Abstract
A new series of 2-alkyloxy-pyridine-3-carbonitrile-benzofuran hybrids (4a–x) was synthesized. All the new derivatives were examined via the standard technique for their vasodilation activity. Some of the investigated compounds exhibited a remarkable activity, with compounds 4w, 4e, 4r, 4s, 4f and 4g believed to be the most active hits in this study with IC50 values 0.223, 0.253, 0.254, 0.268, 0.267 and 0.275 mM, respectively, compared with amiodarone hydrochloride, the reference standard used (IC50 = 0.300 mM). CODESSA PRO was employed to obtain a statistically significant 2-Dimensional Quantitative Structure Activity Relationship (2D-QSAR) model describing the bioactivity of the newly synthesized analogs (N = 24, n = 4, R2 = 0.816, R2cvOO = 0.731, R2cvMO = 0.772, F = 21.103, s2 = 6.191 × 10−8).
Keywords: benzofuran, vasodilation activity, 2D-QSAR, amiodarone hydrochloride
1. Introduction
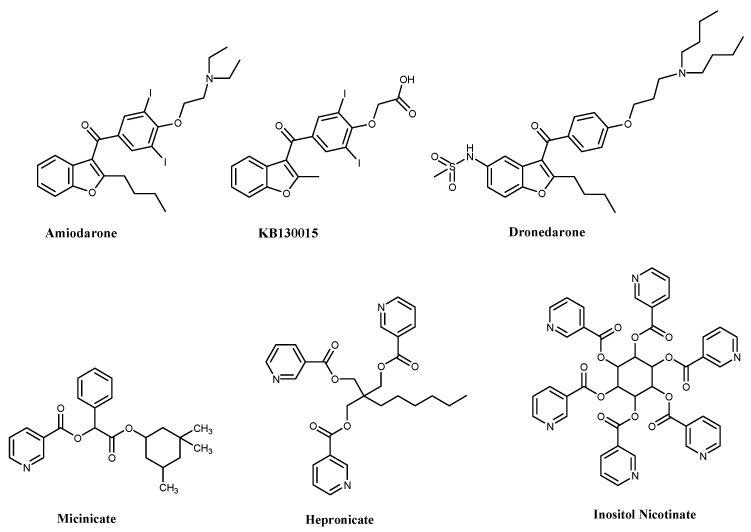
Hypertension is the most common cardiovascular and cerebrovascular disorder representing the major risk factor for endothelial dysfunctions [1,2]. Worldwide, one of every three adults is reported to have high blood pressure, which is responsible for half of the mortalities related to stroke and heart disease [2,3]. Under hypertensive conditions, many functional organs can suffer irreparable lesions [4,5]. Essential hypertension is a common trait caused by many factors and it increases the risk of cardiovascular (heart attacks), cerebrovascular (stroke), peripheral artery, rheumatic heart, congenital heart, heart failure and renal diseases [6,7,8]. A benzofuran-containing compound, amiodarone, is one of the most therapeutically important antiarrhythmic drugs for various types of cardiac dysrhythmias [9,10] (Figure 1). Though the responsible pharmacological mechanisms of amiodarone’s antiarrhythmic effects are not settled [11], it has an extreme effect on various ionic currents [12], as well as sodium, calcium and potassium fluxes. These actions are interrelated in a complex way, but are of prime importance for its activity. Amiodarone also possesses coronary and peripheral vasodilator properties [11]—this appears to be mainly due to a release of nitric oxide (NO). Moreover, it expands the precompressed in vivo human hand veins through the activation of NO synthase and blockade of α-adrenergic mechanisms as a venodilator [13,14], and amiodarone’s analog KB130015 (Figure 1) activates the BKCa channels, which relaxes vascular smooth muscle cells. KB130015 is a novel BKCa activator—its efficacy is based on the subunit composition of the channel complex [15]. Dronedarone as well as KB130015 (Figure 1) is a noniodinated congener of amiodarone that has been developed and approved by FDA (Food and Drug Administration) to avoid the limiting iodine-associated adverse effects of the commercially used amiodarone. Additionally, dronedarone displays antiadrenergic properties, atrial flutter and atrial fibrillation [16]. Consequently, the stimulation of coronary dilation by dronedarone involving a dual mechanism, putative Ca2+ channels inhibition and stimulation of NO synthase pathway [16,17]. Benzofurans are naturally existing scaffolds [18], associated with a broad range of chemotherapeutic properties [19,20,21,22,23,24,25]. Nicotinate esters are very interesting vasodilatory active heterocycles [26,27], also, many nicotinate analogs such as, micinicate, hepronicate and inositol nicotinate are of significant vasodilating activity [28], (Figure 1).
Figure 1.
Structures of some active vasodilating agents.
In the present study, we designed and synthesized some novel hybrids of pyridine-3-carbonitriles and benzofuran-pyrazole functions, attributed to the fact that, pyridine-3-carbonitriles are interesting agents in developing new active hits due to the recognition of bioisosterism with the nicotinate analogs where the acid/ester function is just replaced by a cyano group [28]. Furthermore, it is known that the benzofuran-pyrazole hybrid is of considerable vasorelaxant interest [29], this may due to the belief that the aliphatic secondary amine side chain of amiodarone might be responsible for its vasodilation activity [30,31]. Thus, the insertion of a pyrazole ring system in this scaffold may widen new pharmacological active hits with higher potency and fewer side effects. In addition, studying the two-dimensional quantitative structure activity relationships (2D-QSAR) for the newly synthesized analogs explored the controlling factors governing the observed pharmacological properties as well as validated of the observed activity of the new chemical entities.
2. Results and Discussion
2.1. Chemistry
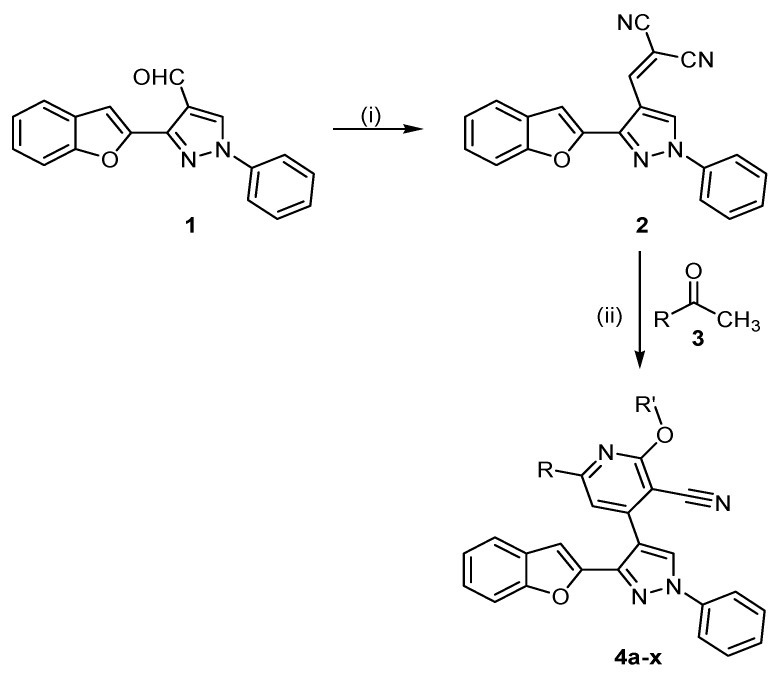
The assumed synthetic approach to obtain target derivatives is illustrated in (Scheme 1). Treating 2-acetylbenzofuran, the key starting compound in this study, with phenyl hydrazine afforded the corresponding semihydrazone derivative which undergo formylation via Vilsmeier–Haack reaction to give 3-(benzofuran-2-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1) [32]. The formed carbaldehyde 1 was treated with malononitrile in refluxing ethanol to give 2-((3-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-methylene)malononitrile (2). Adopting the reported procedure [32], the required 2-alkyloxy-pyridine-3-carbonitrile derivatives 4a–x were synthesized via the condensation reaction of aromatic ketones 3a–l with ylidenemalononitrile 2 and sodium alkoxide of the corresponding alcohol (Scheme 1). As a representative example, the IR spectrum of compound 4a shows a strong stretching vibration band at ν = 2213 cm−1 for nitrile group. 1H-NMR spectrum of 4a exhibits a characteristic signal at δ = 4.23 ppm refering to the methoxide group, while the pyridinyl H-5 appeared as singlet peak at δ = 8.42 ppm. 13C-NMR spectrum of 4a reveals the presence of a methoxide carbon at δ = 54.7 ppm, pyridinyl C-3, C-5 and nitrile carbon signals appeared at δ = 93.5, 105.8, and 114.6 ppm, respectively. Mass spectrum (EI) of 4a reveals the molecular ion peak 482.27 with relative intensity value 30.7%. The established structures of all new chemical entities 4a–x were certified by their microanalyses and spectral data (IR, 1H-NMR, 13C-NMR and EI-MS).
Scheme 1.
