ABSTRACT
Skin wound healing involves Notch/Jagged1 signaling. However, little is known how Jag1 expression level in epidermal stem cells (ESCs) contributes to wound healing and scar formation. We applied multiple cellular and molecular techniques to examine how Jag1 expression in ESCs modulates ESCs differentiation to myofibroblasts (MFB) in vitro, interpret how Jag1 expression in ESCs is involved in wound healing and scar formation in mice, and evaluate the effects of porcine acellular dermal matrix (ADM) treatment on wound healing and scar formation. We found that Jag1, Notch1 and Hes1 expression was up-regulated in the wound tissue during the period of wound healing. Furthermore, Jag1 expression level in the ESCs was positively associated with the level of differentiation to MFB. ESC-specific knockout of Jag1 delayed wound healing and promoted scar formation in vivo. In addition, we reported that porcine ADM treatment after skin incision could accelerate wound closure and reduce scar formation in vivo. This effect was associated with decreased expression of MFB markers, including α-SMA Col-1 and Col-III in wound tissues. Finally, we confirmed that porcine ADM treatment could increase Jag1, Notch1 and Hesl expression in wound tissues. Taken together, our results suggested that ESC-specific Jag1 expression levels are critical for wound healing and scar formation, and porcine ADM treatment would be beneficial in promoting wound healing and preventing scar formation by enhancing Notch/Jagged1 signaling pathway in ESCs.
KEYWORDS: epidermal stem cells, Jag1, scar, porcine acellular dermal matrix, wound healing
Introduction
Formation of scars is one of the most common complications during wound healing.1,2 The success of wound healing process depends on multiple cellular processes involving growth factors, cytokines, and chemokines.3 Given the complexity of the wound repair process, it is necessary to study the mechanisms of scar formation and develop effective therapies to improve wound healing and inhibit scar formation.
Growing evidence has shown that lack of epidermal stem cells (ESCs) and the excessive hyperplasia of myofibroblasts (MFB) are two important factors in scar formation.4-6 ESCs possess strong proliferation and differentiation potential, and are located in the basal layer of the epidermis and the follicle bugle of hair.7 In physiological conditions, ESCs keep the normal structure and function of skin and repair damage by proliferation, migration, and differentiation.8 Our previous study has shown that scar tissue exhibits lower number of ESCs and higher number of MFB than normal skin.9 Pharmacological inhibition of the Notch1/Jagged1 pathway can promote ESC differentiation to MFB.10,11 More recently, we demonstrated that scar formation is attenuated by pharmacological activation of Notch/Jagged1 signaling pathway.12,13 However, it is still not clear that how ESC-specific Notch/Jagged1 signaling pathway involves in scar formation and wound healing.
Porcine acellular dermal matrix (ADM) has been widely used in tissue reconstruction. It has been shown that porcine ADM is useful as a dermal substitute in full-thickness skin defects and can enhance the quality of wound repair.14,15 In our previous studies, we found that porcine ADM treatment is beneficial to burn wound healing.16,17 Importantly, we demonstrated that porcine ADM treatment increased the expression of keratin 19 (K19) and integrin-β1, key biomarkers of keratinocyte stem cells, suggesting that porcine ADM may accelerate skin wound repair.18 However, the effects of porcine ADM on scar formation during skin wound healing process have not been well studied.
Based on these results, we hypothesized that porcine ADM may prevent scar formation by promoting Notch/Jagged1 signaling pathway in the ESCs. Therefore, we designed the study to determine how Jagged1 can regulate the proliferation and differentiation of ESCs and scar formation, and to examine the effects of porcine ADM treatment on wound healing and scar formation in vivo.
Materials and methods
Animals
Breading pairs of Jag1flox/flox mice and K15-CrePR1 mice were acquired from the Jackson Laboratory (Bar Harbor, ME, USA). Eight-week-old athymic nude mice and C57BL/6 mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). Jag1flox/flox mice were crossed with K15-CrePR1 mice. At about 7.5 week of age, dorsal skin from K15-Jag1 mice was shaved and treated with 200 μL of 10 mg/mL mifepristone (Sigma-Aldrich, MO, USA) diluted in 50% DMSO/50% ethanol, applied topically for 5 days to induce Cre-mediated recombination, based on the previously published methods.19 Three days after the final treatment, mice underwent skin wound experiment. Both male and female mice were used, and all skin wound experiment started when mice reached 8 weeks old. All mice were housed in the Animal Resource Facility under 12 h light/dark cycle (lights on/off at 7 am/7 pm) and had free access to food and water. All procedures and experiments involving animals in the present study were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences 2011). All animal experiments were approved by the Institutional Animal Care and Use Committee of The First People's Hospital of Foshan, China.
