ABSTRACT
Mesenchymal stem cells (MSCs) have the ability to differentiate into osteoblasts and chondrocytes. In vitro osteogenic differentiation is critical but the molecular mechanism has yet to be further clarified. The role of TGF-β activated kinase 1 (TAK1) in MSCs osteogenesis differentiation has not been reported. By adding si-TAK1 and rhTAK1, the osteogenic differentiation of MSCs was measured. Expression levels of the osteoblastic marker genes during osteogenic differentiation of MSCs were checked. As well as molecules involved in BMP and Wnt/β-catenin signaling pathways. The phosphorylation of p38 and JNK was also checked. TAK1 is essential for mineralization of MSCs at low concentration, but excessive rhTAK1 inhibits mineralization of MSCs. It up regulates the expression levels of bone sialoprotein (BSP), osteocalcin (OSC), Alkaline phosphatase (ALP), and RUNX2 during osteogenic differentiation of MSCs. It can also promote TGF-β/BMP-2 gene expression and β-catenin expression, and down regulate GSK-3β expression. Meanwhile, TAK1 promotes the phosphorylation of p38 and JNK. Additionally, TAK1 up regulates the expression of BMP-2 at all concentration under the inhibition of p38 and JNK. Our results suggested that TAK1 is essential in MSCs osteogenesis differentiation, and functions as a double-edged sword, probably through regulation of β-catenin and p38/JNK.
KEY WORDS: TAK1, osteogenesis, mesenchymal stem cells, BMP-2, Wnt/β-catenin
INTRODUCTION
Through lifetime, bone undergoes constantly renewal and regeneration.1 Osteoclasts and osteoblasts are two main cell types in bone. They coordinate and contribute to this dynamic process. Sometimes bone regeneration may go beyond its own repair capacity, especially under the situations such as osteolytic bone tumor surgery, osteonecrosis, or after bone fractures.2 Thus bone tissue engineering is of great urgency in medical research.3 Mesenchymal stem cells (MSCs) have the ability of differentiation into multilineage cells during tissue homeostasis in vivo or under certain circumstance in vitro.4 Bone is one of the mesenchymal tissues that MSCs can contribute to the regeneration. Osteoblasts are specialized, terminally differentiated products arising from MSCs.5 The capability of differentiation into bone lineage and the accessibility makes MSCs a good candidate for bone tissue engineering and clinical stem cell therapy. However, many pathways are involved in the tuning of osteogenesis, and the mechanisms between the crosstalk of these pathways still need to be elucidated.
Bone morphogenetic proteins (BMPs) are key growth factor family that functions during endochondral skeletal differentiation.6 Transforming growth factor beta (TGF-β) is part of the superfamily that possesses common signaling elements in the TGF-β signaling pathway.7 TGF-β is especially important in cartilage differentiation,8 which generally precedes bone formation for endochondral ossification. Studies showed that TGF-β1 induces rapid nuclear translocation of β-catenin in MSCs in a Smad3-dependent manner.9 Previous data showed that TGF-β could function together with Wnt5a to regulate Wnt/β-catenin activity,10,11 promoting chondrocyte differentiation at the expense of adipocyte differentiation in hMSCs. Reports have also showed that Wnt3a and Wnt5a play critical roles in TLR4-induced MSC proliferation and osteogenic differentiation.12 By targeting the downstream genes, Wnt/β-catenin pathway is involved in the stimulation of MSC proliferation and the inhibition of MSC osteogenic differentiation by TGF-β1. While the canonical Wnt/β-catenin signaling pathway has been found to play a critical role in skeletal development and osteogenesis, indicating that Wnts can be used to improve de novo bone formation mediated by MSCs. Studies have shown that though Wnt4 did not stabilize β-catenin, it activated p38 MAPK in a novel noncanonical signaling pathway.13 Overall, it seems that BMPs can crosstalk with Wnt/β-catenin and MAPK pathway, thus function in skeletal development and osteogenesis.
