ABSTRACT
Lung transplantation is the last option for the treatment of end stage chronic lung disorders. Because the shortage of donor lung organs represents the main hurdle, lung regeneration has been considered to overcome this hurdle. Recellularization of decellularized organ scaffold is a promising option for organ regeneration. Although detergents are ordinarily used for decellularization, other approaches are possible. Here we used high alkaline (pH12) sodium hydroxide (NaOH)-PBS solution without detergents for lung decellularization and compared the efficacy on DNA elimination and ECM preservation with detergent based decellularization solutions CHAPS and SDS. Immunohistochemical image analysis showed that cell components were removed by NaOH solution as well as other detergents. A Collagen and GAG assay showed that the collagen reduction of the NaOH group was comparable to that of the CHAPS and SDS groups. However, DNA reduction was more significant in the NaOH group than in other groups (p < 0.0001). The recellularization of HUVEC revealed cell attachment was not inferior to that of the SDS group. Ex vivo functional analysis showed 100% oxygen ventilation increased oxygen partial pressure as artificial hemoglobin vesicle-PBS solution passed through regenerated lungs in the SDS or NaOH group. It was concluded that the NaOH-PBS based decellularization solution was comparable to ordinal decellularizaton solutions and competitive in cost effectiveness and residues in the decellularized scaffold negligible, thus providing another potential option to detergent for future clinical usage.
KEYWORDS: lung regeneration, extracellular matrix, decellularization, detergent, sodium hydroxide
INTRODUCTION
The prospect of using decellularized organs that have been recellularized by patient-specific progenitor cells for organ and tissue replacement opens the possibility for future clinical applications wherein an essentially autologous transplant occurs.1-4 Retention of extracellular matrix (ECM) components during the decellularized process is crucial for influencing the behavior of cells that are subsequently placed on the decellularized scaffold.5 ECM components play a major role in the proper migration, protein expression, and active signaling pathways of the donor cells.6,7 Transplantation studies of recellularized lung reveal that the alveolar structure of the bioengineered lung is fragile and the histological integrity of the recellularized lung seems to be determined by damage to the ECM at the thin barrier of the alveoli.8 Thus, the decellularization process has been considered to be crucial for ECM preservation.
The overall decellularization process involves cell destruction by detergents and extensive rinsing of residual cell components and DNA debris. The ability of detergents have been compared because it is one of the important factors for ECM retention. The most commonly used detergents are sodium dodecyl sulfate (SDS), 3-[(3-cholamidopropyl) dimethylammonio]-1- propanesulfonate (CHAPS) or Triton-X100/sodium deoxycholate (Triton/SDC).8,9 There are few direct comparisons of the various protocols utilizing different detergents, but Wallis and colleagues reported Triton/SDC as been less disruptive to native ECM when compared with CHAPS and SDS based approaches.9 Petersen and colleagues reported that CHAPS-based decellularization preserved collagen and elastin compared to SDS-based decellularization and how the mechanical integrity of scaffolds significantly diminishes and that some loss of elasticity occurs using SDS decellularization.8 However, when cells were intratracheally inoculated into the various decellularized lungs, the results of recellularization were comparable for initial binding and short-term (2 wk) proliferation of two different cell types, a stromal progenitor cell and a mouse lung epithelial cell line.9 We have previously explained the influence of pH of CHAPS solution on ECM preservation. In the study, DNA depletion was correlated with high pH levels of CHAPS solution.10 Although there are many decellularization methods available at the time of writing including physical, chemical, enzymatic, and proteinase approaches, however high pH alone may be able to achieve sufficient decellularization.11
Based on this possibility, we evaluated the decellularization ability of high pH sodium hydroxide (NaOH)-PBS solution without using detergents. We showed that high pH NaOH-PBS solution alone has sufficient ability for decellularization and provides comparable ECM preservation when compared to other detergent based decellularization solutions. We also confirmed comparable cell attachment and gas exchange function after the recellularization process in the NaOH group when compared to the SDS group. We collaterally achieved cost reduction in the decellularization process with NaOH-PBS, which will be a significant issue as decellularization based lung regeneration studies are scaled-up for application on larger models such as porcine or human organs and for future clinical usage.
RESULTS
System and gross appearance
For the decellularization of rat lungs, the bioreactor system was established as previously described (Fig. 1A). The gross appearance of decellularized lungs looked similar, white or faintly colored, at the central portions of the organ. (Fig. 1B).
FIGURE 1.
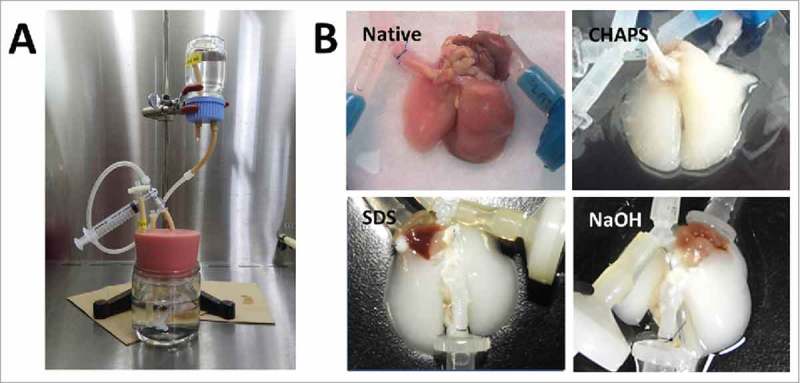
(A) Decellularization system of the lung. (B) Gross findings of decellularization of the lungs.