Synthetic route of 2-alkyloxy-pyridine-3-carbonitrile derivatives 4a–x.
| 3b; R = 4-ClC6H4 | 4b; R = Ph |
| 3c; R = 4-BrC6H4 | 4c; R = 4-ClC6H4 |
| 3d; R = 4-FC6H4 | 4d; R = 4-ClC6H4 |
| 3e; R = 4-H3CC6H4 | 4e; R = 4-BrC6H4 |
| 3f; R = 4-H3COC6H4 | 4f; R = 4-BrC6H4 |
| 3g; R = 1,2,3,4-Tetrahydronaphthalen-6-yl | 4g; R = 4-FC6H4 |
| 3h; R = 2-Pyrrolyl | 4h; R = 4-FC6H4 |
| 3i; R = 2-Furanyl | 4i; R = 4-H3CC6H4 |
| 3j; R = 2-Thienyl | 4j; R = 4-H3CC6H4 |
| 3k; R = 2-Pyridinyl | 4k; R = 4-H3COC6H4 |
| 3l; R = 1-Methyl-1H-benzo[d]imidazol-2-yl | 4l; R = 4-H3COC6H4 |
| 4m; R = 1,2,3,4-Tetrahydronaphthalen-6-yl, R′ = Me | |
| 4n; R = 1,2,3,4-Tetrahydronaphthalen-6-yl, R′ = Et | |
| 4o; R = 2-Pyrrolyl, R′ = Me | |
| 4p; R = 2- Pyrrolyl, R′ = Et | |
| 4q; R = 2-Furanyl, R′ = Me | |
| 4r; R = 2-Furanyl, R′ = Et | |
| 4s; R = 2-Thienyl, R′ = Me | |
| 4t; R = 2-Thienyl, R′ = Et | |
| 4u; R = 2-Pyridinyl, R′ = Me | |
| 4v; R = 2-Pyridinyl, R′ = Et | |
| 4w;R = 1-Methyl-1H-benzo[d]imidazol-2-yl, R′ = Me | |
Reagents and Conditions: (i) CNCH2CN/Ethanol/r.t./6 h, 75%; (ii) alcohol/Na/r.t./33.3–45.3%.
2.2. Biological Evaluation
2.2.1. Vasodilation Properties
Vasodilation properties of the synthesized 2-alkyloxy-pyridine-3-carbonitrile derivatives 4a–x were inspected applying the separated thoracic aortic rings of mice pre-contracted by norepinephrine hydrochloride according to the standard method [33] using amiodarone hydrochloride as a standard reference. The observed data (Table 1), (Figures S1 and S2 of Supplementary Materials) reveal that most the new chemical entities reveal remarkable vasodilation properties. Meanwhile, compounds 4w, 4e, 4r, 4s, 4f and 4g exhibit significant activity (IC50, is the required concentration for 50% lessening of maximal norepinephrine. HCl induced contracture = 0.223, 0.253, 0.254, 0.268, 0.267 and 0.275 mM, respectively), that seems more potent than the used reference standard in the present study (IC50 = 0.300 mM). The 2D-QSAR study was initiated to recognize the observed bioactivities and concluding the most important factors that manage the pharmacological properties. Currently, throughout the observed vasodilator activities of the new chemical entities, few Structure activity relationship (SAR) rules could be achieved, the presence of a methoxy group at the 2-position of 3-pyridinecarbonitriles enhances the vasodilation activity more than the ethoxy group, as shown in all of the tested analogs. Compounds 4k and 4q (IC50 = 0.428, 0.321 mM, respectively) are exceptions. Benzimidazole ring systems attached to pyridine-C5 seems appropriate for designing vasodilation active hits (IC50 = 0.223, 0.299 mM) compared with the corresponding substituted phenyl ring systems or other heterocycles. Thus, the combination of benzimidazole, pyridine and benzofuran has the potential to be developed into potent vasorelaxant active targets.
Table 1.
Vasodilatory activity IC50 (mM) in rat thoracic aortic rings.
| Entry | Compound | R | R′ | Potency (IC50), mM |
|---|---|---|---|---|
| 1 | 4a | Ph | Me | 0.281 |
| 2 | 4b | Ph | Et | 0.343 |
| 3 | 4c | 4-ClC6H4 | Me | 0.295 |
| 4 | 4d | 4-ClC6H4 | Et | 0.397 |
| 5 | 4e | 4-BrC6H4 | Me | 0.253 |
| 6 | 4f | 4-BrC6H4 | Et | 0.267 |
| 7 | 4g | 4-FC6H4 | Me | 0.275 |
| 8 | 4h | 4-FC6H4 | Et | 0.330 |
| 9 | 4i | 4-H3CC6H4 | Me | 0.330 |
| 10 | 4j | 4-H3CC6H4 | Et | 0.452 |
| 11 | 4k | 4-H3COC6H4 | Me | 0.322 |
| 12 | 41 | 4-H3COC6H4 | Et | 0.291 |
| 13 | 4m | 1,2,3,4-Tetrahydronaphthalen-6-yl | Me | 0.286 |
| 14 | 4n | 1,2,3,4-Tetrahydronaphthalen-6-yl | Et | 0.337 |
| 15 | 4o | 2-Pyrrolyl | Me | 0.356 |
| 16 | 4p | 2-Pyrrolyl | Et | 0.400 |
| 17 | 4q | 2-Furanyl | Me | 0.321 |
| 18 | 4r | 2-Furanyl | Et | 0.254 |
| 19 | 4s | 2-Thienyl | Me | 0.268 |
| 20 | 4t | 2-Thienyl | Et | 0.298 |
| 21 | 4u | 2-Pyridinyl | Me | 0.333 |
| 22 | 4v | 2-Pyridinyl | Et | 0.370 |
| 23 | 4w | 1-Methyl-1H-benzo[d]imidazol-2-yl | Me | 0.223 |
| 24 | 4x | 1-Methyl-1H-benzo[d]imidazol-2-yl | Et | 0.299 |
| 25 | Amiodarone.HCl | - | - | 0.300 |
To validate and understand the observed pharmacological activities and to detect the factors that control the activities, the 2D-QSAR study was initiated via the CODESSA PRO package. Molecular descriptors of the 2D-QSAR correlating the chemical structure(s) and property values expressed as 1/IC50 µM are presented in Table 2, arranged on their level of significance (t-criterion). The descriptors were acquired using the BMLR (Best Multiple Linear Regression) method. The first descriptor controlling the BMLR-QSAR model based on its t-criterion value (t = 7.789) is maximum e–e repulsion for bond C–O which is a semiempirical descriptor. Electron–electron repulsion between two given atoms is determined by Equation (1) [34].
| (1) |
where, A stands for a given atomic species, B is another atomic species Pμν, Pλσ is density matrix elements over atomic basis {μνλσ}, is the electron repulsion integrals on atomic basis {μνλσ}. The second important descriptor controlling the BMLR-QSAR model (t = −3.637) is surface-weighted charged partial-negative charged surface area (WNSA1) weighted PNSA (PNSA1 × TMSA/1000) (MOPAC PC) (charge-related descriptor). Surface-weighted charged partial negative-charged surface area (WNSA1) is calculated by Equation (2) [34].
| (2) |
where, PNSA1 stands for partial negatively charged molecular surface area, TMSA for total molecular surface area. The third descriptor controlling BMLR-QSAR model (t = −5.670) is the fractional hydrogen bonding acceptor ability of the molecule FHACA1, which is also a charge-related descriptor determined by Equation (3) [34].
| (3) |
where, HACA1 is hydrogen bonding acceptor ability, TMSA is the total molecular surface area. The fourth descriptor controlling BMLR-QSAR model (t = −6.241) is a semiempirical descriptor, maximum e–n attraction for bond C–N, is determined by Equation (4) [34].
| (4) |
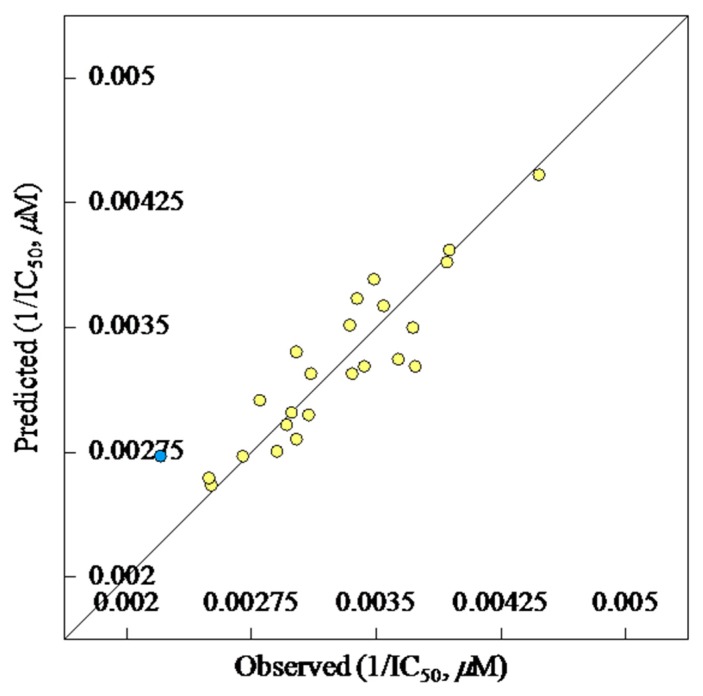
where, A stands for a given atomic species, B is another atomic species Pμν is density matrix elements over atomic basis {μν}, ZB for charge of atomic nucleus B, RiB for distance between the electron and atomic nucleus B, for electron–nuclear attraction integrals on atomic basis {μν}. The correlation between the observed and predicted vasodilation activities is represented in Figure 2. The descriptor values for each respective compound are exhibited in Table S1 of Supplementary Material.
