Abstract
Microangiopathic haemolytic anaemia with thrombocytopenia, called pseudo-thrombotic microangiopathy (TMA), is a clinically important complication in patients with vitamin B12 deficiency. We herein present a case of an 80-year-old woman with pseudo-TMA after gastrectomy. She was initially suspected with thrombotic thrombocytopenic purpura based on rapid progression of anaemia with schistocytes and thrombocytopenia; however, her anaemia and thrombocytopenia were improved by vitamin B12 supplementation alone, with a single session of plasma exchange. Vitamin B12 deficiency was finally confirmed by low vitamin B12 levels from the patient’s initial blood sample. In addition, normal ADAMTS13 activity was proven, lowering the likelihood of thrombotic thrombocytopenic purpura. Therefore, this patient was diagnosed with pseudo-TMA caused by vitamin B12 deficiency. Pseudo-TMA can occur in patients with vitamin B12 deficiency post-gastrectomy.
Keywords: haematology (incl blood transfusion), vitamins and supplements
Background
Vitamin B12 deficiency is relatively common, particularly in people aged over 60 years.1 A wide range of clinical manifestations of vitamin B12 deficiency has been reported, corresponding to clinical severity: fatigue, anaemia, glossitis and subtle neurological disturbance are often observed in patients with mild to moderate deficiency, whereas severe haematologic disturbance, severe neurological features and/or cardiomyopathy are observed with severe deficiency.1 Among haematologic disturbances, microangiopathic haemolytic anaemia with thrombocytopenia or pseudo-thrombotic microangiopathy (TMA)2 is a particularly important clinical complication in patients with vitamin B12 deficiency because distinguishing between this condition and life-threatening thrombotic thrombocytopenic purpura (TTP) is challenging at the time of presentation.3 Although pernicious anaemia is a well-known cause of vitamin B12 deficiency with pseudo-TMA, there is a paucity of data on pseudo-TMA due to vitamin B12 deficiency resulting from other causes. We herein describe a case of an elderly patient with pseudo-TMA whose vitamin B12 deficiency was caused by gastrectomy.
Case presentation
An 80-year-old Japanese woman was referred to our hospital due to suspicion of TTP. Twenty years prior, she underwent total gastrectomy, cholecystectomy and splenectomy for gastric cancer. Macrocytic anaemia was identified by her family physician, and she had been receiving vitamin B12 supplementation for 10 years; however, she stopped her treatment with details unknown. Although she had had no discomfort in daily living, her family noted that her cognitive function had mildly declined beginning 6 months earlier. One month prior, non-palpable purpura appeared on her left anterior thigh. One day before admission, she had headache and lost her appetite. On the day of presentation, her family found her lying on the floor without consciousness, and she was transferred to a hospital by ambulance. Her blood test showed thrombocytopenia, anaemia and elevated creatinine levels. Blood smear showed schistocytes. She was suspected to have TTP and was transferred to our hospital for further evaluation and treatment. Her medical history included type 2 diabetes, hypertension, osteoporosis and chronic constipation. She took ferrous sodium citrate, alfacalcidol, gliclazide, irbesartan, amlodipine and magnesium oxide. Her family history included a brother with leukaemia. She never smoked and had occasional alcohol consumption.
Investigations
On physical examination, she had a Glasgow Coma Scale score of E3V5M6, body temperature of 36.8°C, blood pressure of 142/79 mm Hg, pulse of 67/min, respiratory rate of 13/min and oxygen saturation of 98% with ambient air. Head and neck examination revealed pallor conjunctivae, but there was no icterus or goitre. Heart sound revealed 2/6 early systolic murmur that was best heard at the apex. Lungs were clear to auscultation bilaterally. Abdomen was unremarkable without signs of hepatomegaly. There was no peripheral oedema. There was bruising on her right periorbital area and right lower leg. Neurological examination was unremarkable except for bilateral patellar tendon hyporeflexia.