Transforming growth factor-beta-activated kinase-1 (TAK1) is a MAP3K with high sequence similarity to Raf-1 and MEKK-1.14 It has been identified as a TGF-β/BMP-activated cytosolic component of the MAPK pathways. TAK1 can be activated by growth factors and cytokines such as RANKL and BMPs and its downstream pathways include NF-kappaB and JNK/p38 MAPKs. It is reported that TAK1 regulates osteoclastogenesis by integrating activation of NF-kappaB and derepression of NOTCH/RBPJ in myeloid cells.15 TAK1 is essential for osteoclast differentiation.16 Previous studies have shown that TAK1 is a positive regulator of osteoclast maturation, it functions in osteoclastogenesis in a cell-autonomous manner and in osteoblastogenesis and chondrogenesis in non-cell-autonomous manners.14,17,18 TAK1 interacts with Smad proteins and interferes with osteogenesis in murine mesenchymal progenitors. It is also suggested as a factor that is involved in the fine-tuning of BMP effects during osteogenic development.14 However, no research has been done regarding its role and function in osteogenesis of MSCs. Hereby we investigated the effect of TAK1 on osteogenic differentiation of MSCs.
MATERIALS AND METHODS
Culture of hADMSCs
Normal human Adipose-Derived Mesenchymal Stem Cells (hADMSCs, referred as MSCs in the following text) were purchased from ATCC (PCS-500–011). Cells were seeded into 25-cm2 flasks and incubated at 37°C/5% CO2. After 48 h, non-adherent cells were removed from the flasks by changing the medium. Thereafter, the medium was changed once every 3 days. Typically, cultures reached 90% confluence by 14 days, at which point the cells were trypsinized using 0.25% trypsin-0.53 mM EDTA, counted, and then plated again.
Si-TAK1 Transfection for MSCs
To knockdown TAK1 in MSCs, siRNAs targeting TAK1 were transfected in MSCs using GeneXPlus transfection reagent (ATCC ACS-4004) according to the manufacture's instruction. TAK1 and non-targeting control siRNAs were obtained from Dharmacon (see Table 1, Non-targeting siRNA#1).
TABLE 1.
Primers used.
| Gene Name | Forward | Reverse |
|---|---|---|
| Alkaline phosphatase | 5′-CCTCCTCGGAAGACACTCTG-3′ | 5′-GCAGTGAAGGGC TTCTTGTC-3′ |
| Bone sialoprotein | 5′-AAAGTGAGAACGGGGAACCT-3′ | 5′-GATGCAAAGCCAGAATGGAT-3′ |
| Osteocalcin | 5′-GACTGTGACGAG TTGGCTGA-3′ | 5′-CTGGAGAGGAGCAGAACTGG-3′ |
| Runx2 | 5′-ACAACCACAGAACCACAAG-3′ | 5′-TCTCGGTGGCTGGTAGTGA-3′ |
| BMP-2 | 5′-TGGCCCATTTAGAGGAGAACC-3′ | 5′-AGGCATGATAGCCCGGAGG-3′ |
| TGF-β | 5′- GGCAGGCACCGCCCGCCGGA-3′ | 5′- GGACCCAGGGACCTTCAGG-3′ |
| β-catenin | 5′- GAGGCGGAGACGGATGAAG-3′ | 5′- GTAGCCATTGTCCACGCTGG-3′ |
| GSK-3β | 5′- GCATGGTACTGAATACAGG-3′ | 5′- CTACTGAATGCATATCAC-3′ |
| GAPDH | 5′-GACTTCAACAGCAACTCCCAC-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ |
| Si-TAK1 | 5′-AAUUCUCUUCCAAGAAUGCCUGCAUUCUUGGAAGAGAAUUCC-3′ | |
Osteogenic Differentiation
To induce osteogenic differentiation, MSCs were plated at a density of 2 × 104 cells/cm2 in 6-well plates under normal culture condition. After 2 days, the medium was replaced with an osteogenic-inducing medium (low-glucose DMEM containing 5% FCS, 10 nM DEX, 50 μM L-ascorbic acid-2-phosphate, and 10 mM glycerophosphate), and then fresh differentiation medium was added every other day and the cells were maintained at 37°C in a humidified 5% CO2 atmosphere throughout the experiments until the cells were harvested.
