Summary
The roles of the microbiome and innate immunity in the pathogenesis of multiple sclerosis (MS) remain unclear. We have previously documented abnormally low levels of a microbiome‐derived Toll‐like receptor (TLR)2‐stimulating bacterial lipid in the blood of MS patients and postulated that this is indicative of a deficiency in the innate immune regulating function of the microbiome in MS. We postulated further that the resulting enhanced TLR2 responsiveness plays a critical role in the pathogenesis of MS. As proof‐of‐concept, we reported that decreasing systemic TLR2 responsiveness by administering very low‐dose TLR2 ligands attenuated significantly the mouse model of MS, experimental autoimmune encephalomyelitis. Studies of Toll‐like receptor responses in patients with MS have been conflicting. Importantly, most of these investigations have focused on the response to TLR4 ligation and few have characterized TLR2 responses in MS. In the present study, our goal was to characterize TLR2 responses of MS patients using multiple approaches. Studying a total of 26 MS patients and 32 healthy controls, we now document for the first time that a large fraction of MS patients (50%) demonstrate enhanced responsiveness to TLR2 stimulation. Interestingly, the enhanced TLR2 responders include a significant fraction of those with progressive forms of MS, a subset of patients considered unresponsive to adaptive immune system‐targeting therapies. Our results suggest the presence of a pathologically relevant TLR2 related innate immune abnormality in patients with both relapsing–remitting and progressive MS. These findings may have significant implications for understanding the role of innate immunity in the pathogenesis of MS.
Keywords: innate immunity, microbiome, multiple sclerosis, progressive MS, TLR2
Introduction
A major research focus in multiple sclerosis (MS) involves the role of adaptive immunity in both the pathogenesis and treatment of MS. In contrast, relatively little has been documented about the role of innate immunity in MS. Recently, there have been a number of studies suggesting that environmentally derived pathogen‐associated molecular patterns (PAMPs) that ligate innate immune receptors such as Toll‐like receptors (TLRs) serve an important role in regulating the innate immune system and preventing allergic and autoimmune diseases. Environmentally derived PAMPS are microbial‐derived molecules that are recognized by the mammalian immune system as ‘foreign’, and thus have stimulatory or regulatory effects on the immune system. The ‘environmental’ source of these may be in the external environment, such as in dust particles, or may be the ‘internal environment’, such as the microbiome of the gastrointestinal tract 1, 2, 3, 4. The gastrointestinal microbiome is a large producer of PAMPs and, as such, is probably an important source of ‘environmentally derived’ regulators of innate immunity 3, 5, 6.
We have previously documented abnormally low levels of a microbiome‐derived TLR2‐stimulating bacterial lipid in the blood of MS patients 7, 8. Based on this finding, we postulated that MS patients may have a deficiency in the innate immune‐regulating function of the microbiome. Specifically, we proposed that the microbiome maintains low levels of systemically circulating PAMPs, which mediate tonic systemic TLR signalling and set the threshold and magnitude of innate immune responses via a relative state of TLR tolerance. Our finding of abnormally low levels of a microbiome‐derived TLR2‐stimulating bacterial lipid in the blood of MS patients suggests that MS patients may have a deficiency in microbiome‐induced innate immune regulation. This is predicted to result in enhanced responses to TLR2 ligation, which then play a role in the pathogenesis of the disease. As a proof‐of‐concept we reported recently that decreasing systemic TLR2 responsiveness by administering very low doses of TLR2 ligands significantly attenuated the mouse model of MS, experimental autoimmune encephalomyelitis (EAE) 4, 9.
An integral prediction stemming from our underlying postulate of a deficiency in innate immune regulation in MS is that MS patients will demonstrate enhanced responsiveness to TLR2 stimulation. In the present study our goal was to characterize the TLR2 responsiveness of MS patients using a variety of approaches. We now report for the first time that a large fraction of patients with MS, including those with progressive forms of the disease, have enhanced responsiveness to TLR2 stimulation. These results will now drive investigations aimed at understanding what distinguishes this large fraction of MS patients and the mechanisms underlying their enhanced TLR2 responses.
Materials and methods
Reagents
Lipopolysaccharide A (LPS) (Escherichia coli 0111:B4) was obtained from Sigma‐Aldrich (St Louis, MO, USA). Purified Pam2CSK4 (P2C) and Pam3CSK4 (P3C) were obtained from InvivoGen (San Diego, CA, USA). In some experiments, P3C was obtained from Bachem Americas, Inc. (Torrance, CA, USA).
Patients
All studies were performed using protocols approved by the Institutional Review Board (IRB) at the University of Connecticut Health Center (UCHC). Healthy controls were recruited from volunteer donors at UCHC. None of the 32 control patients had an underlying autoimmune or inflammatory disease by history or treatment, with the exception of one control individual who was being treated for psoriasis. Patients with MS were recruited both from the MS clinic at UCHC as well as from other physicians in the state of Connecticut. Blood samples were drawn after an overnight fast. All healthy controls and MS patients reported no infectious illnesses within 3 months, no antibiotic use within 6 months and no vaccinations within 3 months.
Peripheral blood mononuclear cells (PBMC) and CD14+CD16– monocyte isolation from whole blood
For PBMC isolation, whole blood was diluted 1 : 1 with sterile phosphate‐buffered saline (PBS) and peripheral blood mononuclear cells (PBMC) isolated using Lymphoprep™ density gradient medium (Stem Cell Technologies, Vancouver, Canada). CD14+CD16– monocytes were isolated directly from the whole blood using the EasySep™ Direct Human Monocyte Isolation Kit (Stem Cell Technologies), according to the manufacturer's instructions. This kit enriches by negative selection, allowing the subsequent identification of monocytes by antibody staining.
PBMC: immunological phenotyping
PBMC were cultured for 4 h without any stimulus, blocked with human FcR Blocking Reagent (Miltenyi Biotec, Auburn, CA, USA), and then stained with Live/Dead Near IR (Molecular Probes, Eugene, OR, USA), anti‐human CD14‐allophycocyanin (APC) (Tonbo Biosciences, San Diego, CA, USA), anti‐human CD16‐phycoerythrin‐cyanin 7 (PE‐Cy7) and anti‐CD19‐fluorescein isothiocyanate (FITC) (BD Biosciences, San Jose, CA, USA). All cells were analysed using BD LSRII flow cytometers (BD Biosciences). The frequency of CD14+CD16+ cells within the total PBMC was derived as: (% of CD14+ cells in the PBMC × % of CD16+ cells gated on CD14+ cells).
PBMC and CD14+CD16– monocytes: stimulation with TLR ligands
PBMC (1 × 106 cells/ml; 0·2 ml/well) and CD14+CD16– monocytes (1 × 105/ml; 0·2 ml/well) were cultured in flat‐bottomed 96‐well plates in 10% heat‐inactivated fetal calf serum (FCS) RPMI‐1640 (Gibco, Waltham, MA, USA) and stimulated with either no stimulus, P2C, P3C or LPS for 4 h. Supernatants were collected and frozen until assayed. Human tumour necrosis factor (TNF)‐α was measured in the supernatants using the Ready‐SET‐Go!® enzyme‐linked immunosorbent assay (ELISA) kit from eBiosciences/Affymetrix Inc. (Santa Clara, CA, USA).
CD14+CD16‐ monocytes: TLR2 expression studies
CD14+CD16– monocytes were cultured for 4 h with no added stimulus, blocked with human FcR Blocking Reagent (Miltenyi Biotec) and stained with Live/Dead Near IR (Molecular Probes), anti‐human CD14‐APC (Tonbo Biosciences) and anti‐human TLR2‐PE (eBiosciences/Affymetrix, Inc.). Paired samples were stained with isotype control antibodies and all samples were analysed by flow cytometry.