Skin wound model
Mice were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine, and the hair on their back was shaved. We then made a circular, full-thickness skin excisions of 10 mm in diameter in the middle of back of the spine. Mice were randomly divided into 2 groups; one group was treated with porcine ADM and the other with Povidone Iodine Cream (Control group) for 14 days. Wound area measurement was performed by digital planimetry using ImageJ software version 2.1.4.6 (NIH, Bethesda, MD). The wound residual rate and scar index were calculated using the formula as described previously.20
Isolation of ESCs
We carefully dissected skin samples from the back of C57BL/6 mice from other tissue. The skin samples were then cut into pieces (approximately 0.3 × 0.3 cm2). After incubation in 0.5% Dispase II (17105041; Gibco, Shanghai, China) in PBS at 4 °C overnight, the epidermal sheets were carefully separated from the dermis and digested in 0.25% trypsin (25200-056; Gibco, Shanghai, China) at 37°C for 20 minutes. The trypsin was inactivated in Dulbecco's modified Eagle's medium (DMEM, 12100-046; Gibco, Shanghai, China) containing 10% FBS. Followed by filtering and centrifuge, the cells were resuspended in keratinocyte serum-free medium (K-SFM, 17005042; Gibco, Shanghai, China) and seeded at a density of 105 cells/cm2 in flasks coated with 100 μg/ml collagen IV (ab6586; Abcam, Shanghai, China) to adhere for 15 minutes at 37°C. The rapidly adhering cells were collected and cultured in K-SFM medium at 37 °C in 5% CO2. When the culture reached 70–80% confluence, the cells were digested and passaged at a ratio of 1:2.21 Meanwhile, the cells were identified to be ESCs with primary monoclonal antibodies against keratin 15 (1:100, ab80522, Abcam, Shanghai, China) and β1 Integrin (1:1000, ab179471, Abcam, Shanghai, China) by immunofluorescence staining.22
Viral production
Mouse-specific Hes1 lentiviral construct (LV-Hes1) was purchased from Abm (LV478905; Abmgood, Shanghai, China). Recombinant lentivirus was produced by transient transfection of HEK-293T cells according to Tronolab protocols. Briefly, subconfluent HEK-293T were co-transfected with 20 μg of transfer vector, 15 μg of packaging plasmid (psPAX2) and 6 μg of envelope plasmid (pMD2.G). After 2 days, supernatant was ultra-centrifuged in Beckman L-70 at 26,000 rpm at 4°C for 2 h and viral pellet resuspended in 100 μl of PBS. Twenty μl of fresh viral suspension was used per infection.
Establishment of ESCs with stable expression of Jagged1
ESCs from C57/BL6 mice were transduced with Jag1 lentiviral constructs as previously described.23 ESCs were infected with lentiviral vector overexpressing Jag1 with GFP signals (over 80% of transfected cells) were selected for further experiments.
Knockdown of Jagged1
siRNA for Jag1 was designed and synthesized by Guangzhou RiboBio. The sequence of the negative control (NC) was also designed by RiboBio. Twelve hours prior to transfection, cells were plated on to a six-well plate (Nest Biotech) at 30–50% confluence. TurboFect siRNA Transfection Reagent (Fermentas) was then used to transfect siRNA into cells according to the manufacturer's protocol. Cells were collected after 48–72 h for further experiments.
