Abstract
Objectives
To evaluate the association between high-sensitivity C-reactive protein (hs-CRP) and sarcopenic obesity, and to determine age or sex differences underlying the relationship between hs-CRP and sarcopenic obesity.
Design
Observational study.
Participants
The study included 237 838 participants whose body composition and hs-CRP were analysed at the two health promotion centres in South Korea. Participants were divided into four groups based on body composition: normal, obesity only, sarcopenia only and sarcopenic obesity.
Primary measures
The levels of hs-CRP and proportion of participants with high (≥1.0 mg/L) hs-CRP. Sarcopenic obesity was defined as subjects fulfilling the criteria for sarcopenia (below 2 SD of mean of Skeletal Muscle Mass Index for young adults) and obesity (waist circumference ≥90 cm for men and ≥85 cm for women).
Results
The level of hs-CRP was highest in the sarcopenic obesity group. Following adjustment for various confounders including age, sex, comorbidities, metabolic, health-related behaviour and demographic factors, the adjusted ORs (95% CI) for subjects with high hs-CRP associated with obesity, sarcopenia and sarcopenic obesity compared with normal group (reference) were 1.17 (1.05 to 1.31), 2.23 (1.21 to 4.07) and 3.23 (2.71 to 3.83), respectively. In age subgroup analyses, multivariate logistic regression analysis revealed that the association of high hs-CRP with sarcopenic obesity was stronger in younger (<60 years) participants than in older (≥60 years) participants (p for interaction <0.001). In subgroup analyses for sex, the association of high hs-CRP with sarcopenic obesity was higher in female participants than in males (p for interaction <0.001).
Conclusions
This study demonstrated that high level of hs-CRP was independently associated with sarcopenic obesity in Korean population. We found for the first time that there was a strong association between increased hs-CRP and sarcopenic obesity in female and younger (<60 years) subjects.
Keywords: sarcopenic obesity, sarcopenia, high-sensitivity C reactive protein
Strengths and limitations of this study.
This study was performed with a large sample of participants (n=237 838) to explore the relationship between high-sensitivity C-reactive protein (hs-CRP) and sarcopenic obesity.
For the first time, we demonstrated age and sex differences in association between increased hs-CRP level and sarcopenic obesity.
The association of high hs-CRP level with sarcopenic obesity was investigated after adjusting for potential confounders such as age, sex, comorbidities, metabolic, health-related behaviour and demographic factors. Nevertheless, there is always a potential of residual confounding given the observational design of the study.
The potential for selection bias existed, as elderly subjects were relatively smaller than middle-aged subjects.
Introduction
Sarcopenia, described as the generalised loss of skeletal muscle, is an emerging health challenge in an ageing society.1 2 Recent studies reported that the loss of skeletal muscle is associated with several chronic conditions such as cardiovascular disease, diabetes mellitus (DM), chronic lung disease and metabolic disorders.3 4 However, sarcopenia often coexists with an increased fat mass, so-called sarcopenic obesity (SO).5 Obesity is a well-known risk factor for cardiovascular, endocrine and metabolic disorders.6 7 Therefore, a double metabolic burden due to sarcopenia and obesity (called SO) is assumed to potentiate each other and synergise their adverse health effects.8
C-reactive protein (CRP) is a critical marker of inflammation and a predictor of overall mortality.9–11 However, the standard CRP assay plays a limited role in identifying low-grade inflammation because the standard CRP assay measured down to concentrations of 3–5 mg/L.12 13 High-sensitivity CRP (hs-CRP) enables detection of even mild elevations of hs-CRP, as low as 0.1 mg/L.13 14 Therefore, hs-CRP provides improved sensitivity for detection of low-grade inflammation including subclinical inflammation.14 Low-grade inflammation is a key feature of obesity.15 Furthermore, recent reports demonstrated a strong association between elevated hs-CRP and abdominal obesity.16 17 Sarcopenia is also closely linked to low-grade inflammation in a few studies.18 19 However, few studies have demonstrated the relationship between hs-CRP and sarcopenia. Furthermore, the effects of coexisting sarcopenia and obesity on hs-CRP have been under-recognised. Additionally, because of variation in hs-CRP across age and gender,20 the role of age and gender differences in the association between hs-CRP and SO needs to be established.
The aims of this study were (1) to investigate the association of hs-CRP with SO and (2) to evaluate age or sex differences in the relationship between hs-CRP and SO.
Materials and methods
Study population
A cross-sectional study was conducted to investigate the association of hs-CRP with SO in Korean population. Study participants were recruited from a medical health screening programme at the two health promotion centres of Kangbuk Samsung Hospital in Seoul and Suwon. Between January 2012 and December 2015, a total of 237 838 participants (18–89 years old) were included in the present study, and their hs-CRP and body composition were analysed.
Patient and public involvement
Neither patients nor public were involved in the development of the research question, in the study design and in drawing conclusions from the results.
Laboratory and anthropometric measurements
Data, including demographic characteristics, educational level (<college graduation vs ≥college graduation), alcohol history, smoking status, physical activity, total nutrient intake and medical history of hypertension (HTN), DM, heart disease, stroke and hyperlipidaemia, were collected by the examining physicians using standardised self-administered questionnaires. Subjects with alcohol consumption over 20 g/day were categorised into a heavy drinking group. Individuals with smoking history were grouped into never, former or current smoking categories. Physical activity was assessed using the International Physical Activity Questionnaire-Short Form. Regular physical activity was subjects who performed vigorous exercise >3 times/week for over 20 min/session or moderate exercise >5 times/week for over 30 min/session. Total nutrient intake (kcal/day) was assessed using a 103-item self-administered Food Frequency Questionnaire validated for usage in South Korea, and estimated using a food composition table developed by the Korean Nutrition Society.
