Abstract
Cocaine adulterated levamisole is an increasingly reported cause of skin necrosis, arthralgia and systemic vasculitis, but renal involvement is uncommon. We present a case of a 40-year-old Hispanic man with a history of cocaine abuse who presented with acute kidney injury to the rheumatology clinic where he was being treated for chronic inflammatory arthritis. He was found to have a serum creatinine of 2.5 mg/dL, microscopic haematuria and subnephrotic proteinuria, along with positive proteinase 3, myeloperoxidase, anticardiolipin antibodies and an elevated antinuclear antibody titre. The renal pathology revealed focal necrotising glomerulonephritis with crescentic features and mild immune type deposition. The patient was treated with cocaine abstinence, pulse dose steroids followed by maintenance prednisone, rituximab and cyclophosphamide. His renal function subsequently improved but did not normalise. We believe that his incomplete improvement was due to the degree of kidney injury on presentation as well as recidivism with cocaine use.
Keywords: acute renal failure, chronic renal failure, drug misuse (including addiction), vasculitis, hematuria
Background
Cocaine adulterated levamisole (CAL) intake is associated more commonly with arthralgia, skin lesions (reticular rash, necrosis), cocaine-induced midline destructive lesion and agranulocytosis. Renal involvement is less frequent and, when present, is most commonly associated with Antineutrophil cytoplasmic antibodies (ANCA) vasculitis described under the umbrella of pauci-immune necrotising glomerulonephritis (GN). We describe a patient with CAL who is found to have pauci-immune GN with mild immune complex activity. Our patient did not have skin lesions or blood dyscrasias, but he was found to have arthritis and renal failure. This case highlights the fact that CAL can lead to crescentic GN with dual positivity for myeloperoxidase (MPO) and proteinase 3 (PR3) antibodies.
Case presentation
We present a 40-year-old Hispanic man with a medical history of polysubstance abuse, Fournier gangrene, pubic osteomyelitis, left foot abscess, chronic inflammatory symmetric arthritis, hypertension and steroid-induced diabetes mellitus who was being seen in a rheumatology clinic for chronic joint pain and was referred to nephrology for acute kidney injury. The patient had left hip pain and bilateral lower extremity swelling for 2 weeks and was being treated for chronic inflammatory symmetric arthritis with sulfasalazine and steroids by the rheumatology service. He had denied chest pain, shortness of breath, cough, haemoptysis, weight loss, night sweats, haematuria, dysuria, oral ulcers, red painful eye, rash, epistaxis, hand and foot drop, numbness, tingling and burning sensation. The patient has an extensive history of smoking, alcohol intake and marijuana and cocaine use for approximately 3 years. His last cocaine inhalation was 3 weeks prior to presentation. He denied use of any other illicit substances. He lives with his wife and three children and works as a salesman for electrical supplies.
His medications included hydrochlorothiazide, nifedipine, pantoprazole, sulfasalazine and prednisone. On physical examination, his blood pressure was 157/82 mm Hg, with a heart rate of 97 beats per minute, respiratory rate of 18 with a temperature of 97.9°F. His examination was significant for 1+ bilateral lower extremity oedema with chronic skin changes and a healed scar due to a history of left foot abscess. His cardiovascular, respiratory, abdominal and neurological examinations were otherwise unremarkable.
The urinalysis showed significant 3+ haematuria and >=300 mg/dL of proteinuria. Urine microscopy revealed 5–6 dysmorphic red blood cells/high-power field and one red blood cell cast. His urine protein/creatinine ratio was 2.6 g/g. Inflammatory markers such as erythrocyte sedimentation rate (ESR) and C reactive protein were elevated. Other positive serologies included low positive antinuclear antibody (ANA), high titres of PR3 antibody, MPO antibody and anticardiolipin immunoglobulin M (IgM) antibody (table 1).
Table 1.