Cell preparation for single‐cell analysis
PBMC were isolated by Ficoll‐Paque Plus (GE Healthcare, Chicago, IL, USA) density gradient centrifugation from whole peripheral blood in EDTA anti‐coagulant. Any remaining red blood cells were then removed by treatment with ammonium–chloride–potassium (ACK) lysis buffer (Gibco). CD14+ monocytes were subsequently isolated from purified PBMC by the Pan Monocyte Isolation kit (Miltenyi Biotec). This kit enriches by negative selection, allowing the subsequent identification of monocytes by antibody staining. Isolated CD14+ monocytes were resuspended at a density of 5 × 105 cells/ml in complete RPMI media (Corning, New York, USA) and stimulated with soluble P3C (Pam3CSK4, 2 μg/ml; InvivoGen) or LPS (50 ng/ml; Sigma‐Aldrich) at 37°C, 5% CO2 for 24 h. After stimulation, attached cells were lifted using Accutase (Innovative Cell Technologies, San Diego, CA, USA), then stained with AF647‐conjugated anti‐human CD14 antibody (BioLegend) at room temperature for 10 min, rinsed once with PBS and resuspended in fresh complete RPMI media at a density of 1 × 106 cells/ml. Approximately 30 μl of cell suspension was loaded onto a single‐cell barcode chip (SCBC) for secretomic measurements with a validated 32‐plex antibody panel (Supporting information, Table S1).
Single‐cell barcode chip (SCBC) assay
Each SCBC was assembled with a layer of polydimethyl siloxane (PDMS) containing ∼12000 microchambers and a glass slide prepatterned with the 32‐plex antibody panel. After cell loading onto the microchamber array chip, it was secured to the prepatterned glass slide in a custom clamping system, imaged at both bright and fluorescent fields under a Zeiss fluorescent microscope for the detection of cell number and location within each microchamber, then incubated at 37°C, 5% CO2 for 16 h. Following incubation, the antibody slide was removed from the PDMS microchambers, rinsed with 1% bovine serum albumin (BSA)/PBS and then incubated with 300 μl biotin‐conjugated detection antibody cocktail (Supporting information, Table S1) for 45 min followed by incubation with 300 μl APC–streptavidin solution (BioLegend) for 30 min. The signals of proteins secreted from single cells were captured by a GenePix microarray scanner and analysed by the IsoSpeak software package. Zero‐cell microchambers and areas spatially close to the protein readouts were used to assess cytokine‐specific background. Signals with a signal‐to‐noise ratio (SNR) of at least 2 (relative to the background) and from at least 20 single cells or 2% of all single cells (whichever quantity is larger) are considered secreted significantly. The frequency of all polyfunctional cells that co‐secrete 2+ proteins per cell and the polyfunctional profile (combinations of proteins secreted by individual cells) are determined from the raw data.
Statistical methods
For each of the four TLR2‐response analysing approaches used in this study, an ‘upper threshold’ of control responses was identified based on the interquartile range (IQR) method. This approach allows for the identification of values which represent statistical ‘outliers’ in a given study based on the distribution of control responses in that study. In the IQR method, the upper threshold of control responses is determined using the following equation: Q3 + 1·5 (Q3–Q1), where Q3–Q1 is the IQR; Q3 is the value representing the 75th percentile of the control responses and Q1 is the value representing the 25th percentile of the control responses.
Using this defined upper threshold, responses greater than or equal to the upper threshold were identified as ‘outliers’ or ‘enhanced responses’. The total number of above‐threshold (outlier) responses versus below‐threshold (non‐outlier) responses in the entire study was compared between controls and MS patients using Fisher's exact test. P < 0·05 was considered statistically significant.
All data are expressed as mean ± standard error of the mean (s.e.m.). The normality of all data sets was determined by D'Augustino and Pearson omnibus normality test or Shapiro–Wilk normality test. Parametric data sets were analysed using unpaired two‐tailed Student's t‐test or one‐way analysis of variance (anova). Non‐parametric data sets were analysed using Mann–Whitney test or Kruskal–Wallis test. The statistical analyses were performed using GraphPad Prism version 6 software and P < 0·05 was considered statistically significant.
Study approval
All studies were performed using protocols approved by the Institutional Review Board (IRB no. 10–273SFS‐2) at the University of Connecticut Health Center (UCHC). Healthy controls were recruited from volunteer donors at UCHC. Patients with MS were recruited both from the MS clinic at UCHC as well as from other physicians in the state of Connecticut. Written informed consent was received from all participants prior to inclusion in the study.
Results
Enhanced TLR2 responses in MS patients detected using three approaches
In this study, our goal was to investigate TLR2 responses in patients with MS and specifically to ask whether MS patients had enhanced responses to TLR2. To characterize TLR2 responses in healthy control individuals and patients with MS as completely as possible, we opted to use multiple approaches. These approaches included characterizing the TLR2 responses of: (1) whole PBMC responses; (2) CD14+ monocytes assayed at the population level; and (3) CD14+ monocytes assayed at the single‐cell level. In each of these approaches we analysed approximately 10 controls and 10 MS patients and normalized the results in each approach by determining the percentage of ‘enhanced responses’ in control versus MS patients. In analysing the percentages of enhanced responses throughout our total study, we had results from these three individual approaches and analysed TLR2 responses in a total of 26 MS and 32 control patients.
In each approach, we based our identification of ‘enhanced’ TLR2 responses on the distribution of responses within the control population. Using this distribution, we defined an upper limit (or threshold) of control responses based on the IQR of the control responses (see Methods). This method defines statistically the responses above the threshold as ‘outliers’, and we identified responses equal to or greater than the threshold as ‘enhanced’ responses.
PBMC
We first investigated whether the frequency of the TLR2‐responsive populations within PBMC differed between MS and control patients. PBMC were obtained from nine MS patients and seven controls. Using fluorescence activated cell sorter (FACs) analysis, we assessed the percentages of CD14+ monocytes, CD14+CD16+ monocytes and CD19+ B cells within the PBMC populations. We found that there were no significant differences in the percentages of these populations between MS and control patients. However, there was a trend for a higher percentage of CD14+ monocytes in MS patients (Fig. 1a,b).
Figure 1.
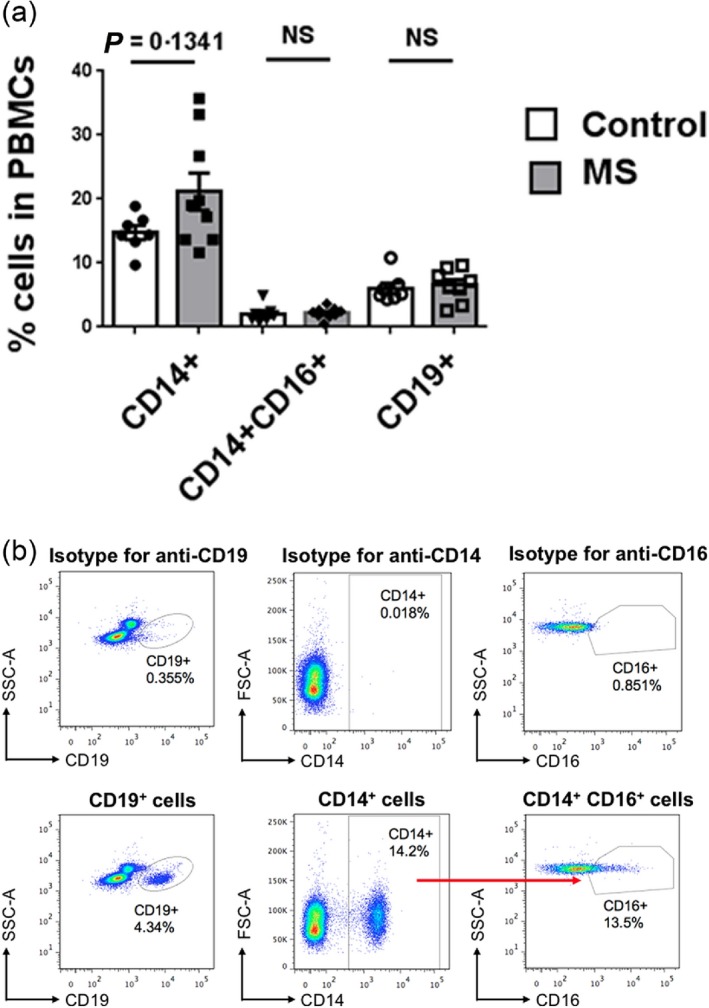
Immunophenotyping of peripheral blood mononuclear cells (PBMC). (a) PBMC from control and multiple sclerosis (MS) patients were cultured for 4 h without any stimulus and stained with anti‐human CD14, CD16 and CD19 antibodies and analysed by flow cytometry. Frequencies of CD14+, CD14+CD16+ and CD19+ cells are shown. The results comparing controls with MS patients for each cell type were analysed by Student's t‐test. Error bars represent standard errors of the mean. (b) Representative flow cytometric plots for determining percentage of CD14+, CD14+CD16+ and CD19+ cells in PBMC.