Histological analyses and scanning electron microscopy (SEM)
Hematoxylin and eosin staining showed that the decellularized scaffolds were completely devoid of any viable cells in each decellularization method using CHAPS, SDS, and NaOH-PBS (Fig. 2). No intact nuclei were observed in decellularized lungs compared to the control native lung. In the CHAPS-decellularized group, however, a faint blue residue was observed in alveolar spaces. Masson's trichrome staining was utilized to evaluate the presence and organization of collagen fiber. Staining indicated that collagen content appeared to be preserved in comparison to native lung (Fig. 2).
FIGURE 2.
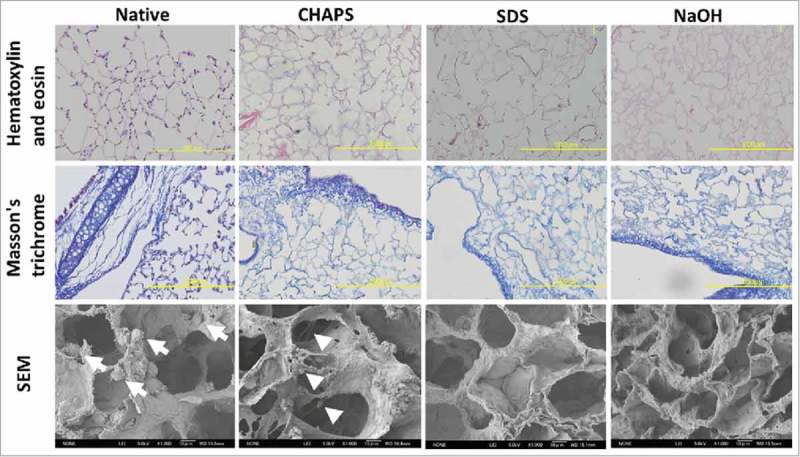
Histopathological examination and Scanning electron microscopy of native, CHAPS-treated, SDS-treated and NaOH-treated decellularized lungs. Scale bar = 200 μm.
Scanning electron microscopy (SEM) showed native alveolar cells attaching to alveolar septa in the control group (Fig. 2; white arrows). Alveolar ducts and alveolar network were completely preserved with an absence of cell bodies in each decellularized group. However, wavy and vesicular residues were observed in the CHAPS group, which reflected the histological findings of HE staining (Fig. 2; white arrow heads).
Immunohistochemistry
Fibronectin and laminin are both important ECM basement membrane proteins for cell adhesion. Immunohistochemical staining revealed that alveolar septa were immunopositive for fibronectin, but weakly positive in each decellularized group compared to native lung (Fig. 3A). Accordingly, quantitative image analysis showed a notable decrease of the fibronectin protein expression in each decellularized group compared to native lung, especially in NaOH group (Fig. 3B). In addition, quantitative image analysis revealed the immunostaining intensities of laminin and β-actin, a cytoskeletal protein, were significantly decreased in each decellularized group compared to native lung (Fig. 3B).
FIGURE 3.
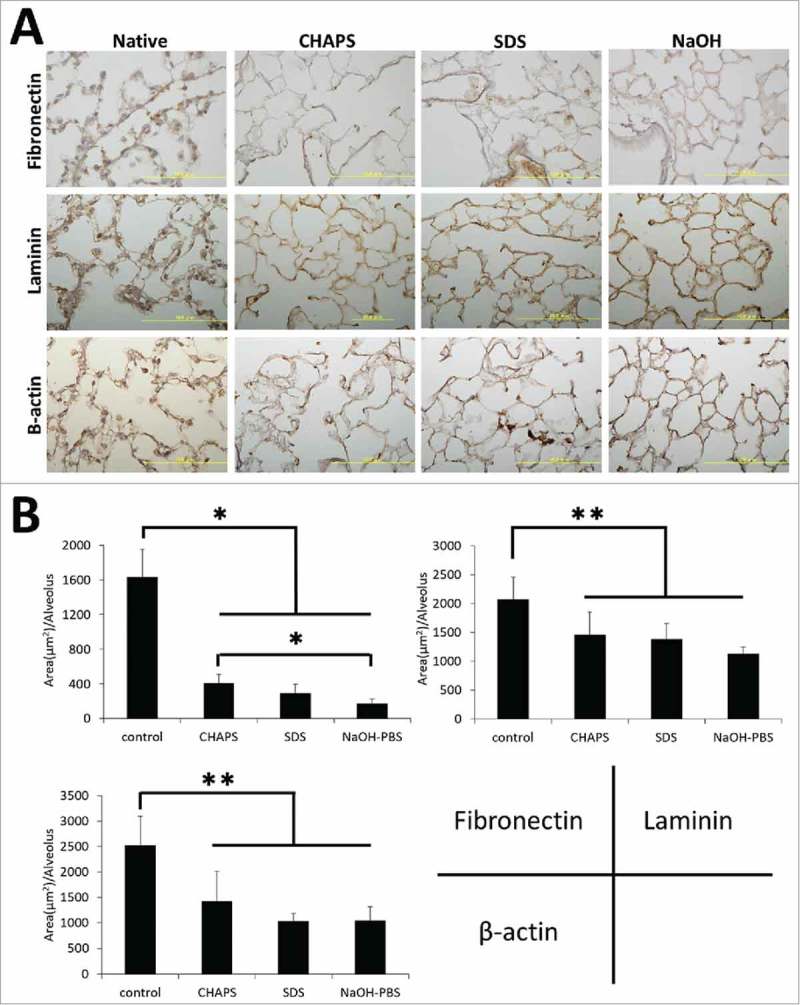
(A) Immunohistochemical staining of native, CHAPS-treated, SDS-treated and NaOH-treated decellularized lungs for fibronectin, laminin and β-actin. Scale bar = 10 μm. (B) Bar graph showing immune-positive area per alveolus of fibronectin, laminin and β-actin, which was calculated in image J software. Asterisk indicates significance of difference between the groups (*P < 0.005, **P < 0.05).