Western blot
Standard western blotting protocols was used as previously described.19 Primary antibodies (Abcam, Cambridge, MA, USA) against TAK1 (ab109526), TGF-β (ab31013), BMP-2 (ab14933), GSK-3β (ab93926), β-catenin (ab32572), P-p38 (ab38238), p38 (ab31828), P-JNK2 (ab4821), JNK2 (ab76125), and GAPDH (ab8245) were used. The blots were incubated after wash with secondary antibodies conjugated with alkaline phosphatase (Abcam, USA). Immunoblots were developed using bromochloroindolyl/phosphate/nitroblue tetrazolium solution (Promega, Madison, WI, USA).
Alizarin Red S Staining
Mineralization was measured using Alizarin red S (Sigma, MO, USA) staining and phase-contrast microscopy. Cells were incubated with 2% Alizarin red at pH 4.2 for 10 min and then washed with distilled water; the sub-cultured cells were observed using phase-contrast microscopy to examine cell morphology and verify the presence of mineralized nodules. Quantitative analysis of Alizarin Red Staining was performed after dye extraction with 200 μL 10% acetic acid during 30 minutes under agitation. Recovered supernatant was further centrifuged, heated to 85°C for 10 minutes and cooled at 4°C. After centrifugation at 20,000 g for 15 minutes pH of supernatant was neutralized using 75 μL of 10% Ammonium hydroxide. Concentrations were calculated by determining OD405 against known Alizarin Red concentrations.
Real time RT-PCR
Cells were collected and total RNAs were extracted with use of RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed using a reverse transcription kit (Applied Biosystem, Waltham, MA, USA) as recently described.12 Real time quantitative PCR reactions were set up in triplicate with Ssofast Master Mix (Bio-Rad, Hercules, CA, USA) and run on a LightCycler 480 (Roche, Penzberg, Upper Bavaria, Germany). Primers listed in Table 1 were used for real time PCR.
Statistic analysis
Statistical evaluation was performed using one or two-way ANOVA analysis following a Tukey's post hoc test. Data were presented as mean ± S.E.M. The significance value was set at p < 0.05.
RESULTS
Effect of TAK1 on the mineralization of MSCs
First we investigated the effect of TAK1 on the mineralization of MSCs by transfecting cells with si-TAK1. Upon successful transfection, the protein content of TAK1 decreased to a significant lower level, compared to control groups (Figure 1A). Cells were cultured in a normal condition for 2 days, and the medium was replaced with an osteogenic-inducing medium. Then, the cells were transfected with si-TAK1, or treated with addition of recombinant human TAK1 (rhTAK1): rh1 (1 ng/ml rhTAK1), rh5 (5 ng/ml rhTAK1), rh50 (50 ng/ml rhTAK1), respectively. At 10 or 20 days after osteogenic inducing mineralization was quantitated (Figure 1B). MSCs transfected with si-TAK1 showed statistically lower mineral deposit compared to the other groups. Addition of rhTAK at low concentration (1 ng/ml, 5 ng/ml) promoted mineralization slightly though not at an obvious dose-dependent manner. Notably, when adding excessive rhTAK1 (50 ng/ml), the mineralization was inhibited. These results suggested that TAK1 is essential for mineralization of MSC but excessive TAK1 would inhibit mineralization of MSC.
FIGURE 1.
