Abstract
Gemcitabine is the first-line treatment for pancreatic ductual adenocarcinoma (PDAC) as well as acts against a wide range of other solid tumors. Patients usually have a good initial response to gemcitabine-based chemotherapy but would eventually develop resistance. To improve survival and prognosis of cancer patients, better understanding of the mechanisms responsible for gemcitabine resistance and discovery of new therapeutic strategies are in great need. Amounting evidence indicate that the developmental pathways, such as Hedgehog (Hh), Wnt and Notch, become reactivated in gemcitabine-resistant cancer cells. Thus, the strategies for targeting these pathways may sensitize cancer cells to gemcitabine treatment. In this review, we will summarize recent development in this area of research and discuss strategies to overcome gemcitabine resistance. Given the cross-talk between these three developmental signaling pathways, designing clinical trials using a cocktail of inhibitory agents targeting all these pathways may be more effective. Ultimately, our hope is that targeting these developmental pathways may be an effective way to improve the gemcitabine treatment outcome in cancer patients.
Keywords: Cancer therapy, Gemcitabine resistance, Hedgehog, Notch, Wnt
Introduction
Gemcitabine (2′,2′-difluoro-2′-deoxycytidine; dFdC)1 is a novel deoxycytidine analogue. Despite of some similarities with other nucleoside analogues such as cytosine arabinoside (AraC), gemcitabine has several unique properties and specific spectrum of activity.2, 3 The cytotoxic lesions caused by gemcitabine include killing cells with active DNA synthesis (S phase) and blocking cell cycle progression at the G1/S phase boundary.4 Gemcitabine was originally used for its antiviral effects but is now widely used as an anti-cancer chemotherapeutic agent. Gemcitabine is recommended as a single agent for first-line chemotherapy for patients with advanced pancreatic cancer.5 It is also used for chemotherapy for patients with non-small cell lung cancer,6 breast cancer,7 bladder cancer8 and ovarian cancer.9
Patients usually have a good initial response to gemcitabine-based chemotherapy but develop resistance by time. Gemcitabine resistance can be either intrinsic or acquired. Resistance can result from molecular and cellular changes, including nucleotide metabolism enzymes, inactivation of the apoptosis pathway, high expression of drug efflux pumps, activation of the cancer stem cells or epithelial-to-mesenchymal transition (EMT) pathway, up- or down-regulated expression of microRNA (miRNA) (Fig. 1). It has been demonstrated that pathways such as Hedgehog (Hh), Wnt and Notch, which regulate embryonic development and somatic stem cells (SCs), can be reactivated in gemcitabine resistance cancer cells (Fig. 2). Herein, we will describe recent advances towards targeting these pathways with a goal to overcome gemcitabine resistance.
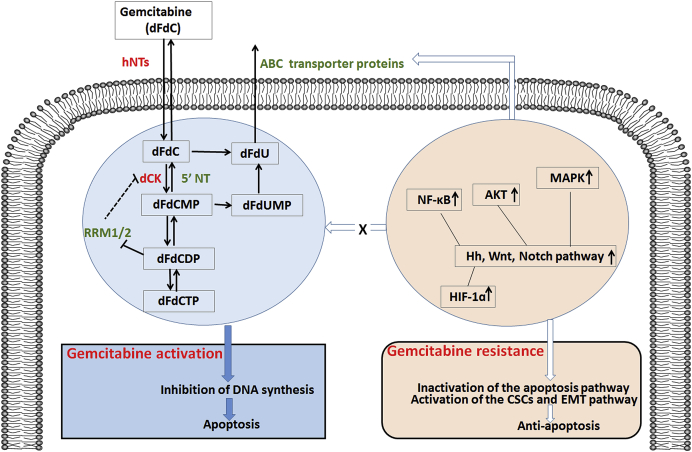
Fig. 1.
A diagram of gemcitabine metabolism and proposed mechanisms of gemcitabine resistance. Gemcitabine (dFdC) is a pro-drug that requires cellular uptake and serial phosphorylation to become pharmacologically active. Gemcitabine can interrupt DNA synthesis and then induce cancer cells apoptosis. Gemcitabine resistance can be either intrinsic or acquired. Resistance can result from molecular and cellular changes, including nucleotide metabolism enzymes, apoptosis pathway, ABC transporter proteins, activation of the CSCs and EMT pathway.
