Abstract
Temozolomide (TMZ) is an oral alkylating agent used to treat glioblastoma multiforme (GBM) and astrocytomas. However, at least 50% of TMZ treated patients do not respond to TMZ. This is due primarily to the over-expression of O6-methylguanine methyltransferase (MGMT) and/or lack of a DNA repair pathway in GBM cells. Multiple GBM cell lines are known to contain TMZ resistant cells and several acquired TMZ resistant GBM cell lines have been developed for use in experiments designed to define the mechanism of TMZ resistance and the testing of potential therapeutics. However, the characteristics of intrinsic and adaptive TMZ resistant GBM cells have not been systemically compared. This article reviews the characteristics and mechanisms of TMZ resistance in natural and adapted TMZ resistant GBM cell lines. It also summarizes potential treatment options for TMZ resistant GBMs.
Keywords: Adaptive, Glioblastoma, Intrinsic, Resistance, Temodar, Temozolomide
Abbreviations: AGT (also known as MGMT), O6-methylguanine-DNA alkyltransferase; APE1, apurinic/apyrimidine endonuclease/redox factor-1; APNG, Alkylpurine-DNA-N-glycosylase; AP-1, activator protein 1; BBB, blood-brain-barrier; BCRP1, breast cancer resistance protein 1; BER, base excision repair; BG, benzylguanine; C8orf4, Chromosome 8 open reading frame 4; EGFR, epidermal growth factor receptor; ERK1/2, Extracellular Signal Regulated Kinases 1 and 2; FDA, Food and Drug Administration; GBM, glioblastoma multiforme or glioblastoma; HDAC, histone deacetylase; IFN-β, Interferon-β; JNK, Jun N-terminal kinase; KDM, Histone lysine demethylase; LC50, 50% cell death concentration; LIF, Leukemia inhibitory factor; MGMT, O6-methylguanine methyltransferase; MSH6, mutS homolog 6; NHA, normal human astrocytes; MMR, DNA mismatch repair; MTIC, 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide; mTOR, mammalian target of rapamycin; NAMPT, nicotinamide phosphoribosyl transferase; NF-κB, nuclear factor-Kappa B; PARP, poly ADP ribose polymerase; SAHA, N-hydroxy-N′-phenyl-octanediamide; STAT3, Signal Transducer and Activator of Transcription 3; TMZ, Temozolomide; TNFAIP3, Tumor necrosis factor-α-induced protein 3; VPA, Valproic acid
What is Temozolomide?
Temozolomide (TMZ) is an imidazotetrazine derivative of the alkylating agent dacarbazine and a prodrug of the anti-cancer drug Temodar.1 The chemical name of TMZ is 3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide (Fig. 1). TMZ is stable at a pH less than 5 but at a pH greater than 7 it is rapidly hydrolyzed to 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC).2 The lipophilic nature of TMZ permits it to penetrate the blood-brain-barrier (BBB) allowing it to be administered orally.
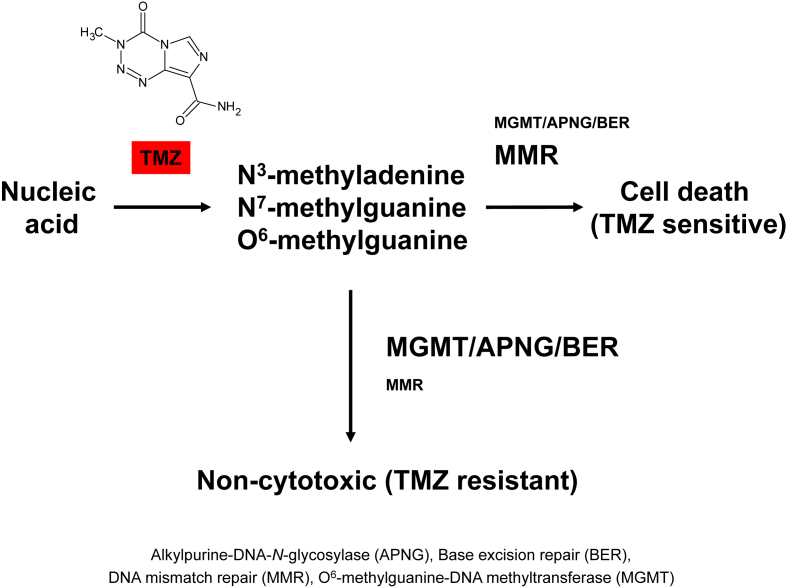
Fig. 1.
Mechanism of Temozolomide and Temozolomide resistance. Temozolomide (TMZ) modifies DNA or RNA at N7 and O6 sites on guanine and the N3 on adenine by the addition of methyl groups. The methylated sites can remain mutated, be fixed by DNA mismatch repair (MMR), be removed by base excision repair (BER) by the action of a DNA glycosylase such as, alkylpurine-DNA-N-glycosylase (APNG), or deakylated by the action of a demethylating enzyme such as O6-methylguanine methyltransferase (MGMT). Cells are TMZ sensitive when MMR is expressed and active. When MGMT, APNG, and BER proteins are expressed, GBM cells are resistant to TMZ.
Use of Temozolomide (sold as Temodar) to treat brain tumors
TMZ is active against human cancers such as melanomas and astrocytomas.3, 4, 5, 6 It was approved by the US Food and Drug Administration (FDA) for use in the treatment of refractory anaplastic astrocytoma in adults in 1999 and newly diagnosed adult glioblastoma (GBM) patients in 2005. The antitumor effect of TMZ is schedule-dependent with multiple administrations being more effective than a single treatment. In a Phase I clinical trial, the recommended dose of TMZ was 750–1000 mg/m2 given orally for 5 days per week for 4 weeks.7 Temodar capsules containing 5–250 mg of TMZ are available for its oral administration to patients and in vials containing 100 mg for those being given TMZ intravenously.
Concomitant therapy using both Temodar and radiation improved overall survival of newly diagnosed adult GBM patients relative to those treated with radiation alone (12.1 → 14.6 months median survival).8 Newly diagnosed GBM patients tend to be given 75 mg/m2/day of Temodar for 6 weeks concomitantly with focal radiotherapy (60 Gy) followed by 6 cycles of in which they are given 150 mg/m2 once daily for 5 days in a row followed by 23 days of no treatment prior to the next cycle. Patients given Temodar iv are injected over a 90 min time period with the same amount that is administered orally.
