xu L.-X., Wei X.-Y. Cyclopeptides from Amanita exitialis. Nat. Prod. Bioprospect. 2011;1:52–56. doi: 10.1007/s13659-011-0013-9. [DOI] [Google Scholar]
- 169.Kim K.H., Choi S.U., Park K.M., Seok S.J., Lee K.R. Cytotoxic constituents of Amanita subjunquillea. Arch. Pharm. Res. 2008;31:579–586. doi: 10.1007/s12272-001-1196-3. [DOI] [PubMed] [Google Scholar]
- 170.Adachi K., Kanoh K., Wisespongp P., Nishijima M., Shizuri Y. Clonostachysins A and B, new anti-dinoflagellate cyclic peptides from a marine-derived fungus. J. Antibiot. 2005;58:145–150. doi: 10.1038/ja.2005.17. [DOI] [PubMed] [Google Scholar]
- 171.Weber D., Erosa G., Sterner O., Anke T. Cylindrocyclin A, a new cytotoxic cyclopeptide from Cylindrocarpon sp. J. Antibiot. 2006;59:495–499. doi: 10.1038/ja.2006.69. [DOI] [PubMed] [Google Scholar]
- 172.Azzolin L., Antolini N., Calderan A., Ruzza P., Sciacovelli M., Marin O., Stefano M., Paolo B., Rasola A. Antamanide, a derivative of Amantia phalloides, is a novel inhibitor of the mitochondrial permeability transition pore. PLoS ONE. 2011;6:e16280. doi: 10.1371/journal.pone.0016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ohyama T., Kurihara Y., Ono Y., Ishikawa T., Miyakoshi S., Hamano K., Arai M., Suzuki T., Igari H., Suzuki Y. Arborcandins A, B, C, D, E and F, novel 1,3-β-glucan synthase inhibitors: Production and biological activity. J. Antibiot. 2000;53:1108–1116. doi: 10.7164/antibiotics.53.1108. [DOI] [PubMed] [Google Scholar]
- 174.Ohyama T., Iwadate-kurihara Y., Ishikawa T., Miyakoshi S., Hamano K., Inukai M. Arborcandins A, B, C, D, E and F, novel 1,3-β-glucan synthase inhibitors: Physico-chemical properties and structure elucidation. J. Antibiot. 2003;56:1024–1032. doi: 10.7164/antibiotics.56.1024. [DOI] [PubMed] [Google Scholar]
- 175.Wieland T. The discovery, isolation, elucidation of structure, and sysnthesis of antamanide. Angew. Chem. 1968;7:204–208. doi: 10.1002/anie.196802041. [DOI] [PubMed] [Google Scholar]
- 176.Survase S.A., Kagliwal L.D., Annapure U.S., Singhal P.S. Cyclosporin A—A review on fermentative production, donstream processing and pharmacological applications. Biotechnol. Adv. 2011;29:418–435. doi: 10.1016/j.biotechadv.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 177.Ismaiel A.A. Production of the immunosuppressant cyclosporine A by a new soil isolate, Aspergillus fumigatus, in submerged culture. Appl. Microbiol. Biotechnol. 2017;101:3305–3317. doi: 10.1007/s00253-016-8052-0. [DOI] [PubMed] [Google Scholar]
- 178.Sallam L.A.R., El-Refai A.H., Hamdi A.A., El-Minofi A.H., Abd-Elsalam S.I. Role of some fermentation parameters on cyclosporine A production by a new isolate of A. terreus. J. Gen. Appl. Microbiol. 2003;49:321–328. doi: 10.2323/jgam.49.321. [DOI] [PubMed] [Google Scholar]
- 179.Margaritis A., Chahal P.S. Development of a fructose based medium for biosynthesis of cyclosporine-A by Bearuveria nivea. Biotechnol. Lett. 1989;11:765–768. doi: 10.1007/BF01026093. [DOI] [Google Scholar]
- 180.Sharmila K., Thillaimaharani K.A., Logesh A.R., Sathishkumar A., Kalaiselvam M. Production of cyclosporin A by saprophytic filamentous fungus Fusarium oxysporum. Int. J. Pharm. Pharm. Sci. 2012;4:149–153. [Google Scholar]
- 181.Traber R., Kuhn M., Loosli H.R., Pache W., Von Wartburg A. Neue cycolpeptide aus Trichoderma polysporum (LINK EX PERS.) RIFAI: Die cyclosporine B, D und E. Helv. Chim. Acta. 1977;60:1568–1578. doi: 10.1002/hlca.19770600513. [DOI] [PubMed] [Google Scholar]
- 182.Kobel H., Traber R. Directed biosynthesis of cyclosporins. Eur. J. Appl. Microbiol. Biotechnol. 1982;14:237–240. doi: 10.1007/BF00498470. [DOI] [Google Scholar]