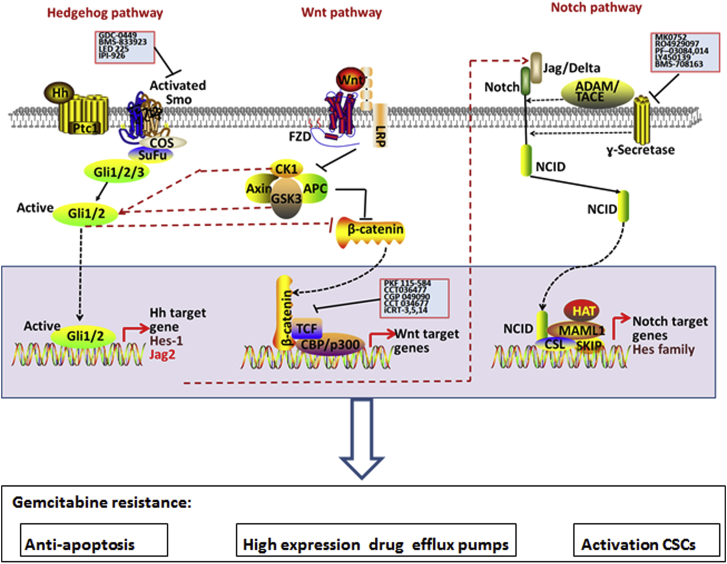
Fig. 2.
Diagrams of the Hedgehog, Wnt, Notch pathways and their roles for gemcitabine resistance. Hedgehog (Hh), Wnt and Notch signaling, which regulate embryonic development and somatic stem cells, may be reactivated in gemcitabine-resistant cancer cells. Elevated expression of the target genes in these pathways may result in inactivation of the apoptosis pathway, high expression of drug efflux pumps or activation of the cancer stem cells (CSCs), ultimately leading to gemcitabine resistance.
Gemcitabine metabolism and mechanism of action
Gemcitabine is transported into cells by sodium-dependent (concentrative nucleoside transporter hCNTs) and sodium-independent (equilibrative nucleoside transporter hENTs) mechanisms.10, 11 Once inside the cell, gemcitabine undergoes a series of phosphorylation by deoxycytidine kinase (dCK) to the monophosphate (dFdCMP) and then by pyrimidine nucleoside monophosphate kinase (UMP–CMP kinase) to give gemcitabine diphosphate (dFdCDP), resulting in formation of gemcitabine triphosphate (dFdCTP) mediated by nucleoside diphosphate kinase (NDPK).12 The first phosphorylation by dCK is considered the rate-limiting step for dFdCDP and dFdCTP production.
Gemcitabine can get inactivated through deamination by cytidine deaminase (CDA), and when in the monophosphate form by deoxycytidylate deaminase (dCTD). Gemcitabine may also become inactivated by dephosphorylation of the monophosphate form by 5′-nucleotidases (5′-NTs), converting nucleotides back to nucleosides. On the other hand, gemcitabine exhibits a unique property called self-potentiation to enhance its activation. The diphosphate form (dFdCDP) inhibits ribonucleotide reductase M1 or M2 (RRM1/RRM2) that convert CDP to dCDP, leading to depletion of dCTP pools and facilitating incorporation of dFdCTP into DNA.13
Known mechanisms of gemcitabine resistance
One mechanism responsible for gemcitabine resistance is the dysregulation of the proteins participating in gemcitabine metabolism pathways, including deficiency of hENT1, down-regulation of rate-limiting enzyme dCK, and up-regulation of RRM1/RRM2.14, 15 Hu antigen R (HuR), which is an RNA-binding protein that post-transcriptionally regulates dCK, is also associated with gemcitabine efficacy. High levels of HuR correlated with a better overall survival in gemcitabine-treated patients.16
Another gemcitabine resistance mechanism is high expression of drug efflux pumps, such as (ABC) transporter family proteins.17 These proteins are highly expressed in cancer stem cells (CSCs) and have been shown to protect CSCs from chemotherapeutic agents.18 Recent evidence has supported the link of gemcitabine resistance and EMT with stem cell phenotype.19 In general, therapeutics directed against CSCs and EMT offer new strategies to chemoresistance.
Gemcitabine resistance is also associated with multiple genetic and epigenetic abnormalities. Changes in one or a few genes remain crucial for maintaining drug resistance cell survival and malignant phenotype. There is evidence to indicate that NF-κB,20, 21, 22, 23 AKT,20, 24, 25 MAPK,25, 26 HIF-1α27pathways are involved in gemcitabine resistance in vitro and some in vivo models (Fig. 1). However, the functional significance of these molecules for gemcitabine resistance remains largely untested.