Extracellular matrix and DNA assay
An ideal scaffold is assumed to combine high ECM preservation and low DNA content. In order to evaluate ECM damage, hydroxyproline collagen assays and sulfated glycosaminoglycans (GAGs) were performed. The collagen assay showed that total collagen content was significantly diminished by decellularization (p < 0.0001) (Fig. 4A). Although the content was significantly lower in decellularized groups compared to that of native lung, the remaining collagen was 30% that of native lung, and the contents were similar in all decellularized groups. GAG assay showed that GAG contents were noticeably diminished compared to native lung in decellularized tissues, especially in the SDS and NaOH groups, which were significantly lower than that of the CHAPS group (p < 0.05) (Fig. 4B).
FIGURE 4.
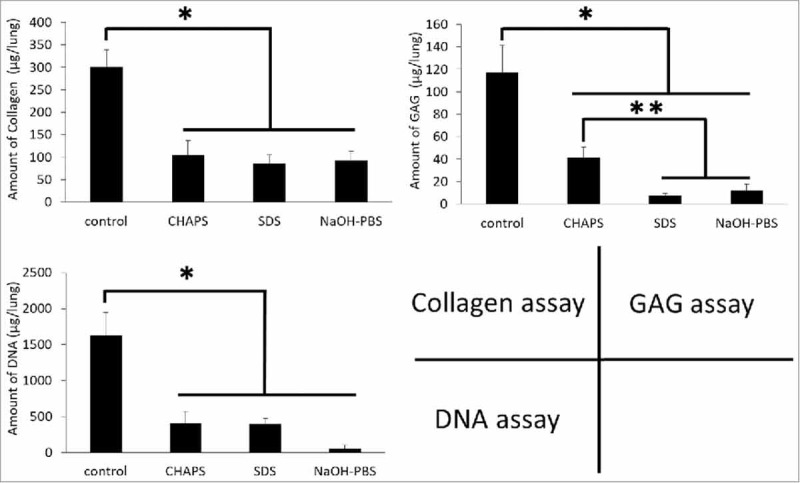
Graphs of matrix protein and DNA quantifications of native, CHAPS-treated, SDS-treated and NaOH-treated decellularized lungs. (A) Collagen assay. (B) GAG assay. (C) DNA assay.
When quantifying DNA by the Quant-iT PicoGreen assay, it appears that the DNA contents in all groups of decellularized scaffolds were significantly lower than that of native lung (p < 0.0001) (Fig. 4C). Further, DNA content of the NaOH group had diminished significantly. The content was relatively high in the SDS and CHPS groups compared to the NaOH group (p < 0.08), suggesting DNA retrieval was not sufficient in both groups.
Histological and functional analysis of recellularized lungs
The most important point of the present study was to confirm whether decellularized lung can be recellularized by live cells. Using HUVEC, the pathological findings of the recellularization group were similar between the NaOH and SDS groups. The recellularized cell number per area is comparable between the groups, suggesting the NaOH group scaffold recellularization ability is similar to that of the SDS group (Fig. 5A).
FIGURE 5.