- 183.Sekar C., Balaraman K. Immobilization of the fungus, Tolypocladium sp. for the production of cyclosporin A. Bioprocess Eng. 1998;19:281–283. doi: 10.1007/PL00009019. [DOI] [Google Scholar]
- 184.Sakamoto K., Tsujii E., Miyauchi M., Nakanishi T., Yamashita M., Shigematsu N., Tada T., Izumi S., Okuhara M. FR901459, a novel immunosuppressant isolated from Stachybotrys chartarum No. 19392. Taxonomy of the producing organism, fermentation, isolation, phytico-chemical properties and biological activities. J. Antibiot. 1993;46:1788–1798. doi: 10.7164/antibiotics.46.1788. [DOI] [PubMed] [Google Scholar]
- 185.Muramatsu Y., Furuichi Y., Tojo N., Moriguchi A., Maemoto T., Nakada H., Hino M., Matsuoka N. Neuroprotective efficacy of FR901459, a novel derivative of cyclosporine A, in vitro mitochondrial damage and in vivo transient cerebral ischemia models. Brain Res. 2007;1149:181–190. doi: 10.1016/j.brainres.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 186.Traber R., Hofmann H., Loosli H.R., Ponelle M., von Warburg A. Neue cyclosprorine aus Tolypocladium inflatum. Die cyclosporine K–Z. Helv. Chim. Acta. 1987;70:13–36. doi: 10.1002/hlca.19870700103. [DOI] [Google Scholar]
- 187.Moussaif M., Jacques P., Schaarwachter P., Budzikiewicz H., Thonart P. Cyclosporin C is the main antifungal compound produced by Acremonium luzulae. Appl. Environ. Microbiol. 1997;63:1739–1743. doi: 10.1128/aem.63.5.1739-1743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Traber R., Loosli H.R., Hofmann H., Kuhn M., Von Wartburg A. Isolation and structure dertermination of the new cyclosporins E, F, G, H and I. Helv. Chim. Acta. 1982;65:1655–1677. doi: 10.1002/hlca.19820650538. [DOI] [Google Scholar]
- 189.Jegorov A., Matha V., Sedmera P., Havlicek V., Josef S., Seidel P., Simek P. Cyclosporins from Tolypocladium terricola. Phytochemistry. 1995;38:403–407. doi: 10.1016/0031-9422(94)00711-2. [DOI] [PubMed] [Google Scholar]
- 190.Song Q.-Y., Nan Z.-B., Gao K., Song H., Tian P., Zhang X.-X., Li C.-J., Xu W.-B., Li X.-Z. Antifungal, phytotoxic, and cytotoxic activities of metabolites from Epichloe bromicola, a fungus obtained from Elymus tangutorum grass. J. Agric. Food Chem. 2015;63:8787–8792. doi: 10.1021/acs.jafc.5b04260. [DOI] [PubMed] [Google Scholar]
- 191.Buchel E., Martini U., Mayer A., Anke H., Sterner O. Omphalotins B, C and D, nematicidal cyclopeptides from Omphalotus olearius. Absolute configuration of omphalotin A. Tetradedron. 1998;54:5345–5352. doi: 10.1016/S0040-4020(98)00209-9. [DOI] [Google Scholar]
- 192.Liermann J.C., Opatz T., Kolshorn H., Antelo L., Hof C., Anke H. Omphalotins E–I, five oxidatively modified nematicidal cyclopeptides from Omphalotus olearius. Eur. J. Org. Chem. 2009:1256–1262. doi: 10.1002/ejoc.200801068. [DOI] [Google Scholar]
- 193.Anke H., Sterner O. Nematicidal metabolites from higher fungi. Curr. Org. Chem. 1997;1:361–374. [Google Scholar]
- 194.Ramm S., Krawczyk B., Muhlenweg A., Poch A., Mosker E., Sussmuth R.D. A self-sacrificing N-methyltransferase is the precursor of the fungal natural product omphalotin. Angew. Chem. Int. Ed. 2017;56:9994–9997. doi: 10.1002/anie.201703488. [DOI] [PubMed] [Google Scholar]
- 195.Aldemir H., Gulder T.A.M. Expanding the structural space of ribosomal peptides: Autocatalytic N-methylation in omphalotin biosynthesis. Angew. Chem. Int. Ed. 2017;56:13570–13572. doi: 10.1002/anie.201708456. [DOI] [PubMed] [Google Scholar]
- 196.Van der Velden N.S., Kalin N., Helf M.J., Piel J., Freeman M.F., Kunzler M. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nat. Chem. Biol. 2017;13:833–835. doi: 10.1038/nchembio.2393. [DOI] [PubMed] [Google Scholar]