Relevance of hedgehog signaling to gemcitabine resistance
As an essential developmental signaling pathway, the Hh pathway is critical for maintaining tissue polarity and stem cell population. Hedgehog signaling molecules in mammal include three ligands (Sonic hedgehog-Shh, Indian hedgehog-Ihh and Desert hedgehog-Dhh), two receptors (Patched1-Ptc1, Patched2-Ptc2), a key signal transducer smoothened (Smo) and three transcription factors (Gli1, Gli2, Gli3). When ligands are not present, Smo function is inhibited by another transmembrane protein (Ptc1, Ptc2). Upon binding of an active Hh ligand, this inhibitory effect is lifted, allowing Smo to signal downstream, eventually leading to active transcription of Gli molecules through binding the specific consensus sequences located in the promoter region of target genes.28, 29 Besides these canonical Hh pathways, there are non-canonical Hh pathways activated directly through Ptc1 or Smo, or other pathways including the PI3K/AKT and MEK signaling cascades.30 Dysregulated signaling through the Hh pathway is implicated in virtually all human cancers. Furthermore, Hh pathway is now also recognized as a major driver of resistance to a number of chemotherapeutical reagents. Smo inhibitors, including vismodegib (GDC-0449), sonidegib (LDE225), BMS-833923, PF-04449913, and LY2940680, are being tested in a range of advanced cancers (NCI Clinical Trail Database (http://www.clinicaltrials.gov/)). To our disappointment, however, the Smo inhibitors seem to be ineffective in most extracutaneous solid tumors.31 Therefore, Smo inhibition alone may not provide sufficient efficacy in these cancers, and combinations with other conventional cytotoxic anti-tumor agents might be needed to achieve maximal benefit. Currently, several Phase II trials of hedgehog pathway inhibitor vismodegib and Phase I trials of LDE225 in combination with gemcitabine are ongoing (http://www.clinicaltrials.gov/).
Activation of Hh signaling may not work alone because cross-talk with other signaling pathways plays critical roles in gemcitabine resistance. For example, MAP3K10 over-expression can result in up-regulation of Gli1 and Gli2, leading to decrease in the gemcitabine sensitivity of in pancreatic cancer cells.32 Perifosine, a novel Akt inhibitor, through suppressing Gli1 activation, can enhance gemcitabine-induced cytotoxicity in pancreatic cancer cells.33 Lithium synergistically enhances the anti-cancer effect of gemcitabine, by inhibiting the activity of glycogen synthase kinase 3β (GSK3β), therefore promoting the ubiquitin-dependent proteasome degradation of Gli1.34 ABCB2, the drug efflux transporters, serves as the downstream target of Shh signaling, and its expression can increase gemcitabine resistance in PDAC cells.35 Understanding how integration of HH signaling with other pathways and discovery and synthesis of Gli specific inhibitors is in great need for effective suppression of non-canonical Hh signaling in cancer.
Recent reports have also indicated that hedgehog signaling may be critical for maintaining a small cell population with stem cell properties, and thus conferring resistance to chemotherapy. There is evidence to indicate that Hh signaling regulates expression of several cancer stem cell-related markers, such as ALDH1, Bmi1, Snail, Wnt2, PDGFRα, Jagged-1, CD44 and c-MET.36, 37, 38, 39, 40, 41, 42 The level of Hh expression is often higher in the cancer stem cell population.43, 44, 45, 46 Thus, we have reasons to believe that inhibition of Hh signaling may be effective in reducing the number of cancer stem cells, which may play an important role for chemotherapy and radiotherapy resistance. Chloroquine treatment in combination with gemcitabine significantly decreased CSCs, via inhibition of hedgehog signaling in the stroma, which supports CSCs and non-CSCs via a paracrine mechanism.47 Arsenictrioxide (ATO) is described as a SHH inhibitor. Combination treatment of ATO and low dose gemcitabine inhibits tumor growth, decreases the expression of CD24, CD44, and aldehyde dehydrogenase 1 family member A1 significantly in mouse model.48 In response to gemcitabine, both Shh and CSCs markers as well as EMT regulators are over-expressed, indicating a protective role of hedgehog signaling against chemotherapeutic drugs.19 Discovery of a more specific and complete set of cell surface markers may help to further characterize CSCs, and the role that Hh pathway played in drug resistance and the in-depth mechanism require further systemic studies.