Mechanism of action of Temozolomide
TMZ is a DNA alkylating agent known to induce cell cycle arrest at G2/M and to eventually lead to apoptosis.9 At physiologic pH it is converted to the short-lived active compound, MTIC. MTIC is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC) and to methylhydrazine. The cytotoxicity of TMZ is mediated by its addition of methyl groups at N7 and O6 sites on guanines and the O3 site on adenines in genomic DNA (Fig. 1). Alkylation of the O6 site on guanine leads to the insertion of a thymine instead of a cytosine opposite the methylguanine during subsequent DNA replication, and this can result in cell death.
Temozolomide resistance in natural TMZ resistant GBM cell lines
To identify TMZ resistant GBM cell lines, we conducted literature searches in PubMed/MEDLINE using the terms Temozolomide/TMZ resistant glioblastoma/GBM and acquired Temozolomide/TMZ resistant glioblastoma/GBM. The searches indicated that several cell lines have been identified as TMZ sensitive or TMZ resistant (Table 1).9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
Table 1.
Characteristics of intrinsic (pre-existing) TMZ resistant GBM and high grade glioma cell lines.
| TMZ sensitive cellsa | TMZ resistant cellsb | IC50 (μM) to TMZ | Molecular events of TMZ resistant cells | TMZ resistant cells are sensitive to | References |
|---|---|---|---|---|---|
|
U87 U373 |
U87: 172 μM U373: 131 μM |
Baer JC et al.10 | |||
| SWB61 | SWB77 | SWB61: 30 μM SWB77: 350 μM |
No clear correlation between MGMT activity and response to TMZ Loss of the p53-inducible cell cycle checkpoint is most effective mechanism for TMZ resistance, but p53 mutation status itself cannot determine the resistance of tumors to TMZ |
Bocangel DB et al.11 | |
| U373 | T98G | U373: <10 μM T98G: >1000 μM |
T98G cells are sensitive to the combination of TMZ and AGT inhibitor O6-benzylguanine (BG) than TMZ treatment alone | Kanzawa T et al.12 | |
|
U87, U373 |
T98G | U87: 100 μM U373: 100 μM T98G: >500 μM |
TMZ suppressed telomerase activity in U87 and U373 cells, but not in T98G cells | AGT inhibitor O6-benzylguanine (BG) sensitized T98G cells to TMZ and also suppressed telomerase activity | Kanzawa T et al.13 |
| AO2 U251 SKMG1 |
T98G U251nu/nu |
AO2, U251: <20 μM SKMG1: <40 μM T98G: >1000 μM U251nu/nu: >1000 μM |
T98G and U251nu/nu cells express high level of MGMT | IFN-β (100 IU/ml) sensitizes T98G and U251nu/nu cells to TMZ (100 μM) via down-regulates MGMT expression and activation of p53 pathway | Natsume A et al.14 |
| CD133 negative primary cultured cells | CD133 positive primary cultured cells | CD133 positive primary cultured cells are resistant to TMZ compared to CD133 negative primary cultured cells established from GBM patients. The resistance is due to higher level of BCRP1, MGMT, and apoptosis inhibition genes mRNA expression | Liu G et al.15 | ||
| LNT-229, U87 |
LN-18, T98G – both cells have mutant p53 | U87: 7 μM LN-18: 511 μM T98G: 502 μM |
LN-18 and T98G cells had high levels of MGMT activity | TMZ resistant cells are sensitive to MGMT inhibitor O6-BG (50 μM) and restores p53 function by p53 rescue agent CP-31398, but not sensitive to PARP inhibitors 3-aminobenzamide (1 mM) and NU1025 (200 μM) | Hermisson M et al.16 |
| U87 | T98G | U87: 204 μM T98G: 1585 μM |
T98G cells express high level of MGMT (wild type promoter), but U87 cells express low level of MGMT (due to partial methylation of the promoter) | Combination of oncolytic adenovirus Δ-24-RGD and TMZ improved the survival of T98G cells via decrease MGMT mRNA | Alonso MM et al.9 |
|
A172, U251 |
Combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (30 ng/ml) and TMZ (100 μM) sensitize A172, U251, and U87 cells, but not effective to TMZ resistant cells | Uzzaman M et al.17 | |||
| U251 | T98G | Combination of IFN-β/TMZ had significant synergistic antitumor effect on the growth of both T98G and U251 subcutaneous tumors, because IFN-β inactivates MGMT via p53 gene induction and enhances the therapeutic efficacy to TMZ | Natsume A et al.18 | ||
| U87, U251, M059K, M059J | U251: 50 μM | Ujifuku K et al.19 | |||
| AMC 3046, Gli6, Hs 683, U251, VU-98, VU-109, VU-122 | T98G, VU-28, VU-110 | U251: <50 μM T98G: >500 μM |
All TMZ-resistant GBM cells express MGMT protein whereas no MGMT protein could be detected in TMZ-sensitive GBM cells | van Nifterik KA et al.20 | |
| T98G | T98G cells have overexpression of MGMT, BER (base excision repair), NAMPT (nicotinamide phosphoribosyl transferase), and NAD+ | Combination of NAD+ biosynthesis inhibition with BER inhibition decreased T98G cell survival | Goellner EM et al.21 | ||
| SW1088, U87, U251 | CCF-STTG1, LN-18, T98G, U343-MG | CCF-STTG1 cells do not express MGMT protein, but express p16INK4A LN-18 and T98G cells express MGMT protein |
Lee SY et al.22 | ||
| A172 | T98G | A172: <100 μM U251: <250 μM T98G: >250 μM NHA: >250 μM |
TMZ resistance is the result of APNG and MGMT activity A172: MGMT/APNG null U251: MGMT null, APNG high T98G: MGMT/APNG high |
Agnihotri S et al.23 | |
| U87, U251 | T98G, U138 | U87: <500 μM U251: <500 μM T98G: >500 μM U138: >500 μM |
T98G and U138 cells express MGMT protein TMZ (50 mg/kg) alone did not decrease the growth of T98G xenograft tumor |
Combination of Valproic acid (VPA) and TMZ, because of via reduced MGMT expression Combination of VPA (300 mg/kg) and TMZ (50 mg/kg) decreased T98G tumor growth |
Ryu CH et al.24 |
| LNT-229, LN-308 |
LN-18 | Upregulation of MGMT | Happold C et al.25 | ||
| KMG4, LN235, LN308, U87 |
T98G, U138, LN382 | MGMT expression | Kohsaka S et al.26 | ||
| U87 | T98G | Significant increase in the miR-9 levels in TMZ treated GBM cells | Munoz JL et al.27 | ||
| U87 | T98G | TMZ treated GBM cells have activated EGFR → Activated JNK-ERK1/2-AP-1 axis → Increased connexin 43 (Cx43) | Munoz JL et al.28 | ||
| A172, LN71, LN405, U373 | LN229 | St-Coeur P-D et al.29 | |||
| U87 | T98G | U87: 10 μM T98G: 600 μM |
T98G cells express MGMT but p53 deficient. U87 cells lack MGMT but p53 proficient | APE1, an essential base excision repair pathway enzyme, knockdown associated with TMZ treatment efficiently reduced cell proliferation and clonogenic survival of resistant cells | Montaldi AP et al.30 |
AGT: O6-methylguanine-DNA alkyltransferase; APNG: alkylpurine-DNA-N-glycosylase; APE1: apurinic/apyrimidine endonuclease/redox factor-1; BCRP1: Breast cancer resistance protein 1; MGMT: O6-methylguanine-DNA methyltransferase; NHA: Normal human astrocytes; O6-BG: O6-benzylguanine; TMZ: Temozolomide; VPA: Valproic acid.