Table 2.
Descriptors of the BMLR-QSAR model for the vasodilatory active compounds.
| Entry | ID | Coefficient | s | t | Descriptor |
|---|---|---|---|---|---|
| N = 24, n= 4, R2 = 0.816, R2cvOO = 0.731, R2cvMO = 0.772, F = 21.103, s2 = 6.191 × 10−8 | |||||
| 1 | 0 | 1.464 | 0.244 | 6.009 | Intercept |
| 2 | D1 | 0.0004 | 4.751 × 10−5 | 7.789 | Max. e–e repulsion for bond C–O |
| 3 | D2 | −7.1825 × 10−6 | 1.975 × 10−6 | −3.637 | WNSA-1 Weighted PNSA (PNSA1*TMSA/1000) (MOPAC PC) |
| 4 | D3 | −0.255 | 0.045 | −5.670 | FHACA Fractional HACA (HACA1/TMSA) (MOPAC PC) |
| 5 | D4 | −0.0043 | 0.001 | −6.241 | Max. e–n attraction for bond C–N |
| 1/IC50 (μM) = 1.464 + (0.0004 × D1) − [(7.1825 × 10−6) × D2] − (0.255 × D3) − (0.0043 × D3) | |||||
Figure 2.
BMLR-QSAR model plot of correlations, the yellow dots are representing the observed vs. predicted vasodilatory active agents for all compounds, while the blue dot is represent an outlier compound (compound 4j).
The reliability and statistical relevance of the attained BMLR-QSAR model is examined by internal validation technique, which is an appropriate technique due to the limited data points of the present study [35,36,37]. Internal validation is applied by the CODESSA PRO employing both Leave One Out (LOO), which involves developing a number of models with one example omitted at a time, and Leave Many Out (LMO) that develops a number of models with many data points omitted at a time (up to 20% of the total data points). The observed correlations attributed to the internal validation techniques are R2cvOO = 0.731, R2cvMO = 0.772. Both of them are significantly correlated with the R2value of the attained QSAR model (R2 = 0.816). Standard deviation of the regressions (s2 = 6.191 × 10−8) and Fisher test value (F = 21.103) are also statistical parameters supporting the QSAR model. The predicted/estimated IC50 values of the new chemical entities according to the achieved BMLR-QSAR model are displayed in Table 3. The obtained results revealed that the most potent analogue among all the tested hybrids, compound 4w, shows error value (difference between estimated and observed IC50 values) = −0.4. Additionally, the high potent analogues synthesized 4a–i, relative to the standard reference, amiodarone hydrochloride (IC50 = 300 µM), also exhibited estimated bioproperties matched with their observed potencies (IC50 = 253–397 µM, 253–392 µM, corresponding to the observed and predicted values respectively, error = 0–−40). Compound 4j is the only exception with high error value = 83 with (IC50 = 452, 369 µM, for observed and predicted bio-data, respectively). This compound is exhibited as an outlier (Figure 2) and shows the lowest vasodilation properties among all the synthesized hybrids. From all the above, it can be concluded that the achieved BMLR-QSAR model is statistically significant and also supported by the matched correlations due to the observed and predicted bio-observations. Success of this study can be attributed to the homogeneity of chemical structural entities. Additionally, the achieved model can be adopted for optimizing hits of high potency relative to the standard reference used based on the hybrid design mentioned in the present study.
Table 3.
Observed and estimated/predicated values of the vasodilatory active compounds according to the BMLR-QSAR model.
| Entry | Compd. | R | R′ | Observed IC50, μM | Estimated IC50, μM | Error |
|---|---|---|---|---|---|---|
| 1 | 4a | Ph | Me | 281 | 276 | 5 |
| 2 | 4b | Ph | Et | 343 | 363 | −20 |
| 3 | 4c | 4-ClC6H4 | Me | 295 | 272 | 23 |
| 4 | 4d | 4-ClC6H4 | Et | 397 | 392 | 5 |
| 5 | 4e | 4-BrC6H4 | Me | 253 | 253 | 0 |
| 6 | 4f | 4-BrC6H4 | Et | 267 | 307 | −40 |
| 7 | 4g | 4-FC6H4 | Me | 275 | 303 | −28 |
| 8 | 4h | 4-FC6H4 | Et | 330 | 354 | −24 |
| 9 | 4i | 4-H3CC6H4 | Me | 330 | 299 | 31 |
| 10 | 4j | 4-H3CC6H4 | Et | 452 | 369 | 83 |
| 11 | 4k | 4-H3COC6H4 | Me | 322 | 337 | −15 |
| 12 | 41 | 4-H3COC6H4 | Et | 291 | 307 | −16 |
| 13 | 4m | 1,2,3,4-Tetrahydronaphthalen-6-yl | Me | 286 | 265 | 21 |
| 14 | 4n | 1,2,3,4-Tetrahydronaphthalen-6-yl | Et | 337 | 344 | −7 |
| 15 | 4o | 2-Pyrrolyl | Me | 268 | 286 | −18 |
| 16 | 4p | 2-Pyrrolyl | Et | 298 | 311 | −13 |
| 17 | 4q | 2-Furanyl | Me | 321 | 310 | 11 |
| 18 | 4r | 2-Furanyl | Et | 254 | 257 | −3 |
| 19 | 4s | 2-Thienyl | Me | 356 | 328 | 28 |
| 20 | 4t | 2-Thienyl | Et | 400 | 386 | 14 |
| 21 | 4u | 2-Pyridinyl | Me | 333 | 336 | −3 |
| 22 | 4v | 2-Pyridinyl | Et | 370 | 369 | 1 |
| 23 | 4w | 1-Methyl-1H-benzo[d]imidazol-2-yl | Me | 223 | 227 | −4 |
| 24 | 4x | 1-Methyl-1H-benzo[d]imidazol-2-yl | Et | 299 | 285 | 14 |
2.2.2. Toxicological Bioassay
The most potent hits in this study, compounds (4a, c, e, f, g, l, m, r, s,t, w and 4x), were tested at 1000 mg kg−1 (mouse body weight), with no toxic symptoms or mortality rates being observed after 24 h post-administrations elucidating the safe behavior of the used doses. Thus, the present study recommended that the benzofuran-containing compounds may have the potential to be developed into potent vasodilatory active agents.
3. Experimental Section
3.1. General Information
Melting points were recorded on a Stuart SMP30 melting point apparatus. IR spectra (KBr) were recorded on a JASCO 6100 spectrophotometer, JASCO, Easton, USA. NMR spectra were recorded on a JEOL AS 500 (DMSO-d6, 1H: 500 MHz, 13C: 125 MHz) spectrometer, JEOL USA, Inc. (Pleasanton, CA, USA). Chemical shifts (δH) are reported relative to Tetramethylsilane (TMS) as the internal standard. All coupling constant (J) values are given in hertz. Chemical shifts (δc) are reported relative to CDCl3 as internal standards. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX (EI, 70 eV) spectrometer, Shimadzu corporation, Kyoto, Japan. Elemental microanalyses were performed by using a Vario Elemental analyzer, Elementar Analysensysteme GmbH, Langenselbold, Germany. 2-Acetylbenzofuran [38], 3-(benzofuran-2-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1) [32] and 2-((3-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)malononitrile (1) [32] were prepared according to the previously reported procedures.
3.1.1. Synthesis of 2-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)malononitrile (2)
The formed carbaldehyde 1 (2.88 g, 10 mmol) was stirred in ethanol at room temperature (25–30 °C) for 6 h with malononitrile (0.66 g, 10 mmol) in the presence of few drops of piperidine, the formed precipitate was filtered, dried and recrystallized from n-butanol, to afford 2.54 g of compound 2 (75% yield).