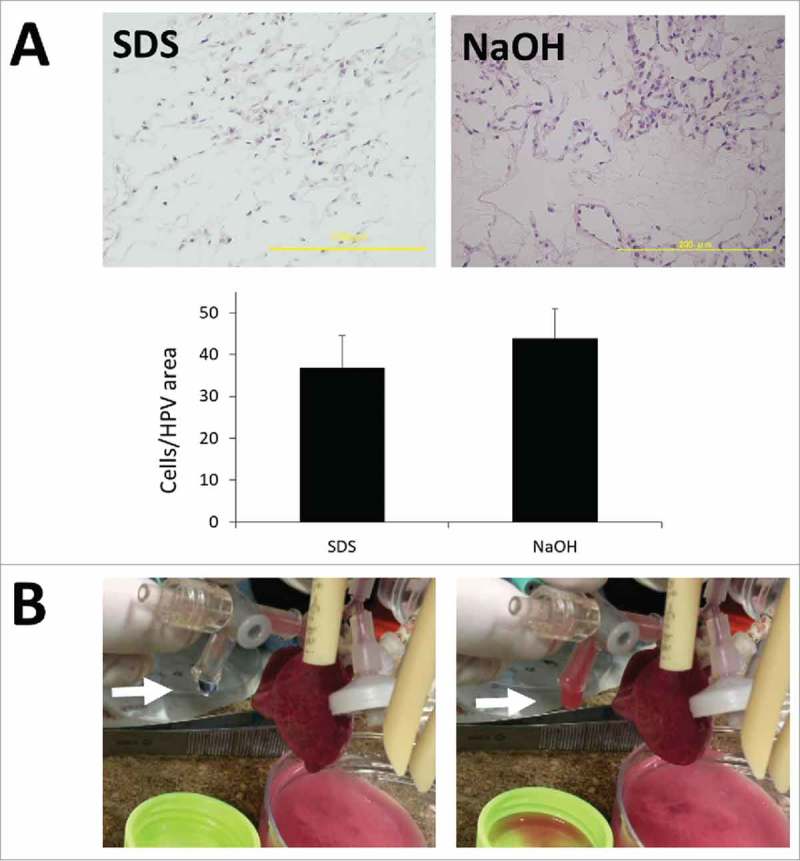
(A) Histopathological findings of SDS-treated and NaOH-treated recellularized lung. Scale bar = 200 μm. Bar graph showing the average number of reseeded HUVECs within a high power view (HPV) area (P = 0.310). (B) Findings of gas exchange functional analysis. Note hemoglobin vesicles-PBS solution was harvested from the cannulated pulmonary vein (white arrow).
We next evaluated the gas exchange ability of recellularized lung endothelialized by rat lung microvascular cells (RLMVEC) and epithelialized by isolated new born rat distal lung epithelial cells. We perfused artificial small size hemoglobin vesicles (HbVs) because native rat red blood cells could not pass the regenerated lung capillaries. In the bioreactor, 100% oxygen ventilation to the regenerated lung increased oxygen partial pressures of HgV-PBS solution, harvested from pulmonary vein, in both groups (Fig. 5B, Table 1).
TABLE 1.
Gas exchange function of regenerated lung with or without ventilation evaluated by HbVs-PBS solution
| Without ventilation | With ventiration (O2; 0.21) | With ventiration (O2; 1.0) | ||||
|---|---|---|---|---|---|---|
| Group |
Pre admin. |
PV |
Pre admin. |
PV |
Pre admin. |
PV |
| SDS | ||||||
| pH | 6.77 | 6.83 | 6.78 | 6.82 | 6.81 | 6.84 |
| PaO2 (mmHg) | 31 | 120 | 26 | 51 | 50 | 289 |
| PaCO2 (mmHg) | 14.8 | 13 | 13.7 | 10.1 | 12 | 9.5 |
| NaOH-PBS | ||||||
| pH | 6.76 | 6.83 | 6.77 | 6.85 | 6.77 | 6.88 |
| PaO2 (mmHg) | 28 | 166 | 29 | 116 | 40 | 283 |
| PaCO2 (mmHg) | 14.4 | 12.2 | 13.3 | 10.1 | 13.2 | 8.2 |
Abbreviations. HbVs; hemoglobin vesicles. Pre admin; pre administration. PV indicates harvested HbVs-PBS solution from pulmonary vein.
Evaluation of cost effectiveness of the respective decellularization methods
In the future, with the prospect of larger animal studies or clinical usage, cost effectiveness will be very important. Thus the cost of the decellularization process was measured. Because of the high cost of the chemical itself, the cost of the CHAPS group was particularly high for the process of decellularization. The cost of the SDS and NaOH groups were similar, they were about 20 times lower than that of CHAPS group (Fig. 6).
FIGURE 6.
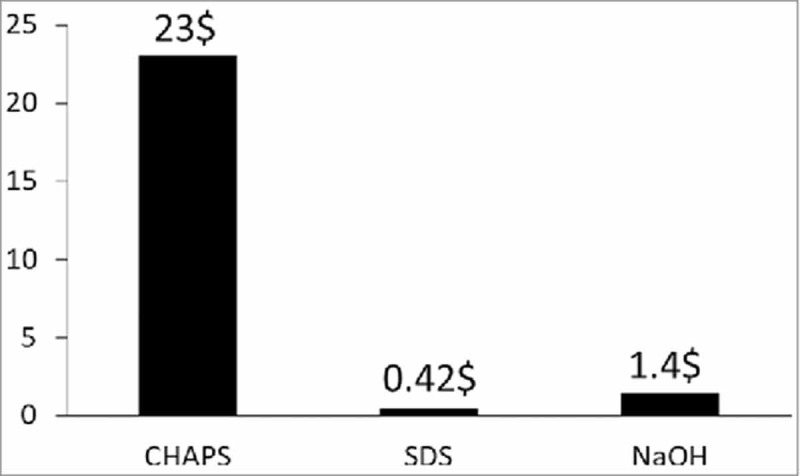
Total costs ($) for the CHAPS-treated, SDS-treated and NaOH-treated decellularization for rat lungs.