Bold indicates TMZ sensitive cell lines which reported at least in two papers.
Bold indicates TMZ resistant cell lines which reported at least in two papers.
Well-known TMZ sensitive GBM cells are A172, U87, U251, and U373. In contrast, the following GBM cells were reported consistently to be TMZ resistant: LN-18, T98G, and U138. We reported that CCF-STTG1 and U343-MG GBM cells are also TMZ resistant.22 The LC50s (50% cell death concentration) published for TMZ on TMZ sensitive GBM cells vary. For example, Hermisson et al.16 and Montaldi et al.30 reported that the LC50s of TMZ on U87 GBM cells were 7 μM and 10 μM, while Baer et al.10 and Ryu et al.24 noted it as 172 μM and <500 μM. Those reported for the lethality of TMZ on U251 GBM cells also varied: <20 μM,14 50 μM,19 <250 μM,23 <500 μM.24 The variations in LC50 may reflect the use of different experimental procedures and cytotoxicity assays (e.g., MTS assay, clonogenic assay). LC50s reported for TMZ on TMZ resistant GBM cells also varied. For TMZ on T98G GBM cells LC50s were found to range from >250 μM23 to 1585 μM.9 The U251 derived U251 nu/nu human glioma cells, which have remarkable transplantability to the nude mouse, behaved analogously to the T98G cells when exposed to TMZ.14 Interestingly, the trend of the cytotoxic effects of TMZ, as indicated by the LC50s, indicates that LC50s for TMZ susceptible cells such as U87 or U251 are lower than those for TMZ resistant ones such as T98G.9, 13, 14, 16, 20, 23, 24, 30 In primary cultured cells, established from GBM patients, CD133 positive cells are more resistant to TMZ than CD133 negative cells.15 While studying the effects of TMZ on normal human astrocytes (NHA) Agnihotri et al.23 found that the LC50 was similar to that for T98G cells (LC50: >250 μM) that are not sensitive to TMZ. In another report,31 immortalized normal human astrocytes which are the most common cell type to cause gliomas had a TMZ LC50 of 500 μM which is similar to that for TMZ resistant GBM cells. Our recent unpublished finding that TMZ is not cytotoxic to NHA as well as TMZ resistant GBM cells such as T98G and CCF-STTG1 agrees with their results.
Molecular events in natural Temozolomide resistant GBM cells
TMZ is an alkylating agent that acts by methylating DNA adenine and guanine residues (∼90%) to form N3-methyladenine and N7-methylguanine, respectively, and to a lesser extent (5–10%) O6-methylguanine (Fig. 1). The methylated DNA can be repaired by base excision or DNA mismatch repair pathways. While O6-methylguanine methyltransferase (MGMT) acts to reverse methylation of the O6 position of guanine, Bocangel et al.11 found no correlation between MGMT activity and response to TMZ in their study of 7 human glial tumor cell lines. Their results indicated that a non-functional p53 response to DNA damage was associated more with TMZ resistance than was MGMT activity. They also suggested that p53 mutation status is not the only factor to determine the resistance of cancer cells to TMZ. However, in other studies natural TMZ resistant GBM cells were found to express higher levels of MGMT protein than TMZ sensitive GBM cells.9, 14, 15, 16, 20, 21, 22, 23, 24, 25, 26, 30 Expression of MGMT protein was found to correlate inversely with the status of MGMT promoter methylation32 although MGMT methylated GBM cells such as T98G cells can express MGMT protein and show TMZ resistance. Based on MGMT protein and the MGMT methylated promoter results different labs hypothesized that the response of GBM cells to TMZ might be best predicted by 1) their expression of the MGMT protein,20 and 2) MGMT promoter methylation status.32 Agnihotri et al. suggested that the TMZ resistance of T98G GBM cells is due not only to expression of the MGMT protein but to that of alkylpurine-DNA-N-glycosylase (APNG) protein as well.23 APNG is a base excision repair enzyme that functions in the repair of N3-methyladenine and N7-methylguanine. A higher expression of nuclear APNG protein correlated with patients having poorer overall survival compared to patients lacking APNG expression.23 Some TMZ resistant GBM cells do not express the MGMT protein. For example, CCF-STTG1 cells do not express MGMT protein and are resistant to TMZ.22 Instead of MGMT expression, the CCF-STTG1 cells express more p16INK4A than TMZ sensitive or other TMZ resistant GBM cells.22
The impact of MGMT promoter methylation on MGMT protein expression has been extensively studied in human GBM patients as well. In studies of GBM patients, MGMT promoter methylation was found to correlate with improved survival when they were treated with alkylating agents like TMZ.33, 34 The finding that not all GBM patients that had MGMT promoter methylation responded to alkylating agents indicates, in agreement with results described above for the cell studies, that there are multiple molecules involved in TMZ resistance.