We next investigated the TLR2 responses of whole PBMC derived from the nine MS patients and seven controls. Whole PBMC were stimulated in culture with 2 or 10 μg/ml of the TLR2 ligand Pam2CSK4 (P2C). Cell supernatants were harvested after 4 h and analysed for TNF‐α via ELISA. Using the IQR‐based upper threshold of control responses to identify enhanced responses, we found that the TLR2 responses from a large subset of the MS cohort were enhanced compared to the healthy cohort. As seen in Fig. 2a, in response to 2 μg/ml of P2C, six of the nine MS patients (66·7%) demonstrated enhanced responses. In contrast, only one of the seven controls (14%) demonstrated enhanced responses. To characterize further these enhanced responses in the MS cohort, we stratified the MS patients into those with relapsing–remitting MS (RRMS) and those with progressive forms of MS. Interestingly, analysing the 2 µg/ml P2C‐stimulated responses based on this stratification of patients revealed that five of the six MS patients with the progressive form of the disease demonstrated enhanced responses (83%), while one of three RRMS patients showed an enhanced response (Fig. 2b). Stimulating the PBMC with 10 µg/ml P2C yielded essentially the same results, with five of the nine total MS patients (56%) demonstrating enhanced responses in contrast to none of seven controls showing enhanced responses (Fig. 2c). Of the MS cohort, four of the six MS patients with the progressive form of the disease (67%) demonstrated enhanced responses, while one of three RRMS patients showed an enhanced response (Fig. 2c).
Figure 2.
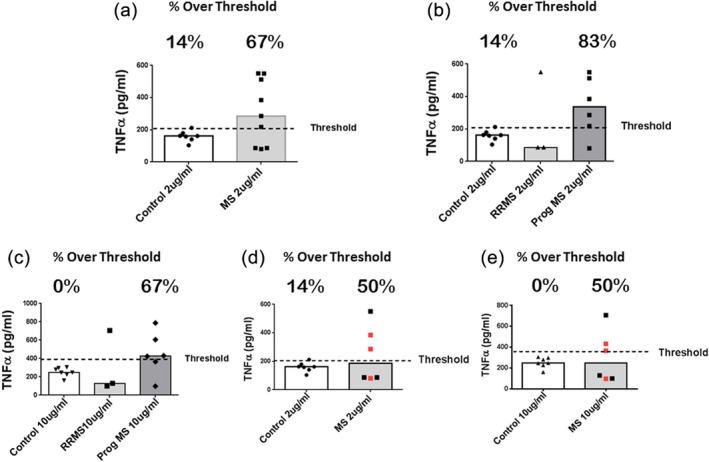
Pam2CSK4 (P2C)‐stimulated responses of peripheral blood mononuclear cells (PBMC). PBMC from multiple sclerosis (MS) patients and controls were cultured at ×1 106/ml (0·2 ml/well) and stimulated with 2 or 10 μg/ml of P2C for 4 h. The levels of tumour necrosis factor (TNF)‐α in the culture supernatants were measured by enzyme‐linked immunosorbent assay (ELISA) and are reported as pg/ml. For each figure, the level of TNF‐α representing the upper threshold of control responses [based on the control interquartile range (IQR)] is depicted by the dashed line. Medians are depicted. The percentage of responses above the threshold is depicted for each cohort. (a) Controls (n = 7) versus MS patients (n = 9) stimulated with 2 μg/ml P2C; control mean response (160 pg/ml) versus total MS mean response (306 pg/ml); P = 0·2914 via Mann–Whitney. (b) Controls versus MS patients stratified into relapsing–remitting MS (RRMS) and progressive forms of MS (MS Prog; n = 6) stimulated with 2 μg/ml P2C; control mean response (160 pg/ml) versus Prog MS mean response (339 pg/ml); P = 0·3930 via Kruskal–Wallis followed by Dunn's multiple comparisons test. (c) Same as (b), but stimulated with 10 μg/ml P2C; control mean response (252 pg/ml) versus Prog MS mean response (452 pg/ml); P = 0·3471 via Kruskal–Wallis followed by Dunn's multiple comparisons test. For (d) and (e), three MS patients with the highest CD14+ percentages (Fig. 1a) have been omitted from the analysis; MS patients (n = 6). (d) Controls versus total MS patients stimulated with 2 μg/ml P2C. Red icons are MS patients with progressive disease. Control mean response (160 pg/ml) versus total MS mean response (246 pg/ml); not significant (n.s.) by Mann–Whitney. (e) Controls versus total MS patients stimulated with 10 μg/ml P2C. Red icons are MS patients with progressive disease. Control mean response (252 pg/ml) versus total MS mean response (305 pg/ml); n.s. by Mann–Whitney.
It was of note that three MS patients had percentages of peripheral blood CD14+ monocytes that were clearly higher than those seen in the control cohort (Fig. 1a). In order to compare the TLR2 responses of MS and control PBMC with similar CD14+ monocyte frequencies, we next omitted these three MS patients and reanalysed the TLR2 responses. Again, we found that the TLR2 responses from three of six (50%) of the MS cohort were enhanced when stimulated with either 2 or 10 μg/ml P2C. This was in contrast to the healthy cohort, that had one of seven (14%) enhanced responses with 2 μg/ml P2C and 0% enhanced responses with 10 μg/ml P2C (Fig 2d,e). In this reanalysis we found that, among patients with the progressive forms of MS, two of three (67%) demonstrated enhanced PBMC TLR2 responses (Fig. 2d,e).
TLR2 responses: CD14+ monocytes
Our finding of enhanced TLR2 responders among the MS patients in the preliminary PBMC studies led us to ask whether the enhanced responses could also be found intrinsically at the level of the CD14+ monocytes. For the remainder of our studies we analysed the response of purified CD14+ monocytes. CD14+CD16– monocytes were derived by negative selection from the whole blood of MS patients (15 patients) and healthy controls (13 individuals) (Tables 1 and 2). We first investigated whether the expression of TLR2 was equivalent in control versus MS CD14+ monocytes. Using flow cytometry, we analysed the mean fluorescent intensity (MFI) of TLR2 expression on the CD14+ cells. As seen in Fig. 3, there was no significant difference in TLR2 expression in the CD14+ populations derived from control versus MS patients.
Table 1.
MS patients
| Age (years) | Enhanced TLR2 response | Tested/response | Whole CD14+ | ||||
|---|---|---|---|---|---|---|---|
| MS | Treatment | M/F | PBMC | Single‐cell | |||
| Pr Prog | None | F | 60 | Yes | E | ||
| Pr Prog | None | F | 68 | N | |||
| Pr Prog | Avonex | F | 65 | Yes | E | ||
| Pr Prog | None | F | 29 | N | N | ||
| Pr Prog | None | F | 60 | N | |||
| Prog Rel | None | F | 51 | Yes | E | E | |
| Sec Prog | Rituximab | M | 36 | Yes | E | E | |
| Sec Prog | Tysabri | F | 61 | Yes | E | N | |
| Sec Prog | None | M | 70 | Yes | E | ||
| Sec Prog | Tysabri | F | 47 | Yes | E | ||
| RRMS | None | F | 39 | N | |||
| RRMS | Tysabri | F | 41 | N | |||
| RRMS | Copaxone | M | 53 | Yes | E | E | E |
| RRMS | Copaxone | F | 59 | Yes | E | ||
| RRMS | Tysabri | F | 58 | N | |||
| RRMS | none | F | 49 | N | |||
| RRMS | Rituximab | F | 55 | N | |||
| RRMS | Rituximab | F | 21 | Yes | E | ||
| RRMS | Avonex | F | 58 | N | |||
| RRMS | Tecfidera | M | 51 | N | |||
| RRMS | None | F | 62 | N | |||
| RRMS | Tysabri | F | 29 | Yes | E | ||
| RRMS | Gilenya | F | 50 | N | |||
| RRMS | Tysabri | F | 49 | N | |||
| RRMS | Gilenya | F | 31 | Yes | E | ||
| RRMS | None | F | 31 | Yes | E |
E = enhanced response; N = normal response; Pr Prog = primary progressive MS; Prog Rel = progressive relapsing MS; RRMS = relapsing–remitting MS; Sec Prog = secondary progressive MS; TLR = Toll‐like receptor; PBMC = peripheral blood mononuclear cells; M/F = male/female.
Table 2.