Serum biochemical parameters and anthropometric measurements were collected by trained nurses. Blood specimens were collected from the antecubital vein in the morning after at least 10 hours of fasting. Serum biochemical parameters included hs-CRP, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, triglycerides, fasting glucose, fasting insulin, glycated haemoglobin, homoeostasis model assessment of insulin resistance (HOMA-IR) and alanine aminotransferase (ALT). Insulin resistance was calculated using the following formula: HOMA-IR=fasting serum insulin (µU/mL) x fasting serum glucose (mg/dL)/405. Serum creatinine assays were analysed using an enzyme caloric method by a Hitachi Automatic Analyzer 7600 (Hitachi, Japan) and calibrated with the isotope dilution mass spectroscopic standard. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) was applied to obtain estimated glomerular filtration rate (eGFR). CKD was defined as eGFR <60 mL/min per 1.73 m2.21 Waist circumference (WC) (cm) was measured as the smallest circumference between the lower end of the sternum (xiphoid process) and the umbilicus. Skeletal muscle mass (kg) was estimated using a bioelectrical impedance analysis (BIA) with eight-point tactile electrodes (InBody 720, Biospace, South Korea). The BIA was calibrated every morning prior to the test and validated for reproducibility and accuracy for analysis of skeletal muscle mass.
Measurement of hs-CRP
Low-grade inflammation was assessed according to serum hs-CRP level, which was analysed using a nephelometric assay with a BN II nephelometer (Dade Behring, Deerfield, Illinois, USA). Participants were grouped into low (<1.0 mg/L) and high (≥1.0 mg/L) hs-CRP groups. Serum hs-CRP below 1 mg/L was considered a healthy level due to its lowest risk for cardiovascular diseases,22 23 and this cut-off value has been widely used in previous studies.24–27 Values of hs-CRP exceeding 10 mg/L were excluded.
Sarcopenia and SO
Skeletal Muscle Mass Index (SMI) was estimated using the following formula: SMI (%)=skeletal muscle mass (kg)/body wt (kg) x 100, according to the established method by Janssen et al.28 Sarcopenia was defined as SMI less than 2 SD below the sex-specific mean of a young adult (20–39 years old).28 The cut-off levels for sarcopenia were 36.7% in men and 32.2% in women, respectively. Obesity was defined as WC ≥90 cm for males and ≥85 cm for females, based on the cut-off points for central obesity in Korean population.29 Thus, SO was defined as sarcopenia combined with WC ≥90 cm in men and ≥85 cm in women, respectively.30 31
Statistical analysis
Baseline demographic characteristics among the study groups of body composition were compared using one-way analysis of variance (ANOVA) for continuous variables and X2 test for categorical variables. Additionally, after adjustments for age, sex, comorbidities (HTN, DM, stroke and heart disease), LDL-C, HOMA-IR, ALT, eGFR, health behavioural (smoking, alcohol drinking, physical and energy intake) and demographic factors (marital status and education level), the adjusted means were compared between the study groups using analysis of covariance.
Multivariate logistic regression analyses were conducted to determine the association between high hs-CRP (≥1.0 mg/L) and the study groups with varying body composition. ORs were calculated as the risks for subjects with high hs-CRP values in obesity only, sarcopenia only and SO compared with normal (reference), respectively. We performed two different models with multivariate adjustments for confounding variables. The first model (model 1) was adjusted for age, sex, comorbidities (HTN, DM, stroke, heart disease), LDL-C, HOMA-IR, ALT and eGFR. The second model (model 2) was additionally adjusted for health behavioural (smoking, alcohol drinking, physical, energy intake) and demographic factors (marital status and education level). Furthermore, hs-CRP was introduced as a continuous variable. Multivariate-adjusted coefficients (95% CI) were estimated according to multivariate general linear models using natural log (hs-CRP +1) as the outcome for increasing hs-CRP in the study groups. We repeated multivariate general linear models for subgroup analyses in study subjects stratified by gender (male and female) and age (<60 years vs ≥60 years). Interactions by subgroup were conducted using likelihood ratio tests comparing models with and without multiplicative interaction terms. The level of statistical significance was set at p<0.05. All analyses were conducted using IBM SPSS V.18.0 (IBM).
Results
Baseline demographic characteristics of study participants
The baseline characteristics of the 237 838 eligible participants according to four different groups of body composition are reported in table 1. The mean age of all participants was 39.0 (SD 8.9) years, and the proportion of males was 53.9%. The proportions of the normal, obesity only, sarcopenia only and SO groups were 77.1%, 19.9%, 0.3% and 2.7%, respectively. The group differences in baseline characteristics according to the body composition were significant for all variables (all p<0.001).
Table 1.