DISCUSSION
The technique of recellularization of the decellularized scaffold has been performed as per current methods for organ engineering. This method has been performed in the heart, trachea, liver, lung, and kidney. The decellularization solutions consist of detergents such as CHAPS, SDS, and Triton/SDC.12 However, many other decellularization methods have been reported for tissue engineering including physical, chemical, and enzymatic based approaches.11
The present results revealed that high alkaline NaOH-PBS solution is comparable with other currently used detergent based decellularization solutions. The HE staining and SEM revealed an absence of cell components in NaOH-PBS- decellularized lung, the histology was similar to CHAPS- and SDS decellularized lung. Immunohistochemistry and quantitative image analysis showed that the staining of ECM proteins reduced after decellularization, but the reduction was comparable between the three decellularized groups. In regard to ECM proteins, fibronectin and laminin serve a prominent role in the normal biological functioning and development of the lung and promote the formation and maintenance of lung epithelium and vasculature.13,14,15 In addition, there appears to be a contrasting relationship between the presence of certain isoforms of laminin (laminin-322) and fibronectin in the promotion of cell migration and adhesion in bronchial epithelial cells.16 Together these prior studies highlight the important role that the ECM plays in the proper maintenance of lung epithelium and also demonstrate the importance of maintaining an adequate ECM structure in tissue-engineered lungs.
The principal determinants of both the tissue integrity and mechanical behavior of lung tissue are collagen and glycosaminoglycans (GAGs). Collagen is the most abundant protein within the mammalian ECM. More than 90% of the dry weight of the ECM from most tissues and organs consists of collagen.17 The main subtypes that make up the lung's structure are collagens I, III, IV, and V. Type I collagen is the major structural protein present in tissues. In nature, collagen is closely associated with glycosylated proteins, growth factors and other structural proteins such as elastin and laminin, which provide unique tissue properties. Thus, retention of collagen may be the most important element in regard to ECM fragility in the decellularization process. In the present study, Masson's trichrome stain showed that the density of collagen fibrils were similar among the decellularized groups. Collagen assay showed that collagen content in each group was almost 30% that of native lung, suggesting tissue fragility was similar among the decellularized groups.
GAGs are found on cell surfaces, within intracellular vesicles, and are incorporated into the ECM.18 They bind growth factors and cytokines, help control macromolecular and cellular movement across the basal lamina, and contribute to the properties of the ECM by using negatively charged ‘tails’ to sequester water.6 Because GAGS are intrinsically part of the cell surface, removal of cells and cell components will ultimately cause the depletion of cell-bound GAGs. Interestingly, GAG content was greater in the CHAPS group. Because CHAPS is a non-denaturing zwitterionic detergent, CHAPS decellularization solution is advantageous in regard to the ECM preservation of such as GAG.
On the other hand, also of great importance, the DNA content was extremely low in the NaOH group, suggesting NaOH-PBS has an advantage in regard to the effective purging of DNA. The pH of the NaOH-PBS based decellularization solution was 12.4. At pH 12, DNA denatures to a single strand (each single strand is free to adopt different random coiled conformations), each with a lower intrinsic viscosity than the double-stranded DNA.19 Therefore, it is possible that the DNA removal observed in NaOH-PBS by washing out was sufficient. Moreover, high alkaline water (pH11.9 or more) can inactivate transmissible spongiform encephalopathies, the pathogens responsible for prion diseases. Since prion infection is a potential risk when using tissue engineered organs,20 to ensure safety from prion disease, NaOH based decellularization solution has a significant advantage in regard to prion inactivation of the biomaterials.21,22
Another important point was to verify the influence of decellularization procedures to the cell seeding efficacy and function of the regenerated lungs. Seeding efficacy of HUVEC in NaOH-PBS decellularized lung was comparable to that of SDS decellularized lung. Moreover, gas exchange functionality verified by oxygenation of HbVs did not vary between the two groups after reseeding with RLMVEC and isolated new born rat distal alveolar cells. HbVs are artificial oxygen carriers that encapsulate purified human Hb solution in phospholipid vesicles (liposomes).23 The safety and efficacy of HbVs as a transfusion alternative and as an oxygen therapeutic agent have been studied extensively using animal models. The smaller average particle size of HbVs (0.25 µm), compared to human red blood cells (8 µm), allows better perfusion through narrow capillaries. Therefore, to evaluate the gas exchange function of the seeded cells, we used small sized HbVs because native rat red blood cells became clogged in the regenerated vasculature (data not shown). It is thought that imperfections in the recellularization process resulted in clogging/impeding the red blood cell flow. Further studies are necessary to improve the recellularization at a capillary level to ensure unimpeded blood cell flow within the lung scaffold.
From 2008, tissue engineering studies using decellularized organ scaffold started in rodent models. However, larger animal studies have begun and clinical usage is expected in the near future. In general, high cost is one of the major hurdles to be addressed for clinical usage and cost reduction is necessary. In our methods, the decellularization cost of the SDS and the NaOH group were approximately 20 times lower than that of the CHAPS group. The other potential problem is the residual detergent in the decellularized scaffold. In a previous report, 50 mg/L (0.005%) of residual detergent did not cause any adverse effects on recellularization.24 However, there is the possibility that accidental insufficient rinsing might cause excessive levels of residual detergents in the scaffold. Because recellularization is similarly achieved after NaOH based decellularization as other detergent based decellularization, overall, an NaOH based solution may be superior in regard to both cost and safety among the studied decellularization solutions.
