Abstract
Five new oleanane-type saponins, named ligushicosides A-E, and three known oleanane-type saponins were isolated from the roots of Ligulariopsis shichuana. Their structures were established by a combination of spectroscopic techniques, including 1D and 2D NMR and high resolution electrospray ionization mass spectroscopy (HR-ESI-MS). Furthermore, all isolates were evaluated for their yeast α-glucosidase inhibitory effects and exhibited potent inhibition against α-glucosidase, while compounds 1 and 2 showed excellent inhibitory activities. The 3-O-glycoside moiety in oleanane-type saponin is important for the α-glucosidase inhibitory effects.
Keywords: Ligulariopsis shichuana, oleanane-type saponins, α-glucosidase inhibitory
1. Introduction
Nowadays, diabetes, especially type II diabetes mellitus, is a significant metabolic disorder that endangers public health, and is regarded as one of the most important public health problems in all nations [1]. One of the effective managements of type II diabetes mellitus is to retard the absorption of glucose by inhibition of carbohydrate hydrolyzing enzymes such as α-glucosidase in the digestive organs, which is the key enzyme catalyzing the final step in the digestive process of carbohydrates. Hence, α-glucosidase inhibitors can retard the liberation of glucose from dietary complex carbohydrates and delay glucose absorption, resulting in reduced postprandial plasma glucose levels and suppression of postprandial hyperglycemia. Among many classes of clinical drugs for diabetes, α-glucosidase inhibitors are useful for preventing the progression of the disease and for treating prediabetic conditions [2]. However, α-glucosidase inhibitors in clinical use usually exhibit decreased efficacy over time, ineffectiveness against some long-term diabetic complications, and low cost-effectiveness [3]. With regard to achieving and maintaining long-term glycemia control, discovering new α-glucosidase inhibitors is an urgent need.
Because of their perceived effectiveness, minimal side effects in clinical experience, and relatively low cost, herbal drugs are recognized as a wonderful source for medicines. Moreover, natural products have always provided new scaffolds for the design and development of new drugs, due to their structural diversity [4,5]. Ligulariopsis shichuana is the only species in genus Ligulariopsis (Compositae) that is endemic to western China. Previous studies on this plant have reported eremophilenolides and triterpenes, showing its chemical similarity with those plants in Ligularia [6,7,8]. Local people currently use the root and rhizome of L. shichuana, as well as some species of Ligularia, as “shanziwan”, a traditional substitute for “ziwan” (root of Aster tataricus L.f.) (Radix Asteris), which is a traditional Chinese medicine used to treat the symptom of xerostomia and frequent drinking caused by diabetes [9].
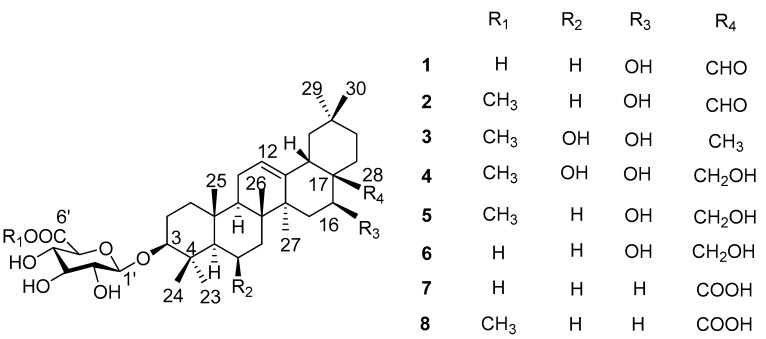
Aiming to elucidate the antidiabetic constituents from L. shichuana, our team carried out phytochemical investigations on L. shichuana. In this study, five new oleanane-type saponins (1–5) and three known oleanane-type saponins (6–8) were found (Figure 1). All isolated compounds were tested for α-glucosidase inhibitory activity.
Figure 1.
Structures of oleanane-type saponins 1–8 from the roots of Ligulariopsis shichuana.
2. Results
Phytochemical study of MeOH extract of the roots of L shichuana led to the isolation of five new ligushicosides A-E (1–5) and three known oleanane-type saponins, longispinogenin 3-O-β-d-glucuronopyranoside (6) [10], calenduloside E (7) [11] and calenduloside E 6′-methyl ester (8) [11] (Figure 1). The known structures were identified by comparison of their spectroscopic data with those reported in the literature. Acid hydrolysis of the saponins mixture A (compounds 1–8, each 1.0 mg) only gave one sugar, which was identified as glucuronic acid (GluA) by TLC, and its absolute configuration was determined as D by measuring its optical rotation [12].