Baseline characteristics of study subjects according to body composition (n=237 838)
| Variables | Normal (n=1 83 326) |
Obesity only (n=47 305) |
Sarcopenia only (n=694) |
Sarcopenic obesity (n=6513) |
P values |
| Demographic factors | |||||
| Age (year) | 38.5 (8.6) | 40.5 (9.2) | 41.6 (12.9) | 40.0 (11.3) | <0.001* |
| Sex | |||||
| Male (%) | 48.6 | 75.0 | 23.6 | 51.3 | <0.001† |
| Female (%) | 51.4 | 25.0 | 76.4 | 48.7 | <0.001† |
| Marital status, married (%) | 79.4 | 82.8 | 69.2 | 71.5 | <0.001† |
| College graduate (%) | 64.3 | 63.4 | 48.3 | 50.8 | <0.001† |
| Height (cm) | 166.4 (8.1) | 171.4 (8.2) | 158.1 (7.4) | 165.6 (9.1) | <0.001* |
| Weight (kg) | 61.2 (9.9) | 79.4 (10.3) | 62.9 (7.7) | 84.7 (14.3) | <0.001* |
| BMI (kg/m2) | 22.0 (2.4) | 26.9 (2.2) | 25.1 (2.2) | 30.7 (3.4) | <0.001* |
| Waist circumference (cm) | 77.8 (6.9) | 93.4 (4.7) | 81.9 (4.2) | 99.7 (8.3) | <0.001* |
| Skeletal muscle mass (kg) | 25.3 (5.6) | 31.7 (5.8) | 19.6 (3.0) | 27.1 (6.2) | <0.001* |
| SMI (%) | 41.4 (3.6) | 39.3 (3.2) | 32.2 (2.2) | 32.9 (2.8) | <0.001* |
| Health behavioural factors | |||||
| Current smoker (%) | 18.8 | 31.6 | 8.5 | 23.0 | <0.001† |
| Heavy drinking (%) | 14.5 | 26.9 | 7.9 | 20.0 | <0.001† |
| Regular physical activity (%) | 15.9 | 15.5 | 10.6 | 10.6 | <0.001† |
| Daily energy intake (cal/day) | 1429.2 (690.0) | 1551.2 (753.6) | 1348.0 (736.1) | 1486.6 (801.8) | <0.001* |
| Comorbidity | |||||
| Hypertension (%) | 5.8 | 16.2 | 12.3 | 22.3 | <0.001† |
| Diabetes mellitus (%) | 1.9 | 4.6 | 3.2 | 6.0 | <0.001† |
| Heart disease (%) | 0.6 | 1.1 | 1.6 | 1.4 | <0.001† |
| Stroke (%) | 0.3 | 0.5 | 1.0 | 0.7 | <0.001† |
| Hyperlipidaemia (%) | 10.3 | 21.4 | 15.3 | 22.9 | <0.001† |
| Chronic kidney disease (%) | 0.2 | 0.6 | 1.0 | 0.7 | <0.001† |
| Laboratory findings | |||||
| Total cholesterol (mg/dL) | 190.4 (32.8) | 203.6 (35.6) | 202.1 (36.2) | 206.1 (36.7) | <0.001* |
| LDL-C (mg/dL) | 115.4 (30.6) | 130.9 (32.1) | 125.9 (33.3) | 132.6 (33.1) | <0.001* |
| HDL-C (mg/dL) | 60.3 (14.8) | 49.9 (12.0) | 59.2 (14.7) | 50.7 (12.1) | <0.001* |
| Triglycerides (mg/dL) | 98.3 (64.6) | 153.6 (97.1) | 109.3 (61.1) | 149.8 (93.0) | <0.001* |
| Fasting glucose (mg/dL) | 93.3 (12.6) | 99.5 (17.6) | 95.4 (15.3) | 102.2 (22.6) | <0.001* |
| Fasting insulin (IU/L) | 5.2 (4.0) | 8.8 (5.7) | 7.1 (3.8) | 12.1 (7.1) | <0.001* |
| HbA1c (%) | 5.5 (0.4) | 5.7 (0.6) | 5.6 (0.5) | 5.8 (0.7) | <0.001* |
| HOMA-IR | 1.2 (1.1) | 2.2 (1.7) | 1.7 (1.0) | 3.1 (2.3) | <0.001* |
| ALT (U/L) | 19.6 (17.3) | 33.7 (27.6) | 22.7 (18.1) | 39.9 (35.0) | <0.001* |
| eGFR (mL/min/1.73 m2) | 100.9 (14.0) | 96.5 (14.1) | 106.6 (15.6) | 101.6 (15.3) | <0.001* |
Data are presented as means (SD) or percentage.
P values for group difference by *one-way ANOVA in continuous variables or by †χ2 test in categorical variables.
ANOVA, analysis of variance; ALT, alanine aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homoeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; SMI, Skeletal Muscle Mass Index.
Comparison of hs-CRP according to body composition
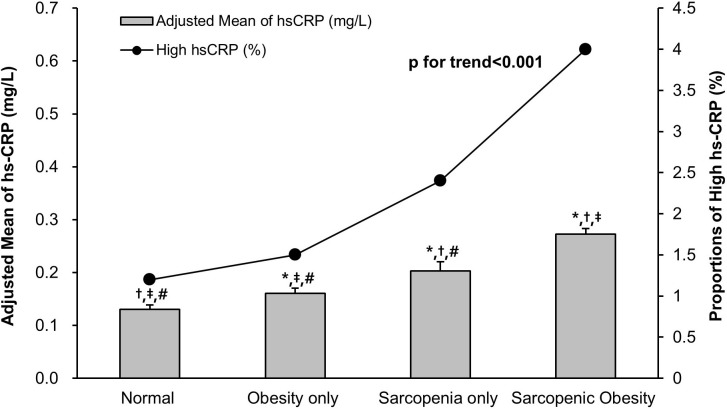
Significant group differences existed in continuous level of hs-CRP (online supplementary table S1; p for one-way ANOVA <0.001; figure 1). Among the four groups, the level of hs-CRP was highest in SO. The level of hs-CRP gradually increased from normal to obesity only group, obesity only to sarcopenia only group and sarcopenia only to SO group, even after adjustment for various confounders (figure 1; adjusted p<0.001; p for trend <0.001). The proportions of subjects with high levels of hs-CRP (≥1.0 mg/L) were 1.2%, 1.5%, 2.4% and 4.0% in normal, obesity only, sarcopenia only and SO groups, respectively, and showed a positive trend along the study groups (p for trend <0.001).
Figure 1.