Investigations
| Laboratory results (normal range) | Admission day | Discharge day | After 1 month |
| Haemoglobin (13.5–17.5 g/dL) | 9.2 | 9.7 | 9.0 |
| White cell count (3.9–10.6 x 10^9/L) | 6.5 | 9.1 | 7.7 |
| Platelet count (150–440 x 10^9/L) | 206 | 307 | 194 |
| Sodium (135–145 mEq/L) | 136 | 137 | 140 |
| Potassium (3.5–5.0 mEq/L) | 4.4 | 4.0 | 4.4 |
| Chloride (98–108 mEq/L) | 100 | 103 | 105 |
| Carbon dioxide (24–30 mEq/L) | 21.3 | 22.7 | 25.6 |
| Blood urea nitrogen (5–26 mg/dL) | 40 | 44 | 41 |
| Creatinine (0.1–1.5 mg/dL) | 2.4 | 1.6 | 1.7 |
| Glucose (70–105 mg/dL) | 184 | 224 | 238 |
| Calcium (9.0–11 mg/dL) | 8.6 | 8.9 | 8.8 |
| Total protein (6.0–8.5 mg/dL) | 7.5 | 6.1 | |
| Albumin (3.3–505 g/dL) | 3.4 | 3.9 | |
| Total bilirubin (0.1–1.2 mg/dL) | 0.2 | 0.5 | |
| Direct bilirubin (0.1–0.3 mg/dL) | 0.1 | ||
| Alkaline phosphatase (30–115 U/L) | 92 | 63 | |
| Aspartate aminotransferase (5–40 U/L) | 17 | 22 | |
| Alanine aminotransferase (1–40 U/L) | 10 | 10 | |
| Hepatitis B | Negative | ||
| Hepatitis C | Negative | ||
| Urine analysis | Large blood (3+), red blood cell: 146, Protein >=300 | Large blood (3+), red blood cell: 224, Protein >=300 | |
| Urine protein to creatinine ratio (g/g) | 2.9 | 3.0 | |
| Erythrocyte sedimentation rate (mm/hour) | 106 | 82 | 47 |
| C reactive protein (CRP mg/dL) | 34.8 | 3.7 | 1.5 |
| Antinuclear antibodies, IU/mL | Positive 1:320 | Positive 1:320 | Negative |
| Antidouble-stranded DNA, (<=99 IU/mL) | 123 (Low Positive) | 199 (Low Positive) | 81 (Negative) |
| Lupus anticoagulant | Positive | ||
| Anti-myeloperoxidase antibody (MPO-ANCA) (<=20) | 127 | 131 | 130 |
| Proteinase 3 autoantibody (PR 3 ANCA) (<=20) | >150 | 144 | 118 |
| Anticardiolipin antibody IgM (<=19.9) | 35.6 | ||
| Anticardiolipin antibody IgG | Negative | ||
| Complement C3 (90–180 mg/dL) | 138 | 130 | |
| Complement C4, mg/dL | 42.8 | 38.7 | |
| Cryoglobulin | Negative | ||
| Serum protein electrophoresis | Biclonal IgG kappa and IgG lambda in gamma region | ||
| Urine protein electrophoresis | Trace amount of all fractions present; no monoclonal bands | ||
| HIV | Negative | ||
| Tuberculin skin test | Negative | ||
| QuantiFERON gold test | Negative |
ANCA, Antineutrophil cytoplasmic antibodies.
The patient’s urine toxicology screen was negative for cocaine while his plasma analysed for levamisole by liquid chromatography/tandem mass spectrometry also returned negative.
Because of clinical and laboratory suspicion of ANCA vasculitis, the patient was prepared for renal biopsy. Prior to biopsy, the patient empirically received pulse dose methylprednisolone (1 gm per day) for 3 days and was continued maintenance prednisone (1 mg/kg) for presumed rapidly progressive G N glomerulonephritis.
Renal biopsy
Of the 21 non-globally sclerotic glomeruli, three showed cellular crescents and/or fibrinoid necrosis, two contained fibrocellular crescents and five displayed fibrous crescents (figure 1). There was focal minimal mesangial expansion without significant mesangial hypercellularity. Immunofluorescence staining for IgG (2+), IgM (trace to 1+), IgA (1+), C3 (trace to 1+), kappa (1–2+) and lambda (1+) and electron microscopy revealing mesangial, segmental 1–2+ and subendothelial trace to 1+ electron dense deposits. Taken together, the renal biopsy was consistent with focal necrotising and crescentic GN with immune type deposition. The immune complex disease was graded as mild given the lack of mesangial or endocapillary proliferation and the restriction of the deposits largely to the mesangium with only a few subendothelial deposits which was out of proportion of the severity of crescentic lesions that involved 10/21 of total sampled glomeruli. Tubular atrophy and interstitial fibrosis involving 50%–60% of cortex was seen along with diffuse inflammation (figure 1).
Figure 1.