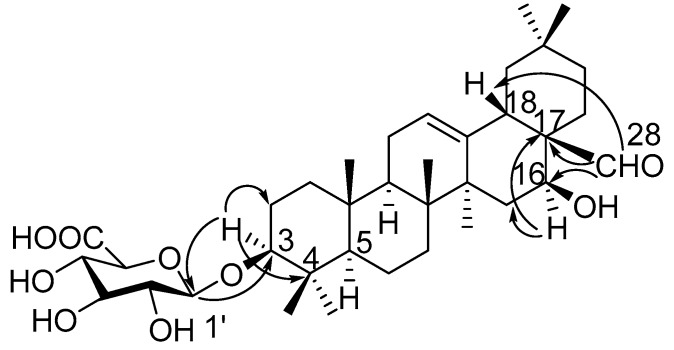
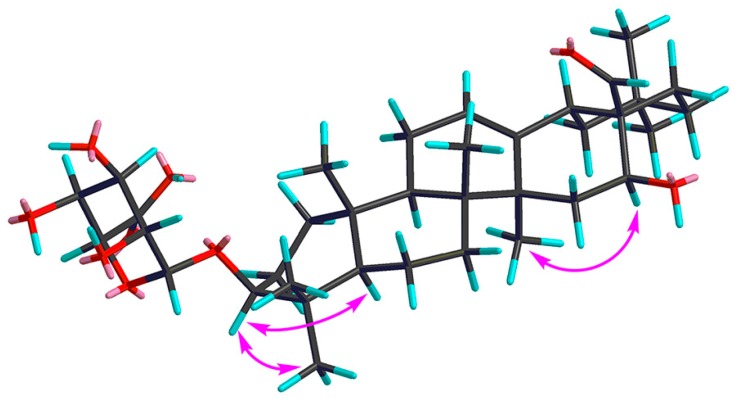
Compound 1 was obtained as a pale yellow powder, and was found to have the molecular formula C36H56O9, as determined through HR-ESI-MS ([M + H]+ peak at m/z 633.3998, calc. for C36H57O9, 633.4003). IR spectrum indicated hydroxyl (3393 cm−1), carbonyl (1715 cm−1) and C=C double bond (1607 cm−1) functions. The 1H-NMR spectrum (Table 1, Figure S1) displayed signals for seven methyl groups at δH 0.83, 0.88, 0.96, 0.96, 0.98, 1.08 and 1.25 (each 3H, s, H3-26, 24, 29, 30, 25, 23, 27). Further features included signals observed at δH 3.19 (1H, dd, J = 11.7, 4.3 Hz, typical for an axial proton attached to a hydroxylate carbon), δH 5.34 (1H, br t, J = 1.5 Hz, assignable to a vinylic proton), and at δH 9.76 (1H, s, an aldehyde proton signal). The 13C NMR spectrum (Table 2, Figure S2) displayed a methine carbon signal at δC 208.0 (supporting the presence of a CHO group), a quaternary carbon signal at δC 175.5 (ester carbon), and two olefinic carbon signals (one quaternary at δC 142.0 and one methine at δC 123.1, suggesting the presence of a double bond). Furthermore, the spectrum also showed a signal at δC 105.3 (CH) assignable to an anomeric carbon in a sugar unit. On the basis of these data, compound 1 was determined to be a triterpene glycoside. Its aglycone was determined to be gummosogenin [13], due to their similar NMR data, except for the presence of one set of resonances attributable to a β-d-glucuronopyranosyl moiety [H-1′ (δH 4.38, 1H, d, J = 8.2 Hz), δC 74.1, 75.3, 76.6, 78.1, 105.3 and 175.5] in 1 [14]. Comparison of the 13C NMR data of 1 with that of gummosogenin showed the downfield shift of C-3 (+11.4 ppm) and upfield shift of C-2 (−2.6 ppm) in 1, indicating glycosylation at C-3. This assignment was further confirmed by the HMBC correlations (Figure 2, Figure S5) between H-3 (δH 3.19, 1H, dd, J = 11.7, 4.3 Hz) in the aglycon and C-1′ (δC 105.3) in β-d-glucuronopyranosyl moiety, and between H-1′ and C-3. The relative configuration of 1 was confirmed by nuclear overhauser enhancement spectroscopy (NOESY) (Figure 3, Figure S6). The α-orientation of H-3 was deduced from the correlations of H-3 with H-5 (δH 0.81, 1H, m) and H3-23 respectively. The α-orientation of H-16 (δH 4.32, 1H, dd, J = 11.9, 4.5 Hz) was deduced from the correlations of H-16 with H3-27. The structure of 1 was confirmed by a complete acid hydrolysis of the saponin mixture A, which afforded D-glucuronic acid, as identified by co-TLC with an authentic sample [12]. On the basis of these findings, the structure of 1 was elucidated and named ligushicoside A.