Comparison of hs-CRP between study groups according to body composition. Adjusted means of hs-CRP in the study groups were estimated from ANCOVA after adjustments for age, sex, comorbidities (HTN, DM, heart disease, stroke), LDL-C, HOMA-IR, ALT, eGFR, health behavioural (smoking, heavy drinking, physical activity, energy intake) and demographic factors (marital status, education level). *Adjusted p<0.001 versus normal group in post hoc analysis. †Adjusted p<0.001 versus obesity only group in post hoc analysis. ‡Adjusted p<0.001 versus sarcopenia only group in post hoc analysis. #Adjusted p<0.001 versus sarcopenic obesity group in post hoc analysis. ANCOVA, analysis of covariance; ALT, alanine aminotransferase; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HOMA-IR, homoeostasis model assessment of insulin resistance; HTN, hypertension; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol.
bmjopen-2017-021232supp001.pdf (252.3KB, pdf)
Relationship between hs-CRP and body composition
Table 2 presents the results of univariate and multivariate logistic regression analyses between the high levels of hs-CRP (≥1.0 mg/L) and the groups with body composition. In the first model (model 1), adjusted for possible confounders (age, sex, HTN, DM, heart disease, stroke, LDL-C, HOMA-IR, ALT and eGFR), adjusted ORs (95% CI) for subjects with high hs-CRP for obesity only, sarcopenia only and SO groups compared with normal group (reference) were 1.19 (1.08 to 1.31), 2.34 (1.43 to 3.79) and 3.39 (2.95 to 3.89) (p for trend <0.001). The second model (model 2) was additionally adjusted for health-related behaviour and demographic factors including smoking, heavy drinking, physical activity, total energy intake, marital status and educational level. Adjusted ORs (95% CI) for subjects with high hs-CRP for obesity only, sarcopenia only and SO groups compared with normal group (reference) were 1.17 (1.05 to 1.31), 2.23 (1.21 to4.07) and 3.23 (2.71 to 3.83) (p for trend <0.001).
Table 2.
Multivariate regression analyses showing associations between high hs-CRP (≥1.0) and the groups of body composition
| Crude ORs | Model 1 | Model 2 | |
| Normal | Reference | Reference | Reference |
| Obesity only | 1.17 (1.07–1.27) | 1.19 (1.08–1.31) | 1.17 (1.05–1.31) |
| Sarcopenia only | 2.23 (1.37–3.62) | 2.34 (1.43–3.79) | 2.23 (1.21–4.07) |
| Sarcopenic obesity | 3.41 (2.98–3.89) | 3.39 (2.95–3.89) | 3.23 (2.71–3.83) |
| P for trend | <0.001 | <0.001 | <0.001 |
Model 1: age, sex, comorbidities (HTN, DM, heart disease, stroke), LDL-C, HOMA-IR, ALT and eGFR.
Model 2: Model 1+ health behavioural (smoking, heavy drinking, physical activity, energy intake) and demographic factors (marital status, education level).
ALT, alanine aminotransferase; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HOMA-IR, homoeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol.
When hs-CRP was introduced as a continuous variable, multivariate (model 2) linear regression analyses between hs-CRP and the study groups were performed (online supplementary figure S1). In the model 2 analyses, the coefficients for obesity only, sarcopenia only and SO groups compared with normal group (reference) were 1.48 (1.45 to 1.52), 1.98 (1.81 to 2.17) and 2.74 (2.60 to 2.90), respectively (p for trend <0.001).
Sex differences in the relationship between hs-CRP and body composition
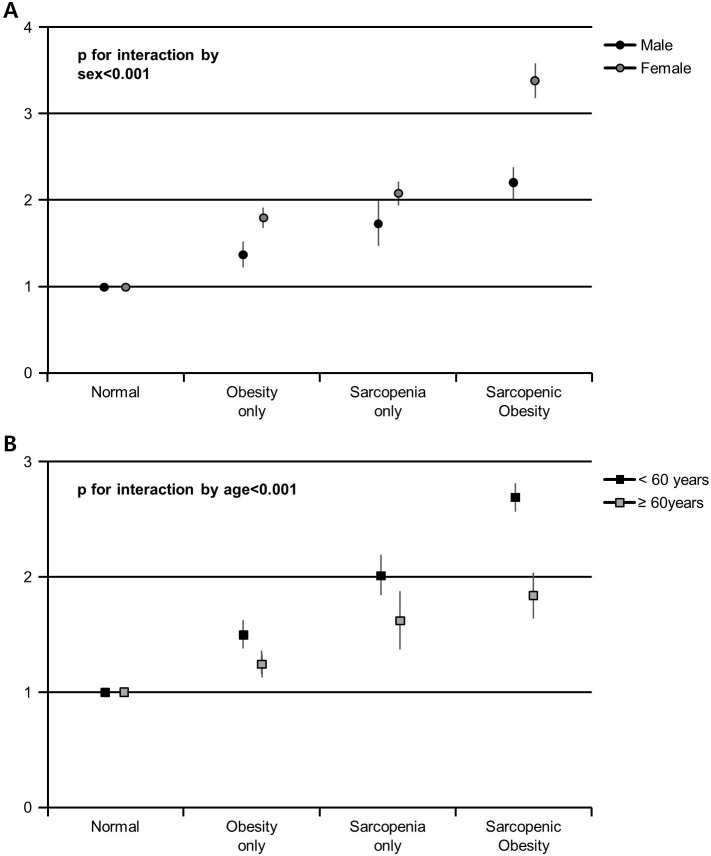
Based on sex subgroup analyses, the associations between hs-CRP and body composition are presented in figure 2A and table 3. In both male and female groups, there was a positive trend in adjusted ORs for the subjects with high hs-CRP (≥1.0) in obesity only, sarcopenia only and SO groups compared with the normal group (all p for trend <0.001). Interestingly, the associations of high hs-CRP (≥1.0) with obesity only, sarcopenia only and SO groups were stronger in females than in male participants (table 3; p for interaction <0.001). When using hs-CRP as a continuous variable, these associations were sustained showing higher coefficients in females than in male groups (figure 2A; p for interaction <0.001).