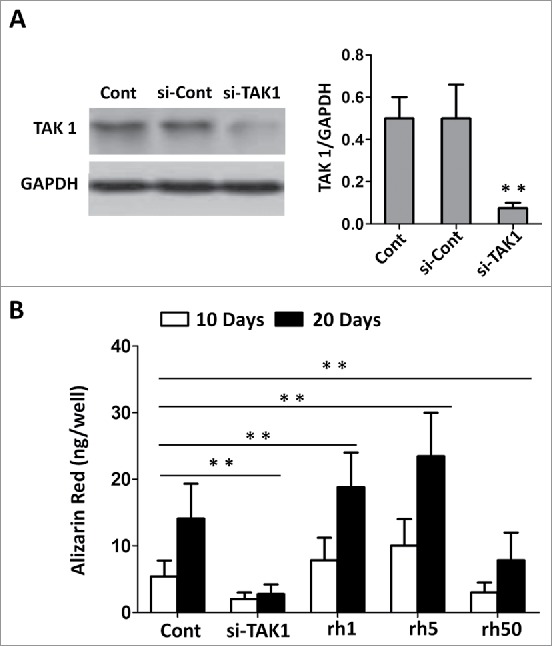
Effect of TAK1 on the mineralization of MSCs. (A) The expression of TAK1 was downregulated by si-TAK1 and analyzed by western blot, with antibodies against TAK1 and GAPDH, respectively. (B) Mineralization was quantitated through the elution of Alizarin Red S from stained mineral deposits. Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 10 and 20 days. Values are expressed as means ± S.E.M. of three independent experiments. **p < 0.001, compared with control group.
Effect of TAK1 on the gene expression during osteogenic differentiation of MSCs
Becasuse TAK1 is involved in osteogenic differentiation of MSCs, thus we checked the expression levels of the osteoblastic marker genes during osteogenic differentiation of MSCs. Bone sialoprotein (BSP), osteocalcin (OSC), Alkaline phosphatase (ALP), and RUNX2 are often used as osteoblastic marker genes, their levels elevate as osteogenesis progress. Expression of all marker genes in cells transfected with si-TAK1 showed significantly down-regulation, compared to the control group (Figure 2). MSCs with addition of rhTAK at low concentration showed slightly increase of expression for BSP (Figure 2A), but significantly increase of expression for OSC (Figure 2B), ALP (Figure 2C), and Runx2 (Figure 2D). However, not surprisingly all marker gene expressions were inhibited in MSCs with addition of high concentration of rhTAK1. These results suggested that TAK1 is essential for the onset of osteogenic differentiation of MSCs at low concentrations, while excessive TAK1 inhibited osteogenic differentiation of MSCs.
FIGURE 2.
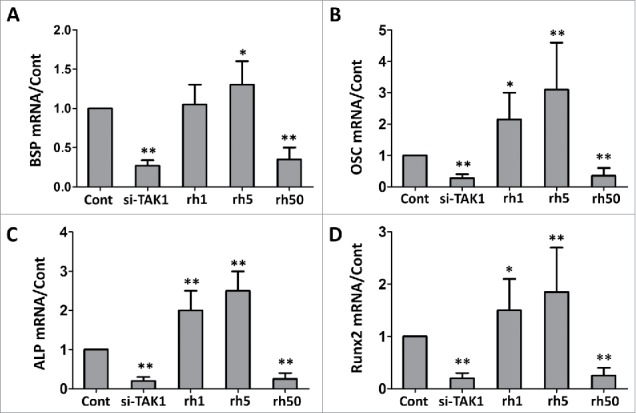
Effect of TAK1 on the expression levels of the osteoblastic marker genes during osteogenic differentiation of MSCs. qRT-PCR analysis of the osteoblastic marker genes bone sialoprotein (BSP), osteocalcin (OSC), Alkaline phosphatase (ALP) and runt-related transcription factor 2 (RUNX2). GAPDH was used as an internal control. Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 14 days. Values are expressed as means ± S.E.M. of three independent experiments. *p < 0.05, **p < 0.001, compared with control.
