Abstract
OBJECTIVE
Sedentary children have greater risk of developing abnormalities in glucose homeostasis. We investigated whether interrupting sedentary behavior (sitting) with very short periods of walking would improve glucose metabolism without affecting dietary intake in children with overweight or obesity. We hypothesized that interrupting sitting with short bouts of moderate-intensity walking would decrease insulin area under the curve (AUC) during an oral glucose tolerance test (OGTT) compared with uninterrupted sitting.
RESEARCH DESIGN AND METHODS
Overweight/obese (BMI ≥85th percentile) children 7–11 years of age underwent two experimental conditions in random order: prolonged sitting (3 h of continuous sitting) and interrupted sitting (3 min of moderate-intensity walking at 80% of ventilatory threshold every 30 min for 3 h). Insulin, C-peptide, and glucose were measured every 30 min for 3 h during an OGTT. Each session was followed by a buffet meal. Primary outcomes were differences in OGTT hormones and substrates and in buffet meal intake by condition.
RESULTS
Among 35 children with complete data, mixed-model results identified lower insulin and C-peptide in the interrupted condition (P = 0.007 and P = 0.029, respectively); the intervention reduced insulin AUC by 21% (P < 0.001) and C-peptide AUC 18% (P = 0.001) and improved estimated insulin sensitivity (P = 0.013). Neither buffet total energy intake (1,262 ± 480 vs. 1,260 ± 475 kcal; P = 0.89) nor macronutrient composition of the meal (P values >0.38) differed between conditions significantly.
CONCLUSIONS
Interrupting sitting with brief moderate-intensity walking improved glucose metabolism without significantly increasing energy intake in children with overweight or obesity. Interrupting sedentary behavior may be a promising intervention strategy for reducing metabolic risk in such children.
Introduction
It is well established that supervised intensive exercise training improves glucose homeostasis in children with overweight or obesity (1); however, the effects of such interventions generally disappear once supervised training ends (2). As a result, there has been a search for lower-cost approaches that could be deployed long-term in school or after-school settings to improve children’s metabolic health.
On average, children remain sedentary during nonsleep periods for at least 6 h/day (3,4). Emerging evidence in adults and children suggests that extended time spent in sedentary behaviors is directly linked to poor metabolic outcomes including abnormalities in glucose homeostasis (5–12). Preliminary studies in adults (13–15) and in children with healthy weight (16) have demonstrated short-term improvements in glucose metabolism through interventions that interrupt sedentary behavior. In a crossover trial, we demonstrated that children of normal body weight, who underwent oral glucose tolerance tests (OGTTs), had improved insulin and glucose concentrations when sitting was interrupted with short (3-min) bouts of moderate-intensity walking (16). However, not all studies of this type of intervention have identified intervention effects (17). Furthermore, there are negligible prior data from children with overweight or obesity to demonstrate benefits from such interventions.
We therefore sought to determine if brief interruptions in sedentary behavior, with moderate intensity walking, acutely improved glucose metabolism in children aged 7–11 years who were overweight/obese. We hypothesized that compared with uninterrupted sedentary behavior, interrupting sedentariness with short bouts of walking would result in lower insulin, C-peptide, and glucose area under the curve (AUC) during an OGTT.
Research Design and Methods
Study Overview
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board approved the randomized crossover trial (https://clinicaltrials.gov/ct2/show/NCT01888939). Participants were seen at the National Institutes of Health (NIH) Hatfield Clinical Research Center for three separate visits. Participants were assessed for eligibility during a screening visit during which written consent and assent were obtained. Eligible participants returned on two separate occasions to complete each experimental condition (uninterrupted sitting and sitting with short walking bouts) in random order. To prevent possible carryover effects (18), test visits were scheduled 7–30 days apart.