- 197.Mayer A., Anke H., Sterner O. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius I. Fermentation and biological activity. Nat. Prod. Lett. 1997;10:25–32. doi: 10.1080/10575639708043691. [DOI] [Google Scholar]
- 198.Sterner O., Etzel W., Mayer A., Anke H. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius II. Isolation and structure determination. Nat. Prod. Lett. 1997;10:33–38. doi: 10.1080/10575639708043692. [DOI] [Google Scholar]
- 199.Mayer A., Kilian M., Hoster B., Sterner O., Anke H. In-vitro and in-vivo nematicidal activities of the cyclic dodecapeptide omphalotin A. Pestic. Sci. 1999;55:27–30. doi: 10.1002/(SICI)1096-9063(199901)55:1<27::AID-PS854>3.0.CO;2-K. [DOI] [Google Scholar]
- 200.Zou X., Niu S., Ren J., Li E., Liu X., Che Y. Verrucamides A–D, antibacterial cyclopeptides from Myrothecium verrucaria. J. Nat. Prod. 2011;74:1111–1116. doi: 10.1021/np200050r. [DOI] [PubMed] [Google Scholar]
- 201.Vanyolos A., Dekany M., Kovacs B., Kramos B., Berdi P., Zupko I., Hohmann J., Beni Z. Gymnopeptides A and B, cyclic octadecapeptides from the mushroom Gymnopus fusipes. Org. Lett. 2016;18:2688–2691. doi: 10.1021/acs.orglett.6b01158. [DOI] [PubMed] [Google Scholar]
- 202.Pan Z., Wu C., Wang W., Cheng Z., Yao G., Liu K., Li H., Fang L., Su W. Total synthesis and stereochemical assignment of gymnopeptides A and B. Org. Lett. 2017;19:4420–4423. doi: 10.1021/acs.orglett.7b01742. [DOI] [PubMed] [Google Scholar]
- 203.Nagano N., Umemura M., Izumikawa M., Kawano J., Ishii T., Kikuchi M., Tomii K., Kumagai T., Yoshimi A., Machida M., et al. Class of cyclic ribosomal peptide synthetic genes in filamentous fungi. Fungal Genet. Biol. 2016;86:58–70. doi: 10.1016/j.fgb.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 204.Kinoshita T., Kono Y., Takeuchi S., Daly J.M. Structure of HV-toxin M, a host-specific toxin-related compound produced by Helminthosporium victoriae. Agric. Biol. Chem. 1989;53:1283–1290. doi: 10.1271/bbb1961.53.1283. [DOI] [Google Scholar]
- 205.Culvenor C.C.J., Edgar J.A., Mackay M.F., Gorst-Allman C.P., Marasas W.F.O., Steyn P.S., Vleggaar R., Wessels P.L. Structure elucidation and absolute configuration of phomopsin A, a hexapeptide mycotoxin produced by Phomopsis leptostromiformis. Tetrahedron. 1989;45:2351–2372. doi: 10.1016/S0040-4020(01)83436-0. [DOI] [Google Scholar]
- 206.Allen J.G., Hancock G.R. Evidence that phomopsins A and B are not the only toxic metabolites produced by Phomopsis leptostromiformis. J. Appl. Toxicol. 1989;9:83–89. doi: 10.1002/jat.2550090203. [DOI] [PubMed] [Google Scholar]
- 207.Schlob S., Hackl T., Herz C., Lamy E., Koch M., Rohn S., Maul R. Dectection of a toxic methylated derative of phomopsin A produced by the legume-infesting fungus Diaporthe toxica. J. Nat. Prod. 2017;80:1930–1934. doi: 10.1021/acs.jnatprod.6b00662. [DOI] [PubMed] [Google Scholar]
- 208.Koiso Y., Li Y., Iwasaki S., Hanaoka K., Kobayashi T., Sonoda R., Fujita Y., Yaegashi H., Sato Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994;47:765–773. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 209.Koiso Y., Morisaki N., Yamashita Y., Mitsui Y., Shirai R., Hashimoto Y., Iwasaki S. Isolation and structure of an antimitotic cyclic peptide, ustiloxin F: Chemical interrelation with a homologous peptide, ustiloxin B. J. Antibiot. 1998;51:418–422. doi: 10.7164/antibiotics.51.418. [DOI] [PubMed] [Google Scholar]