Table 1.
1H-NMR (600 MHz) spectroscopic data for compounds 1–5 in CD3OD (δH, J in Hz).
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1a | 1.62, m | 1.60, m | 1.55, m | 1.55, m | 1.58, m |
| 1b | 1.00, m | 0.98, m | 0.95, m | 0.93, m | 0.95, m |
| 2a | 1.98, m | 1.96, m | 1.78, m | 1.80, m | 1.78, m |
| 2b | 1.72, m | 1.70, m | 1.70, m | 1.76, m | 1.68, m |
| 3 | 3.19, dd (11.7, 4.3) | 3.13, dd (11.8, 4.4) | 3.07, dd (9.9, 5.7) | 3.07, dd (9.9, 5.7) | 3.14, dd (11.2, 4.6) |
| 5 | 0.81, m | 0.77, m | 0.75, m | 0.75, m | 0.76, m |
| 6a | 1.59, m | 1.57, m | 4.51, br s | 4.51, br s | 1.57, m |
| 6b | 1.45, m | 1.43, m | 1.43, m | ||
| 7a | 1.43, m | 1.41, m | 1.72, m | 1.73, m | 1.55, m |
| 7b | 1.27, m | 1.25, m | 1.55, m | 1.58, m | 1.36, m |
| 9 | 1.58, m | 1.56, m | 1.58, m | 1.58, m | 1.54, m |
| 11a | 1.93, m | 1.92, m | 2.03, m | 2.02, m | 1.92, m |
| 11b | 1.92, m | 1.91, m | 1.91, m | 1.90, m | 1.85, m |
| 12 | 5.34, t-like (1.5) | 5.31, t-like (1.5) | 5.27, br s | 5.27, br s | 5.34, br s |
| 15a | 1.82, m | 1.79, m | 1.80, m | 1.40, m | 1.40, m |
| 15b | 1.53, m | 1.51, m | 1.25, m | 1.20, m | 1.21, m |
| 16 | 4.32, dd (11.9, 4.5) | 4.29, dd (12.0, 4.6) | 4.13, dd (11.4, 4.5) | 4.24, dd (11.7, 4.8) | 4.24, dd (12.0, 4.8) |
| 18 | 2.78, dd (14.0, 4.2) | 2.76, dd (14.0, 4.5) | 2.18, m | 2.21, m | 2.18, m |
| 19a | 1.70, m | 1.67, m | 1.73, m | 1.72, m | 1.71, m |
| 19b | 1.19, m | 1.16, m | 1.03, m | 1.06, m | 1.02, m |
| 21a | 1.58, m | 1.56, m | 1.90, m | 1.41, m | 1.41, m |
| 21b | 1.37, m | 1.34, m | 1.15, m | 1.21, m | 1.21, m |
| 22a | 2.09, m | 2.06, m | 1.39, m | 2.21, m | 2.10, m |
| 22b | 1.26, m | 1.23, m | 1.11, m | 1.41, m | 1.40, m |
| 23 | 1.08, s | 1.05, s | 1.13, s | 1.13, s | 1.06, s |
| 24 | 0.88, s | 0.85, s | 1.23, s | 1.23, s | 0.86, s |
| 25 | 0.98, s | 0.95, s | 1.33, s | 1.33, s | 0.97, s |
| 26 | 0.83, s | 0.81, s | 1.28, s | 1.30, s | 1.03, s |
| 27 | 1.25, s | 1.23, s | 1.19, s | 1.20, s | 1.24, s |
| 28a | 9.76, s | 9.73, s | 0.79, s | 3.80, d (9.7) | 3.81, d (9.7) |
| 28b | 3.26, m | 3.27, m | |||
| 29 | 0.96, s | 0.93, s | 0.89, s | 0.90, s | 0.90, s |
| 30 | 0.96, s | 0.94, s | 0.92, s | 0.93, s | 0.92, s |
| GluA-1′ | 4.38, d (8.2) | 4.37, d (7.8) | 4.37, d (7.7) | 4.37, d (7.8) | 4.40, d (7.5) |
| 2′ | 3.24, t (8.3) | 3.22, t (8.5) | 3.24, t (8.4) | 3.24, t (8.4) | 3.26, t (8.4) |
| 3′ | 3.39, t (9.2) | 3.34, t (9.1) | 3.35, t (9.1) | 3.35, t (9.1) | 3.37, t (9.0) |
| 4′ | 3.47, t (8.8) | 3.49, t (9.4) | 3.50, t (9.4) | 3.49, t (9.4) | 3.53, t (9.2) |
| 5′ | 3.62, d (10.0) | 3.81, d (9.8) | 3.80, d (9.8) | 3.80, d (9.7) | 3.83, br d (10.0) |
| 6′-OCH3 | 3.77, s | 3.76, s | 3.76, s | 3.79, s |
Table 2.