The role of Wnt signaling for gemcitabine resistance
The Wnt signaling pathway controls and regulates crucial aspects of development, cell proliferation, survival and polarity.49 Wnts are a family of 19 secreted glycoproteins, binding to a number of 7-transmembrane receptors of the Frizzled (FZD) family.50 These receptors transduce signal to several intracellular proteins, such as Dishevelled (Dsh), glycogen synthase kinase-3β (GSK-3), Axin, Adenomatous Polyposis Coli (APC), and β-Catenin. To date, major signaling branches have been identified including the canonical Wnt/β-Catenin pathway, the non-canonical planar cell polarity pathway, and the non-canonical Wnt/calcium pathway. These pathways are being actively dissected at the molecular and biochemical levels. When FZD cooperates with LRP5/6 (low-density lipoprotein receptor-related proteins 5 and 6), the canonical Wnt/β-Catenin signaling pathway is activated.51 In the absence of the activating Wnt signals, β-Catenin is phosphorylated by a complex of GSK-3β, proteins APC, β-TRCP and Axin. Non-canonical Wnt signaling can phosphorylate small G-proteins and JNK to transmit the signal to the nucleus, or release calcium affecting Nemo-like kinase (NLK) or nuclear factor of activated T-cells (NFAT).52
Emerging evidence indicates that activation of the Wnt pathway is involved in cancer development and drug resistance. Wnt/β-Catenin signaling is activated in gemcitabine-resistant lung cancer cell lines.53 WNT5A expression leads to resistance to gemcitabine-induced apoptosis in a xenograft model of pancreatic ductal adenocarcinoma (PDAC).54 The heparan sulfate mimetic PG545 in combination with gemcitabine exerts anti-tumor activity by disrupting Wnt/β-Catenin signaling.55 Masitinib, a tyrosine kinase inhibitor, can sensitize gemcitabine treatment through down-regulation of the Wnt/β-Catenin signaling pathway in pancreatic cancer cell lines, which is further confirmed in a Phase II clinical study.56 These therapeutic approaches to target upstream or downstream of Wnt signaling pathway indicate that combination therapy may be required to effectively treat gemcitabine-resistant malignancies.
Recent reports have implicated Wnt pathway may be especially important in maintaining the subpopulation of cancer cells with stem cell properties as well as conferring resistance to chemotherapies. Wnt signaling is increased in pancreatic cancer side population (SP) cells. Furthermore, when cultured with increasing concentrations of gemcitabine, the proportion of SP cells were significantly enriched.57 EMT has been implicated as a drug resistance mechanism, allowing tumor cells to escape from cytotoxic and pathway targeted therapies. Activation of Wnt signaling up-regulates the EMT activator ZEB1 and determines response to gemcitabine in mantle cell lymphoma.58 It will be important for future research to unravel the interconnectedness of Wnt pathway with CSCs and EMT in gemcitabine resistance.
The Notch pathway regulates cancer stem cells in gemcitabine resistance
It has been well known that Notch signaling plays important roles for regulating cell–cell communication, cell proliferation, differentiation and apoptosis. Activation of Notch receptors (Notch-1–4) is often achieved by canonical ligands, Delta-like ligands (DLLs) 1, 3 and 4, and Jagged ligands 1 and 2. The binding of ligand–receptor results in γ-secretase-mediated cleavage and nuclear localization of its intracellular domain to regulate notch-specific gene expression.59
Evidence suggests that Notch signaling may play a crucial role in the tumor progression and possibly therapy-resistance.60, 61 Notch-2 and Jagged-1 are highly up-regulated in gemcitabine-resistant pancreatic cancer cells.62 Notch-3 mRNA expression is predicted to be the key predictive biomarker for gemcitabine effect and sensitivity in pre-treatment endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) samples from patients with PDC.63 Inhibition of Notch-1 by siRNA enhances the sensitivity to gemcitabine in pancreatic cancer through activating apoptosis activity.64 Midkine (MK)–Notch-2 interaction increases Notch signaling, elevates EMT, and is associated with gemcitabine resistance.65 Moreover, inhibition of Notch-3 enhances the sensitivity to gemcitabine in pancreatic cancer through inactivation of the PI3K/Akt-dependent pathway.66 The γ-secretase inhibitors DAPT and MRK003 effectively inhibit intra-tumoral Notch signaling in PDA, leading to a remarkable increase in chemosensitivity to gemcitabine.67, 68
Emerging evidence clearly shows that abnormal Notch signaling may contribute to carcinogenesis by deregulating the self-renewal of normal stem cells. Inappropriate activation of Notch signaling could be an early event leading to accumulation of undifferentiated precursor cells in pancreatic cancers.69 MRK-003, γ-secretase inhibitor, treatment results in down-regulation of nuclear Notch-1 intracellular domain, and a decrease in tumor-initiating cells that are capable of extensive self-renewal.70 Furthermore, the proportion of CD24+CD44+ CSCs in pancreatic cancer cells line increased after gemcitabine treatment and this increase was mediated by the Notch pathway.71 A Notch signaling pathway inhibitor (L1790) and Hes-1 siRNA are shown to reverse the chemoresistance induced by PSCs.72 Neutralizing antibodies against human DLL4 reduces the percentage of CSC in gemcitabine-resistant mouse model.73 Through down-regulation of Notch-1, Sulforaphane (SF) prevented gemcitabine-induced selection of ALDH-positive cells.74 As indicated above, Notch signaling may be a promising target to overcome gemcitabine resistance.