3.1.2. Synthesis of 2-Alkoxy-4-(3-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-phenylpyridine-3-carbonitriles (4a–x)
General Procedure
A mixture of equimolar amounts of 2 (3.36 g, 10 mmol) and methyl aryl ketones 3a–l (10 mmol), in the appropriate alcohol (20 mL) containing sodium (0.46 g, 20 mmol) was stirred at room temperature (25–30 °C) for the proper time controlled by Thin-layer chromatography (TLC). The solid separated was collected, washed with water and crystallized from n-butanol to afford the title compounds 4a–x.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-phenylpyridine-3-carbonitrile (4a): yield 1.86 g (39.7%), m.p. 198–200 °C. IR: νmax/cm−1 2213 (C≡N), 1588, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 4.23 (s, 3H), 7.08 (s, 1H), 7.25–7.29 (m, 4H), 7.39–8.04 (m, 6H), 7.09 (d, J = 7.7 Hz, 2H), 8.04 (d, J = 7.7 Hz, 2H), 8.38 (s, 1H), 8.42 (s, 1H). 13C-NMR (CDCl3): δ 54.7 93.5, 105.7, 105.8, 111.6, 111.7, 114.6, 119.8, 120.0, 121.4, 123.3, 125.1, 126.7, 127.4, 127.8, 127.9, 128.4, 128.6, 128.7, 129.0, 129.7, 130.6, 139.3, 154.9, 155.1, 157.8, 165.0. MS: m/z (%) 468.31 (M, 0.47). Anal. for C30H20N4O2 (468.51); Calcd. C, 76.91; H, 4.30; N, 11.96. Found: C, 76.84; H, 4.26; N, 11.87.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-phenylpyridine-3-carbonitrile (4b): yield 1.84 g (39%), m.p. 186–188 °C. IR: νmax/cm−1: 2215 (C≡N), 1590, 1541 (C=N, C=C). 1H-NMR (CDCl3): δ 1.55 (t, J = 7.6 Hz, 3H), 4.69 (q, J = 7.6 Hz, 2H), 7.05 (s, 1H), 7.20–7.53 (m, 10 H), 7.81–8.10 (m, 4 H), 8.40 (s, 1H), 8.50 (s, 1H). 13C-NMR (CDCl3): δ 14.8, 63.5, 93.6, 105.8, 111.6, 114.3, 116.1, 118.1, 119.0, 120.0, 121.4, 123.3, 125.1, 127.3, 127.8, 128.6, 128.7, 128.9, 129.7, 132.5, 135.4, 138.5, 140.0, 143.0, 147.3, 148.9, 155.1, 157.7, 164.8. MS: m/z (%) 482.25 (M, 20.34). Anal. for C31H22N4O2 (482.53); Calcd. C, 77.16; H, 4.60; N, 11.61. Found: C, 77.05; H, 4.57; N, 11.72.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(4-chlorophenyl)-2-methoxypyridine-3-carbonitrile (4c): yield 2.04 g (40.5%), m.p. 259–261 °C. IR: νmax/cm−1 2214 (C≡N), 1588, 1542 (C=N, C=C). 1H-NMR (DMSO-d6): δ 4.14 (s, 3H), 7.08 (s, 1H), 7.20–7.58 (m, 9H), 7.92 (s, 1H), 8.0 (d, J = 8 Hz, 2H), 8.12 (d, J = 8 Hz, 2H), 9.1 (s, 1H). MS: m/z (%) 502.11 (M, 0.47). Anal. for C30H19ClN4O2 (502.95); Calcd. C, 71.64; H, 3.81; N, 11.14. Found: C, 71.56; H, 3.78; N, 11.18.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(4-chlorophenyl)-2-ethoxypyridine-3-carbonitrile (4d): yield 2.27 g (40.4%), m.p. 199–201 °C. IR: νmax/cm−1 2219 (C≡N), 1588, 1541 (C=N, C=C). 1H-NMR (CDCl3): δ 1.54 (t, J = 6.7 Hz, 3H), 4.67 (q, J = 6.7 Hz, 2H), 7.08 (s, 1H), 7.35–7.53 (m, 10H), 7.81–7.84 (m, 4H), 8.43 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 63.5, 93.8, 105.9, 111.5, 114.1, 116.0, 117.5, 119.9, 121.5, 123.4, 125.2, 127.9, 128.4, 128.6, 129.0, 129.2, 129.8, 135.8, 136.7, 139.2, 148.9, 149.0, 154.9, 156.4, 164.7. MS: m/z (%) 516.30, 518.30 (M, M+2, 1.95, 0.68). Anal. for C31H21ClN4O2 (516.98); Calcd. C, 72.02; H, 4.09; N, 10.84. Found: C, 72.14; H, 4.14; N, 10.78.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(4-bromophenyl)-2-methoxypyridine-3-carbonitrile (4e): yield 2.14 g (39%), m.p. 273–275 °C. IR: νmax/cm−1 2217 (C≡N), 1585, 1542 (C=N, C=C). 1H-NMR (DMSO-d6): δ 4.14 (s, 3H), 7.09 (s, 1H), 7.20–7.73 (m, 10H), 7.96–7.16 (m, 4H), 9.10 (s, 1H). MS: m/z (%) 546.16 (M, 3.22). Anal. for C30H19BrN4O2 (547.40): Calcd. C, 65.82; H, 3.50; N, 10.24. Found: C, 65.94; H, 3.38; N, 10.17.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(4-bromophenyl)-2-ethoxypyridine-3-carbonitrile (4f): yield 1.94 g (34.6%), m.p. 207–209 °C. IR: νmax/cm−1 2219 (C≡N), 1588, 1540 (C=N, C=C). 1H-NMR (CDCl3): δ 1.54 (t, J = 6.7 Hz, 3H), 4.67 (q, J = 6.7 Hz, 2H), 7.09 (s, 1H), 7.20–7.52 (m, 10H), 7.77–7.81 (m, 4H), 8.44 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 63.6, 93.9, 105.9, 111.5, 114.1, 115.7, 117.5, 120.0, 121.5, 123.4, 125.2, 127.9, 128.4, 128.8, 129.0, 129.8, 132.2, 136.3, 139.3, 142.4, 147.4, 148.9, 154.9, 156.5, 164.8. MS: m/z (%) 560.40 (M, 0.24). Anal. for C31H21BrN4O2 (561.43); Calcd. C, 66.32; H, 3.77; N, 9.98. Found: C, 66.54; H, 3.84; N, 9.82.
4-(3-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(4-fluorophenyl)-2-methoxypyridine-3-carbonitrile (4g): yield 1.96 g (40.3%), m.p. 237–239 °C. IR: νmax/cm−1 2215 (C≡N), 1590, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 4.22 (s, 3H), 7.07 (s, 1H), 7.25–7.53 (m, 10H), 7.81–7.93 (m, 4H), 8.44 (s, 1H).MS: m/z (%) 486.31 (M, 1.90). Anal. for C30H19FN4O2 (486.5); Calcd. C, 74.06; H, 3.94; N, 11.52. Found: C, 73.95; H, 3.88; N, 11.48.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(4-fluorophenyl)pyridine-3-carbonitrile (4h): yield 2.12 g (42.4%), m.p. 204–206 °C. IR: νmax/cm−1 2218 (C≡N), 1592, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 1.53 (t, J = 7 Hz, 3H), 4.67 (q, J = 7 Hz, 2H), 7.08 (s, 1H), 7.39–7.54 (m, 10 H), 7.81–7.83 (m, 4H), 8.44 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 63.6, 93.5, 105.9, 111.5, 115.9, 116.1, 120.0, 121.4, 123.4, 125.1, 127.9, 129.0, 129.3, 129.4, 129.8, 139.3, 142.4, 147.4, 148.9, 154.9, 156.6, 163.3, 164.8, 165.3. MS: m/z (%) 500.36 (M, 2.71). Anal. for C31H21FN4O2 (500.52); Calcd. C, 74.39; H, 4.23; N, 11.19. Found: C, 74.45; H, 4.18; N, 11.14.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-p-tolylpyridine-3-carbonitrile (4i): yield 2.3 g (47.7%), m.p. 258–260 °C. IR: νmax/cm−1 2218 (C≡N), 1588, 1541 (C=N, C=C). 1H-NMR (CDCl3): δ 2.39 (s, 3H), 4.22 (s, 3H), 7.06 (s, 1H), 7.22–7.52 (m, 10H), 7.58–7.86 (m, 4H), 8.41 (s, 1H). 13C-NMR (CDCl3): δ 21.5, 54.7, 93.1, 105.8, 111.6, 114.2, 115.9, 117.6, 120.1, 121.4, 123.3, 125.1, 127.3, 127.9, 128.4, 129.0, 129.7, 134.5, 139.3, 141.0, 147.2, 148.9, 154.5, 157.9, 165.0. MS: m/z (%) 482.27 (M, 30.7). Anal. for C31H22N4O2 (482.53): Calcd. C, 77.16; H, 4.60; N, 11.61. Found: C, 77.29; H, 4.42; N, 11.51.