Participants
Participants were recruited using social media, community listservs, the NIH Clinical Center ResearchMatch database, mailings, and flyers from December 2014 to December 2016. Participants were eligible if they were 7–11 years old, had fasting plasma glucose <100 mg/dL, were in good health, and had overweight or obesity as determined by BMI ≥85th percentile on the Centers for Disease Control and Prevention growth charts (19). Participants were excluded if they exhibited any signs of chronic disease indicating impaired perfusion, had fasting glucose ≥100 mg/dL, indicated any symptoms of diabetes or other endocrine disorders, or were taking medications that might influence metabolism or cognitive function. Other exclusionary criteria included precocious puberty, a psychiatric disorder that would impede adherence with the study, and below-average cognitive ability as indicated by an age-adjusted score <85 on the NIH Toolbox Picture Vocabulary Test (20).
Screening Visit
Participants were assessed for eligibility through a physical exam, fasting blood analysis, and a 12-lead electrocardiogram. The physical exam included an assessment for pubertal maturation by a trained physician or nurse practitioner via Tanner staging of girls’ breasts and boys’ testicular volumes (21). An exercise test using a modified Balke protocol (22) to estimate peak VO2 was performed. During this examination, the 10-point Borg Scale of Perceived Exertion was used to assess exercise tolerance. Dual-criteria graphs and the V-slope method (23) were used to estimate the ventilatory threshold (VT) achieved by participants. Heart rate and VO2 at 80% of VT were used to estimate the speed and grade for the treadmill during the interrupted sitting condition. Participants also completed the Picture Vocabulary Test, a measure used to assess ability to complete cognitive testing, using the NIH Toolbox (www.nihtoolbox.org). Body composition was assessed using DEXA (Lunar iDXA; GE Healthcare, Madison, WI) and analyzed with GE Encore 15 software.
Study Protocol
Experimental Design Overview (Randomized Crossover Trial)
Randomization was stratified based on sex, and participants were randomized 1:1 using random permutations (www.randomization.com) to the experimental order. Study team members assigned participants to experimental conditions and were thus not blinded to experimental order; participants learned their randomization sequence at their first test visit.
Participants underwent two 3-h OGTTs (1.75 g/kg dextrose with a maximal dose of 75 g) on separate days under the two experimental conditions: uninterrupted sitting (SIT) and sitting with short walking bouts (SIT + WALK), each performed under direct supervision of research staff. In the SIT condition, participants were required to remain seated for 3 h and allowed to rise only to use the bathroom. In the SIT + WALK condition, participants walked on a bedside treadmill for 3 min every 30 min for 3 h and were otherwise sedentary except for bathroom use. Total walking time was 18 min. Speed and grade were individualized to each subject’s target heart rate (80% of VT). Samples to measure hormones and substrates were drawn for 3 h.
Data Collection
Pre-Study Visit.
Participants were given an Actigraph GT3X+ wrist accelerometer to wear on the nondominant wrist for 7–10 days prior to each visit to assess habitual activity and sleep patterns. Data were continuously recorded from all three accelerometer axes at a rate of 80 Hz and later filtered and integrated using the manufacturer software (Actilife V6.11) into 1-min epochs, yielding activity and step counts. The values from each axis were calculated to create a minute-based vector magnitude.
Study Visits.
Participants arrived by 8:00 a.m., in a fasted state (from 10:00 p.m. the night prior). Vital signs, weight (5702; Scale-Tronix, Carol Stream, IL), and height in triplicate (242; Seca, Hanover, MD) were recorded, and a computer-assisted, multiple-pass 24-h dietary recall (Nutrition Data System for Research; Nutrition Coordinating Center, Minneapolis, MN) was conducted by a nutrition staff member at each visit. For blood sampling over the 3-h session, an intravenous line was placed in an antecubital vein at ∼9:00 a.m. Fasting blood samples were collected at −10 and 0 min, after which participants consumed the oral dextrose solution (thus, the OGTT was performed while participants executed the interventions). Blood samples were then collected at 20, 30, 60, 90, 120, 150, and 180 min. For the sitting with short walking bouts (SIT + WALK) condition, blood draws were done immediately after each 3-min walking period. During both conditions, participants were asked to remain sedentary, could watch movies, read, or do homework, and were not allowed to consume any energy-containing food or drink until the OGTT was finished. Once the OGTT completed, a >9,500-kcal standardized buffet-style test meal (51% carbohydrate, 37% fat, and 12% protein) (16) was provided in a confined room without interruptions or audience. Total energy and proportion of macronutrient consumed was calculated by weighing test meal items before and after the meal.