Cross-talk among signaling pathways
Cross-talks among Hh, Notch or Wnt signaling pathways have been reported in a variety of cancer cells. The transcription of Jagged-2, a Notch ligand, can be up-regulated in response to Hh pathway activation.75 Whereas Wnt/β-Catenin can activate Jagged-1,76 Wnt-1 induces transformation and tumorigenesis in human mammary epithelial cells, through up-regulation of Notch pathway. Notch and Hh signaling both can activate Hes-1.77 On the other hand, Hh signaling is found to inhibit Wnt signaling via up-regulation of Wnt-inhibitory factor sFRP1, and to suppress β-Catenin transcriptional activity.78 CK1 and GSK3, the core component of the Wnt pathway, regulate Gli1 proteolytic processing.79 Therefore, combination therapies targeting more than one pathway in cancer may be more effective. Hh may modulate the tumorigenic property in cooperation with Notch in breast cancer. Phase I and Phase Ib/II trials, combining Hh pathway inhibitor vismodegib and Notch pathway inhibitor RO4929097 are being conducted in patients with breast cancer and sarcoma (http://www.clinicaltrials.gov).
Conclusions and future directions
Gemcitabine still has clinical potential to treat a broad spectrum of human cancers, but resistance is probably the major impediment to achieve satisfactory clinical outcomes. As evident in the numerous studies, targeting developmental pathways is quite promising to improve gemcitabine efficacy in cancer (Table 1).
Table 1.
Summary of combination therapies and their mechanism in regulation gemcitabine resistance.
| Pathway | Target | Compound | Mechanism of action |
|---|---|---|---|
| Hh | Smo | Vismodegib, LDE225 | Phase II trials of vismodegib and Phase I trials of LDE225 in combination with gemcitabine are ongoing (http://www.clinicaltrials.gov/). |
| Chloroquine | Chloroquine treatment in combination with gemcitabine significantly decreased CSCs, via inhibition of hedgehog signaling in the stroma.47 | ||
| Gli1 | Perifosine | Perifosine, an Akt inhibitor, through suppressing Gli1 activation, can enhance gemcitabine-induced cytotoxicity in pancreatic cancer cells33 | |
| Lithium | By inhibiting the activity of glycogen synthase kinase 3β (GSK3β), therefore promoting the ubiquitin-dependent proteasome degradation of Gli1.34 | ||
| SHH | Arsenic trioxide (ATO) | Combination treatment of ATO and low dose gemcitabine inhibits tumor growth, decreases the expression of CD24, CD44, and aldehyde dehydrogenase 1 family member A1 significantly in mouse model.48 | |
| Wnt | β-Catenin | PG545 | PG545 in combination with gemcitabine exerts anti-tumor activity by disrupting Wnt/β-Catenin signaling.55 |
| Masitinib | Masitinib, a tyrosine kinase inhibitor, can sensitize gemcitabine treatment through down-regulation Wnt/β-Catenin signaling pathway.56 | ||
| Notch | γ-secretase | DAPT, MRK003 | DAPT and MRK003 effectively inhibit intratumoral Notch signaling in PDA, leading to a remarkable decrease in chemosensitivity to gemcitabine67, 68 |
| L1790 | L1790 are shown to reverse the gemcitabine resistance induced by PSCs.72 | ||
| DLL4 | Neutralizing antibodies | Neutralizing antibodies against human DLL4 reduces the percentage of CSC in gemcitabine-resistant mouse model.73 | |
| Notch-1 | Sulforaphane (SF) | Through down-regulation of Notch-1, Sulforaphane (SF) prevented gemcitabine-induced selection of ALDH1 positive cells74 |
There are a number of challenges in overcoming gemcitabine resistance. First, the mechanism of gemcitabine resistance is still largely unknown. Inhibition of a single signaling pathway is unlikely to result in a substantial improvement in gemcitabine resistance, owing to the cross-talk from various pathways. Second, despite exciting data in various tumor and their disease models, it is still too early to know whether any of these therapeutic agents that specifically target the developmental pathway will be efficacious with an acceptable safety profile. Third, further studies are urgently needed to confirm the relevance of these developmental signaling pathways to gemcitabine resistance and to find reasonable combination for therapy. Despite these future challenges, targeting Hh, Notch and Wnt signaling pathways to improve the gemcitabine treatment outcome in cancer patients is clearly the way for future studies.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Current research in my laboratory is supported by grants from the National Cancer Institute CA155086, Riley Children's Foundation, Wells Center for Pediatric Research and Shandong Provincial Natural Science Foundation of China ZR2015HM018. Due to space limit, we could not include many important findings in this review but want to take this opportunity to thank all the investigators in this field for their works.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yanfei Jia, Email: jiayanfei_@126.com.