Complete blood counts revealed a slightly elevated white cell count (9100×109/L), a decreased haemoglobin level (6.1 g/dL) with elevated mean corpuscular volume (122.6 fL) and a decreased platelet count (72 000×109/L). The absolute reticulocyte count was 5.4020×1012/L (3.7%). Schistocytes (13%), Howell-Jolly bodies and hypersegmented neutrophils were observed on her peripheral blood smear (figure 1). Blood chemistry panel showed hyperkalaemia (5.6 mEq/L) and elevated levels of lactate dehydrogenase (1575 IU/L), aspartate aminotransferase (340 IU/L), alanine aminotransferase (268 IU/L), blood urea nitrogen (55 mg/dL) and creatinine (1.61 mg/dL). Prothrombin time and activated partial thromboplastin time were 18.6 s and 34 s, respectively. D-dimer level was elevated (21 µg/mL). Blood samples for vitamin B12 and folate levels were drawn at this time because vitamin B12 and/or folate deficiency were suspected based on the history of gastrectomy, macrocytic anaemia and the presence of hypersegmented neutrophils. Spot urinalysis revealed mild microscopic haematuria (1+) and mild proteinuria (30 mg/dL). Chest CT without contrast detected ground-glass opacity at the right pulmonary apex. Head and abdominal CTs were normal. Bone marrow aspiration showed normal counts of nuclear cells and megakaryocytes, with elevated prevalence of erythroblasts (43.6%), which resulted in a myeloid-to-erythroblast ratio of 1:1. There were no abnormal blasts.
Figure 1.
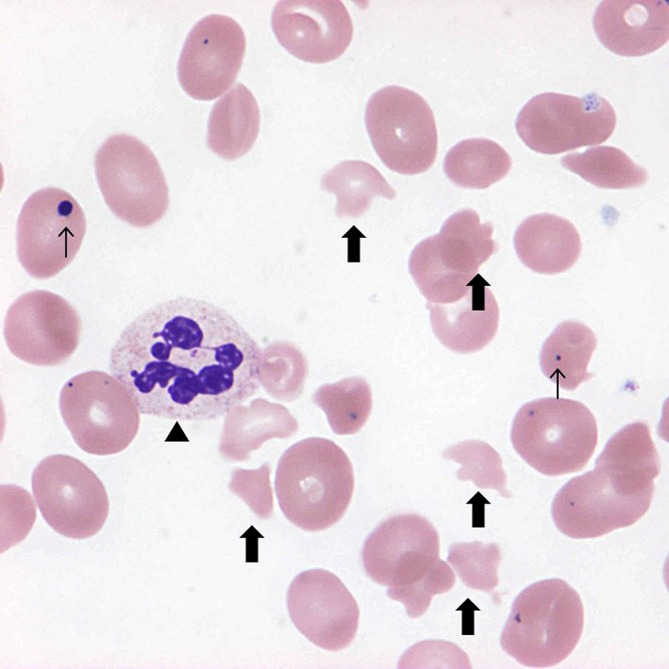
Peripheral blood smear at the time of admission. Peripheral blood smear at the time of admission (Giemsa stain, ×1000) showing multiple schistocytes (wide arrow), hypersegmented neutrophils (arrow head) and Howell-Jolly bodies (narrow arrow).
Differential diagnosis
On the basis of haemolytic anaemia with notable schistocytes on the peripheral blood smear and thrombocytopenia, microangiopathic haemolytic anaemia, particularly TMA, was considered; differential diagnoses were as follows: primary TMA syndromes such as TTP, complement-mediated TMA, Shiga toxin-mediated haemolytic uraemic syndrome, drug-induced TMA, coagulation-mediated TMA and metabolism-mediated TMA; disseminated intravascular coagulation; systemic infection; systemic malignancy; systemic rheumatic diseases such as systemic lupus erythematosus, systemic sclerosis and antiphospholipid syndrome and severe vitamin B12 deficiency. In this patient, primary TMA syndromes other than TTP seemed unlikely, because the patient did not have severe kidney injury, preceding diarrhoea or history of TMA-causing medications.4 The possibility of systemic infection, systemic malignancy or systemic rheumatic diseases also seemed to be low, because there were no specific signs or symptoms indicating these diseases. Disseminated intravascular coagulation was less likely based on the absence of underlying condition and the relatively high schistocytes count in the patient.5 Hence, she was suspected with a diagnosis of TTP and concomitant vitamin B12 deficiency.
Treatment
She was admitted and started on plasma exchange and intravenous vitamin B12 administration in conjunction with two units of pure red blood cell transfusion. Two days after admission, her haemoglobin level was unchanged (6.1 g/dL) and her platelet count was slightly reduced to 49 000/μL. Since her consciousness was clear and her general appearance was good for TTP, vitamin B12 deficiency was suspected as the alternative cause. Plasma exchange was discontinued, and intravenous vitamin B12 supplementation was continued.