Protein extraction and western blot analysis
We extracted total proteins from ESCs or wound skin samples using the ProteoPrep® Total Protein Extraction Kit (PROTTOT-1KT; Sigma-Aldrich, Shanghai, China). Protein concentration was determined by the BCA Assay Kit. After boiling for 10 minutes, equal amounts of protein extract (50 μg) were loaded in 10% SDS-PAGE gels for electrophoresis at 100 V for 2 hours, followed by transferring to PVDF membranes (Millipore, Shanghai, China) at 100 V for 90 minutes. The membranes were then blocked with 5% nonfat milk in TBST (0.1 M, pH = 7.4) and incubated at 4°C overnight with one of the following primary rabbit anti-mouse antibodies: anti-α-SMA (1:1,000, ab32575; Abcam, Shanghai, China), anti-Collagen I (1:1,000, ab34170; Abcam, Shanghai, China), anti-Collagen III (1:1,000, ab7778; Abcam, Shanghai, China), anti-Jagged1 (1:500, ab7771; Abcam, Shanghai, China), anti-Notch1 (1:1000, ab52627; Abcam, Shanghai, China), and anti-Hes1 (1:1,000, ab71559; Abcam, Shanghai, China). After washing with TBS/Tween-20 solution, the membranes were incubated with peroxidase-conjugated secondary antibody IgG (1:2,000, ab6721; Abcam, Shanghai, China). Protein bands were detected by the Odyssey infrared imaging system (LI-COR Biosciences) and analyzed by Image Pro-Plus 6.0 software (Media Cybernetics). Quantitative western blot measurements of target protein were normalized by corresponding measures of GAPDH derived from the same samples in each blot. All experiments were performed in triplicate.
Histological analysis
Skin tissue samples were fixed with formalin, embedded in paraffin, and sectioned at 4 μm thickness. The sections were deparaffinized and stained with hematoxylin and eosin (H&E) and Masson, and were examined under blindfold conditions with standard light microscopy (Olympus, Japan) to observe the skin epidermis, dermis, accessories, inflammation, and scar tissue.
Immunofluorescence analysis
Immunofluorescence staining was performed based on previously published methods.22 In brief, cultured ESCs were fixed with cold methanol at 4°C for 10 minutes. Cells were then permeated with 0.2% Triton X-100 in PBS at room temperature for 10 minutes. After blocking with 10% normal goat serum in PBS for 30 minutes, primary monoclonal antibodies against keratin 15 (1:100, ab80522, Abcam, Shanghai, China) and β1 Integrin (1:1,000, ab179471, Abcam, Shanghai, China) were applied and incubated at room temperature for 2 hours. The sections were then washed with 3% BSA/PBS and incubated with the following secondary antibodies for 1 hour: goat anti-mouse IgG labeled with Alex Fluor 488 (1:200, ab150113; Abcam, Shanghai, China) and goat anti-mouse IgG labeled with Alexa Fluor 594 (1:200, ab150116; Abcam, Shanghai, China), followed by counterstaining Hoechst DNA-binding dye for 5 minutes. Sections were documented with a fluorescence microscope (OLYMPUS, Japan).
Statistical analysis
Data were analyzed with PRISM5.0 software (GraphPad, CA, USA). Values were expressed as the mean ± standard deviation (SD) unless otherwise indicated. Comparisons of expression difference between control and experimental groups were conducted by Student's t test. The differences between multiple groups were compared using one-way analysis of variance (ANOVA), followed by a Bonferroni post-hoc test for pairwise comparisons. P < 0.05 indicates that the difference was statistically significant.
Results
Notch/Jagged1 signaling pathway was enhanced during wound healing
To investigate the role of Notch/Jagged1 signaling pathway in wound healing and scar formation in vivo, we conducted our experiments using a mouse skin wound model to first assess the expression of several key factors of Notch/Jagged1 signaling pathway. We found that skin incision increased the expression of Notch1, Jagged1, and Hes1 in wound tissues during wound healing time as measured by western blot, compared with baseline before skin incision (P < 0.05; Fig. 1).
Figure 1.
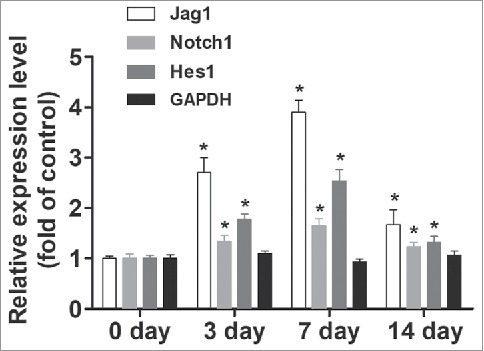
Notch/Jagged1 signaling pathway was enhanced during wound healing. To investigate the role of Notch/Jagged1 signaling pathway in wound healing and scar formation in vivo, we conducted our experiments using a mouse skin wound model to assess the expression of several key factors of Notch/Jagged1 signaling pathway using western blot. N = 10 mice *P < 0.05 compared with day 0.