Figure 2.
*Multivariate-adjusted coefficients (95% CI) for increasing hs-CRP according to groups of body composition (A) in male and female subjects, and (B) in younger (<60 years) and older subjects (≥60 years). The p value for the interaction by sex or age between body composition and increasing hs-CRP was indicated. *Estimated from multivariate general linear models used with natural log (hs-CRP +1) as the outcome. Multivariate model (model 2) was adjusted for sex, comorbidities (HTN, DM, heart disease, stroke), LDL-C, HOMA-IR, ALT, eGFR, health behavioural (smoking, heavy drinking, physical activity, energy intake) and demographic factors (marital status, education level). ALT, alanine aminotransferase; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HOMA-IR, homoeostasis model assessment of insulin resistance; HTN, hypertension; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol.
Table 3.
Multivariate regression analyses showing associations between high hs-CRP (>1.0) and the study groups by sex and age
| Subgroups | Crude ORs | Model 1 | Model 2 |
| Male (n=128 117) | |||
| Normal | Reference | Reference | Reference |
| Obesity only | 1.10 (1.00–1.22) | 1.14 (1.03–1.26) | 1.12 (0.98–1.27) |
| Sarcopenia only | 2.02 (0.83–4.95) | 2.08 (0.85–5.05) | 1.89 (0.60–5.98) |
| Sarcopenic obesity | 2.15 (1.75–2.63) | 2.19 (1.77–2.70) | 2.10 (1.63–2.71) |
| P for trend | <0.001 | <0.001 | <0.001 |
| Female (n=109 721) | |||
| Normal | Reference | Reference | Reference |
| Obesity only | 1.34 (1.12–1.60) | 1.30 (1.08–1.56) | 1.41 (1.12–1.78) |
| Sarcopenia only | 2.48 (1.39–4.41) | 2.47 (1.38–4.40) | 2.41 (1.19–4.91) |
| Sarcopenic obesity | 5.67 (4.76–6.75) | 5.04 (4.20–6.16) | 5.43 (4.26–6.93) |
| P for trend | <0.001 | <0.001 | <0.001 |
| P for interaction by sex | <0.001 | <0.001 | <0.001 |
| Age <60 (n=230 066) | |||
| Normal | Reference | Reference | Reference |
| Obesity only | 1.16 (1.07–1.28) | 1.20 (1.09–1.32) | 1.24 (1.10–1.34) |
| Sarcopenia only | 2.13 (1.25–3.63) | 2.27 (1.33–3.86) | 2.57 (1.21–4.29) |
| Sarcopenic obesity | 3.44 (3.00–3.95) | 3.44 (2.98–4.00) | 3.41 (2.82–3.98) |
| P for trend | <0.001 | <0.001 | <0.001 |
| Age ≥60 (n=7772) | |||
| Normal | Reference | Reference | Reference |
| Obesity only | 1.09 (0.77–1.55) | 1.03 (0.72–1.47) | 1.07 (0.80–1.19) |
| Sarcopenia only | 2.28 (0.70–7.39) | 1.94 (0.60–6.37) | 1.84 (0.88–3.21) |
| Sarcopenic obesity | 2.53 (1.57–4.06) | 2.19 (1.33–3.60) | 2.22 (1.61–2.80) |
| P for trend | <0.001 | <0.001 | <0.001 |
| P for interaction by age | <0.001 | <0.001 | <0.001 |
Model 1: age, sex, comorbidities (HTN, DM, heart disease, stroke), LDL-C, HOMA-IR, ALT and eGFR.
Model 2: Model 1+ health behavioural (smoking, heavy drinking, physical activity, energy intake) and demographic factors (marital status, education level).
ALT, alanine aminotransferase; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HOMA-IR, homoeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol.
Age differences in relationship between hs-CRP and body composition
In age subgroup analyses, the associations between hs-CRP and body composition are presented in figure 2B and table 3. In multivariate logistic regression models, the associations of high hs-CRP (≥1.0) with obesity only, sarcopenia only and SO groups were stronger in younger (<60 years) participants than in older (≥60 years) participants (table 3; p for interaction <0.001). When hs-CRP was introduced as a continuous variable, these associations persisted showing higher coefficients in younger than in older groups (figure 2B; p for interaction <0.001).
Additionally, we performed stratified analyses according to CKD status (present or absent) (online supplementary table S2). In multivariate logistic regression models, only the subgroup without CKD (eGFR ≥60) showed a positive trend in adjusted ORs for the subjects with high hs-CRP (≥1.0) in obesity only, sarcopenia only and SO groups compared with the normal group (p for trend <0.001). Due to a smaller number in the group with CKD, this analysis might be subject to power constraints.
Discussion
The two major findings of the study are as follows. First, the increased level of hs-CRP was independently associated with different groups of body composition, and the association was highest with SO group. Second, in subgroup analyses involving sex and age, we found a considerably strong association between increased hs-CRP and SO among females and younger (<60 years) subjects.