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-p-tolylpyridine-3-carbonitrile (4j): yield 1.92 g (38.7%), m.p. 202–204 °C. IR: νmax/cm−1 2215 (C≡N), 1598, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 1.54 (t, J = 6 Hz, 3H), 2.39 (s, 3H), 4.69 (q, J = 6 Hz, 2H), 7.05 (s, 1H), 7.20–7.56 (m, 10H), 7.81–7.85 (m, 4H), 8.41 (s, 1H). 13C-NMR (CDCl3): δ 14.7, 21.5, 63.4, 93.2, 105.8, 111.6, 114.0, 116.0, 117.7, 120.0, 121.4, 123.3, 125.1, 127.3, 127.8, 128.4, 129.0, 129.7, 134.6, 139.3, 141.0, 143.0, 147.2, 149.0, 154.9, 157.8, 164.7. MS: m/z (%) 496.22 (M, 5.52). Anal. for C32H24N4O2 (496.56): Calcd. C, 77.40; H, 4.87; N, 11.28. Found: C, 77.52; H, 4.76; N, 11.34.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-(4-methoxyphenyl)pyridine-3-carbonitrile (4k): yield 1.86 g (37.3%), m.p. 256–258 °C. IR: νmax/cm−1 2213 (C≡N), 1588, 1543 (C=N, C=C). 1H-NMR (DMSO-d6): δ 3.80 (s, 3H), 4.13 (s, 3H), 7.04 (s, 1H), 7.20–7.62 (m, 10H), 7.88–8.20 (m, 4H), 9.08 (s, 1H).MS: m/z (%) 498.38 (M, 100). Anal. for C31H22N4O3 (498.53); Calcd. C, 74.69; H, 4.45; N, 11.24. Found: C, 74.66; H, 4.38; N, 11.29.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(4-methoxyphenyl)pyridine-3-carbonitrile (4l): yield 2.14 g (41.7%), m.p. 168–170 °C. IR: νmax/cm−1 2219 (C≡N), 1589, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 1H-NMR (CDCl3): δ 1.54 (t, J = 7 Hz, 3H), 3.83 (s, 3H), 4.67 (q, J = 7 Hz, 2H), 6.90 (s, 1H), 7.05 (s, 1H), 7.45–7.52 (m, 9H), 7.81–7.90 (m, 4H), 8.40 (s, 1H). 13C-NMR (CDCl3): δ 14.7, 55.5, 63.4, 92.6, 105.8, 111.6, 113.4, 114.3, 117.8, 120, 121.4, 123.3, 125.1, 127.8, 128.4, 128.9, 129, 129.7, 139.3, 142.4, 147.1, 149, 154.9, 157.4, 161.7, 164.7. MS: m/z (%) 512 (M, 22.4). Anal. for C32H24N4O3 (512.56); Calcd. C, 74.99; H, 4.72; N, 10.93. Found: C, 74.91; H, 4.71; N, 10.89.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(1,2,3,4-tetrahydronaphthalen-6-yl)-2-methoxypyridine-3-carbonitrile (4m): yield 1.78 g (34%), m.p. 255–257 °C. IR: νmax/cm−1 2221 (C≡N), 1591, 1555 (C=N, C=C). 1H-NMR (CDCl3): δ 1.58 (s, 2H), 1.79 (s, 2H), 2.69 (s, 2H), 2.78 (s, 2H), 4.22 (s, 3H), 7.06 (s, 1H), 7.24–7.58 (m, 10H), 7.81–7.83 (m, 4H), 8.43 (s, 1H). 13C-NMR (CDCl3): δ 23.1, 29.6, 54.6, 92.8, 105.9, 111.6, 114.3, 119.9, 121.4, 123.0, 124.5, 125.1, 127.8, 128.1, 128.4, 128.9, 129.7, 129.9, 137.3, 155.0, 158.2, 165.0. Anal. for C34H26N4O2 (522.6); Calcd. C, 78.14; H, 5.01; N, 10.72. Found: C, 73.69; H, 4.14; N, 15.56.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(1,2,3,4-tetrahydronaphthalen-6-yl)pyridine-3-carbonitrile (4n): Yield 2.16 g (40.3%), m.p. 213–215 °C. IR: νmax/cm−1 2213 (C≡N), 1585, 1542 (C=N, C=C). 1H-NMR (CDCl3): δ 1.54 (t, J = 7.7 Hz, 3H), 1.78 (s, 4H), 2.69 (s, 2H), 2.78 (s, 2H), 4.68 (q, J = 7.7 Hz, 2H), 7.05 (s, 1H), 7.12–7.56 (m, 11H), 7.81 (d, J = 8.5 Hz, 2H), 8.42 (s, 1H). 13C-NMR (CDCl3): δ 14.7, 23.1, 23.2, 29.6, 29.6 63.4, 92.9, 105.9, 111.6, 114.0, 116.0, 118.0, 120.0, 121.4, 123.3, 124.5, 125.1, 127.8, 128.1, 128.9, 129.7, 129.8, 135.0, 137.8, 140.3, 148.5, 149.5, 155.0, 158.2, 164.8. MS: m/z (%) 536.31 (M, 100). Anal. for C35H28N4O2 (536.62); Calcd. C, 78.34; H, 5.26; N, 10.44. Found: C, 78.52; H, 5.14; N, 10.57.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-(1H-pyrrol-2-yl)pyridine-3-carbonitrile (4o): yield 1.86 g (40.6%), m.p. 187–189 °C. IR: νmax/cm−1 2218 (C≡N), 1587, 1539 (C=N, C=C). 1H-NMR (CDCl3): δ 3.97 (s, 3H), 7.06 (s, 1H), 7.29–7.55 (m, 10H), 7.59–7.82 (m, 2H), 8.45 (s, 1H), 9.05 (s, 1H). 13C-NMR (CDCl3): δ 54.5, 102.0, 106.3, 111.6, 115.4, 117.0, 120.0, 121.7, 123.6, 125.4, 128.2, 128.4, 129.8, 129.9, 138.9, 139.1, 145.7, 148.9, 155.3, 162.4. MS: m/z (%) 457.25 (M, 1.93). Anal. for C28H19N5O2 (457.48); Calcd. C, 73.51; H, 4.19; N, 15.31. Found: C, 73.69; H, 4.14; N, 15.56.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(1H-pyrrol-2-yl)pyridine-3-carbonitrile (4p): yield 1.84 g (39%), m.p. 208–210 °C. IR: νmax/cm−1 2217 (C≡N), 1588, 1538 (C=N, C=C). 1H-NMR (CDCl3): δ 1.53 (t, J = 7.7 Hz, 3H), 4.64 (q, J = 7.7 Hz, 2H), 7.05 (s, 1H), 7.20–7.30 (m, 4H), 7.46–7.54 (m, 8H), 7.81 (d, 2H), 8.40 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 63.8, 93.0, 105.8, 111.6, 112.7, 120.1, 121.4, 123.3, 125.1, 127.1, 127.9, 128.6, 129.0, 129.7, 130.0, 139.3, 142.4, 143.4, 147.2, 148.8, 152.9, 154.9, 164.7. MS: m/z (%) 471.37 (M, 100). Anal. for C29H21N5O2 (471.51); Calcd. C, 73.87; H, 4.49; N, 14.85. Found: C, 73.75; H, 4.43; N, 14.88.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-6-(furan-2-yl)-2-methoxypyridine-3-carbonitrile (4q): yield 1.88 g (41%), m.p. 246–248 °C. IR: νmax/cm−1 2217 (C≡N), 1588, 1538 (C=N, C=C). 1H-NMR (CDCl3): δ 4.21 (s, 3H), 6.54 (s, 1H), 7.05 (s, 1H), 7.20–7.53 (m, 10H), 7.80 (d, 2H), 8.35 (s, 1H). 13C-NMR (CDCl3): δ 54.7, 93.3, 105.7, 111.6, 112.3, 112.6, 117.4, 120.0, 121.4, 123.2, 125.0, 127.9, 128.4, 129.0, 129.7, 139.3, 142.4, 145.1, 147.6, 148.7, 149.3, 152.5, 154.9, 165.1. MS: m/z (%) 458.21 (M, 100). Anal. for C28H18N4O3 (458.47); Calcd. C, 73.35; H, 3.96; N, 12.22. Found: C, 71.22; H, 4.14; N, 11.49.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(furan-2-yl)pyridine-3-carbonitrile (4r): Yield 2.14 g (45.3%), m.p. 174–176 °C. IR: νmax/cm−1 2216 (C≡N), 1590, 1521 (C=N, C=C). 1H-NMR (CDCl3): δ 1.51 (t, J = 7 Hz, 3H), 4.62 (q, J = 7 Hz, 2H), 6.53 (s, 1H), 7.00 (s, 1H), 7.15–7.30 (m, 3H), 7.39–7.55 (m, 7H), 7.81 (d, 2H), 8.35 (s, 1H). Anal. for C29H20N4O3 (472.49); Calcd. C, 73.72; H, 4.27; N, 11.86. Found: C, 73.65; H, 4.29; N, 11.83.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-(thiophen-2-yl)pyridine-3-carbonitrile (4s): yield 1.58 g (33.3%), m.p. 232–234 °C. IR: νmax/cm−1 2211 (C≡N), 1629, 1523 (C=N, C=C). 1H-NMR (CDCl3): δ 4.19 (s, 3H), 7.06 (s, 1H), 7.25–7.52 (m, 11H), 7.81 (d, 2H), 8.40 (s, 1H). 