Jagged 1 inhibited the differentiation of ESCs to MFB in vitro
We first isolated ESCs and found that keratin 15 (K15) and integrin-β1 are good markers of these ESCs (Fig. 2A). We then treated ESCs with Jag1 siRNA or LV-Jag1 to knockdown or increase the expression of Jag1 I ESCs. We incubated ESCs with Jag1 siRNA or scrambled negative control RNAs for 10 days. To determine ESC differentiation to MFB, we examined the expression of α-SMA, Collagen I, and Collagen III, using Western blot. Col I and Col III are metabolites of MFB, and are also valid markers for MFB.24,25 We found that treatment of Jag1 siRNA significantly enhanced the expression of α-SMA, Collagen I, and Collagen III compared with the control group (P < 0.05; Fig. 2B, C, and D). However, when ESCs were pre-transduced with LV-Jag1, the expression of α-SMA, Collagen I, and Collagen III was significantly decreased compared with the control group (P < 0.05; Fig. 2B, C, and D). These results suggested that Jag 1 expression levels in the ESCs are critical for determine the differentiation of ESC to MFB.
Figure 2.
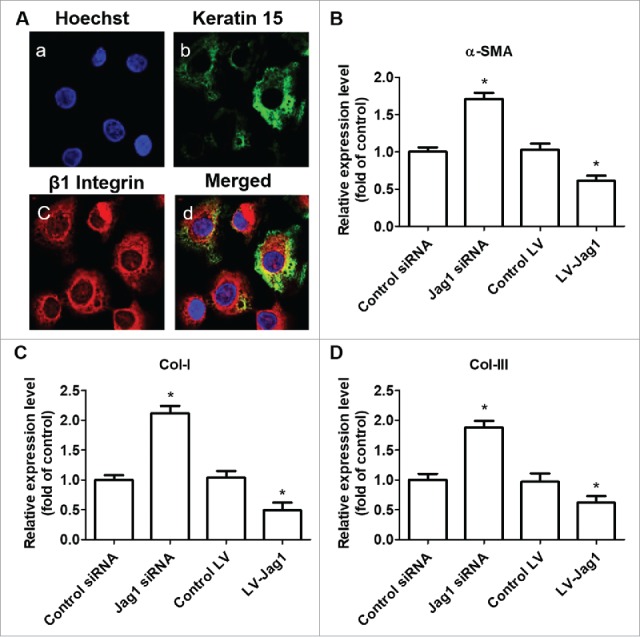
Jagged 1 inhibited the differentiation of ESCs to MFB in vitro. (A) Representative immunofluorescence staining. a) Hoechst counterstaining, b) Keratin 15, c) β1 Integrin, and d) merged image. (B) Western blot analysis of α-SMA expression in ESCs. (C) Western blot analysis of Collagen-I expression in ESCs. (D) Western blot analysis of Collagen-III expression in ESCs. *P < 0.05 compared with control.
ESC-conditional knockout of Jag1 promoted scar formation.
K15 has been demonstrated as biomarker for ESCs. We generated K15-Jag1 mice, and investigate the role of ESC-specific Jag1 in wound healing and scar formation in vivo. We conducted our experiments using the same mouse skin wound model. Meanwhile, we recorded the wound healing time, photographed the wound, measured the wound areas and the thickness of scar tissues, and calculated the residual wound area rate and scar index regularly. We found that K15-Jag1 mice exhibited longer wound healing time (data not shown), and increased skin thickness and scar index (P < 0.05; Fig. 3A), and the residual wound area (P < 0.05; Fig. 3A), compared with control mice (i.e., K15-Cre mice). These results suggested that ESC-specific Jag1 expression after skin wound could increase wound healing and reduce scar formation.
Figure 3.

ESC-conditional knockout of Jag1 promoted scar formation. (A) Representative wound images, (B) Representative H&E staining, (C) Representative Masson staining. N = 10 mice/group.
To further evaluate the quality of wound healing, we observed hair follicle regeneration, re-epithelialization, and the generation and deposition of collagen by H&E and Masson staining. Detailed histopathological analysis of relative sections showed that the neoformative epidermis layer was significantly thinner in K15-Jag1 mice than in control mice, resulting in less cell layers, less epidermal ridges, less formation of primitive hair follicle structures and sebaceous glands, and a less regular and ordered collagen arrangement (Fig. 3B and C).