13C-NMR (150 MHz) spectroscopic data for compounds 1–5 in CD3OD (δC, type).
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.4, CH2 | 38.3, CH2 | 42.1, CH2 | 42.1, CH2 | 39.9, CH2 |
| 2 | 25.5, CH2 | 25.6, CH2 | 27.3, CH2 | 27.3, CH2 | 27.0, CH2 |
| 3 | 89.4, CH | 89.6, CH | 91.3, CH | 91.3, CH | 91.1, CH |
| 4 | 38.8, C | 38.8, C | 41.2, C | 41.2, C | 41.6, C |
| 5 | 55.6, CH | 55.5, CH | 57.3, CH | 57.3, CH | 57.0, CH |
| 6 | 17.9, CH2 | 17.8, CH2 | 68.6, CH | 68.6, CH | 19.3, CH2 |
| 7 | 32.6, CH2 | 32.6, CH2 | 41.6, CH2 | 41.7, CH2 | 33.7, CH2 |
| 8 | 39.5, C | 39.4, C | 40.4, C | 40.4, C | 40.9, C |
| 9 | 46.8, CH | 46.7, CH | 48.7, CH | 48.6, CH | 48.3, CH |
| 10 | 36.4, C | 36.4, C | 37.4, C | 36.7, C | 36.7, C |
| 11 | 23.1, CH2 | 23.1, CH2 | 24.7, CH2 | 24.7, CH2 | 24.1, CH2 |
| 12 | 123.1, CH | 123.0, CH | 123.8, CH | 124.3, CH | 124.0, CH |
| 13 | 142.0, C | 141.9, C | 144.5, C | 143.6, C | 144.3, C |
| 14 | 43.4, C | 43.4, C | 45.4, C | 45.1, C | 44.7, C |
| 15 | 36.5, CH2 | 36.5, CH2 | 36.4, CH2 | 34.9, CH2 | 35.0, CH2 |
| 16 | 63.8, CH | 63.8, CH | 66.4, CH | 68.0, CH | 67.8, CH |
| 17 | 52.8, C | 52.7, C | 38.6, C | 41.5, C | 40.2, C |
| 18 | 41.8, CH | 41.7, CH | 50.8, CH | 45.1, CH | 45.1, CH |
| 19 | 45.7, CH2 | 45.8, CH2 | 48.1, CH2 | 47.9, CH2 | 47.9, CH2 |
| 20 | 30.0, C | 30.0, C | 31.9, C | 30.8, C | 31.7, C |
| 21 | 32.6, CH2 | 32.6, CH2 | 35.4, CH2 | 34.9, CH2 | 34.8, CH2 |
| 22 | 22.3, CH2 | 22.3, CH2 | 31.7, CH2 | 26.0, CH2 | 26.0, CH2 |
| 23 | 27.1, CH3 | 27.0, CH3 | 28.3, CH3 | 28.2, CH3 | 28.5, CH3 |
| 24 | 15.6, CH3 | 15.5, CH3 | 18.5, CH3 | 18.5, CH3 | 17.0, CH3 |
| 25 | 14.6, CH3 | 14.6, CH3 | 17.6, CH3 | 17.5, CH3 | 16.2, CH3 |
| 26 | 16.3, CH3 | 16.2, CH3 | 18.9, CH3 | 18.6, CH3 | 17.7, CH3 |
| 27 | 25.6, CH3 | 25.5, CH3 | 27.8, CH3 | 27.5, CH3 | 27.0, CH3 |
| 28 | 208.0, CH | 208.0, CH | 22.4, CH3 | 69.0, CH2 | 69.0, CH2 |
| 29 | 32.0, CH3 | 32.0, CH3 | 33.9, CH3 | 31.8, CH3 | 33.7, CH3 |
| 30 | 22.6, CH3 | 22.6, CH3 | 24.5, CH3 | 24.3, CH3 | 24.3, CH3 |
| GluA-1′ | 105.3, CH | 105.6, CH | 107.1, CH | 107.1, CH | 107.1, CH |
| 2′ | 75.3, CH | 73.9, CH | 75.4, CH | 75.4, CH | 75.4, CH |
| 3′ | 78.1, CH | 78.1, CH | 77.6, CH | 77.6, CH | 77.6, CH |
| 4′ | 74.1, CH | 71.8, CH | 73.3, CH | 73.3, CH | 73.3, CH |
| 5′ | 76.6, CH | 76.1, CH | 76.7, CH | 76.7, CH | 76.7, CH |
| 6′ | 175.5, C | 170.0, C | 171.5, C | 171.5, C | 171.5, C |
| 6′-OCH3 | 51.4, CH3 | 52.9, CH3 | 52.8, CH3 | 52.8, CH3 |
Figure 2.