Effect of TAK1 on TGF-β/BMP-2 gene expression
Similar to the osteogenesis marker gene expression, expressions of TGF-β/BMP-2 also increase along with osteogenesis process. While transfected with si-TAK1, TGF-β/BMP-2 mRNA expressions decreased significantly (Figure 3A, B). Addition of rhTAK1 promoted TGF-β/BMP-2 gene expression, with slightly dose-dependent manner, though the increase was not statistically significant. Excessive rhTAK1 inhibit TGF-β/BMP-2 gene expression, to a level as low as that in si-TAK1 transfected cells. TGF-β/BMP-2 protein expressions were similar with the mRNA results (Figure 3C). These results implied that TAK1 is the upstream of TGF-β/BMP-2 signaling pathway. Its dysfunction affects TGF-β/BMP-2 gene expression during MSCs osteogenesis.
FIGURE 3.
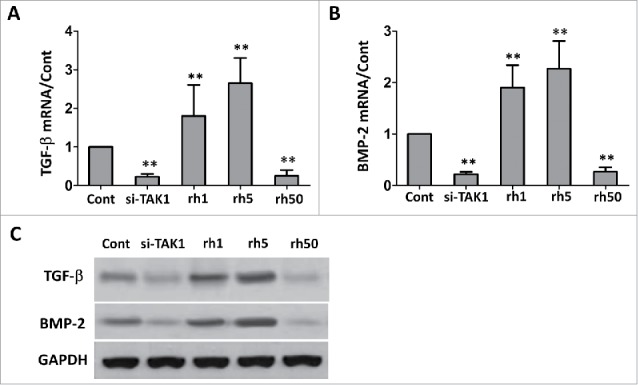
Effect of TAK1 on the expression levels of TGF-β and BMP-2 during osteogenic differentiation of MSCs. qRT-PCR (A,B) and western blot (C) analysis of the genes. GAPDH was used as an internal control. Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 14 days. Values are expressed as means ± S.E.M. of three independent experiments. **p < 0.001, compared with control.
Effect of TAK1 on gene expression of β-catenin/GSK-3β
Wnt/β-catenin signaling pathway is reported to be very important in osteogenesis, and β-catenin is essential for osteoblast differentiation. While MSCs were transfected with si-TAK1, β-catenin expression was greatly down regulated (Figure 4A). Addition of rhTAK1 at low concentration slightly up regulated β-catenin expression, though no more increase at high concentration (50 ng/ml). Not surprisingly, GSK-3β showed a different pattern of expression (Figure 4B). It was up regulated in si-TAK1 transfected cells, and was down regulated in cells with addition of rhTAK1, though no further significant decrease at high concentration (50 ng/ml). Same trend of changes were observed when detected protein expressions (Figure 4C and 4D). These results suggested that TAK1 might be up stream regulator of Wnt/β-catenin signaling pathway regulation.
FIGURE 4.
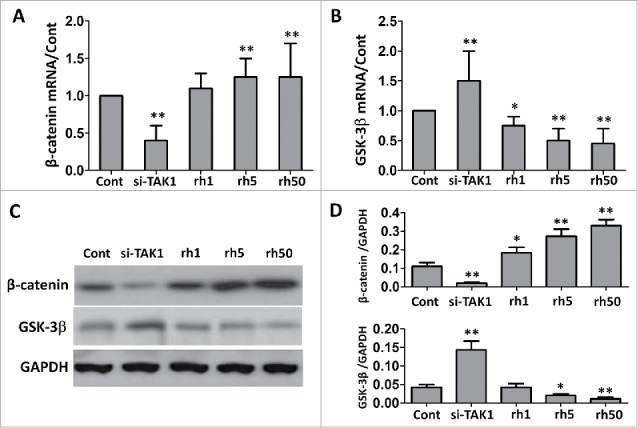
Effect of TAK1 on the expression levels of β-catenin and GSK-3b during osteogenic differentiation of MSCs. qRT-PCR (A, B) and western blot (C, D) analysis of the genes and corresponding proteins. GAPDH was used as an internal control. Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, or added with 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 14 days. Values are expressed as means ± S.E.M. of three independent experiments. *p < 0.05, **p < 0.001, compared with control.