A limitation of the present study was that we have not applied NaOH-PBS decellularization to large scale animal organs. From the results of the present study, we can only conclude that NaOH-PBS solution has been beneficial for a basic study for lung regeneration. While similar methods using such as ammonium hydroxide with Tryton X as a high pH detergent have been successful for larger animal organ decellularization,25 it cannot be presumed that our approach can also be scaled up to produce the same results. Therefore further studies will be necessary to confirm the efficacy of NaOH-PBS for larger scale organ decellularization.
In summary, sodium hydroxide (NaOH) based solution has a comparable effect to the ordinal decellularization solution in regard to ECM damage and the effective elimination of DNA. The cell attachment to NaOH-PBS- decellularized lung was similar to the SDS- decellularized lung and the gas exchange function of the reseeded lung did not change on account of the decellularization procedure. Because the cost of NaOH-PBS solution is lower than that of the CHAPS solution and is safer than the SDS solution, the usage of NaOH-PBS decellularization solution could be significant, particularly in large scale animal studies or clinical usage.
MATERIALS AND METHODS
Organ harvest and decellularization
Lung tissue was obtained from young adult (3-month-old) male F344 rats. All animal work was performed with approval from the Nagasaki University Institutional Animal Care and Use Committee, and all animal care complied with the Guide for the Care and Use of Laboratory Animals. Organ harvest was performed as previously described.4 Briefly, animals were euthanized via intraperitoneal injection of 90 mg/kg body weight ketamine hydrochloride (Daiichi Sankyo Propharma, Tokyo, Japan) and 10 mg/kg body weight ketamine xylazine hydrochloride (Bayer, Leverkusen, Germany) and 250U/kg body weight heparin (Sigma-Aldrich, St. Louis, MO). The lungs were perfused via the right ventricle with PBS containing 50 U/ml heparin and 1 µg/ml sodium nitroprusside. Thereafter, the heart, lungs and trachea were dissected and removed en bloc. Tracheal and pulmonary artery cannulae were inserted and sutured into place, to provide access for perfusion and decellularization. Lungs were decellularized via perfusion of the pulmonary artery while maintaining physiologically appropriate pulmonary aterial pressures (i.e., below 25 mmHg)4 (Figure 1A). The arterial decellularization procedure was similar to a technique described previously.2 Briefly, lungs were cannulated at the pulmonary artery trunk and the vasculature perfused with decellularization fluids at 37℃ with pressures kept below 20 cmH2O. The perfusion time was approximately two and a half hours. After decellularization, tissues were extensively rinsed using vascular perfusion with 2500 mL of PBS.
The 500 ml of decellularization solution consisted of the following materials; CHAPS group: 8mM CHAPS, 1M NaCl, 25 mM EDTA, in 1 × PBS. NaOH group: 0.15M NaOH, 1M NaCl, 25 mM EDTA, in 1 × PBS. SDS group: 0.1% SDS followed by 150ml of 1%Triton X-100.
Histological analysis and immunohistochemistry
Tissues were formalin-fixed, paraffin-embedded and sectioned at a thickness of 5 µm. Analysis was performed with standard hematoxylin and eosin staining. Analysis was also performed with Masson's trichrome stain (for collagen). For immunohistochemistry, tissue sections were deparaffinized, rehydrated, and rinsed in PBS with 0.2% Triton X-100 for 15 minutes. Antigen retrieval was performed with 20ng/ml proteinase K in TE buffer (pH 8.0), at 37°C for 10 minutes. Sections were then blocked with 5% BSA and 0.75% glycine in PBS for one hour at room temperature. Primary antibodies for fibronectin and laminin were applied 1:200 in blocking buffer overnight at 4°C, followed by secondary antibodies in a 1:500 dilution for one hour at room temperature. Primary antibody for β-actin was applied at a dilution of 1:400 in the blocking buffer. Secondary antibodies used were mouse anti-rat antibody.
For the quantitative immunohistochemical analysis, the freeware ImageJ 1.51v 9 as well as the Colour Deconvolution plug-in, both downloaded from the NIH website (http://rsb.info.nih.gov/ij) were used. Five high power view images form alveolar area in each immunostained lung were analysed (n = 3). In each image, immunopositive areas over the threshold level was calculated and divided by the number of the alveoli in the field which were counted by the particle analysis. Thus the protein expression per alveolus in the each image could be validated.
Scanning electron microscopy (SEM)
Samples were fixed using 2% glutaraldehyde and 2.5% paraformaldehyde in 0.1M cacodylate buffer (EMD Biosciences, Gibbstown, NJ) for two hours at room temperature, then rinsed in cacodylate buffer, sliced, and dehydrated through an ethanol gradient. Samples were further dehydrated in hexamethyldisilizane for 10 minutes and dried overnight, then sputter coated with gold and analyzed using a JOEL JXA-8600 microprobe.
Collagen assay
Collagen was quantified with a colorimetric assay to detect hydroxyproline using a modification of Grant's method.18 Lung samples were lyophilized and weighed, then incubated in papain (10U/ml; 25 mg/ml) at 60°C overnight (Sigma). Papain-digested samples were incubated in 6 N HCl at 115° C for 18 h, neutralized, oxidized with chloramine-T and then reacted with p-dimethylaminobenzaldehyde. Absorbance was measured at a wavelength of 550 nm and a 1: 10 w/w ratio of hydroxyproline to collagen was used to calculate the collagen content of the tissue.