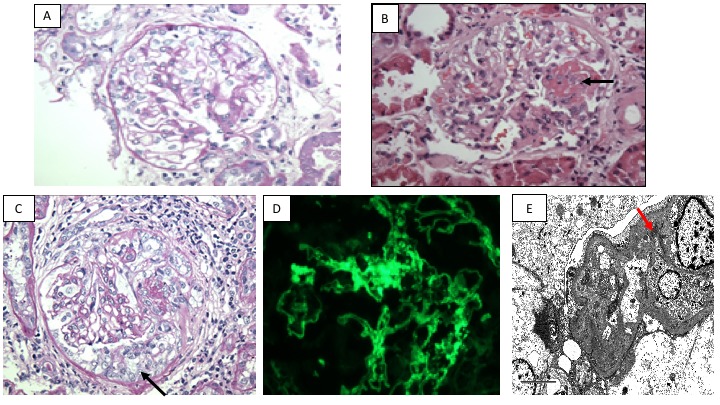
(A) A representative non-affected normocellular glomerulus. The glomerular capillary lumina are largely patent without significant endocapillary, mesangial or extracapillary proliferation (Periodic acid-Schiff, magnification x400). (B) An affected glomerulus displays segmental fibrinoid necrosis (arrow) (H&E, magnification x400). (C) A glomerulus containing cellular crescent (arrow) (Periodic acid-Schiff, magnification x400). (D) The glomeruli display largely mesangial staining for IgG (fluorescence micrograph, magnification x400). (E) Mesangial and subendothelial electron dense immune-type deposits are identified. A focal mesangial electron dense deposit is seen (red arrow). (Electron micrograph, magnification x6000).
Treatment
A transoesophageal echocardiogram excluded the presence of endocarditis prior to starting the patient on additional immunosuppressive treatment. The patient was continued on prednisone and received rituximab 375 mg/m2 every week for four doses. Unfortunately, the patient was lost to follow-up for 3 months before he returned to our clinic. He was then found to have a serum creatinine of 2.1 mg/dL with persistent proteinuria (1.8 g/g) and moderate haematuria. We made a decision to restart immunosuppression with cyclophosphamide for which he received two doses of cyclophosphamide 15 mg/kg body weight, to a maximum dose of 1500 mg intravenous every 2 weeks for two doses. He was then resumed back on prednisone 60 mg daily while we maximised his renin–angiotensin blockade with an ACE inhibitor. Prophylactic measures included dapsone for Pneumocystis jiroveci pneumonia infection, ergocalciferol and calcium supplements for osteoporosis, and a proton pump inhibitor for peptic ulcer disease were administered.
Outcome and follow-up
The patient was intermittently non-compliant with his prednisone and his clinic appointments. One year later, his serum creatinine stabilised at 1.2 mg/dL, but the urinalysis continued to show haematuria and proteinuria with a protein/creatinine of 0.4 g/g. The MPO and PR3 antibodies remained positive without significant change in titres, but his ESR normalised. Despite denying further cocaine use, the patient tested positive for cocaine in his urine at his most recent clinic visit.
Discussion
Levamisole is an antihelminthic agent that has become a common additive to cocaine over the past decade. Previously, it was used in medications for paediatric nephrotic syndrome, colon cancer, inflammatory bowel disease, rheumatoid arthritis and various dermatological pathologies because of its immunomodulatory properties.1 2 However, due to side effects including agranulocytosis, leucopenia and skin vasculitis, it was withdrawn from the market in the USA in 1999.3 In 2013, the Drug Enforcement Administration reported that up to 70% of illicit cocaine samples in the USA contained levamisole. An Australian study in 2018 showed similar results where levamisole was detected in approximately 75% of the urine of cocaine users.4 Animal models show that levamisole potentiates euphoric effects by inhibiting both monoamine oxidase and catechol-O-methyltransferase activity, increasing dopamine, and stimulating the reuptake inhibition effect of cocaine.3 Levamisole is also known to be metabolised into aminorex, an amphetamine-like stimulant.5 It also increases the bulk of cocaine without affecting its morphology.3
Levamisole is rapidly absorbed from the gastrointestinal tract and is mainly metabolised in the liver with only 2%–3% excreted in the urine. The presence of levamisole can be tested in plasma, urine and hair using gas chromatography-mass spectrometry (GC-MS).6 However, plasma and urine test may be negative depending on when the drug was used last since it only has a short half-life of 5–6 hours.7 Hence, it is often difficult to establish that levamisole was indeed the culprit despite the presence of cocaine which lasts for 47–72 hours in the urine. GC-MS or liquid chromatography/MS analysis of the patient’s urine may be used; however, it is recognised that these are specialised techniques that are expensive, labour intensive and not readily available in most clinical laboratories. Corroborative tests such as complete blood count to evaluate for leucopenia, renal function to evaluate for renal failure, antineutrophil cytoplasmic antibodies (see below), antiphospholipid antibodies, cryoglobulins and lupus anticoagulants are both sensitive and specific tests for levamisole-induced vasculitis.8