Taken together, these results suggested that ESC-specific Jag1 expression is critical in the regulation of hair follicle re-formation, re-epithelialization, and the arrangement and deposition of collagen. Lack of ESC-specific Jag1 expression will result in poor-quality wound healing and scar formation.
Porcine ADM treatment accelerated wound closure and reduced scar formation in vivo
To test the effects of porcine ADM on wound healing and scar formation, we used nude mice to avoid possible immune responses to porcine ADM. We recorded the wound healing time, photographed the wound, measured the wound areas and the thickness of scar tissues, and calculated the residual wound area rate and scar index regularly (Fig. 4D and E). We found that porcine ADM treatment reduced wound healing time (data not shown), and reduced skin thickness and scar index (P < 0.05; Fig. 4F), and the residual wound area (P < 0.05; Fig. 4G), compared with control. We also examined the expression of α-SMA, Collagen I, and Collagen III in the wound tissue using western blot analysis. We found that the expression of α-SMA, Col I, and Col III was significantly lower after porcine ADM treatment compared with control (P < 0.05; Fig. 4A, B, and C). Furthermore, porcine ADM treatment resulted in higher levels of expression of Jag1, Notch1, and Hes1 in wound tissues compared with control (P < 0.05; Fig. 5). These results confirmed that porcine ADM treatment could accelerate wound closure and reduce scar formation by inhibiting the differentiation of ESC to MFB.
Figure 4.

Porcine ADM treatment accelerated wound closure and reduced scar formation in vivo. (A) α-SMA expression in wound tissue at day 7. (B) Collagen-I expression in wound tissue at day 7. (C) Collagen-III expression in wound tissue at day 7. (D) Representative wound images. Images were taken after carefully removed porcine ADM. (E) Wound area (%) over time. (F) Scar thickness. (G) Scar index. N = 10 mice/group. (A, B, C) *P < 0.05 compared with day 0, #P < 0.05 compared with VEH. (E, F, G) *P < 0.05 compared with control.
Figure 5.
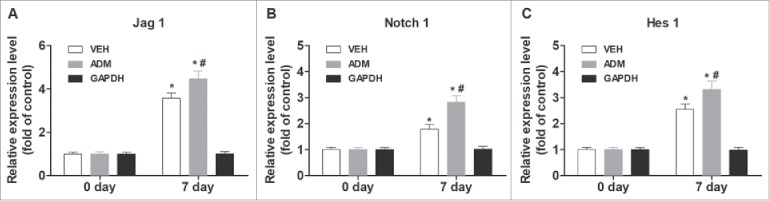
Porcine ADM treatment increased Notch/Jag1 signaling. (A) Jag1 expression in wound tissue at day 7. (B) Notch1 expression in wound tissue at day 7. (C) Hes1 expression in wound tissue at day 7. N = 10 mice/group. *P < 0.05 compared with day 0, #P < 0.05 compared with VEH.
Discussion
The present study was designed to examine the role of Jag1 in ESCs differentiation to MFB and skin wound healing and scar formation and evaluated the effects of porcine ADM treatment on wound healing and scar formation in vivo. Consistent with previous study, we found that Jag1, Notch1 and Hes1 expression is up-regulated in the wound tissue during the period of wound healing. Furthermore, using Western blot analysis, we showed that knockdown of Jag1 expression with Jag1 siRNA increased the percentage of MFB cells, as indicated by MFB biomarkers, including α-SMA Col-1 and Col-III. This phenomenon was reversed in ESCs that has overexpression of Jag1 via lentiviral transduction (LV-Jag1). We further confirmed that ESC-specific knockout of Jag1 delayed wound healing and promoted scar formation in vivo. Specifically, K15-Jag1 mice exhibited less cell layers, less epidermal ridges, less formation of primitive hair follicle structures and sebaceous glands, and a less regular and ordered collagen arrangement during wound healing, suggesting that ESC-specific Jag1 expression is critical in the regulation of hair follicle re-formation, re-epithelialization, the arrangement and deposition of collagen, and scar formation. In addition, we reported that porcine ADM treatment after skin incision could accelerate wound closure and reduce scar formation in vivo. This effect was associated with decreased expression of MFB markers, including α-SMA Col-1 and Col-III in wound tissues. Finally, we confirmed that porcine ADM treatment could increase Jag1, Notch1 and Hesl expression in wound tissues. Taken together, our results suggested that ESC-specific Jag1 expression levels are critical for wound healing and scar formation, and porcine ADM treatment would be beneficial in promoting wound healing and preventing scar formation by enhancing Notch/Jagged1 signaling pathway in ESCs.