The hs-CRP level was significantly increased with altered body composition compared with normal reference group and was highest in subjects with SO. Furthermore, a positive trend for increased hs-CRP along the study groups from normal to obesity, sarcopenia and SO was observed. To our knowledge, this is the largest population study to show an association between hs-CRP and SO spanning the entire age frame of adulthood. Previous studies demonstrated the close association between obesity and increased CRP.16 20 Furthermore, a few studies observed that the levels of CRP were elevated in sarcopenia and SO,32 33 which were consistent with our findings. However, SO is a condition associated with low-grade inflammation. Therefore, a precise measurement tool using hs-CRP rather than standard CRP is useful in determining low-grade inflammation.13 Furthermore, a large epidemiological study confirming a relationship between SO and hs-CRP was lacking. A recent study by Yang et al showed that SO was associated with increased hs-CRP in elderly males but not in elderly females.34 However, the present study demonstrated that there were significant associations between SO and hs-CRP in both male and female subgroups. Furthermore, the association of high hs-CRP with SO was higher in female participants than in males. There are several differences. First, sample size was much higher in the present study than in the previous one. Second, although the previous study recruited only elderly subjects above 65 years, the present study included all adult ages to generalise the result and further stratified the subjects by age of 60 to find out a sex difference in the association of hs-CRP with SO. Moreover, the present study conducted the multivariate regression analyses with various confounding factors (eg, DM, cholesterol level, HOMA-IR, ALT, alcohol drinking, total energy intake and education level), which were not included in the previous study. Additionally, females of SO in the previous study had too low WC (81.9±6.3 cm), which may have diluted the association of SO with hs-CRP. Lastly, Yang et al34 used dual-energy X-ray absorptiometry (DEXA) for analysing body composition rather than BIA. Although recent studies demonstrated that BIA showed good validity and correlated well with DEXA,35 36 different methods may have affected the results of association of SO with hs-CRP. Therefore, further studies are needed to compare the results of the associations between SO and hs-CRP using different evaluation tools of body composition.
An inflammatory status would be expected to be highest among the subjects with SO. Kim et al37 reported that serum CRP levels were independently associated with SO among females, which is consistent with the present study. In our study, the level of hs-CRP was sharply increased and the adjusted ORs for elevated hs-CRP were highest in SO, even after adjustment for possible confounding factors such as age, sex, comorbidities, metabolic, heath-related behavioural and demographic factors. Additionally, we have confirmed significant associations between SO and hs-CRP either as a categorical variable or as a continuous variable. Therefore, our results suggest that hs-CRP represents a useful parameter representing the synergistic association of obesity and sarcopenia with inflammation.
In the present study, female subjects showed a stronger association between high hs-CRP and SO than males. This finding is in line with a previous study, which presented a higher association between CRP and obesity in females than in male subjects.38 However, studies investigating the role of sex differences in the correlation between hs-CRP and SO are unavailable. Although a few reports suggest that hormonal alteration is one of the factors that underlying sarcopenia,20 38 the pathophysiology of sex differences in the relationship between hs-CRP and SO is still unknown. A few possible explanations related to the sex-specific differences are as follows. First, the metabolic activity of adipose tissue in females differs from that of males, which may affect the levels of hs-CRP.39 Second, leptin is an adipocyte-derived hormone, which occurs at a higher level in females than in males. Thus, leptin may be a mediator of sex-specific correlation of hs-CRP with SO.40 41 Additionally, the distribution of body fat and skeletal muscle is different between females and males, which also may influence the levels of CRP.20 38 Therefore, SO may be strongly associated with high hs-CRP levels among females than in male subjects.
The present study demonstrated the strongest association between SO and increased hs-CRP in younger (<60 years) than in elderly (≥60 years) subjects. This is the first study of demonstrating age differences in the relationship between SO and hs-CRP. SO is commonly described as an age-related phenomenon, and is a multifactorial condition, which can occur in young-aged and middle-aged populations.4 Although in the elderly, it is mainly affected by ageing, SO in younger subjects can be triggered by other factors including nutritional deficit, physical activity or endocrine factors rather than ageing.42 43 Therefore, we suspect that the other factors in younger subjects may differently affect the levels of hs-CRP compared with elderly subjects. Furthermore, a previous study reported that the association of obesity with all-cause mortality was increased in younger-aged subjects.44 Considering that CRP is a risk factor for all-cause mortality,45 46 the present finding of strong association of hs-CRP with obesity in younger subjects is in line with a previous report.44 Moreover, we found the strongest association between hs-CRP and SO in younger than in elderly subjects. Although the precise pathophysiology of age differences is unknown, these findings are informative for clinicians when measuring hs-CRP in younger populations with SO.
The present study was conducted by defining obesity using WC. There are some reasons for using this method. First, recent studies demonstrated that SO defined by WC was associated with mortality.47 This is because the mortality associated with sarcopenia is related to increased catabolic metabolism of skeletal muscle in the presence of abdominal obesity.48 Although higher body mass index (BMI) has been known as a risk factor for cardiovascular diseases,44 recent studies presented that underweight BMI or weight loss status in late-aged life were associated with increased long-term mortality.49 50 Thus, whole body obesity by BMI was inversely associated with mortality (called ‘obesity paradox’). Moreover, a few studies revealed that abdominal obesity rather than whole body obesity by BMI was associated with both obesity-related mortality and all-cause mortality.51 52 Second, Batsis et al53 demonstrated that SO defined by per cent body fat mass was not associated with mortality even after adjustments for comorbidities, physical activity, smoking and mobility limitations. This is because per cent body fat mass and BMI could not detect the regional body fat such as visceral fat instead of measuring whole body fat. Additionally, Sanada et al54 investigated the associations between SO and all-cause mortality comparing three different definitions of obesity by BMI, per cent fat mass or WC in a longitudinal cohort study. Sanada et al demonstrated that all-cause mortality was significantly increased in men with SO defined by WC but not by BMI or per cent fat mass, suggesting that WC is a good index of SO compared with other obesity definition variables.54 Therefore, the present study was proceeded by defining obesity by WC.