In addition to MGMT and APNG protein expression, other proteins and factors have been studied for their effect on TMZ resistance. For example, TMZ resistance is associated with increased breast cancer resistance protein 1 (BCRP1), base excision repair (BER), nicotinamide phosphoribosyl transferase (NAMPT), NAD+, CD133 expression, and inhibition of apoptosis.15, 21 Moreover, treatment of U87 and T98G cells with TMZ (200 μM) for 72 h resulted in increased miR-9,27 and increased expression of connexin 43 induced through activation of the epidermal growth factor receptor (EGFR) and Jun N-terminal kinase-Extracellular Signal Regulated Kinases 1 and 2-activator protein 1 (JNK-ERK1/2-AP-1) axis.28 All of these data indicate that TMZ resistance results from a complex cellular response by the GBM cells.
Gene mutations in natural Temozolomide resistant GBM cells
Possible association between a gene mutation and TMZ resistance in GBM has not been interrogated systemically. Although the best studied gene mutation associated with resistance to TMZ is p53, the results have been mixed.11, 35 For example, Blough et al. reported that while expression of wild type p53 conveyed more resistance to TMZ than non-functional mutated p53 in cells of established GBM cell lines, it did not in brain tumor initiating cells.35 In contrast to those results, TMZ sensitive GBM cells (e.g., A172, U87) express wild type of p53 and p53 gene mutations were found in TMZ resistant GBM cells (e.g., LN-18, T98G, U138) as well as TMZ sensitive GBM cells (e.g., U251, U373).36, 37 Therefore, a mutation in the p53 gene does not appear to be a primary indicator of resistance to TMZ. Interestingly, we observed that all but one (LN-18) of the TMZ resistant GBM cells we have studied have a mutation in the HFE gene22 which functions in iron homeostasis. Mutations in the HFE gene lead to iron overload in cells expressing it compared to those expressing wild type HFE. There are two major mutations (H63D, C282Y) of the HFE gene. TMZ resistant GBM cells found to express H63D HFE were T98G, U138, and U343, while the only TMZ resistant GBM cells that expressed C282Y HFE were CCF-STTG1. It is unknown whether increased iron or expression of a HFE gene mutant mediates molecular events associated with TMZ resistance. In a recent study of tumor tissues from GBM patients, Nguyen et al. reported that novel MSH6 (mutS homolog 6) mutations influenced the sensitivity of brain tumor initiating cell lines to TMZ regardless of MGMT promoter methylation status.38
Temozolomide resistance in adapted Temozolomide resistant GBM cell lines
Adapted TMZ resistant GBM cells have been generated from both established GBM cell lines and cells isolated from primary tumors treated with TMZ for different times (72 h–6 months) (Table 2).19, 25, 26, 29, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Cells from known TMZ sensitive GBM cell lines (e.g., A172, SNB-19, U87, U251, and U373) have been frequently used to generate adapted TMZ resistant GBM cells. In studies, known TMZ resistant GBM cell lines (e.g., LN-18, T98G) were also used.25, 44 TMZ resistant cells have been generated by treating them in a step-wise manner with different concentrations (1–1000 μM) of TMZ for various time periods (up to 6 months). Identified acquired TMZ resistant GBM cells were then maintained in medium containing the maximum treated concentration of TMZ or without TMZ.
Table 2.
Characteristics of acquired TMZ resistant GBM cell lines.
| Host cellsa | TMZ resistant cells |
IC50 (μM) to TMZ | Molecular events of adapted TMZ resistant cells | TMZ resistant cells are sensitive to | References | |
|---|---|---|---|---|---|---|
| Selection method | Maintenance method | |||||
| SF188 | Stepwise exposure of the cells to TMZ (50–300 μM) for 6 months | Maintain the TMZ resistant cells in TMZ free cell culture medium, but the cells were frequently incubated with 300 μM TMZ | SF188: 426 μM SF188_R: 1854 μM |
Increased activity of AGT Reduced expression of pro-apoptotic proteins (Bad, Bax, Bcl-Xs) No change for mismatch repair enzymes Not affected by p53 status, because parent and TMZ resistant cells contain mutant p53 |
TMZ resistant cells are sensitive to the AGT inhibitor O6-benzylguanine (BG) | Ma J et al.39 |
| Primary tumor | Incremental concentrations of TMZ (2.5, 5, 7.5, 10 μM) for 1 h for 5 consecutive days. This step was repeated several times until the resulting cell population was resistant | The TMZ resistant cells re-treated with 10 μM TMZ every 8-10 passages | Overexpressed MGMT, Decreased expression of TNFAIP3 (NF-kB pathway modulator, encode the zinc finger protein A20), NFKBIA (NF-kB inhibiting IkB family member), C8orf4, and LIF |
Bredel M et al.40 | ||
| SNB-19 | Incremental concentrations of TMZ (3, 5, 10, 20, 30, 60, 150 μM) | Maintain the TMZ resistant cells in TMZ free cell culture medium | SNB-19: 1.03 μM SNB-19A4: 101 μM SNB-19C1: 55 μM |