Key HMBC correlations of compound 1.
Figure 3.
Key NOESY correlations of compound 1.
The structure determination of compound 2 was performed in a similar manner to that described for 1. Its molecular formula was determined as C37H58O9 on the basis of HR-ESI-MS ([M + Na]+ peak at m/z 669.3972, calc. for C37H58O9Na, 669.3979) and NMR experiments. Compared with the NMR data of 1, the 1H NMR spectrum of 2 (Table 1, Figure S7) displayed a signal for an additional methoxy group at 6′-OCH3 (δH 3.77, 3H, s), and the sugar carbon data revealed a noticeable difference in the δ value of C-6′ (−5.5 ppm). This evidence, together with the observed correlation between 6′-OCH3 and C-6′ (δC 170.0) in its HMBC (Figure S12), suggested that there was a 6′-O-methyl-β-glucuronopyranoside in compound 2 [15]. The relative configuration of 2 was confirmed by the NOESY spectrum, which was identical to that of 1, thus permitting assignment of the molecular structure of 2, and its naming as ligushicoside B.
The HR-ESI-MS ([M + Na]+ peak at m/z 671.4128, calc. for C37H60O9Na, 671.4135) of compound 3 established a molecular formula of C37H60O9. The 1H (Table 1, Figure S14) and the 13C-NMR (Table 2, Figure S15) spectra of 3 were very similar to those of 2, except for the disappearance of the aldehyde group and the appearance of a methyl group and a hydroxy group. The HMBC correlations (Figure S18) between H3-28 (δH 0.79, 3H, s) and C-16 (δC 66.4), C-17 (δC 38.6), C-18 (δC 50.8), and C-22 (δC 31.7) indicated that the C-28 aldehyde group in 2 had been changed into a methyl group in 3. The second hydroxy group in 3 was assigned to C-6 (δC 68.6), due to the COSY correlation (Figure S16) between the protons at H-6 (δH 4.51, 1H, br s) and H-5 (δH 0.75, 1H, m), and between those at H-6 and H2-7 (δH 1.55, 1H, m; 1.72, 1H, m), as well as the HMBC correlation. The relative configuration of 3 was confirmed by its NOESY spectrum (Figure S19), which was identical to that of 1. Furthermore, the β-orientation of OH-6 was deduced from the correlations of H-6 and H-5, H3-23 (δH 1.13, 3H, s), and the α-orientation of H-16 (δH 4.13, 1H, dd, J = 11.4, 4.5 Hz) was deduced from the correlations of H-16 and H3-27 (δH 1.19, 3H, s) in the NOSEY spectrum. Thus, the structure of ligushicoside C was assigned to 3.
The molecular formula of compound 4 was established as C37H60O10 ([M + Na]+ peak at m/z 687.4076, calc. for C37H60O10Na, 687.4084) by the HR-ESI-MS and NMR data. The 1H (Table 1, Figure S21) and 13C NMR (Table 2, Figure S22) spectra of 4 were similar to those of olean-12-en-3β,6β,16β,28-tetraol, previously obtained from this plant [8]. The presence of a 6′-O-methyl-β-glucuronopyranoside moiety [H-1′ (δH 4.37, 1H, d, J = 7.8 Hz) and 6′-OCH3 (δH 3.76, 3H, s); δC 52.8, 73.3, 75.4, 76.7, 77.6, 107.1 and 171.5] in 4, which was attached to the C-3 (δC 91.3) supported by the glycosylation shift at the C-3 position (+11.5 ppm) in its 13C NMR spectra. On the basis of these findings, the structure of 4 was elucidated and named ligushicoside D.
The molecular formula of compound 5 was determined as C37H60O9 ([M + Na]+ peak at m/z 671.4128, calc. for C37H60O9Na, 671.4135) by its HR-ESI-MS and NMR data. The NMR signals (Table 1 and Table 2, Figures S28 and S29) of 5 were similar to those of longispinogenin 3-O-β-d-glucuronopyranoside [10], except for an additional methoxy group at 6′-OCH3 (δH 3.79, 3H, s). The HMBC correlation (Figure S32) between 6′-OCH3 and C-6′ (δC 171.5) suggested that there was a 6′-O-methyl-β-glucuronopyranoside in compound 5. Thus, 5 was named ligushicoside E.