Effects of TAK1 on phosphorylation of p38 and JNK
TAK1 is closely related with the phosphorylation of p38 and JNK, we sought to check their expression level in the situation of absence or presence of TAK1. In si-TAK1 transfected cells, we could hardly detect their phosphorylated protein (P-p38, P-JNK2), while p38 and JNK2 level was not changed compared to control group (Figure 5A-C). However, P-p38 and P-JNK2 levels were up regulated with the addition of rhTAK1 on a nearly dose-dependent manner. Notably, total protein level of p38 or JNK2 was not affected. These results suggested that TAK1 promote the phosphorylation of p38 and JNK.
FIGURE 5.
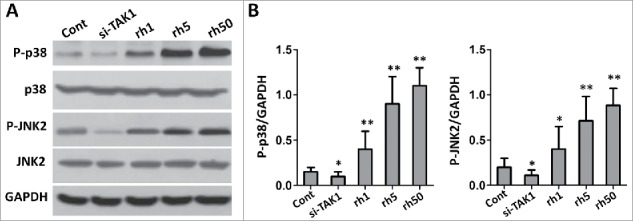
Effects of TAK1 on the expression levels of p38 and JNK during osteogenic differentiation of MSCs. (A) Western blot analysis of the protein expression levels. (B) The bar graph showed the intensities of protein expressions compared with GAPDH. Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 14 days. Values are expressed as means ± S.E.M. of three independent experiments. *p < 0.05, **p < 0.001, compared with control.
Effect of p38 and JNK on BMP-2
BMP signaling pathway is another important pathway involved in osteogenesis. We wanted to check the effect of inhibition of p38 or JNK or together on the expression of BMP-2. Transfection of si-TAK1 led to down regulation of BMP-2, addition of p38 inhibitor (SB203580) rescued some of BMP-2 expression, though couldn't get to the level as high as the control (Figure 6A). With the presence of rhTAK1, BMP-2 expression was up regulated at low concentration of rhTAK1, despite the addition of p38 inhibitor. However at high concentration of rhTAK1, the addition of p38 inhibitor rescued BMP-2 expression to a level as high as that at low concentration of rhTAK1. We did the same experiment by adding JNK inhibitor (SP600125), and BMP-2 expression showed the same trend of regulation (Figure 6B). When adding both inhibitors, the expression level of BMP-2 at high concentration of 50 ng/ml rhTAK1 showed even higher than that at low concentration of 5 ng/ml rhTAK1 (Figure 6C). These results implied that TAK1 could up regulate the expression of BMP-2 at all concentration with the inhibition of p38 and JNK, suggesting that p38 and JNK inhibited BMP-2 expression in cells with excessive rhTAK (50 ng/ml).
FIGURE 6.
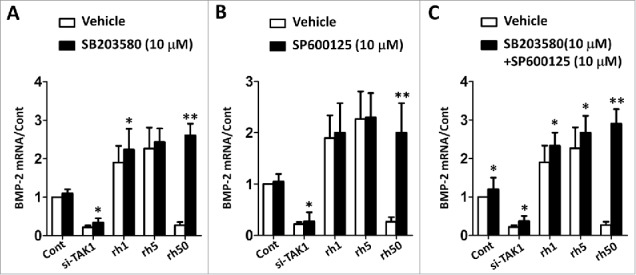
p38 and JNK down regulated the effect of TAK1 on the osteogenic differentiation of MSCs. (A) The mRNA level of BMP-2 with p38 inhibitor (SB203580). (B) The mRNA level of BMP-2 with JNK inhibitor (SP600125). (C) The mRNA level of BMP-2 with p38 inhibitor (SB203580) and JNK inhibitor (SP600125). Cells were plated at a density of 2 × 104 cells/cm2 in 6-well plates. After 2 days, the medium was replaced with an osteogenic -inducing medium. Then, the cells were transfected with si-TAK1, 1 ng/ml rhTAK1 (rh1), 5 ng/ml rhTAK1 (rh5) or 50 ng/ml rhTAK1 (rh50), respectively, for 14 days. Values are expressed as means ± S.E.M. of three independent experiments. *p < 0.05, **p < 0.001, compared with control.