No detectable MGMT expression in TMZ resistant cells Gene alteration (loss of 2p16.1-2p25.3, loss of partial amplification of the 4p14.4-4q21.22, loss of amplification of the 16q12.1–16q22.1 and 1p13.2-1q21.1) |
Auger N et al.41 | |
| U87, U251, M059K, M059J | 100 μM TMZ for 2 weeks | U251: ∼50 μM U251_R: >300 μM |
MGMT expression is not involved in the acquisition of TMZ resistance in U251_R cells Up-regulated microRNA such as miR-10a, miR-195, miR-455-3p |
miR-195 knockdown showed moderate growth inhibition to U251_R cells combined treatment with both miR-10a or miR-455-3p inhibitors with TMZ showed better cytotoxicity |
Ujifuku K et al.19 | |
| SNB19, U373 | Stepwise increment TMZ concentrations (1, 2, 5, 10, 20, 50, 100 μM) for 6 months | Maintain the TMZ resistant cells in 100 μM TMZ | SNB19: 36 μM U373: 68 μM SNB19_R: 280 μM U373_R: 289 μM |
SNB19_R & U373_R cells acquired TMZ resistance via distinct mechanisms. SNB19_R: down-regulation of MSH6 message and protein (under continued presence of TMZ), up-regulation of BER gene NTL1 U373_R: MGMT expression, but its expression requires the selective pressure of continued TMZ presence |
Zhang J et al.42 | |
| U251 | Stepwise 2 fold increase of TMZ concentration from 2.5 μM to 1 mM for 6 months | Maintain the TMZ resistant cells in 160 μM TMZ. For over 50 passages, the resistance to TMZ was retained | Decreased MGMT expression Decreased mitochondrial DNA copy number Large heteroplasmic mtDNA deletions Remodeling of the entire electron transport chain (Significant decreases of complexes I and V and increases of complexes II/III and IV) |
Oliva CR et al.43 | ||
|
U251, U373, T98G |
TMZ concentration is 2 fold increased every two passages from 12.5 μM to 800 μM for 2 months | U251: 100 μM U373: 50 μM U251_R: 1000 μM U373_R: 800 μM |
TMZ resistant cells do not have altered MGMT expression, but have upregulation of STAT3 and pSTAT3 (Ser727) while pSTAT3 (Tyr705) was decreased | STAT3 siRNA sensitize TMZ resistant cells | Lee E-S et al.44 | |
| LN-18, LNT-229, LN-308 | 24 h TMZ exposure every 2 weeks. Increasing concentration of TMZ for 6 months | LN-308: <40 μM LN-18: ∼400 μM LN-308_R: >400 μM LN-18_R: ∼800 μM |
LN-18_R: up-regulation of MGMT LNT-229_R: down-regulation of DNA mismatch-repair protein LN-308_R: reduced methylation of LINE-1 repetitive elements |
Happold C et al.25 | ||
| U87 | Culture the cells for 3 weeks with a low dose of TMZ | U87: <40 μM (growth inhibition) or <10 μM (clonogenic assay) U87_R: 150 μM (growth inhibition) or >400 μM (clonogenic assay) |
Upregulation of MGMT and STAT3 | STAT3 inhibitor | Kohsaka S et al.26 | |
| U343 | Culture the cells with 200 μM of TMZ for 1 month. Continuous TMZ (150 μM) treatment for at least 5 months. | U343: <50 μM U343_R: 280.63 μM | Increased invasiveness Increased JNK signaling pathway Activation of known JNK effector paxillin |
JNK inhibitor SP600125 or JNK siRNA suppressed up-regulation of invasiveness | Ueno H et al.45 | |
| U373 | At every two passages, TMZ concentration is increased subsequently from 12.5 μM to 500 μM for months | TMZ resistant cells have upregulation of glucose, citrate, and isocitrate levels TMZ sensitive cells have upregulation of alanine, choline, creatine, and phosphorylcholine |
A glucose analog (2-Deoxy-d-glucose) alone or with TMZ is cytotoxic to TMZ resistant cells | St-Coeur P-D et al.29 | ||
| U251 | Stepwise 2 fold increase of TMZ concentration from 1.25 μM to 160 μM for 10 months | U251: 58 μM U251_R: 271 μM | Upregulation of MGMT and phosphorylated-p65 | IkBα inhibitor BAY 11-7082 sensitize TMZ resistant cells growth Combination effect of IkBα inhibitor and TMZ on TMZ resistant cells |
Wang X et al.46 | |
|
A172, LN229, U87 |
Culture the cells with 20 μM of TMZ → Culture the survival cells with 40 μM of TMZ → Culture the survival cells with 40 μM of TMZ | TMZ resistance is not due to increased repair of O6-methylguanin but is correlated to decreased mismatch repair (MMR) activity such as MSH2 and MSH6 | McFaline-Figueroa JL et al.47 | |||
|
A172, GBM cancer stem cells |
Culture the cells with continuous TMZ (200 or 400 μM) for 30 days | GBM3: 98 μM GBM3_R: >1000 μM GBM5: 634 μM GBM5_R: 1115 μM |
TMZ resistant cells grow slowly Increased histone lysine demethylase (KDMs) gene expression, especially KDM5A No change in MGMT and drug efflux mechanisms |
TMZ resistant cells are sensitive to the histone deacetylase (HDAC) inhibitor (SAHA 1 μM) and TMZ (200 μM) combination treatment | Banelli B et al.48 | |
AGT: O6-methylguanine-DNA alkyltransferase; C8orf4: Chromosome 8 open reading frame 4; HDAC: histone deacetylase; KDM: Histone lysine demethylase; LIF: Leukemia inhibitory factor; MGMT: O6-methylguanine-DNA methyltransferase; MMR: Mismatch repair; O6-BG: O6-benzylguanine; SAHA: N-hydroxy-N′-phenyl-octanediamide; STAT3: Signal transducers and activators of transcription 3; TNFAIP3: Tumor necrosis factor-α-induced protein 3.
Bold indicates that these cell lines were commonly used as host cells to generate acquired TMZ resistant cells.