In our previous study, we found that pharmacological activation of the Notch1/Jagged1 pathway contributed to promoting ESC proliferation and inhibiting the cells' differentiation.10 However, these manipulations are not specific to ESCs per se. Adding to this literature, the present study first demonstrated that ESC-specific Jag1 expression level is critical for wound healing and scar formation. While in this study, we generated ESC-specific Jag1 conditional knockout mice based on the assumption that K15 is a reliable ESC marker. In fact, the reliability of using K15 as ESC marker is still in debate. Studies have reported the specific localization of K15 in stem cells residing in the bulge,26,27 and all components of skin epithelium.28 K15 positive cells in the mouse epidermal bulge also co-localized with Lgr5 positive cells, which were able to regenerate new hair follicles and maintain all cell lineages of the follicle.29 Additionally, K15 positive bulge cells from human skin also express CD200, another ESC marker.30 However, other studies have also shown that K15 could be expressed in the stem cells as well as in differentiated cells. Therefore, use of this marker on its own may not provide conclusive information about the stem cell population in a tissue. Therefore, future studies will be necessary to use additional ESC markers.
While our study demonstrated the critical role of ESC-specific Jag1 expression in regulation of wound healing and scar formation, skin wound healing involves multiple cellular signaling. Many studies have identified various miRNAs involved in cell differentiation. Specifically, when Jagged1 binds to Notch1 receptors, the Notch intracellular domain (NICD) can be released and translocated to the nucleus and can induce the activation of Hairy/Enhancer of split-1 gene (Hes1), which play a key role in promoting proliferation and inhabiting differentiation of epidermal stem cells.31 Recent studies have shown that miR-203 can suppress Hesl expression.32 miR-203 has potent antiproliferative function and regulates the balance between stem cell proliferation and terminal differentiation in skin cells, by targeting p63 when stem cells in the epidermis are proliferating and differentiating into stratified epithelium.33 miR-203 has the highest level of expression in the skin.34 It induces cell cycle exit and represses “stemness' in epidermal progenitors, suggesting its involvement in keratinocyte differentiation.33,35 Importantly, the expression of miR-203 is drastically enhanced in the wound surrounding tissue.36-39 Therefore, it will be important to evaluate the interactions of miR-203 and Notch1/Jagged1 pathway during wound healing and scar formation.
Previous studies have identified three populations of stem cells in epidermis. These include the interfollicular (IF) ESCs in the epidermal basal layer, the hair follicle (HF) stem cells of the bulge, and the sebaceous gland (SG) stem cells located immediately above the hair bulge as well. Our previous studies have shown that porcine ADM treatment promoted the expression of integrin-β1 and keratin 19, which are informative in defining the stem cell phenotype and have been used as potential markers.40-42 Adding to these findings, the present study demonstrated that porcine ADM treatment accelerated wound healing and inhibited scar formation. This phenomenon was associated with decreased expression of MFB markers and increased expression of Jag1, Notch1, and Hes1. These results indicated that porcine ADM treatment was effective in inhibiting ESCs differentiation to MFB in wound healing.
In addition to Notch/Jagged1 signaling pathway, several other molecules may be also involved in regulation of wound healing and scar formation. The proliferation and activation of pathological scar fibroblasts are regulated and controlled by various cytokines, among which transforming growth factor-β1 (TGF-β1)/Smad3 pathway has been widely accepted to be a major factor leading to scar formation.43 Therefore, a reasonable hypothesis is that porcine ADM treatment may suppress TGF-β1/Smad3 pathway to inhibit scar formation. Hence, future studies will be necessary to evaluate the effects of porcine ADM treatment on many other factors that are involved in regulation of wound healing and scar formation comprehensively. This line of research will be not only important for understanding the mechanism of scar formation, but also critical for identification of effective therapeutic target that can promote wound healing process without scar formation.