DISCUSSION
By loss of function (siTAK1) and gain of function (rhTAK1) experiments, we checked the effect of TAK1 on osteogenesis and the involved pathways: TGF-β pathway, Wnt/β-catenin pathway, and MAPK pathway. We found that TAK1 is essential for mineralization of MSCs but excessive TAK1 would inhibit mineralization of MSCs, by up regulating the expression levels of the osteoblastic marker genes during osteogenic differentiation of MSCs. We also showed that TAK1 is the upstream of TGF-β/BMP-2 signaling pathway and Wnt/β-catenin signaling pathway..Of note, previous studies suggested that TAK1 is also a target gene of TGF-β/BMP-2 signaling pathway.20 Therefore TAK1 and TGF-β/BMP-2 signaling pathway form a positive feedback loop to facilitate osteogenesis of MSCs. MAPK pathway is also involved because TAK1 can promote the phosphorylation of p38 and JNK, for TAK1 is p38 MAPK upstream components and it is important for activation of JNK.21 There is also report showing that TAK1 and MKK6 constitute the p38 signaling pathway to participate in osteoclast differentiation.22 Many reports showed that β-catenin pathway is involved in osteogenesis and β-catenin is essential for osteogenesis. Upon the effect of si-TAK1, there is hardly any β-catenin expression. With the addition of TAK1, the expression level increases along with the dose increase of TAK1, even at an excessive level. It is not controversial that GSK-3β expression level decreases because it functions in an opposite way of β-catenin, for GSK-3β represses the expression of β-catenin.23 However, it is controversial to the effect that excessive TAK1 inhibit oeteogenesis. There is unknown underlying mechanism that needs to be elucidated in the future.
Our data demonstrated that TAK1 upregulates β-catenin and represses GSK-3β in a dosage-dependent manner, indicating that TAK1 activates WNT signaling pathway. However, how does TAK1 enhance WNT signaling pathway is not clear yet. Previous studies have shown that TAK1 interacts with the receptor tyrosine kinase Ror2 and modulates canonical Wnt-signaling.24 Interestingly, it was also reported that Wnt activates the Tak1/Nemo-like kinase pathway,25 indicating the existence of a TAK1-Wnt feedback loop. Further studies are needed to reveal the mechanisms underlying TAK1 regulates distinct Wnt genes and WNT signaling pathways.
It is interesting that high excessive rhTAK1 (50 ng/ml) inhibits BMP-2 expression and osteogenesis. Of note, the inhibition of BMP-2 by the high level of rhTAK1 is JNK/p38 dependent; while the activation of BMP-2 by low or moderate level of rhTAK1 is JNK/p38 independent. We propose that low or moderate level rhTAK1 facilitates BMP-2 expression through Wnt signaling pathway but not JNK/p38. On the other hand, high excessive rhTAK1, through JNK/p38, activates a BMP-2 repressor, such as p21 or Gli3,26,27 therefore inhibits BMP-2 expression. Notably, rhTAK1-mediated Wnt signaling activation was saturated at 50 ng/ml. Therefore, the BMP-2 repression induced by JNK/p38 pathway was not compensated by Wnt-mediated BMP-2 activation. However, we could not rule out the possibility that low or moderate level of rhTAK1 could also activate BMP-2 via other pathways, such as Akt/NFkb pathway.28 Nevertheless, our study provides a new sight into the mechanisms of TAK1 regulating osteogenesis and a guideline for the usage of TAK1 in MSC-driven osteogenesis.
MSCs are the top candidate for in vitro engineering of osteogenesis and clinical application.29 As many signaling pathways are participating in osteogenesis, making it is complicated. It is of great interest to basic research and clinical research to investigate the involved essential factors and signaling pathways, which is important for the in vitro osteogenic differentiation of MSCs. Therefore the role of TAK1 in osteogenic differentiation is of great significance for the clinical application of MSCs.