In the current study, we investigated the independent association of SO with hs-CRP in Korean population. Based on our results, we conclude that increased hs-CRP was associated with the presence of SO. Furthermore, there was a strong relationship between SO and high hs-CRP among females and younger subjects. Previous studies reported a higher prevalence of sarcopenia-related oxidative stress in healthy males and older subjects, which would be related to low-grade inflammation.55 56 However, our findings suggest that females and younger subjects had stronger associations of increased hs-CRP with SO than males and older subjects. Furthermore, the associations persisted even after adjustment for various confounding factors such as comorbidities, demographic, metabolic and health-related behavioural factors, which supported the significance of the current findings. To the best of our knowledge, this is the first study reporting the role of sex and age differences in the association between SO and hs-CRP using a large study sample. Since the increased level of hs-CRP is considered as a significant risk factor for cardiovascular illness and all-cause mortality, this measurement may be useful to screen for inflammatory markers in female and younger subjects diagnosed with SO.
There are some limitations in this study. First, subjects aged 60 years and above were relatively fewer in number than subjects aged below 60, which may suggest a selection bias. However, we addressed this issue by analysing the data after adjustments for age. Second, because this was a cross-sectional study, cause–effect associations cannot be inferred. Third, the skeletal muscle mass was measured using BIA. Nevertheless, recent studies demonstrated that BIA showed good validity and correlated well with DEXA.35 36 Additionally, BIA is usefully applied for large-scale health screening purposes due to its speed and simplicity without the need for a specialised radiologist.57
In conclusion, we demonstrated that a high level of hs-CRP was associated with SO in Korean adults. Furthermore, SO in female and younger (<60 years) subjects had a stronger association with the high level of hs-CRP than in male and elderly subjects.
Supplementary Material
Footnotes
Y-TL and KJY contributed equally.
Contributors: C-HP: manuscript drafting, critical revision and study design. JGD: study supervision and data interpretation. Y-TL: study concepts, design and manuscript revision for important intellectual content. KJY: study concepts, design and critical revision of manuscript. All authors: final version approval.
Funding: This study was supported by a grant from Minister of Education (NRF-2017R1D1A1B03032899).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study protocol was approved by the Institutional Review Board of the Sungkyunkwan University School of Medicine.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–56. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr 1993;123:465–8. 10.1093/jn/123.suppl_2.465 [DOI] [PubMed] [Google Scholar]
- 3. Hyun YY, Lee KB, Rhee EJ, et al. Chronic kidney disease and high eGFR according to body composition phenotype in adults with normal BMI. Nutr Metab Cardiovasc Dis 2016;26:1088–95. 10.1016/j.numecd.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 4. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol 2012;24:623–7. 10.1097/BOR.0b013e328358d59b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prado CM, Wells JC, Smith SR, et al. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr 2012;31:583–601. 10.1016/j.clnu.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998;97:2099–100. 10.1161/01.CIR.97.21.2099 [DOI] [PubMed] [Google Scholar]
- 7. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 8. Kob R, Bollheimer LC, Bertsch T, et al. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology 2015;16:15–29. 10.1007/s10522-014-9539-7 [DOI] [PubMed] [Google Scholar]
- 9. Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lagrand WK, Visser CA, Hermens WT, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation 1999;100:96–102. 10.1161/01.CIR.100.1.96 [DOI] [PubMed] [Google Scholar]
- 11. Koenig W, Sund M, Fröhlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999;99:237–42. 10.1161/01.CIR.99.2.237 [DOI] [PubMed] [Google Scholar]
- 12. Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem 2001;47:418–25. [PubMed] [Google Scholar]
- 13. Kimberly MM, Vesper HW, Caudill SP, et al. Standardization of immunoassays for measurement of high-sensitivity C-reactive protein. Phase I: evaluation of secondary reference materials. Clin Chem 2003;49:611–6. 10.1373/49.4.611 [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001;103:1813–8. 10.1161/01.CIR.103.13.1813 [DOI] [PubMed] [Google Scholar]
- 15. Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 2010;87:407–16. 10.1038/clpt.2009.311 [DOI] [PubMed] [Google Scholar]
- 16. Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol 2010;106:56–61. 10.1016/j.amjcard.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 17. Nishida M, Moriyama T, Sugita Y, et al. Abdominal obesity exhibits distinct effect on inflammatory and anti-inflammatory proteins in apparently healthy Japanese men. Cardiovasc Diabetol 2007;6:27 10.1186/1475-2840-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med 2001;137:231–43. 10.1067/mlc.2001.113504 [DOI] [PubMed] [Google Scholar]
- 19. Malafarina V, Uriz-Otano F, Iniesta R, et al. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 2012;71:109–14. 10.1016/j.maturitas.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 20. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013;14:232–44. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- 21. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline:behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014. 49:e61. [DOI] [PubMed] [Google Scholar]