Control patients
| Enhanced | |||
|---|---|---|---|
| M/F | Age (years) | TLR2 response | |
| M | 53 | ||
| F | 42 | ||
| F | 57 | ||
| M | 37 | ||
| F | 28 | ||
| F | 62 | ||
| F | 66 | ||
| F | 50 | ||
| F | 58 | Yes | |
| F | 53 | ||
| M | 40 | ||
| M | 65 | ||
| F | 55 | Yes | |
| F | 42 | ||
| F | 47 | ||
| F | 30 | ||
| M | 27 | ||
| M | 46 | ||
| F | 51 | ||
| F | 57 | ||
| F | 48 | Yes | |
| M | 58 | ||
| M | 41 | ||
| M | 27 | ||
| M | 64 | ||
| F | 53 | ||
| F | 39 | ||
| F | 31 | ||
| M | 47 | ||
| F | 21 | ||
| F | 52 | ||
| F | 23 | ||
F = female; M = male; TLR = Toll‐like receptor.
Figure 3.
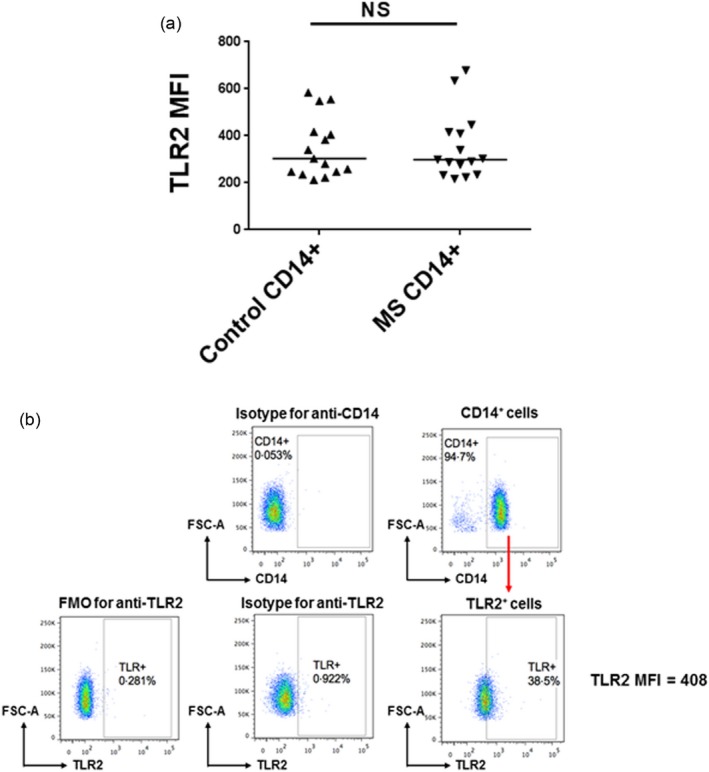
Toll‐like receptor (TLR)2 expression on CD14+ monocytes. (a) CD14+ monocytes from multiple sclerosis (MS) patients and controls were cultured for 4 h without stimulation and then stained with anti‐human CD14 and TLR2 antibodies. Mean fluorescence intensity (MFI) of TLR2 staining on CD14+ monocytes is shown. Medians are depicted. The results were analysed by Mann–Whitney test. (b) Representative flow cytometric plots for determining MFI of TLR2 staining on purified CD14+ monocytes.
We next stimulated the purified CD14+CD16– monocytes for 4 h using two different TLR2 ligands [P2C and Pam3CSK4 (P3C)] and assayed culture supernatants by ELISA for TNF‐α. We analysed the TLR2 responses of 15 MS patients (16 total responses; one MS patient was tested with both ligands) and 13 control individuals (15 total responses; two controls were tested with both ligands). As seen in Fig. 4a,b, we observed that of the 16 total MS responses tested (10 P2C‐stimulated and six P3C‐stimulated), seven of 16 (43·7%) were enhanced responses. This is in contrast to the 15 control responses tested (nine P2C‐stimulated and six P3C‐stimulated), in which two of 15 (13·3%) were enhanced responses. The MS patient stimulated with both P2C and P3C was an enhanced responder in both instances. The two control patients stimulated with both P2C and P3C were not enhanced responders in either instance. Among the MS patients with progressive forms of the disease four of seven responses (57%) were enhanced. In patients with RRMS, three of nine (33%) responses were enhanced (Fig. 4b and Table 1). Finally, we noted that one of three MS patients demonstrating an enhanced PMBC TLR2 response subsequently showed a normal CD14+ monocyte response (Table 1). This probably reflects the occasional lack of stability of an enhanced TLR2 response over time, a finding we have noted in preliminary studies (data not shown). Overall, these results suggest: (1) that a large fraction of MS patients have enhanced responses to TLR2 that are CD14+ monocyte‐intrinsic; and (2) that enhanced responses to TLR2 stimulation are found among MS patients with both RRMS and the progressive forms of the disease.
Figure 4.
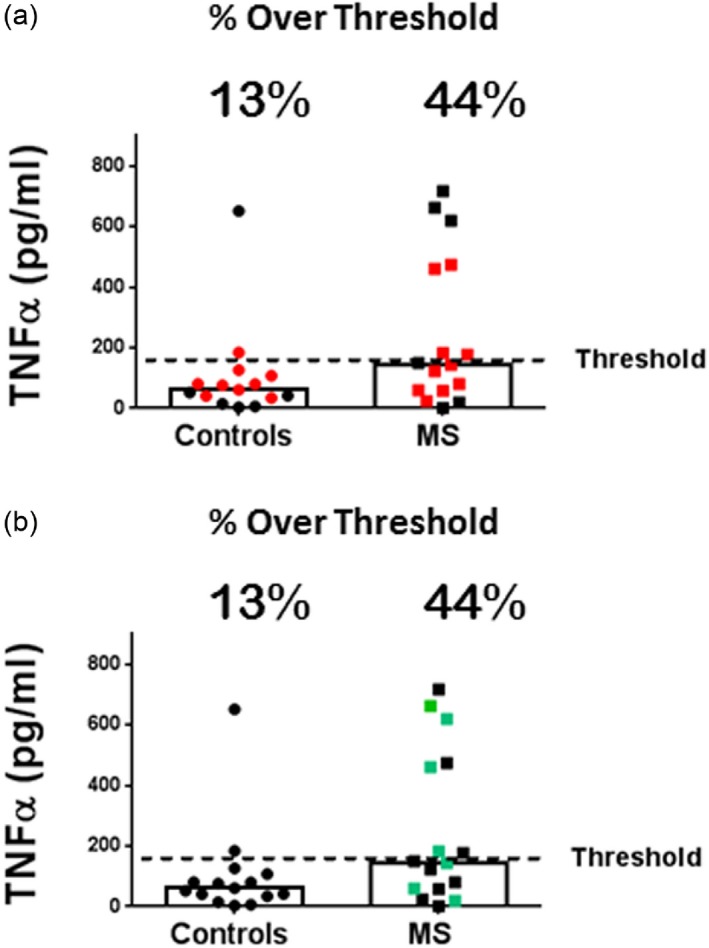
Pam2CSK4 (P2C) and Pam3CSK4 (P3C)‐stimulated responses of CD14+ monocytes. CD14+CD16– monocytes were derived by negative selection from the whole blood of multiple sclerosis (MS) patients (15 patients;16 total responses) and healthy controls (13 individuals; 15 total responses), cultured at 1 × 105/ml (0·2ml/well), stimulated with 2 μg/ml of P2C or P3C for 4 h, and tumour necrosis factor (TNF)‐α in the culture supernatants measured by enzyme‐linked immunosorbent assay (ELISA). The level of TNF‐α representing the upper threshold of control responses [based on the control interquartile range (IQR)] is depicted by the dashed line. The percentage of responses above the threshold is depicted for controls and total MS patients. Medians are depicted. (a) Red icons represent P2C stimulation and black icons represent P3C stimulation; (b) same results as in (a), but green icons represent patients with progressive MS. The mean TNF‐α level for the control cohort was 104 pg/ml and the mean TNF‐α level for the total MS cohort was 247 pg/ml; P = 0·0712 via Mann–Whitney. The percentage of responses above threshold: 57% of Prog MS patients, 33% of relapsing–remitting MS (RRMS) patients.