CONCLUSION
We conclude that TAK1 can increase the expression of β-catenin, reduce the expression of GSK-3β to promote osteogenic differentiation. TAK1 is also an upstream gene of p38 and JNK that increase their expression. P38 and JNK have a negative effect for osteogenesis differentiation. As a result, osteogenesis is promoted when β-catenin occupies, however osteogenesis is inhibited when p38 and JNK occupies. Our study provides a new vision into the mechanism how TAK1 regulates osteogenesis, suggesting a clue for the usage of TAK1 in osteogenic differentiation of MSCs.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare that there is no conflict of interests.
REFERENCES
- [1].Gittens SA, Uludag H. Growth factor delivery for bone tissue engineering. J Drug Target 2001;9:407–29. [DOI] [PubMed] [Google Scholar]
- [2].Nauth A, Schemitsch EH. Stem cells for the repair and regeneration of bone. Indian J Orthop. 2012;46:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135–50. [DOI] [PubMed] [Google Scholar]
- [4].Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3:131–45. [DOI] [PubMed] [Google Scholar]
- [6].Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al.. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chaudhury A, Howe PH. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life 2009;61:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009;11:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou S. TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011;112:1651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].He X, Wang H, Jin T, Xu Y, Mei L, Yang J. TLR4 Activation Promotes Bone Marrow MSC Proliferation and Osteogenic Differentiation via Wnt3a and Wnt5a Signaling. PLoS One 2016;11:e0149876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, et al.. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–48. [DOI] [PubMed] [Google Scholar]
- [14].Hoffmann A, Preobrazhenska O, Wodarczyk C, Medler Y, Winkel A, Shahab S, et al.. Transforming growth factor-beta-activated kinase-1 (TAK1), a MAP3K, interacts with Smad proteins and interferes with osteogenesis in murine mesenchymal progenitors. J Biol Chem. 2005;280:27271–83. [DOI] [PubMed] [Google Scholar]
- [15].Swarnkar G, Karuppaiah K, Mbalaviele G, Chen TH, Abu-Amer Y. Osteopetrosis in TAK1-deficient mice owing to defective NF-kappaB and NOTCH signaling. Proc Natl Acad Sci U S A 2015;112:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33:582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, et al.. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2010;25:1784–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qi B, Cong Q, Li P, Ma G, Guo X, Yeh J, et al.. Ablation of Tak1 in osteoclast progenitor leads to defects in skeletal growth and bone remodeling in mice. Sci Rep. 2014;4:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;4:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–8. [DOI] [PubMed] [Google Scholar]
- [22].Huang H, Ryu J, Ha J, Chang EJ, Kim HJ, Kim HM, et al.. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ 2006;13:1879–91. [DOI] [PubMed] [Google Scholar]
- [23].Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 2006;116:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Winkel A, Stricker S, Tylzanowski P, Seiffart V, Mundlos S, Gross G, et al.. Wnt-ligand-dependent interaction of TAK1 (TGF-beta-activated kinase-1) with the receptor tyrosine kinase Ror2 modulates canonical Wnt-signalling. Cell Signal 2008;20:2134–44. [DOI] [PubMed] [Google Scholar]
- [25].Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem. 2004;279:17232–40. [DOI] [PubMed] [Google Scholar]
- [26].Porlan E, Morante-Redolat JM, Marques-Torrejon MA, Andreu-Agullo C, Carneiro C, Gomez-Ibarlucea E, et al.. Transcriptional repression of Bmp2 by p21(Waf1/Cip1) links quiescence to neural stem cell maintenance. Nat Neurosci. 2013;16:1567–75. [DOI] [PubMed] [Google Scholar]
- [27].Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 2000;100:423–34. [DOI] [PubMed] [Google Scholar]
- [28].Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ. TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp Cell Res. 2008;314:2725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62. [DOI] [PMC free article] [PubMed] [Google Scholar]