- 22. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 2004;116 Suppl 6A:9S–16. 10.1016/j.amjmed.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 23. Wilson PW, Pencina M, Jacques P, et al. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes 2008;1:92–7. 10.1161/CIRCOUTCOMES.108.831198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu K, Kim C, Sung E, et al. Association of Serum Total Bilirubin with Serum High Sensitivity C-reactive Protein in Middle-aged Men. Korean J Fam Med 2011;32:327–33. 10.4082/kjfm.2011.32.6.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higashiyama A, Wakabayashi I, Kubota Y, et al. Does high-sensitivity C-reactive protein or low-density lipoprotein cholesterol show a stronger relationship with the cardio-ankle vascular index in healthy community dwellers?: the KOBE study. J Atheroscler Thromb 2012;19:1027–34. 10.5551/jat.13599 [DOI] [PubMed] [Google Scholar]
- 26. Tsunoda K, Arita M, Yukawa M, et al. Retinopathy and hypertension affect serum high-sensitivity C-reactive protein levels in Type 2 diabetic patients. J Diabetes Complications 2005;19:123–7. 10.1016/j.jdiacomp.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 27. Sung KC, Ryu S, Lee JY, et al. All cause mortality and body mass index in a young Asian occupational cohort without baseline metabolic syndrome components. Int J Cardiol 2016;224:271–8. 10.1016/j.ijcard.2016.09.056 [DOI] [PubMed] [Google Scholar]
- 28. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–96. 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 29. Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75:72–80. 10.1016/j.diabres.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 30. Hwang B, Lim JY, Lee J, et al. Prevalence rate and associated factors of sarcopenic obesity in korean elderly population. J Korean Med Sci 2012;27:748–55. 10.3346/jkms.2012.27.7.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim YS, Lee Y, Chung YS, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci 2012;67:1107–13. 10.1093/gerona/gls071 [DOI] [PubMed] [Google Scholar]
- 32. Alemán H, Esparza J, Ramirez FA, et al. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing 2011;40:469–75. 10.1093/ageing/afr040 [DOI] [PubMed] [Google Scholar]
- 33. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017;35:200–21. 10.1016/j.arr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 34. Yang CW, Li CI, Li TC, et al. Association of sarcopenic obesity with higher serum high-sensitivity c-reactive protein levels in chinese older males-a community-based study (Taichung Community Health Study-Elderly, TCHS-E). PLoS One 2015;10:e0132908 10.1371/journal.pone.0132908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donini LM, Poggiogalle E, Del Balzo V, et al. How to estimate fat mass in overweight and obese subjects. Int J Endocrinol 2013;2013:1–9. 10.1155/2013/285680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dicembrini I, Pala L, Cresci B, et al. Predictors of weight loss in the clinical management of obese patients: The relevance of body composition. Obe Metab 2010;6:29–33. [Google Scholar]
- 37. Kim TN, Park MS, Lim KI, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin Endocrinol 2013;78:525–32. 10.1111/j.1365-2265.2012.04433.x [DOI] [PubMed] [Google Scholar]
- 38. Clark DO, Unroe KT, Xu H, et al. Sex and race differences in the relationship between obesity and C-Reactive protein. Ethn Dis 2016;26:197–204. doi:10.18865/ed.26.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001;4:499–502. 10.1097/00075197-200111000-00006 [DOI] [PubMed] [Google Scholar]
- 40. Couillard C, Mauriège P, Prud’homme D, et al. Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia 1997;40:1178–84. 10.1007/s001250050804 [DOI] [PubMed] [Google Scholar]
- 41. Ostlund RE, Yang JW, Klein S, et al. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 1996;81:3909–13. 10.1210/jcem.81.11.8923837 [DOI] [PubMed] [Google Scholar]
- 42. Ko BJ, Chang Y, Jung HS, et al. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler Thromb Vasc Biol 2016;36:1016–21. 10.1161/ATVBAHA.116.307156 [DOI] [PubMed] [Google Scholar]
- 43. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third national health and nutrition examination survey. J Clin Endocrinol Metab 2011;96:2898–903. 10.1210/jc.2011-0435 [DOI] [PubMed] [Google Scholar]
- 44. Stevens J, Cai J, Pamuk ER, et al. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998;338:1–7. 10.1056/NEJM199801013380101 [DOI] [PubMed] [Google Scholar]
- 45. Yeun JY, Levine RA, Mantadilok V, et al. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000;35:469–76. 10.1016/S0272-6386(00)70200-9 [DOI] [PubMed] [Google Scholar]
- 46. Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J 2000;21:1584–90. 10.1053/euhj.1999.1982 [DOI] [PubMed] [Google Scholar]
- 47. Atkins JL, Whincup PH, Morris RW, et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc 2014;62:253–60. 10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 2000;55:M716–M724. 10.1093/gerona/55.12.M716 [DOI] [PubMed] [Google Scholar]
- 49. Fleischmann E, Teal N, Dudley J, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 1999;55:1560–7. 10.1046/j.1523-1755.1999.00389.x [DOI] [PubMed] [Google Scholar]
- 50. Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 2006;83:202–10. 10.1093/ajcn/83.2.202 [DOI] [PubMed] [Google Scholar]
- 51. McNeely MJ, Shofer JB, Leonetti DL, et al. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes Care 2012;35:296–8. 10.2337/dc11-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saito I, Kokubo Y, Kiyohara Y, et al. Prospective study on waist circumference and risk of all-cause and cardiovascular mortality: pooled analysis of Japanese community-based studies. Circ J 2012;76:2867–74. [DOI] [PubMed] [Google Scholar]
- 53. Batsis JA, Mackenzie TA, Barre LK, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001–7. 10.1038/ejcn.2014.117 [DOI] [PubMed] [Google Scholar]
- 54. Sanada K, Chen R, Willcox B, et al. Association of sarcopenic obesity predicted by anthropometric measurements and 24-y all-cause mortality in elderly men: The Kuakini Honolulu Heart Program. Nutrition 2018;46:97–102. 10.1016/j.nut.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 55. Fulle S, Belia S, Di Tano G. Sarcopenia is more than a muscular deficit. Arch Ital Biol 2005;143:229–34. [PubMed] [Google Scholar]
- 56. Fanò G, Mecocci P, Vecchiet J, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil 2001;22:345–51. 10.1023/A:1013122805060 [DOI] [PubMed] [Google Scholar]
- 57. Norman K, Pirlich M, Sorensen J, et al. Bioimpedance vector analysis as a measure of muscle function. Clin Nutr 2009;28:78–82. 10.1016/j.clnu.2008.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-021232supp001.pdf (252.3KB, pdf)