The pathophysiology involves binding of ANCA antibodies (IgG) to autoantigens which activate neutrophils causing release of the reactive oxygen species, degranulation with release of lytic enzymes, activating alternative complement pathway and forming neutrophil extracellular traps (NETs).9 NETs consist of various proinflammatory proteins and cause direct vessel inflammation by attacking endothelial cells and activating the complement system and indirectly link the innate and adaptive immune systems by the production of ANCAs leading to ANCA-associated vasculitis (AAV).10 Levamisole was found to cause increased NET formation through muscarinic M3 subtype receptors which was toxic to endothelial cells causing impaired vasorelaxation.11
There are a few case reports (table 2) of CAL-associated renal involvement in the form of pauci-immune GN,2 12 immune complex GN13 14 and in combination with membranous nephropathy.15 16 The first case was reported by Hansen et al in 1978 in a patient with rheumatoid arthritis treated with levamisole. After 10 months of treatment, the patient exhibited up to 1.8 g protein in a 24-hour urine collection while his kidney biopsy showed immune complex GN (which may be due to rheumatoid arthritis). The proteinuria subsided within 6 months after stopping levamisole.14 A large case series of 30 patients with cocaine and ANCA-associated disease from the Massachusetts General Hospital ANCA laboratory found that the most common presentations involved were arthralgia (83%) and skin lesions (61%).2 A unique feature of CAL-associated renal vasculitis is the combination of abnormal serologies. A sizeable number of patients tested positive for both MPO and PR3 antibodies, along with other positive autoantibodies including ANA, anticardiolipin antibody and lupus anticoagulant. Treatments in these case reports have ranged from the cessation of cocaine use to a number of different immune suppression regimens including plasmapheresis, high-dose steroids, cyclophosphamide, rituximab, tacrolimus or azathioprine (table 2). Patients with CAL-associated renal vasculitis who presented with the more severe disease had a worse prognosis for renal recovery despite aggressive immunosuppression.2 12–16
Table 2.
Case reports
| Articles | Cases | Biopsy result | Treatment |
| Garg et al13 | 40-year-old man Serum creatinine of 20.8 mg/dL Urine Pro:Cr ratio of 4.7 g/g positive PR3-ANCA and MPO-ANCA Low complement C3 and C4 High ESR and CRP |
Diffuse tubulointerstitial fibrosis with majority of glomeruli globally sclerosed | High-dose steroids tapered to prednisone 40 mg/day. CRRT followed by IHD. |
| McGrath et al2 | Two patients with renal failure out of a series of 30 patients 100% MPO-ANCA positive 50% with PR3-ANCA positive First patient had Serum creatinine 7.7mg/dL Second patient had Serum creatinine of 5.6 mg/dL. UA for both patients with haematuria and proteinuria |
One patient underwent renal biopsy which was suggestive of pauci-immune focal necrotising and crescentic GN. | Treated with immunosuppression (specific treatment not mentioned in the article) |
| Carrara et al15 | 34-year-old woman Serum creatinine of 4.2 mg/dL Urine Pro:Cr ratio of 2.4 g/g UA with haematuria and proteinuria. MPO-ANCA positive and PR3-ANCA negative. |
Crescentic glomeruli (30%–40%), mild interstitial fibrosis. Granular staining for IgG kappa and lambda light chains. Numerous immune type electron dense deposits in intramembranous and subepithelial locations. | Pulse methyl prednisolone (1 g daily x 3 days). PLEX: seven sessions. Rituximab infusions (Two doses of 1 g each) and IHD. |
| Liu et al17 | 48-year-old woman Serum creatinine of 3.17 mg/dL Urine Pro:Cr ratio of 2 g/g Urine sediment with dysmorphic RBCs PR3-ANCA positive Low C3 and C4 |