Outcome and follow-up
Her reticulocyte count began to rise from day 3. On day 4, the result of the blood test that was taken on the day of admission returned, showing markedly low vitamin B12 level (under 50 pg/mL) with relatively high folate level (31.8 ng/mL) and normal ADAMTS13 activity (50%), which were consistent with vitamin B12 deficiency and suggested against TTP. Since there was haematological dysfunction consistent with vitamin B12 deficiency with sufficiently low vitamin B12 level, we decided that additional investigation of plasma total homocysteine, methylmalonic acid or methylene tetrahydrofolate reductase mutations were not necessary. Vitamin B12 replacement was continued. Folic acid supplementation was not started, since the patient’s serum folate level seemed sufficiently high. Haemoglobin level and platelet count gradually recovered, and lactate dehydrogenase level and the degree of schistocytes gradually decreased (figure 2). She was finally discharged home 1 month after admission with a haemoglobin level of 10.7 g/dL and a platelet count of 103 000/μL. At several follow-up visits for 1 year after discharge, she had no recurrence of decreased haemoglobin levels and platelet counts. Regarding peripheral blood smear, while abnormal red cells were still detected at 9 months after discharge (figure 3A), they disappeared at 1 year after discharge (figure 3B).
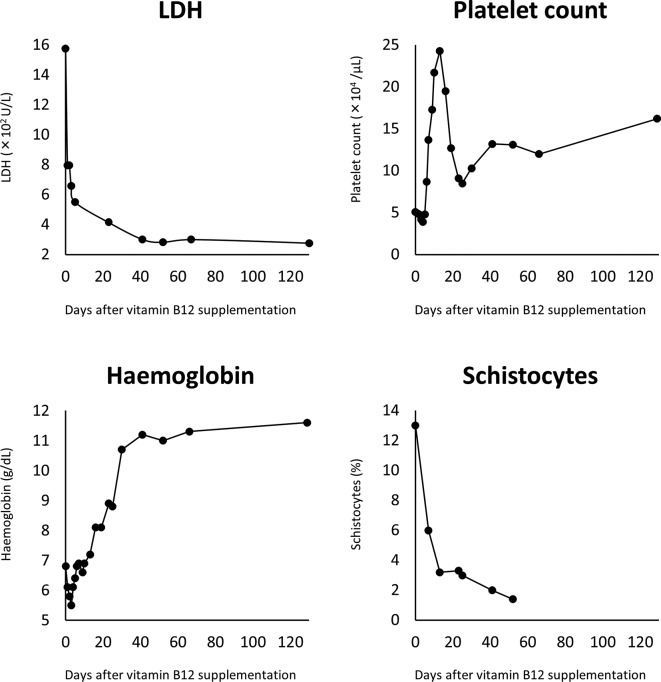
Figure 2.
Changes in lactate dehydrogenase, haemoglobin, platelet count and schistocytes following vitamin B12 supplementation. Responses to therapy are observed within 10 days following the initiation of vitamin B12 supplementation and all indicators reach nadir around day 40. LDH, lactate dehydrogenase.
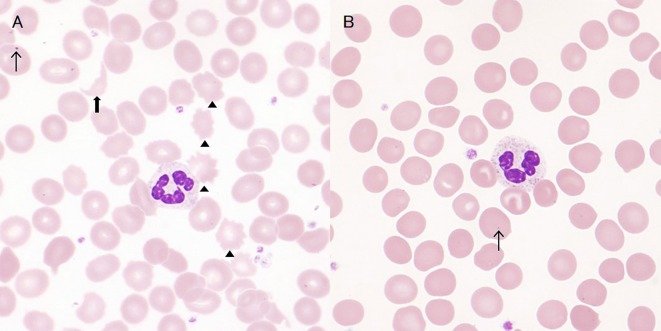
Figure 3.
Peripheral blood smears at 9 months (A, Giemsa stain, ×1000) and 1 year after discharge (B, Giemsa stain, ×1000). Peripheral blood smear at 9 months showing a few schistocytes (wide arrow) and acanthocytes (arrow head); these abnormal red cells disappeared at 1 year; Howell-Jolly bodies were identified in both smears (narrow arrow).
Discussion
We reported a case of pseudo-TMA due to severe vitamin B12 deficiency following total gastrectomy. To the best of our knowledge, this case is the first case of pseudo-TMA in a post-gastrectomy patient.
Pseudo-TMA seems to occur in patients with any type of vitamin B12 deficiency. Most frequently, pernicious anaemia has been reported as the cause of vitamin B12 deficiency in patients with pseudo-TMA3; however, other conditions, including malnutrition,3 6–9 food-cobalamin malabsorption,2 3 post-small bowel resection10 and post-gastric bypass surgery have also been considered as the causes of vitamin B12 deficiency.11 Therefore, vitamin B12 deficiency should be considered in patients who are suspected with TTP and are at risk of such deficiency.