Funding Statement
This work was supported by the National Natural Science Foundation of China (NSFC) (81671970). National Science Foundation of Guangdong Province (2017ZC0466). National Natural Science Foundation of China (NSFC) (81671935). National Natural Science Foundation of China (NSFC) (81772136).
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Acknowledgments
This paper was supported by the National Natural Science Foundation of China (Grant No. 81671970, 81671935, 81772136) and Natural Science Foundation of Guangdong Province, China (Grant No. 2017ZC0466).
REFERENCES
- 1.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283–9. [DOI] [PubMed] [Google Scholar]
- 3.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.xWRR410[pii]. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G-Y, Li X, Chen X-L, Li Z-J, Yu Q, Jiang L-F, Ding J, Gao W-Y. Contribution of epidermal stem cells to hypertrophic scars pathogenesis. Medical hypotheses. 2009;73(3):332–3. [DOI] [PubMed] [Google Scholar]
- 5.Li-Tsang CW, Feng B, Huang L, Liu X, Shu B, Chan YT, Cheung K-K. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns. 2015;41(5):1008–16. [DOI] [PubMed] [Google Scholar]
- 6.Hu MS, Rennert RC, McArdle A, Chung MT, Walmsley GG, Longaker MT, Lorenz HP. The role of stem cells during scarless skin wound healing. Adv Wound Care (New Rochelle). 2014;3(4):304–14. doi: 10.1089/wound.2013.047110.1089/wound.2013.0471[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charruyer A, Ghadially R. What's new in dermatology: epidermal stem cells. Giornale Italiano Di Dermatologia E Venereologia: Organo Ufficiale, Societa Italiana Di Dermatologia E Sifilografia. 2011;146(1):57–67. [PubMed] [Google Scholar]
- 8.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Cai J, Niu F, Zong X, Xu J, Du L, Chen G. Comparison of biological characteristics and quantity of epidermal stem cells from hypertrophic scar skin and normal skin of human beings. Zhonghua Yi Xue Za Zhi. 2014;94(14):1097–100. [PubMed] [Google Scholar]
- 10.Yang R-H, Qi S-H, Shu B, Ruan S-B, Lin Z-P, Lin Y, Shen R, Zhang F-G, Chen X-D, Xie J-L. Epidermal stem cells (ESCs) accelerate diabetic wound healing via the Notch signalling pathway. Biosci Reports. 2016;36(4):e00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Sato C, Cerletti M, Wagers A. Chapter twelve-notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Topics Dev Biol. 2010;92:367–409. [DOI] [PubMed] [Google Scholar]
- 12.Shu B, Yang R, Shi Y, Xu Y, Liu J. Notch1 Signaling Regulates Wound Healing via Changing the Characteristics of Epidermal Stem Cells. J Stem Cell Res Ther. 2016;6(348):2. [Google Scholar]
- 13.Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z, Chen L, Zhao J, Liu X, Qi S. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res Therapy. 2017;8(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava A, DeSagun EZ, Jennings LJ, Sethi S, Phuangsab A, Hanumadass M, Reyes HM, Walter RJ. Use of porcine acellular dermal matrix as a dermal substitute in rats. Annals Of Surg. 2001;233(3):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z, Chai J, Yang H, Liu Q, Xu M, Yin H. Acellular porcine dermal matrix produced with different methods and an experimental study on its transplantation to skin wound. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue = Chinese Critical Care Medicine = Zhongguo Weizhongbing Jijiuyixue. 2005;17(2):92–4. [PubMed] [Google Scholar]
- 16.Feng X, Shen R, Tan J, Chen X, Pan Y, Ruan S, Zhang F, Lin Z, Zeng Y, Wang X. The study of inhibiting systematic inflammatory response syndrome by applying xenogenic (porcine) acellular dermal matrix on second-degree burns. Burns. 2007;33(4):477–9. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Tan J, Pan Y, Wu Q, Ruan S, Shen R, Chen X, Du Y. Control of hypertrophic scar from inception by using xenogenic (porcine) acellular dermal matrix (ADM) to cover deep second degree burn. Burns. 2006;32(3):293–8. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Shi Y, Shu B, Xie X, Yang R, Zhang L, Ruan S, Lin Y, Lin Z, Shen R. The effect of porcine ADM to improve the burn wound healing. Int J Clin Exp Pathol. 2013;6(11):2280. [PMC free article] [PubMed] [Google Scholar]