During each experimental visit, participants wore two accelerometer devices: one on the nondominant wrist and one on the right hip to track in-laboratory activity. Heart rate was monitored using a Polar RS800CX Watch plus chest-band electrode (Polar Electro Inc., Lake Success, NY) during the visits to ensure participants reached their designated target heart rate. Hip and wrist accelerometer data were aligned based on the position of maximum cross-correlation between the two series using custom software written in MATLAB (MathWorks Inc., Natick, MA). The aligned activity series were further integrated into 60-s epochs, and vector magnitude was calculated on a per-minute basis. Step counts were cumulated in 60-s epochs and based on hip-mounted data on the vertical axis.
Assays
Blood was collected at each timepoint for glucose, insulin, C-peptide, cortisol, free fatty acids (FFAs), and triglycerides. Catecholamines were also measured at the 0- and 30-min time points only. All samples were assayed by the NIH Clinical Center Department of Laboratory Medicine. Insulin and C-peptide were measured with Roche Diagnostics reagents on a Roche Cobas 6000 instrument (model e601; Roche Diagnostics, Indianapolis, IN) via electrochemiluminescence immunoassay. For insulin, the analytical sensitivity was 0.2 μU/mL, cross-reactivity with proinsulin was 0.05%, average intra-assay coefficient of variation (CV) was 1.1%, and average interassay CV was 4.3%. For C-peptide, the analytical sensitivity was 0.01 ng/mL, cross-reactivity with proinsulin was 32.5%, and average intra- and interassay CV were 1.1% and 4.1%, respectively. Plasma glucose was collected, kept on ice in tubes with powdered sodium fluoride until centrifugation, and determined using a Roche/Hitachi instrument (model c502; Roche Diagnostics). Total cholesterol and triglycerides were also analyzed using the Roche/Hitachi c502 instrument. Cortisol was measured on an Immulite 2000 XPi (Siemens Medical Solutions, Inc., Tarrytown, NY) via chemiluminescent enzyme immunoassay. The analytical sensitivity was 0.2 μg/dL, and average intra- and interassay CV were 6.8% and 9.9%, respectively. FFAs were measured on a Roche/Hitachi Cobas 6000 instrument (model c501; Roche Diagnostics) using the Wako enzymatic method. The analytical sensitivity was 0.01 mEq/L, and average intra- and interassay CV were 1.6% and 5.1%, respectively. Catecholamines were measured on a Bio Advantage Basic C18, 5-μm column (Thomson Instrument Company, Clear Brook, VA) via high-performance liquid chromatography with electrochemical detection. For norepinephrine, the intra- and interassay CV were 2.9% and 5.2%, respectively. For epinephrine, the intra- and interassay CV were 4.2% and 6.6%, respectively. For dopamine, the intra- and interassay CV were 5.8% and 6.4%, respectively.
The average of samples collected at −10 and 0 min is reported as baseline. The Matsuda index of insulin sensitivity was calculated using the OGTT results (24). Glucose effectiveness (GE) was calculated as previously described (25,26). See Supplementary Data for calculation details.
Statistical Analysis
Statistical analyses for descriptive characteristics, biomarker AUC, catecholamines, nutrition, and free-living comparisons were performed using SPSS Statistics 24 (IBM Corporation, Armonk, NY). SAS v9.3 (SAS Institute, Cary, NC) was used for mixed models of the primary outcomes. The power calculation to determine sample size was based on a previous trial in adults (13) determining the effect of moderate-intensity walking on insulin AUC. The calculations assumed a correlation coefficient of 0.5 between repeated outcome measures and a Cohen d of 0.40 (moderate effect size) and suggested a total sample size of 27 paired observations would supply a power of 0.80. The protocol therefore allowed for up to 50 participants to be randomized to ensure at least 30 (15 participants per crossover order group) had sufficient data available for analysis.