Resistance to TMZ of the adapted GBM cells was 2–98 times greater than that of the host cells. Different comparisons of the response to TMZ as measured by the TMZ LC50s of adaptive TMZ resistant GBM cells and host cells gave variable results. For example, Auger et al.41 reported that the LC50 obtained for TMZ when it was used to treat TMZ-resistant SNB-19 cells was 55–101 μM, while in a study by Zhang et al.42 it was 280 μM. This difference may reflect the fact that the original responses of the host cells to TMZ differed in the two laboratories: 1.03 μM vs 36 μM. U87 and U251 GBM cells have also been commonly used to generate adaptive TMZ resistant ones. Kohsaka et al.26 found that the LC50 for TMZ on acquired TMZ resistant U87 cells, isolated after 3 weeks of exposure to low doses of TMZ, was increased 4 fold, from 40 μM to 150 μM, when measured using a growth inhibition assay, but by 40 fold, from 10 μM to 400 μM, using a clonogenic assay. Similarly, Ujifuku et al.19 found that the LC50 for the effect of TMZ on acquired TMZ resistant U251 cells, isolated after a 2 weeks exposure to TMZ, increased at least 6 fold from 50 μM to >300 μM. Some adaptive TMZ resistant GBM cells were generated using known TMZ resistant cell lines such as LN-18. After adaptation by exposure to TMZ for 6 months, the LC50 of TMZ on adapted resistant LN-18 cells increased 2 fold to ∼800 μM.25
Mechanisms of Temozolomide resistance in adapted TMZ resistant GBM cells
Adaptive TMZ resistant GBM cells differ from their parent cells at the molecular level. Of particular interest in regard to TMZ resistance are alterations related to known TMZ resistance molecules such as MGMT and DNA repair. Increased MGMT protein expressions were observed in adaptive TMZ resistant SF188, LN-18, U87, U251, and primary tumor derived GBM cells.25, 26, 39, 40, 46 In addition to an increased expression of MGMT in adaptive TMZ resistant GBM cells, they have been reported to show a decrease in the nuclear factor-Kappa B (NF-kB) pathway modulator Tumor Necrosis Factor-Alpha-Induced Protein 3 (TNFAIP3) and up-regulation of p-p65.40, 46 Increased expression of Signal Transducer and Activator of Transcription 3 (STAT3) is also found in adaptive TMZ resistant U87 cells.26
Analyses of MGMT protein expression in adaptive TMZ resistant SNB-19, LNT-229, U251, U343 and U373 cells indicated no change or a decrease in MGMT protein expression.19, 41, 42, 43, 44 In adaptive TMZ resistant GBM cells that did not over-express MGMT, expression of other molecules found to participate in TMZ resistance were observed. For example, adaptive TMZ resistant SNB-19 cells showed a loss of gene amplification41 or down-regulation of MSH6 and up-regulation of BER gene NTL1.42 Down-regulation of DNA mismatch-repair protein was also found in adaptive TMZ resistant LNT-229 cells.25 The adaptive TMZ resistant U251 cells had an up-regulation of microRNA,19 up-regulation of STAT3/p-STAT3,42 decreased mitochondrial DNA copy number and remodeling of the mitochondria electron transport chain.43 Adaptive TMZ resistant GBM cells also have been reported to show activation of JNK,45 up-regulation of metabolisms of glucose, citrate, and isocitrate,29 and an increase in histone demethylase KDM5A gene expression.48
Temozolomide resistance in animal models
Not many studies have been done to generate TMZ resistant xenograft animal models (Table 3).43, 49, 50, 51 These tumors can be generated by successive injections of TMZ into the flanks of animals bearing TMZ sensitive GBM cell xenografts. When the flank tumors are not inhibited by administration of 120 mg/kg/day of TMZ for 5 days, the tumors are classified as TMZ resistant. The TMZ resistant xenografts had higher mitochondrial complex II–IV activities and decreased mitochondrial complex I/V activity.43, 49 Similar molecular events were observed in in vitro studies of adaptive TMZ resistant U251 cells.43 Like some of the adaptive TMZ resistant GBM cells, strongly induced and prolonged MGMT protein expression was found in TMZ resistant xenografts.51
Table 3.
Characteristics of intrinsic or acquired TMZ resistant xenograft animal models.
| Host cell | TMZ resistant cells |
Molecular events of TMZ resistant GBM xenograft | TMZ resistant cells are sensitive to | References | |
|---|---|---|---|---|---|
| Selection method | Maintenance method | ||||
| GBM Xenograft lines | Subjecting mice with established flank tumors to successively higher doses of TMZ until tumor growth was no longer inhibited by 120 mg/kg/day TMZ for 5 days | By serial passage of subcutaneous tumor in mice | Higher complex II–IV activities and decreased activities of complex I/V | Giannini C et al.49 Oliva CR et al.43 |
|
| TMZ sensitive GBM Xenograft lines (GBM12, GBM22, GBM39) | Subjecting mice with established flank tumors to successively higher doses of TMZ until tumor growth was no longer inhibited by TMZ 120 mg/kg/day for 5 days | By serial passage of subcutaneous tumor in mice | Combination of PARP inhibitor and TMZ is effective to primary GBM lines that have not been previously exposed to TMZ | Clarke MJ et al.50 | |
| GBM Xenograft lines | GBM43, GBM44 xenograft lines | Direct implantation of patient samples and subsequent serial subcutaneous propagation in nude mic | TMZ treatment induce robust and prolonged induction of MGMT expression | Kitange GJ et al.51 | |
Treatment for Temozolomide resistant GBM
Intrinsic TMZ resistant GBM cells are not treated with just a single agent but with different drug combinations. The most widely used single drugs for naturally TMZ resistant GBM cells are a MGMT inhibitor or a drug to decrease activity/inhibit expression of MGMT since the level of MGMT protein expression is closely related to TMZ resistance. Treatment with the MGMT inhibitor O6-benzylguanine (O6-BG) sensitizes TMZ resistant LN-18 and T98G cells to TMZ.12, 16 Interferon-β (IFN-β) also sensitizes TMZ resistant T98G and U251nu/nu cells via down-regulation of MGMT expression.14 IFNs are a family of cytokines that have immunomodulatory, cell differentiation, antiangiogenic, and antiproliferative effects. The effect of IFN-β on TMZ resistant GBM cells may be due to its anti-tumor activity (e.g., cytostatic effects, antitumor immune response). Treatment with IFN-β also activates the p53 pathway that can sensitize TMZ resistant GBM cells to TMZ. Treatment of TMZ resistant tumors with a combination of TMZ and either O6-benzylguanine or IFN-β was shown to make the cells more vulnerable to TMZ and synergistic anti-tumor effects than treatment with TMZ alone.12, 18 The effectiveness of using an oncolytic adenovirus or valproic acid in combination with TMZ has also been studied.9, 24 Knockdown of an essential base excision repair pathway enzyme apurinic/apyrimidine endonuclease/redox factor-1 (APE1) also sensitized TMZ resistant T98G cells to TMZ.30