13C-NMR (CDCl3): δ 54.8, 92.9, 105.8, 111.6, 112.9, 120.1, 121.4, 123.3, 127.2, 127.9, 128.6, 129.1, 129.7, 130.1, 139.3, 142.4, 143.2, 148.8, 153.0, 154.9, 165.0. MS: m/z (%) 474.18 (M, 100). Anal. for C28H18N4O2S (474.53); Calcd. C, 70.87; H, 3.82; N, 11.81. Found: C, 70.79; H, 3.74; N, 11.74.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(thiophen-2-yl)pyridine-3-carbonitrile (4t): Yield 2.06 g (42.2%), m.p. 202–204 °C. IR: νmax/cm−1 2217 (C≡N), 1591, 1546 (C=N, C=C). 1H-NMR (CDCl3): 1H-NMR (CDCl3): δ 1.52 (t, J = 7 Hz, 3H), 4.63 (q, J = 7 Hz, 2H), 6.35 (s, 1H), 6.65 (s, 1H), 7.26–7.52 (m, 3H), 7.64–7.83 (m, 7H), 8.25 (d, J = 8 Hz, 2H), 8.36 (s, 1H), 9.65 (s, 1H). 13C-NMR (CDCl3): δ 14.6. 63.8, 93.0, 105.8, 111.6, 112.7, 115.5, 117.5, 120.1, 121.4, 123.4, 125.1, 127.1, 127.9, 128.6, 129.0, 129.7, 130.0, 139.3, 142.4, 143.4, 147.2, 148.8, 153.0, 155.0, 165.0. MS: m/z (%) 488.34 (M, 100). Anal. for C29H20N4O2S (488.56); Calcd. C, 71.29; H, 4.13; N, 11.47. Found: C, 71.22; H, 4.14; N, 11.49.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-(pyridin-2-yl)pyridine-3-carbonitrile (4u): Yield 1.85 g (39.4%), m.p. 275–277 °C. IR: νmax/cm−1 2218 (C≡N), 1588, 1545 (C=N, C=C). 1H-NMR (DMSO-d6): δ 4.18 (s, 3H), 7.09 (s, 1H), 7.25–7.62 (m, 10H), 7.98 (d, J = 6.7 Hz, 2H), 8.26 (s, 1H), 8.66 (s, 1H), 9.15 (s, 1H).MS: m/z (%) 469.39 (M, 100). Anal. for C29H19N5O2 (469.49); Calcd. C, 74.19; H, 4.08; N, 14.92. Found: C, 74.24; H, 4.15; N, 14.86.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(pyridin-2-yl)pyridine-3-carbonitrile (4v): yield 1.75 g (36.2%), m.p. 204–206 °C. IR: νmax/cm−1 2219 (C≡N), 1589, 1543 (C=N, C=C). 1H-NMR (CDCl3): δ 1.55 (t, J = 6.7 Hz, 3H), 4.69 (q, J = 6.7 Hz, 2H), 7.03 (s, 1H), 7.19–7.53 (m, 13H), 7.82 (d, J = 8.6 Hz, 2H), 8.27–8.35 (m, 2H), 8.65 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 63.7, 95.8, 104.9, 105.5, 111.6, 119.8, 120.0, 121.3, 123.1, 124.8, 128.9, 129.7, 137.0, 139.4, 142.5, 142.6, 148.1, 149.6, 154.3, 156.5, 164.6. MS: m/z (%) 483.25 (M, 100). Anal. for C30H21N5O2 (483.52); Calcd. C, 74.52; H, 4.38; N, 14.48. Found: C, 74.36; H, 4.24; N, 11.56.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-methoxy-6-(1-methyl-1H-benzo[d]imidazole-2-yl)pyridine-3-carbonitrile (4w): yield 2.05 g (39.2%), m.p. 295–297 °C. IR: νmax/cm−1 2214 (C≡N), 1586, 1538 (C=N, C=C). 1H-NMR (CDCl3): δ 4.18 (s, 3H), 4.35 (s, 3H), 7.14 (s, 1H), 7.27–8.01 (m, 13H), 8.26 (s, 1H), 9.16 (s, 1H).MS: m/z (%) 522.1 (M, 20.5). Anal. for C32H22N6O2 (522.56); Calcd. C, 73.55; H, 4.24; N, 16.08. Found: C, 73.49; H, 4.22; N, 16.13.
4-(3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-2-ethoxy-6-(1-methyl-1H-benzo[d]-imidazol-2-yl)pyridine-3-carbonitrile (4x): yield 2.12 g (39.5%), m.p. 247–249 °C. IR: νmax/cm−1 2218 (C≡N), 1587, 1539 (C=N, C=C). 1H-NMR (CDCl3): δ 1.56 (t, J = 7 Hz, 3H), 4.36 (q, J = 7 Hz, 2H), 4.64 (s, 3H), 7.1 (s, 1H), 7.20–7.53 (m, 11H, Ar–H), 7.77, 7.83 (dd, 2H, J = 7.65, J = 7.65, Ar–H), 8.33 (s, 1H), 8.39 (s, 1H). 13C-NMR (CDCl3): δ 14.6, 33.2, 64.1, 95.9, 105.4, 110.1, 111.7, 118.7, 120.0, 120.6, 121.4, 123.1, 123.3, 124.4, 124.8, 127.8, 128.9, 129.7, 137.5, 139.3, 142.5, 142.7, 148.1, 148.3, 148.9, 150.7, 155.0, 164.3. MS: m/z (%) 536.44 (M, 100). Anal. for C33H24N6O2 (536.58); Calcd. C, 73.87; H, 4.51; N, 15.66. Found: C, 73.91; H, 4.45; N, 15.58.
3.2. Vasodilation Activity Screening
The vasodilation activity screening procedures were carried out according to the standard reported techniques [35] by testing the effects of the synthesized 2-alkoxy-4-aryl-6-(benzofuran-2-yl)-3-pyridinecarbonitriles 4a–x on isolated thoracic aortic rings of male Wistar rats (250–350 g). After light ether anesthesia, the rats were sacrificed by cervical dislocation. The aortae were immediately excised, freed of extraneous tissues and prepared for isometric tension recording. Aorta was cut into (3–5 mm width) rings and each ring was placed in a vertical chamber “10 mL jacketed automatic multi-chamber organ bath system (Model no. ML870B6/C, Panlab, Spain)” filled with Krebs solution composed of (in mM): NaCl, 118.0; KCl, 4.7; NaHCO3, 25.0; CaCl2, 1.8; NaH2PO4, 1.2; MgSO4, 1.2; glucose, 11.0 and oxygenated with carbogen gas (95% O2/5% CO2) at 37 ± 0.5 °C. Each aortic ring was mounted between two stainless steel hooks passed through its lumen. The lower hook was fixed between two plates, while the upper one was attached to a force displacement transducer (Model no. MLT0201, Panlab, Spain) connected to an amplifier (PowerLab, AD Instruments Pty., Ltd. Victoria, Australia), which is connected to a computer. The chart for Windows (v 3.4) software was used to record and elaborate data. Preparations were stabilized under 2 g resting tension during 2 h and then the contracture response to norepinephrine hydrochloride (10−6 M) was measured before and after exposure to increasing concentrations of the tested synthesized compounds. The tested compounds were dissolved in dimethylsulfoxide (DMSO) as stock solution (10 mL of 0.005 M). Control experiments were performed in the presence of DMSO alone, at the same concentrations as those used with the derivatives tested, which demonstrated that the solvent did not affect the contractile response of isolated aorta. The observed vasodilation activity screening data are reported and the potency (IC50, concentration necessary for 50% reduction of maximal norepinephrine hydrochloride induced contracture) was calculated in three successful replicates and the observed vasodilation activity data expressed as IC50 has determined mathematically from the dose response curve of each tested compound (Table 1), (Figures S1 and S2 of Supplementary Materials) and the potency (IC50, concentration necessary for 50% reduction of maximal norepinephrine hydrochloride-induced contracture) was determined. This experiment was carried out in according to recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication No. 85–23, revised 1996) and under regulations of the Animal Care and Use of National Research Centre in Egypt.