For the primary outcomes, mixed models assessed the effect of experimental condition on log-transformed insulin, glucose, C-peptide, and additional exploratory outcomes (FFAs, triglycerides, and cortisol) measured at 30-min intervals over the 3 h. Variance components were estimated using the restricted maximum likelihood estimate method to control for within-individual correlations and an unstructured covariance structure. Time-invariant covariates were baseline serum values, age (years), sex (male = 0 and female = 1), fat mass (kilograms), randomization order (uninterrupted sitting [SIT] at first visit, SIT + WALK at second visit vs. SIT + WALK at first visit, and SIT at second visit), visit condition (SIT and SIT + WALK experimental conditions), and pubertal stage. Race and Picture Vocabulary Test score were evaluated as covariates, not associated with any outcome, and therefore not included in the final models. Time-variant variables were time (minutes) and serum values. Repeated-measures ANOVA, controlling for randomization order in addition to the same covariates as the primary outcome, was also used for a post hoc analysis comparison of insulin sensitivity calculated with the Matsuda index (24) and calculated GE between conditions. Peak VO2 was also considered in this analysis, had no statistical contribution, and thus was removed. Repeated-measures ANOVAs, controlling for randomization order, were additionally used to compare insulin, C-peptide, and glucose AUC as well as the exploratory outcomes of differences in triglycerides, cortisol, FFA AUC, baseline and 30-min concentrations of catecholamines, and energy intake from the test meal between conditions. Repeated-measures ANOVAs controlling for randomization order were also used to investigate differences between conditions on free-living calorie consumption, sleep, and activity data obtained during the week before each test visit. Data in text are reported as means ± SD unless noted otherwise.
Results
Of 48 children screened, 43 were randomized, and 35 completed all assessments necessary for primary analyses (Supplementary Fig. 1). Table 1 presents baseline characteristics of randomized children. The sample demonstrated relatively equal male/female participation and included a notable variety of race, with only 40% identifying as white and the remaining identifying with a race of minority background. All had normal fasting glucose at screening; 53% had obesity, and 47% were overweight. There were no statistically significant demographic or anthropometric differences between those who completed and those who did not complete the study and additionally no difference in peak VO2. There was an average of 22 days between the two experimental visits. During the week before experimental visits, free-living energy intake, activity, and sleep did not differ significantly by condition (Table 2). There were no adverse events recorded during the study.
Table 1.
Baseline characteristics of randomized participants (n = 43)
| Variable | |
|---|---|
| Female sex, n (%) | 20 (46.5) |
| Race, n (%) | |
| White | 17 (39.5) |
| Asian | 2 (4.6) |
| Black or African American | 17 (39.5) |
| Multiple races | 3 (7.0) |
| Unknown/declined | 3 (7.0) |
| Pacific Islander | 1 (2.3) |
| Ethnicity, n (%) | |
| Latino/Hispanic | 4 (9.3) |
| Non-Latino/Hispanic | 39 (90.7) |
| Age (years) | 9.6 ± 1.3 |
| Pubertal maturation | |
| Male, testis volume (mL) | 4 (1–15) |
| Female, Tanner breast stage | 2 (1–4) |
| Picture Vocabulary Test score1 | 106.8 ± 11.3 |
| Fat mass (kg)2 | 20.3 ± 8.5 |
| Lean mass (kg)2 | 30.0 ± 6.3 |
| Waist-to-height ratio | 0.5 ± 0.1 |
| BMI (kg/m2) | 24.5 ± 4.5 |
| BMI Z score | 1.8 ± 0.5 |
| Fasting glucose (mg/dL) | 90.0 ± 5.4 |
| Systolic BP (mmHg) | 112.2 ± 9.8 |
| Diastolic BP (mmHg) | 61.5 ± 8.8 |
| Peak VO2 (mL/kg/min) | 26.2 ± 8.0 |
| HOMA-IR3 | 5.3 ± 9.4 |
| Matsuda index3 (during sedentary OGTT) | 3.6 ± 2.1 |
Data are mean ± SD or median (range) unless otherwise indicated.