- 19.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc Natl Acad Sci U S A. 2011;108(10):4093–8. doi: 10.1073/pnas.10130981081013098108[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, Chen L, Qi S, Liu X, Wang P. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Therapy. 2015;6(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiisi S, Esmaeili F, Shirazi A. Isolation, culture and identification of epidermal stem cells from newborn mouse skin. Vitro Cell Dev Biol Animal. 2010;46(1):54–9. [DOI] [PubMed] [Google Scholar]
- 22.Eckert RL, Adhikary G, Balasubramanian S, Rorke EA, Vemuri MC, Boucher SE, Bickenbach JR, Kerr C. Biochemistry of epidermal stem cells. Biochim Et Biophys Acta (BBA)-General Subjects. 2013;1830(2):2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, et al. . Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008;27(13):1886–95. doi: 10.1038/emboj.2008.113emboj2008113[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Su D, Li S, Gao L. The expression of alpha-smooth muscle actin in primary cultural fibroblasts of rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chinese J Applied Physiol. 2009;25(3):339–43. [PubMed] [Google Scholar]
- 25.Al-Qattan MM, Abd-Elwahed MM, Hawary K, Arafah MM, Shier MK. Myofibroblast expression in skin wounds is enhanced by collagen III suppression. BioMed Res Int. 2015;2015:958695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waseem A, Dogan B, Tidman N, Alam Y, Purkis P, Jackson S, Lalli A, Machesney M, Leigh IM. Keratin 15 expression in stratified epithelia: downregulation in activated keratinocytes. J Invest Dermatol. 1999;112(3):362–9. doi: 10.1046/j.1523-1747.1999.00535.xS0022-202X(15)40426-9[pii]. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121(5):963–8. [DOI] [PubMed] [Google Scholar]
- 28.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411–7. doi: 10.1038/nbt950nbt950[pii]. [DOI] [PubMed] [Google Scholar]
- 29.Jaks V, Barker N, Kasper M, Van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genetics. 2008;40(11):1291–9. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Suga H, Eto H, Araki J, Inoue K, Yoshimura K, Aoi N, Sato T, Yamauchi Y. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest. 2009;89(8):844. [DOI] [PubMed] [Google Scholar]
- 31.Yeo S-Y, Chitnis AB. Jagged-mediated Notch signaling maintains proliferating neural progenitors and regulates cell diversity in the ventral spinal cord. Proc Natl Acad Sci. 2007;104(14):5913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diao Y, Guo X, Jiang L, Wang G, Zhang C, Wan J, Jin Y, Wu Z. miR-203, a tumor suppressor frequently down-regulated by promoter hypermethylation in rhabdomyosarcoma. J Biol Chem. 2014;289(1):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452(7184):225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PloS One. 2007;2(7):e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight R, Melino G, Candi E. miR-203 represses stemness by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–95. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Xu Y, Shu B, Wang P, Tang J, Chen L, Qi S, Liu X, Xie J. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. Int J Clin Exp Pathol. 2015;8(10):13416–20. [PMC free article] [PubMed] [Google Scholar]
- 37.Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, Kirsner RS, Jimenez JJ, Leslie C, Tomic-Canic M. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287(35):29324–35. doi: 10.1074/jbc.M112.382135M112.382135[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell. 2011;8(3):294–308. doi: 10.1016/j.stem.2011.01.014S1934-5909(11)00015-4[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi R, Fuchs E. A miR image of stem cells and their lineages. Curr Topics Dev Biol. 2012;99:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel M, Torok N, Godbout M-J, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109(5):1017–28. [DOI] [PubMed] [Google Scholar]
- 41.Fu X, Sun X, Li X, Sheng Z. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358(9287):1067–8. [DOI] [PubMed] [Google Scholar]
- 42.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80(1):83–93. [DOI] [PubMed] [Google Scholar]
- 43.Zunwen L, Shizhen Z, Dewu L, Yungui M, Pu N. Effect of tetrandrine on the TGF-β-induced smad signal transduction pathway in human hypertrophic scar fibroblasts in vitro. Burns. 2012;38(3):404–13. [DOI] [PubMed] [Google Scholar]