Sulfated GAG assay
Sulfated GAGs were quantified using the Blyscan GAG assay kit (Biocolor, Northern Ireland, UK). Lung samples were lyophilized and weighed, then incubated in 25 mg/ml body weight papain (Sigma-Aldrich, St. Louis, MO) at 60° C overnight. Papain-digested samples (prepared as described for the collagen assay, above) were assayed according to the manufacturer's instructions. Absorption was measured at a wavelength of 650 nm, and GAG content was quantified using a standard curve.
DNA assay
DNA content of tissues was quantified using the Quant-iT PicoGreen dsDNA assay kit (Thermo Fisher Scientific, Waltham, MA), following the manufacturer's instructions. Briefly, tissue samples were weighed and lyophilized, diluted in TE buffer and mixed with the Quant-iT PicoGreen reagent. Fluorescence was measured at 535 nm with excitation at 485 nm, and DNA content was quantified using a standard curve.
Rat lung scaffold re-cellularization and gas exchange functional analysis
Lung recellularization was performed using a previously published but modified protocol.2 Immediately after trypsinization, 4 × 107 HUVEC were diluted in 100 mL of medium. All cell-seeding experiments were conducted in the bioreactors allowing cell delivery and perfusion from both the pulmonary artery (PA) and pulmonary vein (PV). The PA and trachea were cannulated to the inside of the bioreactor through a fixed port. Decellularized lung scaffolds were primed by perfusion with 100 mL of PBS and equilibrated in the respective culture medium for at least 3 h before cell seeding. For re-endothelialization, in vitro expanded cells were resuspended in a single seeding chamber with a mixture of 50 mL of EGM-2MV and 50 mL of ADSC-GM medium, and seeded simultaneously by means of gravity perfusion through the PA and PV at a rate of 1:1. Two-hour static culture was then performed allowing cell attachment, and then perfusion was initiated at 1 mL/min from both the PA and PV, and continued for one day after seeding. During vasculature perfusion, the airway branches were filled with EC medium from the airway reservoir via trachea cannulation to avoid exposure to air. At day 1, the PV cannula was released and perfusion was changed to 4 mL/min from the PA only until the end of the culture period. The media was refreshed every other day. Seven days after the recellularization, the recellularized lung was histologically evaluated. For the quantitative analysis, the numbers of nuclei were quantified from 40 × magnification fields. Five high power view images in each lung were analysed (n = 3).
In order to evaluate the gas exchange ability of the recellularized lung, endothelial and epithelial cells were both seeded on the decellularized lung and an artificial oxygen carrying haemoglobin vesicle (0.25 µm)23 was perfused. Firstly, 4.5 × 106 of rat microvascular cells (RLMVEC) were seeded from PA and PV in the bioreactor. Thereafter, 3 × 107 of rat distal lung epithelial cells, which were isolated from new born F344 rats as described previously,26 were seeded from the trachea. Seven days after the recellularization, the recellularized lungs were ventilated (45 breaths per 1 min) using room air or 100% oxygen via the trachea under the HbVs mixed PBS perfusion. The final Hb concentration of HbVs mixed PBS was adjusted to 5 g/dL and deoxygenated. The blood gas of the pre-administrated HbVs-PBS and post-administrated HbVs-PBS collected from the pulmonary vein were analysed.
Statistical analysis
For comparisons among groups, ANOVA was performed to determine the differences between median values using standard deviation. If a difference was significant, the Tukey's HSD test was performed. Statistical significance between the two groups was evaluated using an unpaired Student's t-test. Values of P < 0.05 were considered significant. All statistical analyses were performed using JMP software (version 13).
Funding Statement
Funding of this project was provided by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (T.T., grant numbers 23592067 and 15H04944; T.M, grant number 16K10686).