Jingwu Xie, Email: jinxie@iupui.edu.
References
- 1.Bianchi V., Borella S., Calderazzo F., Ferraro P., Chieco Bianchi L., Reichard P. Inhibition of ribonucleotide reductase by 2'-substituted deoxycytidine analogs: possible application in AIDS treatment. Proc Natl Acad Sci U. S. A. 1994;91:8403–8407. doi: 10.1073/pnas.91.18.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi V., Plunkett W. Modulatory activity of 2',2'-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res. 1990;50:3675–3680. [PubMed] [Google Scholar]
- 3.Hertel L.W., Boder G.B., Kroin J.S. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) Cancer Res. 1990;50:4417–4422. [PubMed] [Google Scholar]
- 4.Huang P., Chubb S., Hertel L.W., Grindey G.B., Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–6117. [PubMed] [Google Scholar]
- 5.Burris H.A., 3rd, Moore M.J., Andersen J. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol – Off J Am Soc Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A.B., Nemunaitis J., Denham C. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol – Off J Am Soc Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 7.Nagourney R.A., Flam M., Link J. Carboplatin plus gemcitabine repeating doublet therapy in recurrent breast cancer. Clin Breast Cancer. 2008;8:432–435. doi: 10.3816/CBC.2008.n.052. [DOI] [PubMed] [Google Scholar]
- 8.von der Maase H., Hansen S.W., Roberts J.T. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 9.Pfisterer J., Plante M., Vergote I. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 10.Rauchwerger D.R., Firby P.S., Hedley D.W., Moore M.J. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000;60:6075–6079. [PubMed] [Google Scholar]
- 11.Spratlin J., Sangha R., Glubrecht D. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 12.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17:v7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 13.Heinemann V., Xu Y.Z., Chubb S. Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res. 1992;52:533–539. [PubMed] [Google Scholar]
- 14.Zhou B.S., Tsai P., Ker R. Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clin Exp Metastasis. 1998;16:43–49. doi: 10.1023/a:1006559901771. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J., Oliveira P., Li X., Chen Z., Bepler G. Modulation of the ribonucleotide reductase-antimetabolite drug interaction in cancer cell lines. J Nucleic Acids. 2010;2010:597098. doi: 10.4061/2010/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costantino C.L., Witkiewicz A.K., Kuwano Y. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69:4567–4572. doi: 10.1158/0008-5472.CAN-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M., Xue X., Wang F. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol Rep. 2012;27:265–269. doi: 10.3892/or.2011.1475. [DOI] [PubMed] [Google Scholar]
- 18.Zinzi L., Contino M., Cantore M., Capparelli E., Leopoldo M., Colabufo N.A. ABC transporters in CSCs membranes as a novel target for treating tumor relapse. Front Pharmacol. 2014;5:163. doi: 10.3389/fphar.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quint K., Tonigold M., Di Fazio P. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int J Oncol. 2012;41:2093–2102. doi: 10.3892/ijo.2012.1648. [DOI] [PubMed] [Google Scholar]
- 20.Arlt A., Gehrz A., Muerkoster S. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 21.Pan X., Arumugam T., Yamamoto T. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res – Off J Am Assoc Cancer Res. 2008;14:8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung H., Kim J.S., Kim W.K. Intracellular annexin A2 regulates NF-kappaB signaling by binding to the p50 subunit: implications for gemcitabine resistance in pancreatic cancer. Cell Death Dis. 2015;6:e1606. doi: 10.1038/cddis.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y., Wang J., Xia N., Li B., Jiang X. Maslinic acid potentiates the antitumor activities of gemcitabine in vitro and in vivo by inhibiting NF-kappaB-mediated survival signaling pathways in human gallbladder cancer cells. Oncol Rep. 2015;33:1683–1690. doi: 10.3892/or.2015.3755. [DOI] [PubMed] [Google Scholar]
- 24.Simon P.O., Jr., McDunn J.E., Kashiwagi H. Targeting AKT with the proapoptotic peptide, TAT-CTMP: a novel strategy for the treatment of human pancreatic adenocarcinoma. Int J Cancer J. 2009;125:942–951. doi: 10.1002/ijc.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trehoux S., Duchene B., Jonckheere N., Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42-44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophysical Res Commun. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Yang X.L., Lin F.J., Guo Y.J., Shao Z.M., Ou Z.L. Gemcitabine resistance in breast cancer cells regulated by PI3K/AKT-mediated cellular proliferation exerts negative feedback via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 2014;7:1033–1042. doi: 10.2147/OTT.S63145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R., Cheng L., Xia J., Wang Z., Wu Q., Wang Z. Gemcitabine resistance is associated with epithelial-mesenchymal transition and induction of HIF-1alpha in pancreatic cancer cells. Curr Cancer Drug Targets. 2014;14:407–417. doi: 10.2174/1568009614666140226114015. [DOI] [PubMed] [Google Scholar]