Regarding the mechanism of pseudo-TMA due to vitamin B12 deficiency, hyperhomocysteinaemia has been recognised as a key factor. Hyperhomocysteinaemia has potential haemolytic effect by mechanisms involving both lipid peroxidation and membrane/cytoskeletal protein derangement,12 13 it activates coagulation and platelet aggregation and it impairs endothelial functions.14 Because hyperhomocysteinaemia occurs in most patients with functional vitamin B12 deficiency15 and higher susceptibility of structurally defective megaloblastic erythrocytes to damaged endothelium can be assumed in patients with vitamin B12 deficiency,16 it seems possible for microangiopathic haemolytic anaemia to occur in patients with vitamin B12 deficiency through hyperhomocysteinaemia. In addition, coexistence of methylene tetrahydrofolate reductase mutations with vitamin B12 deficiency seems related to pseudo-TMA due to vitamin B12 deficiency though hyperhomocysteinaemia16; conversely, one case of pseudo-TMA due to vitamin B12 deficiency without methylene tetrahydrofolate reductase mutations was also reported.17
Measurement of plasma homocysteine and methylmalonic acid levels can play an important role for the diagnosis of pseudo-TMA due to vitamin B12 deficiency. In general, elevated levels of homocysteine and methylmalonic acid can be considered as sensitive indicators of functional vitamin B12 deficiency, because these have been proven as the markers of insufficient intracellular vitamin B12.15 In addition, based on a systematic review of case reports, elevated levels of homocysteine and methylmalonic acid were observed in around 94% of cases with pseudo-TMA due to vitamin B12 deficiency.18 Although these levels were not obtained in our case, plasma homocysteine and methylmalonic acid levels may be required in case of ambiguity for the diagnosis of pseudo-TMA due to vitamin B12 deficiency.
TTP is the most important differential diagnosis of pseudo-TMA. Considering that TTP can be fatal without appropriate treatment, initiating both plasmapheresis therapy for TTP and vitamin B12 replacement therapy for vitamin B12 deficiency seems to be a reasonable option for patients suspected with pseudo-TMA.19 Still, rapid diagnosis of pseudo-TMA is important to limit the potential adverse reaction associated with plasmapheresis. For this reason, useful clinical decision rules to distinguish TTP from pseudo-TMA are essential. When concerning the possibility of TTP, the PLASMIC score, which was recently proposed as a predictive tool for assessing the likelihood of severe ADAMTS13 deficiency in patients with TMA, may be useful.20 The details on PLASMIC score include that one point each awarded for the following: Platelet count <30,000/µL; Evidence of hemolysis (reticulocyte count >2.5%; undetectable haptoglobin, or indirect bili >2.0); No active cancer; No history of solid-organ or stem-cell transplant MCV <90 fl; INR <1.5; Creatinine <2.0 mg/dL. A score of 0-4 indicates low risk of severe ADAMTS13 deficiency; Score of 5 is intermediate risk; Score of 6 or 7 indicates high risk, respectively. This patient fulfilled four items of the PLASMIC score, which indicated low risk of severe ADAMTS13 deficiency (0%–4%). Moreover, the typical characteristics at presentation of pseudo-TMA reported by Andrès et al, such as haemolytic anaemia, haemoglobin level (range: 5.1–10 g/dL), mean corpuscular volume (range: 112–124 fL), platelet count (range: 25 000–110 000/μL) and vitamin B12 level (range: 12–70 pg/mL) seem to be important.21 Indeed, most findings in our case were consistent with those aforementioned characteristics. Hence, PLASMIC score and the characteristics reported by Andrès et al may be useful for the swift diagnosis of pseudo-TMA.
In conclusion, pseudo-TMA due to vitamin B12 deficiency can occur in patients post-gastrectomy. Rapid diagnosis of pseudo-TMA using useful tools for correctly distinguishing this condition from TTP may avoid the risks of plasmapheresis, and concurrent initiation of the treatment for both TTP and vitamin B12 deficiency may sometimes be inevitable in real clinical settings.
Learning points.
Microangiopathic haemolytic anaemia with thrombocytopenia due to vitamin B12 deficiency can occur in patients post-gastrectomy.
Peripheral blood smear is an important diagnostic tool for microangiopathic haemolytic anaemia and vitamin B12 deficiency.
Measurement of serum vitamin B12 level and supplementation of vitamin B12 should be considered for patients suspected with thrombotic microangiopathy whose PLASMIC score is low and who are at risk of vitamin B12 deficiency.