There are only a few studies of the effects of different drug combinations lacking TMZ on TMZ resistant GBM cells. Results of one such study in which an inhibitor of NAD+ biosynthesis was used in combination with a BER inhibitor indicated that the combination could inhibit TMZ resistant GBM cells.21 Recent report showed that the combined treatment of monoclonal antibody against EGFR (Nimotuzumab) and mammalian target of rapamycin (mTOR) inhibitor (rapamycin) was more cytotoxic than single treatments including TMZ on patient derived human glioma cells.31
Like intrinsically TMZ resistant GBM cells, acquired TMZ resistant GBM cells are also vulnerable to O6-benzylguanine.39 Studies of a combination of TMZ and other targeted drugs on acquired TMZ resistant GBM cells indicated that the miR-455-3p inhibitor and TMZ were more cytotoxic than TMZ alone on acquired TMZ resistant U251 cells.19 The combination of an IkBα inhibitor and TMZ also decreased their survival.46 Acquired TMZ resistant GBM cells derived from GBM cancer stem cells, were sensitive to treatment with a combination of the histone deacetylase (HDAC) inhibitor (SAHA 1 μM) and TMZ (200 μM).48 Other drugs analyzed for their effects on acquired TMZ resistant GBM cells are inhibition of microRNA-195,19 an inhibitor of STAT3 or STAT3 knockdown,26, 44 an inhibitor of JNK or JNK siRNA,45 and a glucose analog.29 In an animal study, a combination of TMZ and a poly ADP ribose polymerase (PARP) inhibitor was found to extend survival of the GBM orthotopic xenografts.50
In addition to the in vitro and in vivo xenograft studies, the effectiveness of combination treatments comprised of TMZ and a pharmacologic agent (e.g., O6-benzylguanine) on TMZ resistant GBM has been studied in clinical trials. In a phase I clinical trial, patients with recurrent GBM showed a marginal response when treated with a combination of TMZ and dendritic cell vaccination.52 In a phase II clinical trial, the combination of TMZ and O6-BG was ineffective on TMZ resistant GBM patients, but was found to restore TMZ sensitivity in TMZ resistant anaplastic glioma patients.53 The combination of imatinib and hydroxyurea did not improve recurrent GBM patient survival in phase III clinical study.54
Conclusions
TMZ resistance is a major problem in the treatment of malignant brain tumors. Studies of numerous intrinsic and acquired TMZ resistant GBM cells indicate that TMZ resistance is associated with the expression levels of DNA alkylating proteins and DNA repair enzymes. Results obtained from studies of intrinsic and acquired TMZ resistant GBM cells support the conclusion that TMZ resistance is not mediated by a single molecular event but by multiple ones. Therefore, identification of GBM patients based on the patient's gene/protein profiling data could be beneficial for selecting drugs for their treatment. A potential problem with the use of TMZ to treat GBM patients is that their tumors may acquire TMZ resistance through alteration not only in their expression of DNA alkylating proteins and DNA repair enzymes but in cell signaling pathways as well. While TMZ resistance in GBM has been primarily studied in vitro using different cell models, more studies of TMZ resistance need to be done using patient derived GBM xenograft animal models or tumor tissues of TMZ resistant patients in order to better interrogate potential targets and therapeutic options to pursue for human study.
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
Acknowledgments
These studies were supported from the Gittlen Foundation. The author thanks Dr. Cara-Lynne Schengrund for providing comments and critical reading on the manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Moody C.L., Wheelhouse R.T. The medicinal chemistry of imidazotetrazine prodrugs. Pharmaceuticals (Basel) 2014;7:797–838. doi: 10.3390/ph7070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid J.M., Stevens D.C., Rubin J., Ames M.M. Pharmacokinetics of 3-methyl-(triazen-1-yl)imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin Cancer Res. 1997;3:2393–2398. [PubMed] [Google Scholar]
- 3.Middleton M.R., Grob J.J., Aaronson N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Quirt I., Verma S., Petrella T., Bak K., Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12:1114–1123. doi: 10.1634/theoncologist.12-9-1114. [DOI] [PubMed] [Google Scholar]
- 5.Yung W.K., Prados M.D., Yaya-Tur R. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 6.Hart M.G., Garside R., Rogers G., Stein K., Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;4:CD007415. doi: 10.1002/14651858.CD007415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newlands E.S., Blackledge G.R., Slack J.A. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65:287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M.H., Johnson J.R., Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 9.Alonso M.M., Gomez-Manzano C., Bekele B.N., Yung W.K., Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- 10.Baer J.C., Freeman A.A., Newlands E.S., Watson A.J., Rafferty J.A., Margison G.P. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer. 1993;67:1299–1302. doi: 10.1038/bjc.1993.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocangel D.B., Finkelstein S., Schold S.C., Bhakat K.K., Mitra S., Kokkinakis D.M. Multifaceted resistance of gliomas to temozolomide. Clin Cancer Res. 2002;8:2725–2734. [PubMed] [Google Scholar]
- 12.Kanzawa T., Bedwell J., Kondo Y., Kondo S., Germano I.M. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg. 2003;99:1047–1052. doi: 10.3171/jns.2003.99.6.1047. [DOI] [PubMed] [Google Scholar]
- 13.Kanzawa T., Germano I.M., Kondo Y., Ito H., Kyo S., Kondo S. Inhibition of telomerase activity in malignant glioma cells correlates with their sensitivity to temozolomide. Br J Cancer. 2003;89:922–929. doi: 10.1038/sj.bjc.6601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natsume A., Ishii D., Wakabayashi T. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005;65:7573–7579. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- 15.Liu G., Yuan X., Zeng Z. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermisson M., Klumpp A., Wick W. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 17.Uzzaman M., Keller G., Germano I.M. Enhanced proapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on temozolomide-resistant glioma cells. J Neurosurg. 2007;106:646–651. doi: 10.3171/jns.2007.106.4.646. [DOI] [PubMed] [Google Scholar]
- 18.Natsume A., Wakabayashi T., Ishii D. A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol. 2008;61:653–659. doi: 10.1007/s00280-007-0520-x. [DOI] [PubMed] [Google Scholar]
- 19.Ujifuku K., Mitsutake N., Takakura S. miR-195, miR-455-3p and miR-10a( *) are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010;296:241–248. doi: 10.1016/j.canlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 20.van Nifterik K.A., van den Berg J., van der Meide W.F. Absence of the MGMT protein as well as methylation of the MGMT promoter predict the sensitivity for temozolomide. Br J Cancer. 2010;103:29–35. doi: 10.1038/sj.bjc.6605712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goellner E.M., Grimme B., Brown A.R. Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res. 2011;71:2308–2317. doi: 10.1158/0008-5472.CAN-10-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.Y., Liu S., Mitchell R.M. HFE polymorphisms influence the response to chemotherapeutic agents via induction of p16INK4A. Int J Cancer. 2011;129:2104–2114. doi: 10.1002/ijc.25888. [DOI] [PubMed] [Google Scholar]