Glomeruli have crescents and segmental sclerosis. 80% interstitial fibrosis. EM with unusual deposits of granules and rare single fibrils. |
Methylprednisolone 50 mg intravenously/day (duration not mentioned) with 50 mg/day prednisone taper for 6 weeks |
| Carlson et al12 | Patient 1: 60-year-old male Serum creatinine of 1.6 mg/dL Urine sediment with dysmorphic RBCs Urine Pro:Cr ratio of 2.0 g/g |
Necrotising pauci-immune GN | Prednisone, intravenous cyclophosphamide |
| Patient 2: 49-year-old woman Serum creatinine of 7.31 g/dL Urine sediment with dysmorphic RBCs Urine Pro:Cr ratio of 4.9 g/g |
Focal segmental and global sclerosing glomerulopathy with cellular crescents | Prednisone, plasmapheresis, intravenous cyclophosphamide | |
| Patient 3: 63-year-old woman Serum creatinine of 3.25 mg/dL Urine Pro:Cr ratio of 0.7 g/g. |
Necrotising pauci-immune GN | Prednisone, oral cyclophosphamide. | |
| Patient 4: 43-year-old woman Serum creatinine of 14.2 mg/dL Anuric |
Necrotising pauci-immune GN. | No treatment. | |
| Collister et al16 | Patient 1: 53-year-old man Serum creatinine of 5.08 mg/dL UA with haematuria and proteinuria Urine Pro:Cr ratio of 1.65 g/g PLA2R: Negative |
Active focal crescentic and necrotising GN, diffuse glomerular capillary wall thickening with epimembranous spikes. | Cyclosporine and ACE inhibitor. |
| Patient 2: 35-year-old woman with haemoptysis, arthritis Serum creatinine of 1.7 mg/dL Urine Pro:Cr ratio of 5.24 g/g. |
Active segmental fibrinoid necrosis. Diffuse foot process effacement and subepithelial and intramembranous immune deposits on EM. | Prednisone 40 mg oral twice a day. Cyclophosphamide 1 g intravenously followed by 750 mg intravenously every month for 6 months. Maintenance with azathioprine which he did not tolerate. Enalapril 10 mg daily. |
|
| Patient 3: 34-year-old male with CAL-induced AAV being treated with immunosuppression developed new-onset proteinuria. Serum creatinine of 0.8 mg/dL Urine sediment with dysmorphic RBCs Urine Pro:Cr ratio of 3.98 g/g. |
Focal intervening spikes of new basement membrane material. EM showed three glomeruli with subepithelial immune complex deposits. | Azathioprine and Irbesartan 150 mg oral daily for secondary membranous nephropathy. Azathioprine replaced with oral cyclophosphamide 150 mg and prednisone 60 mg daily due to lack of response. Cyclophosphamide changed to tacrolimus 4 mg twice daily due to complications from cyclophosphamide. Immunosuppression continued for 2 years, stopped 3 months after complete remission, prednisone taper continued for 9 more months. |
AAV, ANCA-associated vasculitis; CRP, C reactive protein; EM, electron microscopy; ESR, erythrocyte sedimentation rate; GN, glomerulonephritis; MPO, myeloperoxidase; PR3, proteinase 3; RBC, red blood cell; Urine Pro:Cr, Urine protein to creatinine ratio; ANCA, Anti neutrophil cytoplasmic antibodies; RRT, Renal replacement therapy; IHD, Intermittent Hemodialysis; UA, Urine analysis; PLEX, Plasma exchange; PLA2R, Anti Phospholipase A2 receptor antibody; CRRT, Continuous renal replacement therapy.
Our patient presented only with arthralgia (no cutaneous findings) for 1 year for which he was being treated with sulfasalazine, prednisone and ibuprofen until he developed renal failure with ANCA positivity. Given our patient’s recent cocaine use and findings of dual positivity of ANCAs to both MPO and PR3 along with positive ANA and anticardiolipin antibody, we conclude that his pauci-immune crescentic, necrotising GN was associated with CAL.
The prognosis of CAL appears to be similar to that of drug-induced AAV. Patients who present with elevated baseline glomerular filtration rate (eGFR) >30 mL/min/m2 and low degree of chronicity/interstitial fibrosis fare better as compared with patients who present with low eGFR (<30 mL/min/m2) and moderate to severe chronicity/fibrosis. The most challenging aspect of managing this group of patients is to ensure proper follow-up and CAL abstinence as it has been shown that neither of those two situations is commonly achieved.15 16 It is also important to specifically inquire about cocaine use and prompt testing for urine cocaine in a patient who has high titres of MPO and/or PR3 antibodies.