- 28.Kinzler K.W., Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10:634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki H., Hui C., Nakafuku M., Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 31.LoRusso P.M., Rudin C.M., Reddy J.C. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res – Off J Am Assoc Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An Y., Cai B., Chen J. MAP3K10 promotes the proliferation and decreases the sensitivity of pancreatic cancer cells to gemcitabine by upregulating Gli-1 and Gli-2. Cancer Lett. 2013;329:228–235. doi: 10.1016/j.canlet.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Xin Y., Shen X.D., Cheng L., Hong D.F., Chen B. Perifosine inhibits S6K1-Gli1 signaling and enhances gemcitabine-induced anti-pancreatic cancer efficiency. Cancer Chemother Pharmacol. 2014;73:711–719. doi: 10.1007/s00280-014-2397-9. [DOI] [PubMed] [Google Scholar]
- 34.Peng Z., Ji Z., Mei F., Lu M., Ou Y., Cheng X. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS One. 2013;8:e61457. doi: 10.1371/journal.pone.0061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M., Li L., Liu Z. ABCB2 (TAP1) as the downstream target of SHH signaling enhances pancreatic ductal adenocarcinoma drug resistance. Cancer Lett. 2013;333:152–158. doi: 10.1016/j.canlet.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Gu D., Liu H., Su G.H. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038–1048. doi: 10.1158/1535-7163.MCT-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaguma S., Kasai K., Hashimoto M., Ikeda H. GLI1 modulates EMT in pancreatic cancer–letter. Cancer Res. 2012;72:3702–3703. doi: 10.1158/0008-5472.CAN-12-0379. [author reply 3704–3705] [DOI] [PubMed] [Google Scholar]
- 38.Liu S., Dontu G., Mantle I.D. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Z., Yue W., Wei B. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takebe N., Harris P.J., Warren R.Q., Ivy S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H., Nakamura M., Kameda C. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24-/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 42.Takahashi T., Kawakami K., Mishima S. Cyclopamine induces eosinophilic differentiation and upregulates CD44 expression in myeloid leukemia cells. Leukemia Res. 2011;35:638–645. doi: 10.1016/j.leukres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Bar E.E., Chaudhry A., Lin A. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C., Heidt D.G., Dalerba P. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 45.Visbal A.P., LaMarca H.L., Villanueva H. Altered differentiation and paracrine stimulation of mammary epithelial cell proliferation by conditionally activated Smoothened. Dev Biol. 2011;352:116–127. doi: 10.1016/j.ydbio.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su W., Meng F., Huang L., Zheng M., Liu W., Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through beta-catenin signaling. Exp Hematol. 2012;40:418–427. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Balic A., Sorensen M.D., Trabulo S.M. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther. 2014;13:1758–1771. doi: 10.1158/1535-7163.MCT-13-0948. [DOI] [PubMed] [Google Scholar]
- 48.Han J.B., Sang F., Chang J.J. Arsenic trioxide inhibits viability of pancreatic cancer stem cells in culture and in a xenograft model via binding to SHH-Gli. Onco Targets Ther. 2013;6:1129–1138. doi: 10.2147/OTT.S49148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 50.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 51.Veeman M.T., Axelrod J.D., Moon R.T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 52.Janikova M., Skarda J. Differentiation pathways in carcinogenesis and in chemo- and radioresistance. Neoplasma. 2012;59:6–17. doi: 10.4149/neo_2012_002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H.H., Zhang Z.Y., Che C.L., Mei Y.F., Shi Y.Z. Array analysis for potential biomarker of gemcitabine identification in non-small cell lung cancer cell lines. Int J Clin Exp Pathology. 2013;6:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 54.Griesmann H., Ripka S., Pralle M. WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic cancer. Neoplasia. 2013;15:11–22. doi: 10.1593/neo.121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung D.B., Yun M., Kim E.O. The heparan sulfate mimetic PG545 interferes with Wnt/beta-catenin signaling and significantly suppresses pancreatic tumorigenesis alone and in combination with gemcitabine. Oncotarget. 2015;6:4992–5004. doi: 10.18632/oncotarget.