BP, blood pressure; HOMA-IR, HOMA of insulin resistance.
1Proxy of intelligence quotient.
2n = 42.
3n = 32.
Table 2.
Dietary recall and activity prior to visit, biomarker AUC, visit activity, and energy intake comparison by condition
| Variable | SIT | SIT + WALK | P |
|---|---|---|---|
| Total 24-h diet recall | |||
| Total energy intake (kcal/day) | 1,951.3 (651.6) | 2,047.5 (570.0) | 0.413 |
| Carbohydrate intake (%)1 | 52.7 (9.3) | 51.9 (7.9) | 0.697 |
| Fat intake (%)1 | 33.1 (8.5) | 32.5 (7.5) | 0.766 |
| Protein intake (%)1 | 14.1 (4.2) | 15.6 (4.5) | 0.051 |
| Wrist accelerometry (week prior to testing) | |||
| Number of valid days of data | 6.9 (2.2) | 6.6 (2.4) | 0.982 |
| Wear time (h/day) | 22.1 (2.0) | 22.2 (1.6) | 0.988 |
| Daily activity (3-D counts/day × 10−6) | 3.2 (0.61) | 3.20 (0.59) | 0.606 |
| Total sleep time (h/night) | 7.5 (0.7) | 7.6 (0.7) | 0.146 |
| Sleep efficiency (%) | 86.7 (4.0) | 86.5 (4.7) | 0.850 |
| Metabolic outcomes from OGTT | |||
| Insulin AUC (mIU/dL ∙ min)2,3 | 21,145.9 (18,280.7) | 16,629.9 (17,563.0) | <0.001 |
| C-peptide AUC (ng/dL ∙ min)2,3 | 14,778.5 (900.9) | 1,205.7 (527.8) | 0.001 |
| Glucose AUC (mg/dL ∙ min)2,3 | 21,587.7 (2,215.5) | 21,106.5 (2,630.4) | 0.143 |
| FFA AUC (mEq/L ∙ min)2,3 | 34.6 (11.0) | 31.1 (11.4) | 0.074 |
| Cortisol AUC (µg/dL ∙ min)2,3 | 1,165.0 (229.2) | 1,175.6 (292.0) | 0.973 |
| Triglycerides AUC (mg/dL ∙ min)2,3,4 | 16,033.6 (10,580.3) | 15,969.0 (8,857.3) | 0.442 |
| Matsuda index of insulin sensitivity3 | 3.64 (2.18) | 4.26 (2.51) | 0.013 |
| GE index3 | 4.04 (1.0) | 3.99 (1.02) | 0.124 |
| Catecholamines during OGTT (pg/dL)5 | |||
| Norepinephrine baseline3 | 255.0 (65.9) | 243.7 (75.9) | 0.527 |
| Norepinephrine at 30 min3 | 268.6 (62.1) | 401.4 (128.4) | <0.001 |
| Epinephrine baseline3 | 32.9 (17.3) | 34.6 (16.4) | 0.591 |
| Epinephrine at 30 min3 | 26.0 (10.1) | 33.7 (25.3) | 0.283 |
| Dopamine baseline | 25.9 (3.9) | 25.2 (0.64) | 0.384 |
| Dopamine at 30 min | 25.8 (2.6) | 25.6 (2.9) | 0.779 |
| Buffet energy intake after OGTT | |||
| Total energy intake (kcal) | 1,261.9 (480.4) | 1,259.6 (474.9) | 0.890 |
| Carbohydrate intake (%)1 | 49.7 (9.4) | 49.2 (8.4) | 0.383 |
| Fat intake (%)1 | 38.2 (7.7) | 38.7 (6.7) | 0.379 |
| Protein intake (%)1 | 12.1 (2.9) | 12.2 (2.9) | 0.558 |
Data are mean (SD). P value determined with repeated-measures ANOVA controlling for randomization order, age, sex, fat mass, and puberty stage. n = 32–35 unless noted. Boldface type indicates significant P values.