Footnotes
Contributors: YH and IK contributed to the conception and design, acquisition of data and interpretation of data. All authors contributed to the drafting the article or revising it critically for important intellectual content, final approval of the manuscript for submission and agreed to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved. Also, TS revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ 2014;349:g5226 10.1136/bmj.g5226 [DOI] [PubMed] [Google Scholar]
- 2.Andrès E, Affenberger S, Federici L, et al. Pseudo-thrombotic microangiopathy related to cobalamin deficiency. Am J Med 2006;119:e3 10.1016/j.amjmed.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM 2013;106:1017–22. 10.1093/qjmed/hct142 [DOI] [PubMed] [Google Scholar]
- 4.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654–66. 10.1056/NEJMra1312353 [DOI] [PubMed] [Google Scholar]
- 5.Lesesve JF, Martin M, Banasiak C, et al. Schistocytes in disseminated intravascular coagulation. Int J Lab Hematol 2014;36:439–43. 10.1111/ijlh.12168 [DOI] [PubMed] [Google Scholar]
- 6.Bayle S, Tardy B, Page Y, et al. When vitamin deficiency mimics thrombotic thrombocytopenic purpura. J Am Geriatr Soc 2006;54:369–70. 10.1111/j.1532-5415.2005.00592_3.x [DOI] [PubMed] [Google Scholar]
- 7.Asano T, Narazaki H, Kaizu K, et al. Neglect-induced pseudo-thrombotic thrombocytopenic purpura due to vitamin B12 deficiency. Pediatr Int 2015;57:988–90. 10.1111/ped.12718 [DOI] [PubMed] [Google Scholar]
- 8.Keskin EY, Keskin M. Severe vitamin B₁₂ deficiency in a 15-year-old boy: presentation with haemolysis and pancytopenia. BMJ Case Rep 2015;2015:bcr2015209718 10.1136/bcr-2015-209718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra VA, Harbada R, Sharma A. Vitamin B12 and vitamin d deficiencies: an unusual cause of fever, severe hemolytic anemia and thrombocytopenia. J Family Med Prim Care 2015;4:145–8. 10.4103/2249-4863.152276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimond A, George JN, Hastings C. Severe vitamin B-12 deficiency in a child mimicking thrombotic thrombocytopenic purpura. Pediatr Blood Cancer 2009;52:420–2. 10.1002/pbc.21788 [DOI] [PubMed] [Google Scholar]
- 11.Green R. Anemias beyond B12 and iron deficiency: the buzz about other B’s, elementary, and nonelementary problems. Hematology Am Soc Hematol Educ Program 2012;2012:492–8. 10.1182/asheducation-2012.1.492 [DOI] [PubMed] [Google Scholar]
- 12.Olinescu R, Kummerow FA, Handler B, et al. The hemolytic activity of homocysteine is increased by the activated polymorphonuclear leukocytes. Biochem Biophys Res Commun 1996;226:912–6. 10.1006/bbrc.1996.1449 [DOI] [PubMed] [Google Scholar]
- 13.Ventura P, Panini R, Tremosini S, et al. A role for homocysteine increase in haemolysis of megaloblastic anaemias due to vitamin B(12) and folate deficiency: results from an in vitro experience. Biochim Biophys Acta 2004;1739:33–42. 10.1016/j.bbadis.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 14.Nappo F, De Rosa N, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 1999;281:2113–8. 10.1001/jama.281.22.2113 [DOI] [PubMed] [Google Scholar]
- 15.Salinas M, Flores E, López-Garrigós M, et al. Vitamin B12 deficiency and clinical laboratory: lessons revisited and clarified in seven questions. Int J Lab Hematol 2018;40(Suppl 1):83–8. 10.1111/ijlh.12833 [DOI] [PubMed] [Google Scholar]
- 16.Acharya U, Gau JT, Horvath W, et al. Hemolysis and hyperhomocysteinemia caused by cobalamin deficiency: three case reports and review of the literature. J Hematol Oncol 2008;1:26 10.1186/1756-8722-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panchabhai TS, Patil PD, Riley EC, et al. When the picture is fragmented: vitamin B12 deficiency masquerading as thrombotic thrombocytopenic purpura. Int J Crit Illn Inj Sci 2016;6:89–92. 10.4103/2229-5151.183026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran PN, Tran MH. Cobalamin deficiency presenting with thrombotic microangiopathy (TMA) features: a systematic review. Transfus Apher Sci 2018;57:102–6. 10.1016/j.transci.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 19.Walter K, Vaughn J, Martin D. Therapeutic dilemma in the management of a patient with the clinical picture of TTP and severe B12 deficiency. BMC Hematol 2015;15:16 10.1186/s12878-015-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol 2017;4:e157–64. 10.1016/S2352-3026(17)30026-1 [DOI] [PubMed] [Google Scholar]
- 21.Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol 2006;28:50–6. 10.1111/j.1365-2257.2006.00755.x [DOI] [PubMed] [Google Scholar]