Single‐cell analysis
As our third approach, we utilized technology that allowed for analysis of cytokine secretion at the single‐cell level. We used the IsoCode microchip technology, a single‐cell barcode chip (SCBC) system which allows for highly multi‐plexed profiling of immune effector function proteins at the single‐cell level (IsoPlexis, Branford, CT, USA). The SCBC system uses rigorous quantitation of ELISA antibody‐based capture (measuring 32 cytokines/chemokines/growth factors) applied to single‐cell detection to map each cell's cytokine secretion repertoire. This system has been well validated in various immune‐related disease settings, including cancer and infectious disease 10, 11, 12, 13, 14, 15. Prior reports of the use of this technology demonstrated the power of single‐cell high‐plex protein secretion profiling for deep functional phenotyping and comprehensive dissection of immune functional states of single cells 10, 16, 17. Moreover, it was reported that applying this platform to profiling phenotypically similar macrophages revealed a previously unobserved deep functional heterogeneity 18.
We utilized the SCBC technology to capture single‐cell, secretomic, cytokine profiles from CD14+ monocytes (including the CD14+CD16+ subset) purified from the PBMC of MS patients and controls. The SCBC approach yields an overall summary metric of single‐cell responses termed the ‘polyfunctional strength index’ (PSI). The PSI metric is derived from the percentage of individual cells secreting two or more cytokines (termed ‘polyfunctionality’) and the intensity of the cytokines secreted. Specifically, PSI = % polyfunctional cells × average signal intensity of secreted cytokines in fluorescent units. The functional relevance of PSI as a metric in this single‐cell assay has been documented in its ability to predict relapse after initial disease remission in patients with melanoma who had an adoptive transfer of melanoma‐associated antigen recognizing T cells (MART‐1) TCR T cells 11.
In this study we analysed eight MS patients and eight controls. To confirm the validity of this single‐cell approach in the context of our previous ‘population‐based’ approaches, three of the eight MS patients represented patients studied previously and two of these three had been enhanced responders in the earlier studies (Table 1). CD14+ monocytes were derived from fresh PBMC and stimulated for 24 h in the continuous presence of 2 µg/ml of P3C. Of the 32 cytokines/chemokines/growth factors assayed, we found seven that were secreted consistently at levels above the threshold values needed for accurate detection: interleukin (IL)‐6, IL‐8, macrophage inflammatory protein (MIP)‐1α, MIP‐1β, monocyte chemoattractant protein (MCP)‐1, macrophage migration inhibitor factor (MIF) and TNF‐α. In these single‐cell studies, the most prevalent cytokine detected in terms of both frequency of cells secreting and intensities was IL‐8. We determined the PSI of the eight control and eight MS responses and identified enhanced responses based on the IQR of the control PSI values.
We found that once again a large fraction of the MS patients demonstrated enhanced responses compared to the control responses. As seen in Fig. 5a, five of eight MS patients (62·5%) demonstrated a PSI that was above the IQR‐derived upper threshold of control responses. This was in contrast to none of eight control responses that were above the threshold. These results were thus consistent with our CD14+ monocyte whole population studies in documenting a large percentage of MS patients with enhanced TLR2 responses compared to control responses. Importantly, the single‐cell results for the three repeated MS patients were consistent with the results of earlier assays: one of two progressive MS patients repeated as an enhanced responder and one RRMS patient repeated as an enhanced responder (Table 1). Five additional RRMS patients tested in the single‐cell analysis were not tested previously in the CD14+ whole population assays. Importantly, the single‐cell approach demonstrated that three of these five newly tested RRMS patients were enhanced responders (Table 1).
Figure 5.
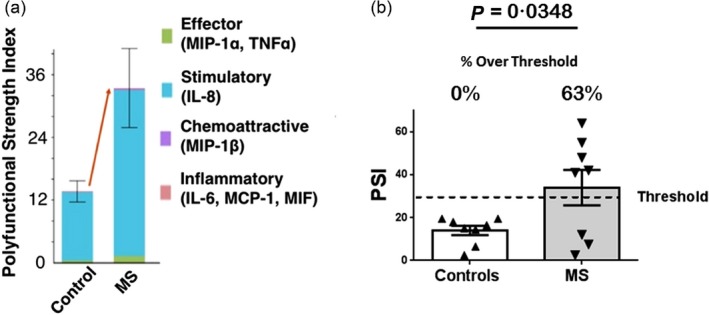
Pam3CSK4 (P3C)‐stimulated single‐cell analysis; polyfunctional strength index (PSI). CD14+ monocytes from controls (n = 8) and multiple sclerosis (MS) patients (n = 8) were stimulated for 24 h with P3C and assayed for PSI. (a) The PSI values of single‐cell CD14+ monocytes from control versus MS patients were stratified into contributing functional subgroups of cytokines. Effector cytokines: macrophage inflammatory proteins (MIP)‐1α and tumour necrosis factor (TNF)‐α; stimulatory cytokines: interleukin (IL)‐8; chemoattractive chemokines: MIP‐1β; inflammatory cytokines: IL‐6, monocyte chemoattractant protein (MCP)‐1 and macrophage migration inhibitor factor (MIF). Error bars represent standard errors of the mean. (b) PSI values, depicted in (a), of controls (mean = 14) versus MS patients (mean = 34); P = 0·0348 via Student's t‐test. Error bars represent standard errors of the mean. The level of PSI representing the upper threshold of control responses [based on the control interquartile range (IQR)] is depicted by the dashed line. The percentage of responses above threshold is depicted for controls and total MS patients.
The PSI is a value derived from the percentage of individual cells secreting two or more cytokines (termed ‘polyfunctionality’) and the intensity of the cytokines secreted. As seen in Fig. 6a, the major difference between MS patients and controls was the higher percentage of cells secreting two or three cytokines among the MS cohort. Of the five MS patients with enhanced PSI values (Fig. 5), four had enhanced polyfunctionality responses (percentage of cells secreting two or more cytokines) (Fig. 6b). One MS patient who had an enhanced PSI did not demonstrate an enhanced polyfunctionality, and instead had enhanced cytokine intensities (data not shown). Overall, the single‐cell analyses confirm our findings with the PBMC and CD14+ monocyte whole population assays, showing that a large subset of MS patients demonstrate enhanced responses to TLR2 stimulation.
Figure 6.
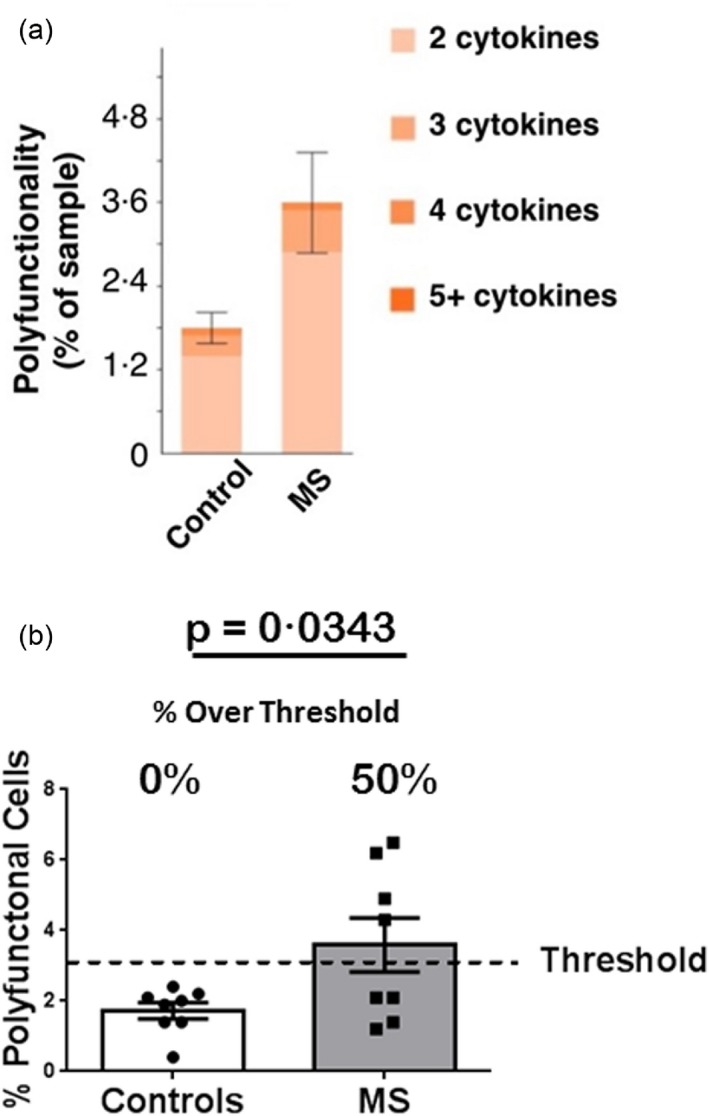
Pam3CSK4 (P3C)‐stimulated single‐cell analysis. Polyfunctionality. CD14+ monocytes from the subjects depicted in Fig. 5 were stimulated for 24 h with P3C and assayed for polyfunctionality (cells secreting two or more cytokines). (a) The mean percentage of CD14+ monocytes secreting two or more cytokines in the control and total multiple sclerosis (MS) cohorts are stratified further by the percentage of cells secreting two, three, four or five cytokines. Error bars represent standard errors of the mean. (b) The percentage of CD14+ monocytes that are polyfunctional (secrete two or more cytokines); mean = 1·725% for controls; mean = 3·587% for total MS patients; P = 0·0343 via Student's t‐test. Error bars represent standard errors of the mean. The percentage of polyfunctional cells representing the upper threshold of control responses [based on the control interquartile range (IQR)] is depicted by the dashed line. The percentage of responses above threshold is depicted for controls and total MS patients.