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humbert M., Casteran N., Letard S. Masitinib combined with standard gemcitabine chemotherapy: in vitro and in vivo studies in human pancreatic tumour cell lines and ectopic mouse model. PLoS One. 2010;5:e9430. doi: 10.1371/journal.pone.0009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niess H., Camaj P., Renner A. Side population cells of pancreatic cancer show characteristics of cancer stem cells responsible for resistance and metastasis. Target Oncol. 2015;10:215–227. doi: 10.1007/s11523-014-0323-z. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Tillo E., Fanlo L., Siles L. The EMT activator ZEB1 promotes tumor growth and determines differential response to chemotherapy in mantle cell lymphoma. Cell Death Differ. 2014;21:247–257. doi: 10.1038/cdd.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiuza U.M., Arias A.M. Cell and molecular biology of Notch. J Endocrinol. 2007;194:459–474. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 60.Meng R.D., Shelton C.C., Li Y.M. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69:573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Li Y., Ahmad A. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochimica Biophysica Acta. 2010;1806:258–267. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z., Li Y., Kong D. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eto K., Kawakami H., Kuwatani M. Human equilibrative nucleoside transporter 1 and Notch3 can predict gemcitabine effects in patients with unresectable pancreatic cancer. Br J Cancer. 2013;108:1488–1494. doi: 10.1038/bjc.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du X., Wang Y.H., Wang Z.Q. Down-regulation of Notch1 by small interfering RNA enhances chemosensitivity to gemcitabine in pancreatic cancer cells through activating apoptosis activity. J Zhejiang Univ Med Sci. 2014;43:313–318. doi: 10.3785/j.issn.1008-9292.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Gungor C., Zander H., Effenberger K.E. Notch signaling activated by replication stress-induced expression of midkine drives epithelial-mesenchymal transition and chemoresistance in pancreatic cancer. Cancer Res. 2011;71:5009–5019. doi: 10.1158/0008-5472.CAN-11-0036. [DOI] [PubMed] [Google Scholar]
- 66.Yao J., Qian C. Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol. 2010;27:1017–1022. doi: 10.1007/s12032-009-9326-5. [DOI] [PubMed] [Google Scholar]
- 67.Cook N., Frese K.K., Bapiro T.E. Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J Exp Med. 2012;209:437–444. doi: 10.1084/jem.20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du X., Zhao Y.P., Zhang T.P. Alteration of the intrinsic apoptosis pathway is involved in Notch-induced chemoresistance to gemcitabine in pancreatic cancer. Archives Med Res. 2014;45:15–20. doi: 10.1016/j.arcmed.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Lomberk G., Urrutia R. Primers on molecular pathways–notch. Pancreatol – Off J Int Assoc Pancreatol. 2008;8:103–104. doi: 10.1159/000123603. [DOI] [PubMed] [Google Scholar]
- 70.Mizuma M., Rasheed Z.A., Yabuuchi S. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol Cancer Ther. 2012;11:1999–2009. doi: 10.1158/1535-7163.MCT-12-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J.Y., Song S.Y., Park J.Y. Notch pathway activation is associated with pancreatic cancer treatment failure. Pancreatology. 2014;14:48–53. doi: 10.1016/j.pan.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Cao F., Li J., Sun H., Liu S., Cui Y., Li F. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol Rep. 2015;33:1883–1889. doi: 10.3892/or.2015.3789. [DOI] [PubMed] [Google Scholar]
- 73.Yen W.C., Fischer M.M., Hynes M. Anti-DLL4 has broad spectrum activity in pancreatic cancer dependent on targeting DLL4-Notch signaling in both tumor and vasculature cells. Clin Cancer Res. 2012;18:5374–5386. doi: 10.1158/1078-0432.CCR-12-0736. [DOI] [PubMed] [Google Scholar]
- 74.Kallifatidis G., Labsch S., Rausch V. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19:188–195. doi: 10.1038/mt.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rabadan M.A., Cayuso J., Le Dreau G. Jagged2 controls the generation of motor neuron and oligodendrocyte progenitors in the ventral spinal cord. Cell Death Differ. 2012;19:209–219. doi: 10.1038/cdd.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X., Stoeck A., Lee S.J., Shih Ie M., Wang M.M., Wang T.L. Jagged1 expression regulated by Notch3 and Wnt/beta-catenin signaling pathways in ovarian cancer. Oncotarget. 2010;1:210–218. doi: 10.18632/oncotarget.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schreck K.C., Taylor P., Marchionni L. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He J., Sheng T., Stelter A.A. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 79.Ramaswamy B., Lu Y., Teng K.Y. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72:5048–5059. doi: 10.1158/0008-5472.CAN-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]