- 23.Agnihotri S., Gajadhar A.S., Ternamian C. Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest. 2012;122:253–266. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu C.H., Yoon W.S., Park K.Y. Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/987495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Happold C., Roth P., Wick W. Distinct molecular mechanisms of acquired resistance to temozolomide in glioblastoma cells. J Neurochem. 2012;122:444–455. doi: 10.1111/j.1471-4159.2012.07781.x. [DOI] [PubMed] [Google Scholar]
- 26.Kohsaka S., Wang L., Yachi K. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11:1289–1299. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 27.Munoz J.L., Bliss S.A., Greco S.J. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz J.L., Rodriguez-Cruz V., Greco S.J. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014;5:e1145. doi: 10.1038/cddis.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St-Coeur P.D., Poitras J.J., Cuperlovic-Culf M., Touaibia M., Morin P., Jr. Investigating a signature of temozolomide resistance in GBM cell lines using metabolomics. J Neurooncol. 2015;125:91–102. doi: 10.1007/s11060-015-1899-6. [DOI] [PubMed] [Google Scholar]
- 30.Montaldi A.P., Godoy P.R., Sakamoto-Hojo E.T. APE1/REF-1 down-regulation enhances the cytotoxic effects of temozolomide in a resistant glioblastoma cell line. Mutat Res Genet Toxicol Environ Mutagen. 2015;793:19–29. doi: 10.1016/j.mrgentox.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Chong D.Q., Toh X.Y., Ho I.A. Combined treatment of Nimotuzumab and rapamycin is effective against temozolomide-resistant human gliomas regardless of the EGFR mutation status. BMC Cancer. 2015;15:255. doi: 10.1186/s12885-015-1191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uno M., Oba-Shinjo S.M., Camargo A.A. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo) 2011;66:1747–1755. doi: 10.1590/S1807-59322011001000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 34.Hegi M.E., Liu L., Herman J.G. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 35.Blough M.D., Beauchamp D.C., Westgate M.R., Kelly J.J., Cairncross J.G. Effect of aberrant p53 function on temozolomide sensitivity of glioma cell lines and brain tumor initiating cells from glioblastoma. J Neurooncol. 2011;102:1–7. doi: 10.1007/s11060-010-0283-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Zhu S., Cloughesy T.F., Liau L.M., Mischel P.S. p53 disruption profoundly alters the response of human glioblastoma cells to DNA topoisomerase I inhibition. Oncogene. 2004;23:1283–1290. doi: 10.1038/sj.onc.1207244. [DOI] [PubMed] [Google Scholar]
- 37.Koul D., Fu J., Shen R. Antitumor activity of NVP-BKM120–a selective pan class I PI3 kinase inhibitor showed differential forms of cell death based on p53 status of glioma cells. Clin Cancer Res. 2012;18:184–195. doi: 10.1158/1078-0432.CCR-11-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen S.A., Stechishin O.D., Lchman H.A. Novel MSH6 mutations in treatment-naïve glioblastoma and anaplastic oligodendroglioma contribute totemozolomide resistance independently of MGMT promoter methylation. Clin Cancer Res. 2014;20:4894–4903. doi: 10.1158/1078-0432.CCR-13-1856. [DOI] [PubMed] [Google Scholar]
- 39.Ma J., Murphy M., O'Dwyer P.J., Berman E., Reed K., Gallo J.M. Biochemical changes associated with a multidrug-resistant phenotype of a human glioma cell line with temozolomide-acquired resistance. Biochem Pharmacol. 2002;63:1219–1228. doi: 10.1016/s0006-2952(02)00876-6. [DOI] [PubMed] [Google Scholar]
- 40.Bredel M., Bredel C., Juric D. Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24:274–287. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 41.Auger N., Thillet J., Wanherdrick K. Genetic alterations associated with acquired temozolomide resistance in SNB-19, a human glioma cell line. Mol Cancer Ther. 2006;5:2182–2192. doi: 10.1158/1535-7163.MCT-05-0428. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Stevens M.F., Laughton C.A., Madhusudan S., Bradshaw T.D. Acquired resistance to temozolomide in glioma cell lines: molecular mechanisms and potential translational applications. Oncology. 2010;78:103–114. doi: 10.1159/000306139. [DOI] [PubMed] [Google Scholar]
- 43.Oliva C.R., Nozell S.E., Diers A. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. J Biol Chem. 2010;285:39759–39767. doi: 10.1074/jbc.M110.147504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee E.S., Ko K.K., Joe Y.A., Kang S.G., Hong Y.K. Inhibition of STAT3 reverses drug resistance acquired in temozolomide-resistant human glioma cells. Oncol Lett. 2011;2:115–121. doi: 10.3892/ol.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno H., Tomiyama A., Yamaguchi H. Augmentation of invadopodia formation in temozolomide-resistant or adopted glioma is regulated by c-Jun terminal kinase-paxillin axis. Biochem Biophys Res Commun. 2015;468:240–247. doi: 10.1016/j.bbrc.2015.10.122. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Jia L., Jin X. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol Lett. 2015;9:2586–2590. doi: 10.3892/ol.2015.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McFaline-Figueroa J.L., Braun C.J., Stanciu M. Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res. 2015;75:3127–3138. doi: 10.1158/0008-5472.CAN-14-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banelli B., Carra E., Barbieri F. The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma. Cell Cycle. 2015;14:3418–3429. doi: 10.1080/15384101.2015.1090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giannini C., Sarkaria J.N., Saito A. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke M.J., Mulligan E.A., Grogan P.T. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Mol Cancer Ther. 2009;8:407–414. doi: 10.1158/1535-7163.MCT-08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitange G.J., Carlson B.L., Schroeder M.A. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009;11:281–291. doi: 10.1215/15228517-2008-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunn M.K., Bauer E., Wood C.E. Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. J Neurooncol. 2015;121:319–329. doi: 10.1007/s11060-014-1635-7. [DOI] [PubMed] [Google Scholar]
- 53.Quinn J.A., Jiang S.X., Reardon D.A. Phase II trial of temozolomide plus O6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27:1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dresemann G., Weller M., Rosenthal M.A. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96:393–402. doi: 10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]