Compilation: enhanced TLR2 responses in patients with MS
To examine the percentages of enhanced responses across our entire study, we had results from three individual approaches studying a total of 26 MS patients and 32 controls. In addition to the three major approaches, in the PBMC approach we had results from responses to a low and a high concentration of the TLR2 ligand. Finally, we also analysed MS patients not only as a total population, but also stratified them into those with progressive forms of the disease and those with the relapsing–remitting form of the disease.
We began our evaluation of the entire study by tabulating the number of controls and MS patients who demonstrated enhanced TLR2 responses. Overall, in studying a total of 26 MS patients and 32 controls, we found that 13 of 26 (50%) MS patients demonstrated enhanced responses (Table 1). In contrast, in the control patients, we found that only three of 32 (9·3%) were enhanced responders (Table 2). Interestingly, seven of 10 (70%) of MS patients with progressive forms of the disease demonstrated enhanced responses and this included four of four patients with secondary progressive MS (Table 1). Of patients with RRMS, six of 16 (37·5%) were enhanced responders.
To evaluate these results further, we separated the total responses from all approaches into those responses above or below the assay‐specific thresholds (37 total control TLR2 responses; 36 total MS TLR2 responses). The differences between MS and control responses were then analysed using Fisher's exact test. We found that the differences in the proportion of responses above and below the threshold between controls and the total cohort of MS patients were highly significant (Fig. 7a–c). This was also true comparing controls with both RRMS patients and patients with the progressive forms of the disease (Fig. 7a–c).
Figure 7.
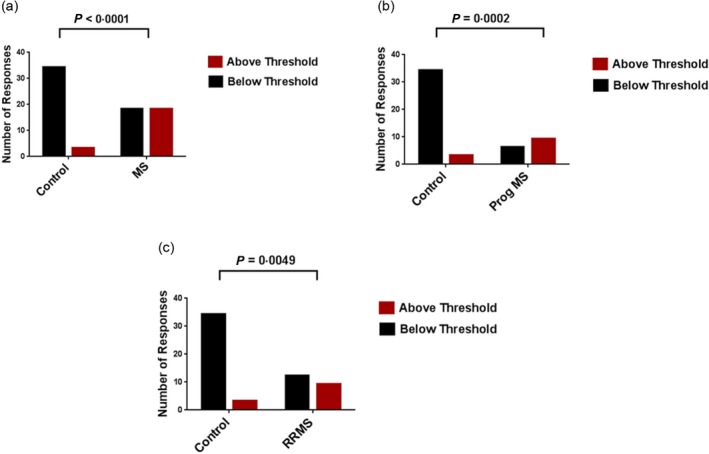
Total Toll‐like receptor (TLR)2 responses above and below the interquartile range (IQR)‐based threshold of control responses. Total TLR2 responses were compiled from controls and multiple sclerosis (MS) patients throughout all approaches (37 total control TLR2 responses; 36 total MS TLR2 responses) and separated into those above or below the upper threshold in each assay [the interquartile range (IQR)‐based upper threshold of control responses defined in each study]. The proportion of responses above and below the threshold were then compared between: (a) control responses and total MS patient responses; (b) control responses and progressive (Prog) MS patient responses; and (c) control responses and relapsing–remitting MS (RRMS) patient responses. The results were analysed using Fisher's exact test.
We also evaluated these results as percentages of total responses that were above the threshold (enhanced responses) in each cohort in each of the approaches. As seen in Fig. 8, we found a large increase in the mean percentage of enhanced responses in all MS patients compared to those from the control responses. The percentages of enhanced responses from patients with progressive forms of the disease (mean = 60·13%) was statistically greater than that of the controls (mean = 6·87%), while the percentages of enhanced responses among the total MS patients (mean = 51·55%) and the RRMS patients (mean = 41·65%) were also increased compared to the controls (6.87%), but did not quite reach statistical significance (Fig. 8). Overall, these results (Tables 1 and 2; Figs 7 and 8) suggest that a large fraction of MS patients demonstrate enhanced responses to TLR2 stimulation when compared to controls, and that this fraction includes both RRMS and patients with progressive forms of MS.
Figure 8.
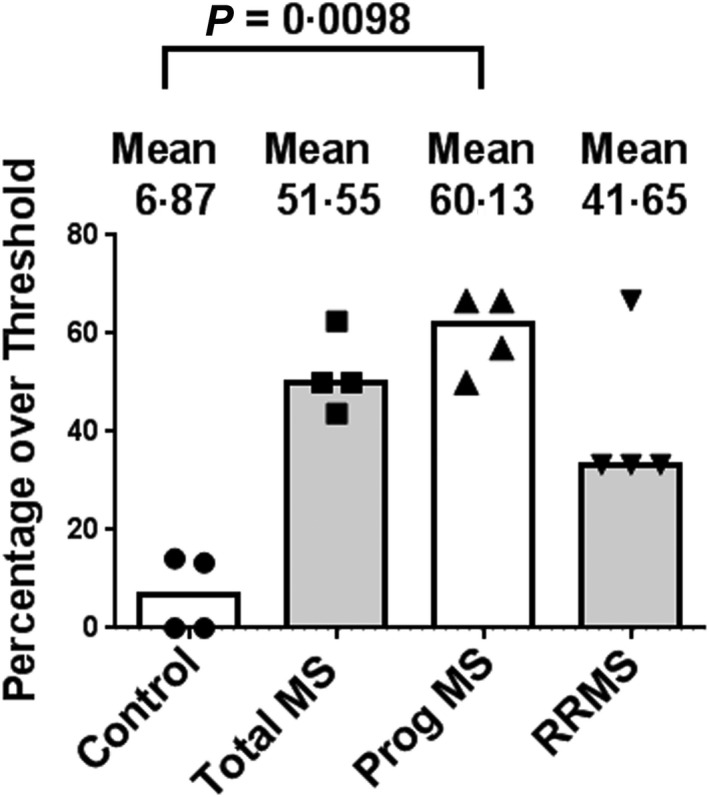
Compilation of percentages of Toll‐like receptor (TLR)2 responses above the interquartile range (IQR)‐based threshold of control responses. Compilation of percentages of control and multiple sclerosis (MS) patients with enhanced TLR2 responses as assessed in peripheral blood mononuclear cells (PBMC), CD14+ whole population and CD14+ single‐cell analyses. These studies encompass 26 MS patients (Table 1) and 32 healthy controls (Table 2). The percentages of progressive MS patients (Prog MS) with enhanced responses (mean = 60·13%) were significantly greater than those of the control patients (mean = 6·87%); P = 0.0098 via Kruskal–Wallis followed by Dunn's multiple comparisons test. The percentages of total MS patients (51·55%) and relapsing–remitting MS (RRMS) patients (41·65%) with enhanced responses were greater than those of the control patients, but did not reach statistical significance. Medians are depicted.