2D-QSAR Study
The QSAR study was undertaken using comprehensive descriptors for structural and statistical analysis (CODESSA PRO) software employing the synthesized compounds 4a–x of the present study (Table 3). Geometry of the compounds was optimized using molecular mechanics force field (MM+) followed by the semiempirical AM1 method implemented in the HyperChem 8.0 package. The structures were fully optimized without fixing any parameters, thus bringing all geometric variables to their equilibrium values. The energy minimization protocol employed the Polake–Ribiere conjugated gradient algorithm. Convergence to a local minimum was achieved when the energy gradient was ≤0.01 kcal/mol. The Restricted Hartree–Fock (RHF) method was used in spin-pairing for the two semiempirical tools [39,40,41,42,43,44]. The resulting output files were exported to CODESSA PRO that includes MOPAC capability for final geometry optimization. CODESSA PRO calculated 797 molecular descriptors including constitutional, topological, geometrical, charge-related, semiempirical, thermodynamic, molecular-type, atomic-type and bond-type descriptors for the exported 24 bioactive benzofuran-based hybrids 4a–x, which were used in the present study. Different mathematical transformations of the experimentally observed property/activity (IC50, µM, which is the concentration necessary for 50% reduction of maximal norepinephrine hydrochloride induced contracture) of the training set compounds were utilized for the present QSAR modeling determination including property (IC50, µM), 1/property, log(property) and 1/log(property) values in searching for the best QSAR models. Best multilinear regression (BMLR) was utilized, which is a stepwise search for the best n-parameter regression equations (where, n stands for the number of descriptors used), based on the highest R2 (squared correlation coefficient), R2cvOO (squared cross-validation “leave one-out, LOO” coefficient), R2cvMO (squared cross-validation “leave many-out, LMO” coefficient), F (Fisher statistical significance criteria) values, and s2 (standard deviation). The QSAR models, with up to four descriptor models describing the bioactivity of the vasodilatory active agents were generated (obeying the thumb rule of 6:1, which is the ratio between the data points and the number of QSAR descriptor models). Statistical characteristics of the QSAR models are presented in Table 2. The established QSAR model is statistically significant. The descriptors are sorted in descending order of the respective values of the Student’s t-criterion, which is a widely accepted measure of statistical significance of individual parameters in multiple linear regressions. Figure 2 exhibits the QSAR multilinear model plot of correlation representing the observed vs. predicted 1/IC50 values for vasodilatory active agents. The scattered plots are uniformly distributed, covering ranges, Observed 0.00221–0.00448; Predicted 0.00255–0.004411/IC50 units.
3.3. Toxicological Bioassay
Toxicological bioassay of the most promising vasodilatory active compounds (4a, c, e, f, g, l, m, r, s, t, w and 4x) was determined using the standard reported method in mice [45]. Albino mice weighing 25–30 g were divided in 13 groups of 6 mice each. Administrations of the tested compounds dissolved in saline solution (0.9%) by the aid of few drops of Tween 80 were given intraperitoneally in 1000 mg kg−1 (mouse body weight). The control group was given saline solution only with few drops of Tween 80. The toxic symptoms and mortality rates were recorded 24 h post-administration in each group.
4. Conclusions
The required 2-alkyloxy-pyridine-3-carbonitrile hybrids (4a–x) were designed and synthesized via the condensation reaction of aromatic ketones 3a‒l with 2-((3-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)malononitrile (2) in the presence of sufficient amount of sodium alkoxide in the corresponding alcohol. The compounds have evaluated for their vasodilation activity adopting the standard technique “using isolated thoracic aortic rings of rats precontracted with norepinephrine hydrochloride”. Some compounds revealed a noteworthy activity, with compounds 4w, 4e, 4r, 4s, 4f and 4g believed to be the most active hits in this study. “IC50, concentration necessary for 50% reduction of maximal norepinephrine hydrochloride induced contracture = 223, 253, 254, 268, 267 and 275 µM, respectively”, compared with amiodarone hydrochloride, the reference standard used (IC50 = 300 μM). The CODESSA PRO program was utilized to achieve a statistically significant 2D-QSAR model describing the bioactivity of the newly synthesized analogues 4a–x, and afforded an excellent predictive and statistically vital four-crucial 4 descriptor model (R2 = 0.816, R2observed = 0.731, R2pridicted = 0.772). It is obvious that the 2D-QSAR study supported the attained model, so the applicability of benzofuran-based hybrids incorporating the 3-pyridinecarbonitrile function have potential to be developed into vasorelaxant active agents.
Acknowledgments
The project was financially supported by King Saud University, Vice Deanship of Research Chairs. The project was also financially supported by National Research Centre, Dokki, Cairo 12622, Egypt, under project No. 110-10-312.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
The listed authors contributed to this work as described in the following. A.M.S. and S.S.A.E.-k. carried out the synthetic work, interpreted the results and prepared the manuscript; N.M.K. and M.A.A.-O. interpreted the results and cooperated in the preparation of the manuscript, and D.O.S. carried and interpreted the results of the biological activities. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest
Footnotes
Sample Availability: Samples of which all the compounds are available from the authors.
References
- 1.Garovic V.D., Hayman S.R. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat. Clin. Pract. Nephrol. 2007;3:613–622. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 2.Kummerle A.E., Raimundo J.M., Leal C.M., Da Silva G.S., Balliano T.L., Pereira M.A., De Simone C.A., Sudo R.T., Zapata-Sudo G., Fraga C.A., et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009;44:4004–4009. doi: 10.1016/j.ejmech.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Media Centre. [(accessed on 14 February 2016)]; Available online: http://www.who.int/mediacentre/news/releases/2012/world_health_statistics_20120516/en/4004-4009.
- 4.Da Silva V.J., Viana P.C., Alves R.M., Salgado H.C., Montano N., Fazan R.J. Antihypertensive action of amiodarone in spontaneously hypertensive rats. Hypertension. 2001;38:597–601. doi: 10.1161/hy09t1.096187. [DOI] [PubMed] [Google Scholar]
- 5.Almeida M.R., Lima E.O., da Silva V.J., Campos M.G., Antunes L.M., Salman A.K., Dias F.L. Genotoxic studies in hypertensive and normotensive rats treated with amiodarone. Mutat. Res. 2008;657:155–159. doi: 10.1016/j.mrgentox.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Cardiovascular Disease. [(accessed on 22 March 2016)]; Available online: http://www.who.int/topics/cardiovascular_diseases/en/
- 7.Kashyap M.K., Yadav V., Sherawat B.S., Jain S., Kumari S., Khullar M., Sharma P.C., Nath R. Different antioxidants status, total antioxidant power and free radicals in essential hypertension. Mol. Cell. Biochem. 2005;277:89–99. doi: 10.1007/s11010-005-5424-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang R., Shamloul R., Wang X., Meng Q., Wu L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension. 2006;48:685–692. doi: 10.1161/01.HYP.0000239673.80332.2f. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson B., Singh B.N., Temciuc M., Nilsson S., Li Y.-L., Mellin C., Malm J. Synthesis and preliminary characterization of a novel antiarrhythmic compound (KB130015) with an improved toxicity profile compared with amiodarone. J. Med. Chem. 2002;45:623–630. doi: 10.1021/jm001126+. [DOI] [PubMed] [Google Scholar]
- 10.Mubagwa K., Macianskiene R., Viappiani S., Gendviliene V., Carlsson B., Brandts B. KB130015, a new amiodarone derivative with multiple effects on cardiac ion channels. Cardiovasc. Drug Rev. 2003;21:216–235. doi: 10.1111/j.1527-3466.2003.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 11.Guiraudou P., CosnierPucheu S.C., Gayraud R., Gautier P., Roccon A., Herbert J.M., Nisato D. Involvement of nitric oxide in amiodarone- and dronedarone-induced coronary vasodilation in guinea pig heart. Eur. J. Pharmacol. 2004;496:119–127. doi: 10.1016/j.ejphar.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Kodama I., Kamiya K., Toyama J. Amiodarone: Ionic and cellular mechanisms of action of the most promising class III agent. Am. J. Cardiol. 1999;84:20–28. doi: 10.1016/S0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann M., Dobrev D., Kirch W. Amiodarone causes endothelium-dependent vasodilation in human hand veins in vivo. Clin. Pharmacol. Ther. 1998;64:302–311. doi: 10.1016/S0009-9236(98)90179-5. [DOI] [PubMed] [Google Scholar]