- 210.Shan T., Sun W., Wang X., Fu X., Sun W., Zhou L. Purification of ustiloxins A and B from rice false smut balls by macroporous resins. Molecules. 2013;18:8181–8199. doi: 10.3390/molecules18078181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang X., Fu X., Lin F., Sun W., Meng J., Wang A., Lai D., Zhou L., Liu Y. The contents of ustiloxins A and B along with their distribution in rice false smut balls. Toxins. 2016;8:262. doi: 10.3390/toxins8090262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang X., Wang J., Lai D., Wang W., Dai J., Zhou L., Liu Y. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins. 2017;9:54. doi: 10.3390/toxins9020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Umemura M., Nagano N., Koike H., Kawano J., Ishi T., Miyamura Y., Kikuchi M., Tamano K., Yu J., Shin-ya K., et al. Characterization of the biosynthetic gene cluster for the ribosomally synthesized cyclic peptide ustiloxin B in Aspergillus flavus. Fungal Genet. Biol. 2014;68:23–30. doi: 10.1016/j.fgb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 214.Ye Y., Minami A., Igarashi Y., Izumikawa M., Umemura M., Nagano N., Mashida M., Kawahara T., Shin-ya K., Gomi K., et al. Unveiling the biosynthetic pathway of the ribosomally synthesized and post-trnslationally modified peptide ustiloxin B in filamentous fungi. Angew. Chem. Int. Ed. 2016;55:8072–8075. doi: 10.1002/anie.201602611. [DOI] [PubMed] [Google Scholar]
- 215.Wolpert T.J., Macko V., Acklin W., Jaun B., Seibl J., Meili J., Arigoni D. Structure of victorin C, the major host-selective toxin from Cochliobolus victoriae. Experientia. 1985;41:1524–1529. doi: 10.1007/BF01964789. [DOI] [Google Scholar]
- 216.Hallen H.E., Luo H., Scott-Craig J.S., Walton J.D. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA. 2007;104:19097–19101. doi: 10.1073/pnas.0707340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hershenhorn J., Casella F., Vurro M. Weed biocontrol with fungi: Past, present and future. Biocontrol Sci. Technol. 2016;26:1313–1328. doi: 10.1080/09583157.2016.1209161. [DOI] [Google Scholar]
- 218.Brindisi M., Maramai S., Brogi S., Fanigliulo E., Butini S., Guarino E., Casagni A., Lamponi S., Bonechi C., Nathwani S.M., et al. Development of novel cyclic peptides as pro-apoptotic agens. Eur. J. Med. Chem. 2016;117:301–320. doi: 10.1016/j.ejmech.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 219.Bills G., Li Y., Chen L., Yue Q., Niu X.-M., An Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat. Prod. Rep. 2014;31:1348–1375. doi: 10.1039/C4NP00046C. [DOI] [PubMed] [Google Scholar]
- 220.Denning D.W. Echinocandin antifngal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 221.Enoch D.A., Idris S.F., Aliyu S.H., Micallef C., Sule O., Karas J.A. Micafungin for the treatment of invasive apsergillosis. J. Infect. 2014;68:507–526. doi: 10.1016/j.jinf.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 222.Abdalla M.A., Matasyoh J.C. Endophytes as producers of peptides: An overview about the recently discovered peptides from endophytic microbes. Nat. Prod. Bioprospect. 2014;4:257–270. doi: 10.1007/s13659-014-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Newman D.J., Cragg G.M. Drugs and drug candidates from marine source: An assessment of the current “State of Play”. Planta Med. 2016;82:775–789. doi: 10.1055/s-0042-101353. [DOI] [PubMed] [Google Scholar]
- 224.Lee Y., Phat C., Hong S.-C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94–105. doi: 10.1016/j.peptides.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 225.Martinez-Klimova E., Rodriguez-Pena K., Sanchez S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017;134:1–17. doi: 10.1016/j.bcp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 226.Zhao J., Shan T., Mou Y., Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011;11:159–168. doi: 10.2174/138955711794519492. [DOI] [PubMed] [Google Scholar]