In evaluating potential correlates of enhanced TLR2 responses within the MS cohort we found that the MS patients had an average age of 49·8 years, while the control cohort had an average age of 45·9 years (P = 0·257; Student's t‐test). Within the MS cohort, the mean age of the enhanced responders was 47·46 years, while the mean age of those MS patients with non‐enhanced responses was 52·07 years (P = 0·3828; Student's t‐test). The MS cohort consisted of 15·5% males while the control cohort was comprised of 34·3% males (Tables 1 and 2). The lower percentage of male subjects in the MS cohort may not be surprising, given the female predominance in MS, especially true in the RRMS form of the disease. Nevertheless, while all three enhanced responders in the control cohort were females, three of 13 enhanced responders in the MS cohort were males (Tables 1 and 2). Our data to date thus suggest that neither age nor gender appear to account for the increase in enhanced responders among the MS patients. Treatment modality also does not appear to be associated with enhanced TLR2 responses within our MS cohort. Enhanced responders included patients treated with Avonex, Tysabri, Rituximab, Copaxone and Gilenya, as well as patients on no treatment (Table 1). Finally, among the MS patients with progressive forms of the disease, seven of 10 (70%) were enhanced responders. Moreover, of the four patients with secondary progressive MS, four of four were enhanced responders. Among the RRMS patients, six of 16 (37·5%) were enhanced responders (Table 1). Thus, our study suggests that the highest frequency of enhanced TLR2 responders within the MS cohort may be found among the patients with the progressive forms of the disease.
Using the same cohort of control and MS patients, we also examined simultaneously the lipopolysaccharide (LPS)‐stimulated TLR4 responses in our PBMC and CD14+ monocytes (both at the whole‐population and single‐cell levels). In these assays we stimulated the cells using 10 ng/ml or 50 ng/ml LPS (for the PBMC and CD14+ monocyte single‐cell assay) or 100 ng/ml LPS (for the CD14+ monocyte population assay). We found that while there was a tendency for a small number of MS patients to have enhanced responses to LPS, the percentages of such responses did not reach the level seen with TLR2 stimulation (Supporting information, Fig. S1). We found that the MS patients with enhanced TLR4 responses were, with one exception, always enhanced responders to TLR2 (the one exception was an RRMS patient in the single‐cell analysis). Thus, while there is some increase in TLR4 responsiveness in our MS cohort, our findings suggest a more specific enhancement in response to TLR2 stimulation.
Discussion
In this study our goal was to investigate the TLR2 responses of patients with MS using a variety of approaches. Our motivation for characterizing TLR2 responses in MS stemmed from our postulate that patients with MS have a deficiency in innate immune regulation leading to hyper‐responsiveness to TLR2 stimulation 4, 7, 9. There have been a number of studies attempting to identify the role of TLR2 signalling in the pathogenesis of EAE and MS 19, 20, 21, 22, 23, 24, 25. Although still controversial, most of these suggest that TLR2 signalling plays an important role in mediating the disease process. Additionally, studies from our laboratory and others have suggested the potential for targeting TLR2 as a therapeutic approach in MS 9, 26. However, there is a lack of consistency in the literature concerning the level of response of MS patients to Toll‐like receptor stimulation and there has been little characterization of the TLR2 responses in MS patients. In the present studies we sought a more definitive characterization of the TLR2 response in MS.
To accomplish this, we chose to characterize the TLR2 responsiveness of MS patients using multiple approaches. These approaches included characterizing the TLR2 responses of: (1) whole PBMC; (2) CD14+ monocytes assayed at the population level; and (3) CD14+ monocytes assayed at the single‐cell level. In each of these approaches we analysed six to 10 controls and six to 10 MS patients and normalized the results in each approach to the percentage of ‘enhanced responses’ in control versus MS patients. In total, we analysed TLR2 responses from 26 MS and 32 control patients. In each approach, we identified ‘enhanced’ responses based on the IQR of the control cohort responses.
In these studies we have documented, for the first time to our knowledge, that a large percentage of MS patients show enhanced TLR2 responsiveness. We found that 13 of 26 (50%) of MS patients demonstrated enhanced TLR2 responses (Table 1, Figs 7 and 8). In contrast, in the control cohort, only three of 32 (9·3%) demonstrated enhanced TLR2 responses (Table 2). Importantly, we found enhanced responsiveness in MS patients in each of the approaches we used to investigate TLR2 responses. Among patients with RRMS, six of 17 (35%) were enhanced responders. Interestingly, seven of 10 (70%) MS patients with progressive forms of the disease were enhanced responders, and this included four of four patients with secondary progressive MS (Table 1). This result may be of special interest, given the lack of therapeutic response of progressive patients to most of the adaptive immune‐targeting drugs used currently to treat RRMS patients. This lack of therapeutic response has led to the suggestion that progressive forms of MS do not have an immunological basis to their pathogenesis. Our results may now suggest that there is an involvement of the innate immune system in the pathogeneses of the progressive forms of MS. To investigate the specificity of the enhanced TLR2 responses, we also analysed responses to TLR4 stimulation. We found that the percentages of MS patients with enhanced TLR4 responses did not reach the level seen with TLR2 stimulation. Thus, our findings suggest a more specific enhancement in response to TLR2 stimulation.
It has been reported that there is enhanced cellular expression of TLR2 in MS patients, e.g. on neutrophils 27, T cells 28 and in the central nervous system (CNS) 29. However, in the present study we did not find an enhanced expression of TLR2 on CD14+ monocytes in patients with MS. Importantly, there has been significant variability in the literature concerning the state of Toll‐like receptor responsiveness in patients with MS, although the overwhelming majority of these studies have examined TLR4 responses or other non‐TLR2 responses 30, 31, 32, 33, 34, 35, 36. Our present studies, documenting enhanced TLR2 responses in a significant percentage of MS patients, now establish a new baseline for interpreting studies of the role of Toll‐like receptors in the pathogenesis of MS.
The question of what distinguishes the subset of enhanced responder MS patients from other MS patients remains unanswered. We found no association of enhanced TLR2 responses with age, gender, patient‐reported disease activity or mode of treatment (Table 1). None of our RRMS patients were in an exacerbation as determined by clinical history. Nevertheless, parameters such as age and disease duration could potentially affect TLR responsiveness, and future studies analysing these and other patient characteristics will be required to identify factors that associate with enhanced TLR2 responders. Interestingly, the greatest percentage of enhanced responders was found among MS patients with the progressive forms of the disease. Future studies will focus on this relationship, including the concept that an innate immune abnormality underlies the pathogenesis of progressive MS and the possibility that enhanced TLR2 responsiveness may signal a change from RRMS to progressive forms of the disease.
It is theoretically possible that enhanced TLR2 responses in MS patients stem from a genetic alteration in the TLR signalling pathway. However, a number of points argue against this possibility: (1) we have documented that 50% of our total MS patient cohort and 70% of the patients with progressive forms of MS demonstrate enhanced responses. Given that our MS patients were selected randomly, this frequency is too high to be entirely a result of genetic cell‐signalling aberrations; and (2) a number of prior studies have reported no difference in TLR responses (non‐TLR2) between MS patients and controls and we have found this to be the case in our TLR4 studies. MS‐associated genetically based signalling aberrations would be expected to be noted in such studies. These points suggest that the enhanced TLR2 responses noted in our study are not a result of genetically based signalling aberrations but, rather, stem at least partially from environmental influences.
In sum, in this study we document for the first time that a large percentage of MS patients, including patients with progressive forms of the disease, have enhanced responses to TLR2 stimulation. The motivation for our study stemmed from our postulate that patients with MS have defective regulation of TLR2 responses due to a deficiency in chronic exposure to microbiome‐derived TLR2 ligands 4, 7, 9. Linking the present results with the microbiome will depend upon future studies that correlate low levels of serum microbiome‐derived TLR2 ligands (e.g. Lipid 654 7, 8) with enhanced TLR2 responses in MS patients.
Disclosure
B. F., K. M, C. Ng, P. P. J. Z. and S. M. are employees of IsoPlexis and have competing interests with IsoPlexis. S. M. and P. P. are employed by, have equity ownership in and are patent holders with IsoPlexis. K. M., B. F., C. N. and J. Z. are employed by and have equity ownership in IsoPlexis.