All isolated compounds 1–8 were tested for α-glucosidase inhibitory activity. The IC50 values, defined as the compound concentration that inhibits α-glucosidase activity by 50%, are summarized in Table 3. The results showed that all of the oleanane-type saponins isolated from the roots of L. shichuana displayed inhibitory activity against α-glucosidase, with IC50 values in the range of 18.7–154.3 μM, which was obviously stronger than the positive control of acarbose (IC50 = 190.5 μM). This result was in agreement with a report suggesting that the 3-O-glycoside moiety was essential to the inhibition activity of oleanane-type glycosides [16]. Furthermore, compounds 1 (IC50 = 18.7 μM), 6 (IC50 = 42.6 μM) and 7 (IC50 = 57.6 μM) showed stronger activities than compounds 2 (IC50 = 37.9 μM), 5 (IC50 = 154.3 μM) and 8 (IC50 = 133.7 μM), respectively, suggesting that the 6′-methyl ester of the glucuronic acid moiety reduced the α-glucosidase inhibitory activities [17]. Compared to 6 and 5, respectively, both 1 and 2 were more potent inhibitors, and the formyl group at C-28 may be responsible for their significant inhibitory properties. Compound 4, containing the 6-OH group, showed more potent α-glucosidase inhibitory activity (IC50 = 89.7 μM) than compound 5 (IC50 = 154.3 μM), indicating that 6-OH may increase the activity. Interestingly, according to previous literature, compounds 7 and 8 have also shown significant effects on the incensement of serum glucose levels in glucose-loaded rats, especially compound 7, which strongly inhibited the incensement in serum glucose levels [18].
Table 3.
Inhibitory effects of compounds 1–8 and acarbose against α-glucosidase.
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | 18.7 ± 1.7 | 5 | 154.3 ± 11.2 |
| 2 | 37.9 ± 3.6 | 6 | 42.6 ± 5.9 |
| 3 | 104.9 ± 4.3 | 7 | 57.6 ± 6.8 |
| 4 | 89.7 ± 5.1 | 8 | 133.7 ± 3.3 |
| Acarbose | 190.5 ± 3.1 |
3. Materials and Methods
3.1. General Experimental Material
Optical rotations were measured on a Perkin-Elmer 241 polarimeter (Perkin-Elmer, Waltham, MA, USA). IR data were recorded using a Nicolet Magna-IR 750 spectrophotometer (Nicolet Instrument Company, Madison, WI, USA). 1D and 2D NMR spectra were recorded on a Bruker AV-600 spectrometer (600 MHz for 1H and 150 MHz for 13C, respectively, Bruker Biospin, Fallanden, Switzerland), using tetramethylsilane (TMS) as an internal standard. HR-ESI-MS were measured by an Agilent 6520 Q-TOF LC-MS mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Analytical TLC were run on silica gel plates (GF254, Yantai Institute of Chemical Technology, Yantai, China). Spots were observed under UV light and visualized by spraying with 10% H2SO4 in ethanol, followed by heating. Column chromatography (CC) was performed on silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden). Acarbose was purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Plant Material
The roots of L. shichuana were collected in Baoji, Shaanxi Province, People’s Republic of China, in August 2011, and identified by one of the authors (W.-S.W.). A voucher specimen (No. 20110802) was deposited in the herbarium of the College of Life and Environmental Sciences, Minzu University of China, Beijing, People’s Republic of China.
3.3. Extraction and Isolation
The chopped, dried roots of L. shichuana (1.5 kg) were pulverized and extracted three times with MeOH (each for 7 days) at room temperature. After filtration, the filtrate was concentrated under reduced pressure to yield a residue (135.0 g), and then was suspended in water and partitioned successively with petroleum ether, CHCl3, EtOAc, and n-butanol, to afford five fractions. The n-butanol fraction (15.5 g) was subjected to silica gel CC (500.0 g) eluting with CHCl3–MeOH (15:1–0:1, gradient system). On the basis of TLC analysis, seven fractions A-G were obtained. Fraction B (1.1 g) was eluted with CHCl3–MeOH (8:1) on CC, further purified by Sephadex LH-20 (MeOH) to afford 2 (16.3 mg). Fraction C (0.9 g) was subjected to CC eluting with CHCl3–MeOH (5:1) to afford 3 (2.6 mg) and 5 (9.1 mg). Fraction D (0.5 g) was subjected to CC eluting with CHCl3–MeOH (4:1) to afford 4 (20.6 mg). Fraction E (1.3 g) was subjected to CC eluting with CHCl3–MeOH (3:1) to afford 1 (9.7 mg) and 7 (7.3 mg). Fraction F (0.7 g) was subjected to Sephadex LH-20 (MeOH) and then was subjected to CC eluting with EtOAc–MeOH (1:1) to afford 6 (5.1 mg). Fraction G (2.4 g) was eluted with CHCl3–MeOH (1:1) on CC, further purified by Sephadex LH-20 (MeOH) to afford 8 (7.2 mg).