- 14.Massie B.M., Fisher S.G., Deedwania P.C., Singh B.N., Fletcher R.D., Singh S.N. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. Circulation. 1996;93:2128–2134. doi: 10.1161/01.CIR.93.12.2128. [DOI] [PubMed] [Google Scholar]
- 15.Gessner G., Heller R., Hoshi T., Heinemann S.H. The amiodarone derivative 2-methyl-3-(3,5-diiodo-4-carboxymethoxybenzyl)benzofuran (KB130015) opens large- conductance Ca2+-activated K+ channels and relaxes vascular smooth muscle. Eur. J. Pharm. 2007;555:185–193. doi: 10.1016/j.ejphar.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Hanafy D.A., Chen Y., Chang S., Lu Y., Lin Y., Kao Y., Chen S., Chen Y. Different effects of dronedarone and amiodarone on pulmonary vein electrophysiology, mechanical properties and H2O2-induced arrhythmogenicity. Eur. J. Pharm. 2013;702:103–108. doi: 10.1016/j.ejphar.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Stewart S., Hart C.L., Hole D.J., McMurray J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am. J. Med. 2002;113:359–364. doi: 10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 18.Hayta S.A., Arisoy M., Arpaci O.T., Yildiz I., Aki E., Ozkan S., Kaynak F. Synthesis, antimicrobial activity, pharmacophore analysis of some new 2-(substitutedphenyl/benzyl)-5-[(2-benzofuryl)carboxamido]benzoxazoles. Eur. J. Med. Chem. 2008;43:2568–2578. doi: 10.1016/j.ejmech.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Manna K., Agrawal Y.K. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity. Bioorg. Med. Chem. Lett. 2009;19:2688–2692. doi: 10.1016/j.bmcl.2009.03.161. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y., Kumar D., Bodduri V.D., Tarani P.S., Zhao B., Miao J., Jang K., Shin D. Microwave-assisted parallel synthesis of benzofuran-2-carboxamide derivatives bearing anti-inflammatory, analgesic and antipyretic agents. Tetrahed. Lett. 2014;55:2796–2800. doi: 10.1016/j.tetlet.2014.02.116. [DOI] [Google Scholar]
- 21.El-Sawy E.R., Ebaid M.S., Abo-Salem H.M., Al-Sehemi A.G., Mandour A.H. Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of some new 4,6-dimethoxy-5- (heterocycles)benzofuran starting from naturally occurring. Arab. J. Chem. 2014;7:914–923. doi: 10.1016/j.arabjc.2012.12.041. [DOI] [Google Scholar]
- 22.Thevenin M., Thoret S., Grellier P., Dubois J. Synthesis of polysubstitutedbenzofuran derivatives as novel inhibitors of parasitic growth. Bioorg. Med. Chem. 2013;21:4885–4892. doi: 10.1016/j.bmc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Feher D., Barlow R.S., McAtee J., Hemscheidt T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010;73:1963–1966. doi: 10.1021/np100506z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galal S.A., Abd El-All A.S., Abdallah M.M., El-Diwani H.I. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett. 2009;19:2420–2628. doi: 10.1016/j.bmcl.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 25.Xie F., Zhu H., Zhang H., Lang Q., Tang L., Huang Q., Yu L. In vitro and in vivo characterization of a benzofuran derivative, a potential anticancer agent, as a novel Aurora B kinase inhibitor. Eur. J. Med. Chem. 2015;89:310–319. doi: 10.1016/j.ejmech.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Kleemann A., Engel J., Kutscher B., Reichert D. Pharmaceutical Substances: Syntheses Patents Applications. 3rd ed. Thieme, Stuttgart; New York, NY, USA: 1999. [Google Scholar]
- 27.Girgis A.S., Kalmouch A., Ellithey M. Synthesis of novel vasodilatory active nicotinate esters with amino acid function. Bioorg. Med. Chem. 2006;14:8488–8494. doi: 10.1016/j.bmc.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 28.Nofal Z.M., Srour A.M., El-Eraky W.I., Saleh D.O., Girgis A.S. Rational design, synthesis and QSAR study of vasorelaxant active 3-pyridinecarbonitriles incorporating 1H-benzimidazol-2-yl function. Eur. J. Med. Chem. 2013;63:14–21. doi: 10.1016/j.ejmech.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Hassan G.S., Abdel Rahman D.E., Saleh D.O., Abdel Jaleel G.A. Benzofuran–Morpholinomethyl–Pyrazoline Hybrids as a New Class of Vasorelaxant Agents: Synthesis and Quantitative Structure–Activity Relationship Study. Chem. Pharm. Bull. 2013;62:1238–1251. doi: 10.1248/cpb.c14-00572. [DOI] [PubMed] [Google Scholar]
- 30.Matsui A., Matsuo H., Takanaga H., Sasaki S., Maeda M., Sawada Y. Prediction of catalepsies induced by amiodarone, aprindine and procaine: Similarity in conformation of diethylaminoethyl side chain. J. Pharmacol. Exp. Ther. 1998;287:725–732. [PubMed] [Google Scholar]
- 31.Srour A.M., Abd El-Karim S.S., Saleh D.O., El-Eraky W.I., Nofal Z.M. Rational design, synthesis and 2D-QSAR study of novel vasorelaxant active benzofuran-pyridine hybrids. Bioorg. Med. Chem. Lett. 2016;26:2557. doi: 10.1016/j.bmcl.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 32.El-Zahar M.I., Abd El-Karim S.S., Anwar M.M. Synthesis and cytotoxicity screening of some novel benzofuranoyl-pyrazole derivatives against liver and cervix carcinoma cell lines. S. Afr. J. Chem. 2009;62:189–199. [Google Scholar]
- 33.Oliferenko P.V., Oliferenko A.A., Girgis A.S., Saleh D.O., Srour A.M., George R.F., Pillai G.G., Panda C.S., Hall C.D., Katritzky A.R. Synthesis, bioassay, and molecular field topology analysis of diverse vasodilatoryheterocycles. J. Chem. Inf. Model. 2014;54:1103–1116. doi: 10.1021/ci400723m. [DOI] [PubMed] [Google Scholar]
- 34.University of Florida 2001–2005, CODESSA PRO. [(accessed on 20 July 2017)]; Available online: http://www.codessa-pro.com/index.htm.
- 35.Panda S.S., Liaqat S., Girgis A.S., Samir A., Hall C.D., Katritzky A.R. Novel antibacterial active quinolone–fluoroquinolone conjugates and 2D-QSAR studies. Bioorg. Med. Chem. Lett. 2015;25:3816–3821. doi: 10.1016/j.bmcl.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 36.Girgis A.S., Pand S.S., Ahmed Farag I.S., El-Shabiny A.M., Moustafa A.M., Pillai G.G., Panda C.S., Hall C.D., Katritzky A.R. Synthesis, and QSAR analysis of anti-oncological active spiro-alkaloids. Org. Biomol. Chem. 2015;13:1741–1753. doi: 10.1039/C4OB02149E. [DOI] [PubMed] [Google Scholar]
- 37.Girgis A.S., Mishriky N., Farag A.M., El-Eraky W.I., Farag H. Synthesis of new 3-pyridinecarboxylates of potential vasodilation properties. Eur. J. Med. Chem. 2008;43:1818–1827. doi: 10.1016/j.ejmech.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Raj P.A., Suddendra G., Shakeel A.S., Girish M. Synthesis of new benzofuran derivatives and their in vitro evaluation for antimicrobial activity. IJDFR. 2012;3:135–147. [Google Scholar]
- 39.Girgis A.S., Panda S.S., Srour A.M., Farag H., Ismail N.M., Elgendy A.M., Abdel-Aziz M., Katritzky A.R. Rational design, synthesis and molecular modeling studies of novel anti-oncological alkaloids against melanoma. Org. Biomol. Chem. 2015;13:6619–6633. doi: 10.1039/C5OB00410A. [DOI] [PubMed] [Google Scholar]
- 40.Girgis A.S., Panda S.S., Aziz M.N., Steel P.J., Hall C.D., Katritzky A.R. Rational design, synthesis, and 2D-QSAR study of anti-oncological alkaloids against hepatoma and cervical carcinoma. RSC Adv. 2015;5:28554–28569. doi: 10.1039/C4RA16663A. [DOI] [Google Scholar]
- 41.Girgis A.S., Panda S.S., Shalaby E.M., Mabied A.F., Steel P.J., Hall C.D., Katritzky A.R. Regioselective synthesis and theoretical studies of an anti-neoplastic fluoro-substituted dispiro-oxindole. RSC Adv. 2015;5:14780–14787. doi: 10.1039/C4RA13433H. [DOI] [Google Scholar]
- 42.Girgis A.S., Aziz M.N., Shalaby E.M., Saleh D.O., Mishriky N., El-Eraky W.I., Farag I.S. Molecular structure studies of novel bronchodilatory active 4-azafluorenes. Z. Kristallog. 2016;231:179–187. doi: 10.1515/zkri-2015-1892. [DOI] [Google Scholar]
- 43.Girgis A.S., Mabied A.F., Stawinski J., Hegazy L., George R.F., Farag H., Shalaby E.M., Farag I.S. Synthesis and DFT studies of an antitumor active spiro-oxindole. N. J. Chem. 2015;39:8017–8027. doi: 10.1039/C5NJ01109D. [DOI] [Google Scholar]
- 44.Shalaby E.M., Girgis A.S., Moustafa A.M., ElShaabiny A.M., El-Gendy B.E., Mabied A.F., Farag I.S. Regioselective synthesis, stereochemical structure, spectroscopic characterization and geometry optimization of dispiro[3H-indole-3,2′-pyrrolidine-3′,3″-piperidines] J. Mol. Struct. 2014;1075:327–334. doi: 10.1016/j.molstruc.2014.07.014. [DOI] [Google Scholar]
- 45.Tiwari A.D., Panda S.S., Girgis A.S., Sahu S., George R.F., Srour A.M., La Starza B., Asiri A.M., Hall C.D., Katritzky A.R. Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers. Org. Biomol. Chem. 2014;12:7238–7249. doi: 10.1039/C4OB01281J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.