Author contributions
M. F., E. A., B. F., J. Z., S. M. and R. C. designed the experiments. M. F., E. A., N. W., B. F. and K. M. conducted the experiments. M. F, E. A., B. F., K. M., C. N., P. P., J. Z. and S. M. acquired the data. M. .F, E. A., B. F., K. M., C. N., P. P., J. Z., S. M., F. N. and R. B. C. analysed the data. M. F., E. A., B. F., P. P., J. Z., S. M. and R. C. wrote the manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Compilation: percentages of Toll‐like receptor (TLR)4 responses above the interquartile range (IQR)‐based threshold of control responses. Compilation of percentages of control and multiple sclerosis (MS) patients with enhanced TLR4 responses as assessed in peripheral blood mononuclear cells (PBMC) [10 and 50 ng lipopolysaccharide (LPS) stimulation], CD14+ whole population and CD14+ single‐cell analyses. These studies encompass 25 MS patients (Table 1) and 29 controls (Table 2). The mean percentages of control patients with enhanced responses was 2·75%, while that of the total MS patients was 12·28%, the progressive MS patients 16·65% and the relapsing–remitting (RR)MS patients 4·15%. There were no statistically significant differences between the percentages of enhanced responses in the control cohort and either the total MS patients, the progressive (Prog) patients or the RRMS patients. Statistical significance was determined via Kruskal–Wallis test followed by Dunn's multiple comparisons test. Medians are depicted.
Table S1. Single‐cell barcode chip (SCBC) assay pairs of capture and biotin‐conjugated detection antibodies
Acknowledgements
We thank all the healthy volunteers and the MS patients for their participation in our study; the staff of the Lowell P. Weicker Jr Clinical Research Center, UConn Health; Kelly Hawley PhD, Sara Paveglio PhD and Evan Jellison PhD for providing technical assistance. We also thank Dr Kenneth Clark and Dr Chia‐ling Kuo for their assistance with data analysis and to Matthew Tremblay for acquiring the data. This work was supported by a grant from the Connecticut Bioscience Innovation Fund (Grant no. 517).
References
- 1. Schuijs MJ, Willart MA, Vergote K et al Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015; 349:1106–10. [DOI] [PubMed] [Google Scholar]
- 2. Stein MM, Hrusch CL, Gozdz J et al Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 2016; 375:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vatanen T, Kostic AD, d'Hennezel E et al Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016; 165:1551. [DOI] [PubMed] [Google Scholar]
- 4. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 2018; 18:105–20. [DOI] [PubMed] [Google Scholar]
- 5. Balmer ML, Schürch CM, Saito Y et al Microbiota‐derived compounds drive steady‐state granulopoiesis via MyD88/TICAM signaling. J Immunol 2014; 193:5273–83. [DOI] [PubMed] [Google Scholar]
- 6. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010; 16:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrokhi V, Nemati R, Nichols FC et al Bacterial lipodipeptide, Lipid 654, is a microbiome‐associated biomarker for multiple sclerosis. Clin Transl Immunol 2013; 2:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark RB, Cervantes JL, Maciejewski MW et al Serine lipids of porphyromonas gingivalis are human and mouse toll‐like receptor 2 ligands. Infect Immun 2013; 81:3479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anstadt EJ, Fujiwara M, Wasko N, Nichols F, Clark RB. TLR tolerance as a treatment for central nervous system autoimmunity. J Immunol 2016; 197:2110–8. [DOI] [PubMed] [Google Scholar]
- 10. Ma C, Fan R, Ahmad H et al A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nat Med 2011; 17:738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma C, Cheung AF, Chodon T et al Multifunctional T‐cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov 2013; 3:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu Y, Xue Q, Eisele MR et al Highly multiplexed profiling of single‐cell effector functions reveals deep functional heterogeneity in response to pathogenic ligands. Proc Natl Acad Sci USA 2015; 112:E607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleppe M, Kwak M, Koppikar P et al JAK‐STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 2015; 5:316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou J, Kaiser A, Ng C et al CD8+ T‐cell mediated anti‐malaria protection induced by malaria vaccines; assessment of hepatic CD8+ T cells by SCBC assay. Hum Vaccin Immunother 2017; 13:1625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan R, Vermesh O, Srivastava A et al Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat Biotechnol 2008; 26:1373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei W, Shin YS, Ma C et al Microchip platforms for multiplex single‐cell functional proteomics with applications to immunology and cancer research. Genome Med 2013; 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwak M, Mu L, Lu Y, Chen JJ, Brower K, Fan R. Single‐cell protein secretomic signatures as potential correlates to tumor cell lineage evolution and cell–cell interaction. Front Oncol 2013; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue Q, Lu Y, Eisele MR et al Analysis of single‐cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation. Sci Signal 2015; 8:ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll‐like receptor 2 and CD14 in the mouse CNS. Brain Pathol 2006; 12:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miranda‐Hernandez S, Gerlach N, Fletcher JM et al Role for MyD88, TLR2 and TLR9 but not TLR1, TLR4 or TLR6 in experimental autoimmune encephalomyelitis. J Immunol 2011; 187:791–804. [DOI] [PubMed] [Google Scholar]
- 21. Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll‐like receptor 2 and poly(ADP‐ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol 2009; 10:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hossain MJ, Tanasescu R, Gran B. TLR2: an innate immune checkpoint in multiple sclerosis. Oncotarget 2015; 6:35131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersson A, Covacu R, Sunnemark D et al Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol 2008; 84:1248–55. [DOI] [PubMed] [Google Scholar]
- 24. Ochoa‐Reparaz J, Mielcarz DW, Wang Y et al A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 2010; 3:487–95. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Telesford KM, Ochoa‐Reparaz J et al An intestinal commensal symbiosis factor controls neuroinflammation via TLR2‐mediated CD39 signalling. Nat Commun 2014; 5:4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gambuzza M, Licata N, Palella E et al Targeting Toll‐like receptors: emerging therapeutics for multiple sclerosis management. J Neuroimmunol 2011; 239:1–12. [DOI] [PubMed] [Google Scholar]
- 27. Hertwig L, Pache F, Romero‐Suarez S et al Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica. Mult Scler 2016; 22:160–73. [DOI] [PubMed] [Google Scholar]
- 28. Zastepa E, Fitz‐Gerald L, Hallett M et al Naive CD4 T‐cell activation identifies MS patients having rapid transition to progressive MS. Neurology 2014; 82:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci USA 2010; 107:11555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farrokhi M, Etemadifar M, Jafary Alavi MS et al TNF‐alpha production by peripheral blood monocytes in multiple sclerosis patients and healthy controls. Immunol Invest 2015; 44:590–601. [DOI] [PubMed] [Google Scholar]
- 31. Johnson TP, Tyagi R, Patel K, Schiess N, Calabresi PA, Nath A. Impaired toll‐like receptor 8 signaling in multiple sclerosis. J Neuroinflammation 2013; 10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chuluundorj D, Harding SA, Abernethy D, La Flamme AC. Expansion and preferential activation of the CD14(+)CD16(+) monocyte subset during multiple sclerosis. Immunol Cell Biol 2014; 92:509–17. [DOI] [PubMed] [Google Scholar]
- 33. Imamura K, Suzumura A, Hayashi F, Marunouchi T. Cytokine production by peripheral blood monocytes/macrophages in multiple sclerosis patients. Acta Neurol Scand 1993; 87:281–5. [DOI] [PubMed] [Google Scholar]
- 34. Saresella M, Gatti A, Tortorella P et al Toll‐like receptor 3 differently modulates inflammation in progressive or benign multiple sclerosis. Clin Immunol 2014; 150:109–20. [DOI] [PubMed] [Google Scholar]
- 35. Chiurchiù V, Leuti A, Cencioni MT et al Modulation of monocytes by bioactive lipid anandamide in multiple sclerosis involves distinct Toll‐like receptors. Pharmacol Res 2016; 113:313–9. [DOI] [PubMed] [Google Scholar]
- 36. Nyirenda MH, Morandi E, Vinkemeier U et al TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J Immunol 2015; 194:5761–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Compilation: percentages of Toll‐like receptor (TLR)4 responses above the interquartile range (IQR)‐based threshold of control responses. Compilation of percentages of control and multiple sclerosis (MS) patients with enhanced TLR4 responses as assessed in peripheral blood mononuclear cells (PBMC) [10 and 50 ng lipopolysaccharide (LPS) stimulation], CD14+ whole population and CD14+ single‐cell analyses. These studies encompass 25 MS patients (Table 1) and 29 controls (Table 2). The mean percentages of control patients with enhanced responses was 2·75%, while that of the total MS patients was 12·28%, the progressive MS patients 16·65% and the relapsing–remitting (RR)MS patients 4·15%. There were no statistically significant differences between the percentages of enhanced responses in the control cohort and either the total MS patients, the progressive (Prog) patients or the RRMS patients. Statistical significance was determined via Kruskal–Wallis test followed by Dunn's multiple comparisons test. Medians are depicted.
Table S1. Single‐cell barcode chip (SCBC) assay pairs of capture and biotin‐conjugated detection antibodies