Patient’s perspective.
The patient said at follow-up: ‘I am very thankful. Steroids and immunosuppression were scary.’
Learning points.
Levamisole can be associated with arthritis, focal necrotising and crescentic immune complex glomerulonephritis with the presence of both proteinase and myeloperoxidase antibodies.
Urine cocaine and plasma levamisole levels can be negative in these patients. Thus, awareness of this unusual entity in a susceptible patient will allow for a timely diagnosis and aggressive treatment to avoid permanent renal failure.
Cocaine abstinence is essential for a better prognosis.
Footnotes
Twitter: Dileep Kumar @dileeplakhani
Contributors: DK participated in the renal care for the patient and prepared the manuscript, IB contributed to writing of the manuscript and the assessment of the renal pathology, BM participated in the rheumatologic care for the patient and contributed to writing manuscript, BJ contributed to the writing of the manuscript and KA supervised the writing and clinical care of the patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol 2015;10:1300–10. 10.2215/CJN.01910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrath MM, Isakova T, Rennke HG, et al. . Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 2011;6:2799–805. 10.2215/CJN.03440411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010;88:408–11. 10.1038/clpt.2010.156 [DOI] [PubMed] [Google Scholar]
- 4.Pope JD, Drummer OH, Schneider HG. The cocaine cutting agent levamisole is frequently detected in cocaine users. Pathology 2018;50:536–9. 10.1016/j.pathol.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Hofmaier T, Luf A, Seddik A, et al. . Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters. Neurochem Int 2014;73:32–41. 10.1016/j.neuint.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karch SB, Busardò FP, Vaiano F, et al. . Levamisole adulterated cocaine and pulmonary vasculitis: Presentation of two lethal cases and brief literature review. Forensic Sci Int 2016;265:96–102. 10.1016/j.forsciint.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 7.Kouassi E, Caillé G, Léry L, et al. . Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine. Biopharm Drug Dispos 1986;7:71–89. 10.1002/bdd.2510070110 [DOI] [PubMed] [Google Scholar]
- 8.Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc 2012;87:581–6. 10.1016/j.mayocp.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennette JC, Nachman PH. ANCA Glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 2017;12:1680–91. 10.2215/CJN.02500317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Söderberg D, Segelmark M. Neutrophil extracellular traps in ANCA-Associated Vasculitis. Front Immunol 2016;7:256 10.3389/fimmu.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmona-Rivera C, Purmalek MM, Moore E, et al. . A role for muscarinic receptors in neutrophil extracellular trap formation and levamisole-induced autoimmunity. JCI Insight 2017;2:e89780 10.1172/jci.insight.89780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson AQ, Tuot DS, Jen KY, et al. . Pauci-immune glomerulonephritis in individuals with disease associated with levamisole-adulterated cocaine: a series of 4 cases. Medicine 2014;93:290–7. 10.1097/MD.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg L, Gupta S, Swami A, et al. . Levamisole/cocaine induced systemic vasculitis and immune complex glomerulonephritis. Case Rep Nephrol 2015;2015:1–5. 10.1155/2015/372413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen TM, Petersen J, Halberg P, et al. . Levamisole-induced nephropathy. Lancet 1978;2(8092 Pt 1):737 10.1016/S0140-6736(78)92737-X [DOI] [PubMed] [Google Scholar]
- 15.Carrara C, Emili S, Lin M, et al. . Necrotizing and crescentic glomerulonephritis with membranous nephropathy in a patient exposed to levamisole-adulterated cocaine. Clin Kidney J 2016;9:234–8. 10.1093/ckj/sfv141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collister D, Sathianathan C, Ryz K, et al. . ANCA ASsociated vasculitis secondary to levamisole-adultered cocaine with associated membranous nephropathy: a case series. Am J Nephrol 2017;45:209–16. 10.1159/000456553 [DOI] [PubMed] [Google Scholar]
- 17.Liu YW, Mutnuri S, Siddiqui SB, et al. . Levamisole-adulterated cocaine nephrotoxicity: ultrastructural features. Am J Clin Pathol 2016;145:720–6. 10.1093/ajcp/aqw029 [DOI] [PubMed] [Google Scholar]