3.4. Spectroscopic Data
Ligushicoside A (1): Pale yellow, amorphous powder; [α] + 5.1 (c = 0.023, MeOH); IR (KBr) νmax 3393, 2952, 1715, 1607, 1459, 1033 cm−1; 1H-NMR (CD3OD, 600 MHz) data see Table 1, Figure S1; 13C-NMR (CD3OD, 150 MHz) data see Table 2, Figure S2; HR-ESI-MS m/z 633.3998 (calcd. for C36H57O9, 633.4003, Figure S7).
Ligushicoside B (2): Pale yellow, amorphous powder; [α] + 10.0 (c = 0.032, MeOH); IR (KBr) νmax 3404, 2943, 1601, 1456, 1027 cm−1; 1H-NMR (CD3OD, 600 MHz) data see Table 1, Figure S8; 13C-NMR (CD3OD, 150 MHz) data see Table 2, Figure S9; HR-ESI-MS m/z 669.3972 (calcd. for C37H58O9Na, 669.3979, Figure S13).
Ligushicoside C (3): Pale yellow, amorphous powder; [α] + 7.8 (c = 0.026, MeOH); IR (KBr) νmax 3413, 2952, 1604, 1459, 1024 cm−1; 1H-NMR (CD3OD, 600 MHz) data see Table 1, Figure S14; 13C-NMR (CD3OD, 150 MHz) data see Table 2, Figure S15; HR-ESI-MS m/z 671.4128 (calcd. for C37H60O9Na, 671.4135, Figure S20).
Ligushicoside D (4): Pale yellow, amorphous powder; [α] + 8.1 (c = 0.031, MeOH); IR (KBr) νmax 3403, 2942, 1608, 1461, 1029 cm−1; 1H-NMR (CD3OD, 600 MHz) data see Table 1, Figure S21; 13C-NMR (CD3OD, 150 MHz) data see Table 2, Figure S22; HR-ESI-MS m/z 687.4076 (calcd. for C37H60O10Na, 687.4084, Figure S27).
Ligushicoside E (5): Pale yellow, amorphous powder; [α] + 7.3 (c = 0.017, MeOH); IR (KBr) νmax 3433, 2952, 1605, 1464, 1032 cm−1; 1H-NMR (CD3OD, 600 MHz) data see Table 1, Figure S28; 13C-NMR (CD3OD, 150 MHz) data see Table 2, Figure S29; HR-ESI-MS m/z 671.4128 (calcd. for C37H60O9Na, 671.4135, Figure S33).
3.5. Acid Hydrolysis of Saponins
The crude saponin mixture A (compounds 1–8, each 1.0 mg) were treated with 5 N TFA (trifluoroacetic acid, aqueous solution, 3 mL) at 90 °C for 6 h. After extraction with CHCl3 (3 × 3 mL), the aqueous layer was neutralized with 0.1 M NaOH and freeze-dried. The sugar was analyzed by TLC (silica gel, CHCl3–MeOH–AcOH–H2O, 60:32:12:8) for glucuronic acid (Rf 0.30) in comparison with standard sugar. Moreover, the optical rotations of the purified sugar were measured as for d-glucuronic acid, [α] + 8.1 (c = 0.26, H2O) [12].
3.6. α-Glucosidase Inhibitory Activity Assay
α-Glucosidase inhibitory activity was examined by the method described by Omar et al. [19]. Acarbose, a definite α-glucosidase inhibition, was used as the positive drug.
4. Conclusions
In this study, eight oleanane-type saponins, including five new ones, were isolated from the roots of L. shichuana. All the structures were established by extensive spectroscopic analysis. The isolates were evaluated on the basis of their α-glucosidase inhibitory activity assays. All of the oleanane-type saponins exhibited significant inhibitory activities, with IC50 values in the range of 18.7–154.3 μM, which were obviously stronger than the positive control of acarbose (IC50 = 190.5 μM). Compounds 1 and 2 showed excellent inhibitory activity against α-glucosidase, which might be useful for further developing α-glucosidase inhibitors.
Acknowledgments
This work was supported by the National Science Foundation of China (No. 31570407 and 31670564); Building World-Class University (Discipline) and Characteristic Development Special Funds (No. YDZXXK201618 and 2017MDYL33), together with 111 Project (No. B08044); Collaborative Innovation Center for Ethnic Minority Development; The Fundamental Research Funds for the Central Universities (No. 2016SHXY07); The National College Students Innovation and Entrepreneurship Training Program (No. GCCX2017110012).
Supplementary Materials
Supplementary data (Figures S1–S33) associated with this article, including HRESIMS, 1D and 2D NMR spectra data (1–5) can be found in the online version.