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical support of Toshimitsu Komatsu (Department of Pathology), Kazuo Yamamoto (Biomedical Research Center) and staff at the Laboratory Animal Center of Nagasaki University.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- [1].Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Future prospects for tissue engineered lung transplantation: Decellularization and recellularization-based whole lung regeneration. Organogenesis. 2014;10:37–41. http://www.ncbi.nlm.nih.gov/pubmed/24488093 doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Petersen T, Calle E, Zhao L, Lee E, Gui L, Raredon M, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al.. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41 http://www.sciencemag.org/content/329/5991/538.short doi: 10.1126/science.1189345. PMID:20576850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21 http://www.nature.com/nm/journal/v14/n2/abs/nm1684.html doi: 10.1038/nm1684. PMID:18193059 [DOI] [PubMed] [Google Scholar]
- [4].Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, Fontana G, Sibila O, Rombol G, Jungebluth P, et al.. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet. 2014;383:238–44 http://www.sciencedirect.com/science/article/pii/S0140673613620334 doi: 10.1016/S0140-6736(13)62033-4. PMID:24161821 [DOI] [PubMed] [Google Scholar]
- [5].Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE. Epithelial Cell Differentiation of Human Mesenchymal Stromal Cells in Decellularized Lung Scaffolds. Tissue Eng Part A. 2014;20:1735–46 http://online.liebertpub.com/doi/abs/10.1089/ten.tea.2013.0647 doi: 10.1089/ten.tea.2013.0647. PMID:24393055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77 http://www.ncbi.nlm.nih.gov/pubmed/15157928 doi: 10.1016/j.trim.2003.12.016. PMID:15157928 [DOI] [PubMed] [Google Scholar]
- [7].Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol. 1996;270:L3–27 http://www.ncbi.nlm.nih.gov/pubmed/8772523 PMID:8772523 [DOI] [PubMed] [Google Scholar]
- [8].Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–31. doi: 10.1159/000324896. PMID:21502745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative Assessment of Detergent-Based Protocols for Mouse Lung De-Cellularization and Re-Cellularization. Tissue Eng Part C Methods. 2012;18:420–32 http://online.liebertpub.com/doi/abs/10.1089/ten.tec.2011.0567 doi: 10.1089/ten.tec.2011.0567. PMID:22165818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsuchiya T, Balestrini JL, Mendez J, Calle EA, Zhao L, Niklason LE. Influence of pH on Extracellular Matrix Preservation During Lung Decellularization. Tissue Eng Part C Methods. 2014;20:1028–36 http://online.liebertpub.com/doi/abs/10.1089/ten.tec.2013.0492 doi: 10.1089/ten.tec.2013.0492. PMID:24735501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83 http://www.ncbi.nlm.nih.gov/pubmed/16519932 PMID:16519932 [DOI] [PubMed] [Google Scholar]
- [12].Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods [Internet]. 2012;18:420–32 http://www.ncbi.nlm.nih.gov/pubmed/22165818%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3358122 doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53 http://www.ncbi.nlm.nih.gov/pubmed/21417722 doi: 10.1146/annurev-bioeng-071910-124743. PMID:21417722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol. 2006;294:271–9 http://www.ncbi.nlm.nih.gov/pubmed/16643883 doi: 10.1016/j.ydbio.2006.03.032. PMID:16643883 [DOI] [PubMed] [Google Scholar]
- [15].Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84 http://www.ncbi.nlm.nih.gov/pubmed/15473841 doi: 10.1146/annurev.cellbio.20.010403.094555. PMID:15473841 [DOI] [PubMed] [Google Scholar]
- [16].Kligys K, Wu Y, Hamill KJ, Lewandowski KT, Hopkinson SB, Budinger GRS, Jones JCR. Laminin-332 and α3β1 integrin-supported migration of bronchial epithelial cells is modulated by fibronectin. Am J Respir Cell Mol Biol. 2013;49:731–40. doi: 10.1165/rcmb.2012-0509OC. PMID:23590307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rest M Van der, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–23 http://www.fasebj.org/content/5/13/2814.short doi: 10.1096/fasebj.5.13.1916105. PMID:1916105 [DOI] [PubMed] [Google Scholar]
- [18].Ferdous Z, Grande-Allen KJ. Utility and control of proteoglycans in tissue engineering. Tissue Eng. 2007;13:1893–904 http://www.ncbi.nlm.nih.gov/pubmed/17518731 doi: 10.1089/ten.2006.0056. PMID:17518731 [DOI] [PubMed] [Google Scholar]
- [19].Paul E, Paul D. The Alkaline Denaturation of Deoxyribose Nucleic Acid. J Am Chem Soc. 1958;80:4251–5. doi: 10.1021/ja01549a033. [DOI] [Google Scholar]
- [20].Lemmer K, Mielke M, Kratzel C, Joncic M, Oezel M, Pauli G, Beekes M. Decontamination of surgical instruments from prions. II. In vivo findings with a model system for testing the removal of scrapie infectivity from steel surfaces. J Gen Virol. 2008;89:348–58. doi: 10.1099/vir.0.83396-0. PMID:18089760 [DOI] [PubMed] [Google Scholar]
- [21].Kim Y, Nowzari H, Rich SK. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin Implant Dent Relat Res. 2013;15:645–53. PMID:22171533 [DOI] [PubMed] [Google Scholar]
- [22].Fichet G, Comoy E, Duval C, Antloga K, Dehen C, Charbonnier A, McDonnell G, Brown P, Ida Lasmézas C, Deslys JP. Novel methods for disinfection of prion-contaminated medical devices. Lancet. 2004;364:521–6. doi: 10.1016/S0140-6736(04)16810-4. PMID:15302195 [DOI] [PubMed] [Google Scholar]
- [23].Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Haemoglobin-vesicles as artificial oxygen carriers: present situation and future visions. J Intern Med. 2008;263:4–15. PMID:18042220 [DOI] [PubMed] [Google Scholar]
- [24].Kawasaki T, Kirita Y, Kami D, Kitani T, Ozaki C, Itakura Y, Toyoda M, Gojo S. Novel detergent for whole organ tissue engineering. J Biomed Mater Res – Part A. 2015;103:3364–73. doi: 10.1002/jbm.a.35474. [DOI] [PubMed] [Google Scholar]
- [25].Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods. 2013;19:642–51. doi: 10.1089/ten.tec.2012.0334. PMID:23270591 [DOI] [PubMed] [Google Scholar]
- [26].Calle EA, Mendez JJ, Ghaedi M, Leiby KL, Bove PF, Herzog EL, Sundaram S, Niklason LE. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix. Tissue Eng Part A. 2015;21(11-12):1916–28. doi: 10.1089/ten.TEA.2014.0511. doi:. PMID:25789725 [DOI] [PMC free article] [PubMed] [Google Scholar]