Author Contributions
Hai-Bo Wu conceived and designed the experiments; Hai-Bo Wu and Ting-Ting Liu performed the experiments; Hong-Mei Tian analyzed the data; Hai-Bo Wu, Wen-Shu Wang and Jin-Chao Feng wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Mata R., Cristians S., Escandón-Rivera S., Juárez-Reyes K., Rivero-Cruz I. Mexican antidiabetic herbs: Valuable sources of inhibitors of α-glucosidases. J. Nat. Prod. 2013;76:468–483. doi: 10.1021/np300869g. [DOI] [PubMed] [Google Scholar]
- 2.Kim D.H., Jung H.A., Sohn H.S., Kim J.W., Choi J.S. Potential of icariin metabolites from Epimedium Koreanum Nakai as antidiabetic therapeutic agents. Molecules. 2017;22:986. doi: 10.3390/molecules22060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002;81:81–100. doi: 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W.S., Gao K., Jia Z.J. New eremophilenolides from Ligulariopsis shichuana. J. Nat. Prod. 2002;65:714–717. doi: 10.1021/np010535l. [DOI] [PubMed] [Google Scholar]
- 7.Wang W.S., Gao K., Jia Z.J. New sesquiterpenes from Ligulariopsis shichuana. J. Chin. Chem. Soc. 2004;51:417–422. doi: 10.1002/jccs.200400064. [DOI] [Google Scholar]
- 8.Wang W.S., Gao K., Wang C.M., Jia Z.J. Cytotoxic triterpenes from Ligulariopsis shichuana. Int. J. Pharm. Sci. 2003;58:148–150. doi: 10.1002/chin.200324151. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M., Wei M.X., Ding W., Zhang Q.D. Effects of Chinese herbs on salivary fluid secretion by isolated and perfused rat submandibular glands. World J. Gastroenterol. 2009;15:3908–3915. doi: 10.3748/wjg.15.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye W.C., Zhang Q.W., Liu X., Che C.T., Zhao S.X. Oleanane saponins from Gymnema sylvestre. Phytochemistry. 2000;53:893–899. doi: 10.1016/S0031-9422(99)00483-5. [DOI] [PubMed] [Google Scholar]
- 11.Yin M., Wang X., Wang M., Chen Y., Dong Y., Zhao Y., Feng X. A new triterpenoid saponin and other saponins from Salicornia europaea. Chem. Nat. Compd. 2012;48:258–261. doi: 10.1007/s10600-012-0216-2. [DOI] [Google Scholar]
- 12.Tapondjou A.L., Miyamoto T., Lacaille-Dubois M.A. Glucuronide triterpene saponins from Bersama engleriana. Phytochemistry. 2006;67:2126–2132. doi: 10.1016/j.phytochem.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Takizawa T., Kinoshita K., Koyama K., Takahashi K., Kondo N., Yuasa H. A new triterpene from Rathbunia alamosensis. J. Nat. Prod. 1995;58:1913–1914. doi: 10.1021/np50126a018. [DOI] [Google Scholar]
- 14.Kim K.H., Lee I.K., Choi S.U., Lee J.H., Moon E., Kim S.Y., Lee K.R. New triterpenoids from the tubers of Corydalis ternata: Structural elucidation and bioactivity evaluation. Planta Med. 2011;77:1555–1558. doi: 10.1055/s-0030-1270781. [DOI] [PubMed] [Google Scholar]
- 15.Melek F.R., Miyase T., El-Gindi O.D., Abdel-Khalik S.M., Haggag M.Y. Saponins from Fagonia mollis. Phytochemistry. 1996;42:1405–1407. doi: 10.1016/0031-9422(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa M., Murakami T., Harada E., Murakami N., Yamahara J., Matsuda H. Bioactive saponins and glycosides. VII. On the hypoglycemic principles from the root cortex of Aralia elata Seem.: Structure related hypoglycemic activity of oleanolic acid oligoglycoside. Chem. Pharm. Bull. 1996;44:1923–1927. doi: 10.1248/cpb.44.1923. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda H., Li Y., Murakami T., Matsumura N., Yamahara J., Yoshikawa M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem. Pharm. Bull. 1998;46:1399–1403. doi: 10.1248/cpb.46.1399. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa M., Matsuda H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors. 2000;13:231–237. doi: 10.1002/biof.5520130136. [DOI] [PubMed] [Google Scholar]
- 19.Omar R., Li L.Y., Yuan T., Seeram N.P. α-Glucosidase inhibitory hydrolyzable tannins from Eugenia jambolana seeds. J. Nat. Prod. 2012;75:1505–1509. doi: 10.1021/np300417q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.