1Arcsine (square root) transformed for analysis.
2AUC by trapezoidal rule.
3Log transformed for analysis.
4Additionally controlled for race.
5n = 24.
During the SIT + WALK OGTT test visits, participants demonstrated increased activity counts from wrist- and hip-mounted accelerometers (Supplementary Fig. 2A and B). A greater number of steps were taken during the SIT + WALK condition (10.9 ± 2.1 steps/min; 1,962 ± 378 total steps) than the SIT condition (1.2 ± 0.7 steps/min; 216 ± 126 total steps; P ≤ 0.001) (Supplementary Fig. 2C). Measured heart rate during walking periods (132.8 ± 12.9 bpm) confirmed that participants reached 80% of VT (131.6 ± 13.6 bpm; P = 0.06). Participants stood for unscheduled walking (most commonly to use the restroom) on average one time per session with no difference between conditions (P = 0.47).
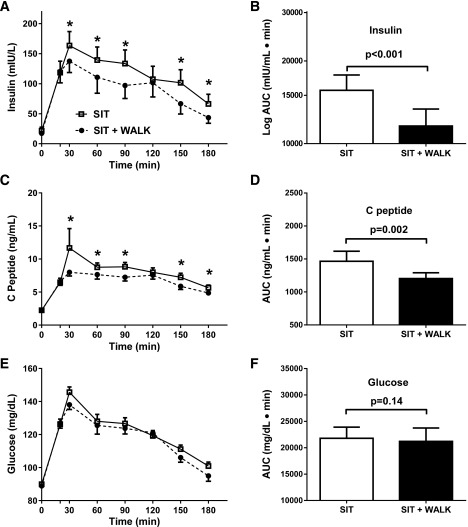
Insulin secretion over the 3-h test was significantly lower in the SIT + WALK versus the SIT condition (Table 3) (F = 2.8; P = 0.007). OGTT mean insulin concentrations were lower at time points 60, 90, 150, and 180 min (P values <0.01) in the SIT + WALK condition (Fig. 1A). Mean insulin AUC was 21% lower in the SIT + WALK condition compared with the SIT condition (Table 2 and Fig. 1B) (P < 0.001), yielding a Cohen d coefficient of 0.24 (small-moderate effect). C-peptide was also lower in SIT + WALK versus the SIT condition (Table 3) (F = 2.26; P = 0.03), with lower C-peptide at most OGTT time points (Fig. 1C). Overall, the mean C-peptide AUC was 18% lower in the SIT + WALK condition compared with the SIT condition (Table 2 and Fig. 1D) (P = 0.002), with a Cohen d coefficient of 0.36 (moderate effect).
Table 3.
Results from six separate mixed models predicting change in substrate/hormone concentrations over time by visit type: sitting interrupted with 3 min of moderate-intensity walking every half hour (SIT + WALK) or uninterrupted sitting (SIT)
| Variable | Insulin1 |
C-peptide1 |
Glucose1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | F2 | P | Estimate | SE | F2 | P | Estimate | SE | F2 | P | |
| Intercept | 1.533 | 0.251 | 6.12 | <0.001 | 0.539 | 0.121 | 4.46 | <0.001 | 1.8191 | 0.072 | 25.42 | <0.001 |
| Age (years) | −0.052 | 0.028 | 3.51 | 0.062 | −0.017 | 0.0133 | 1.67 | 0.198 | −0.013 | 0.005 | 5.51 | 0.020 |
| Sex (reference = male) | −0.075 | 0.070 | 1.15 | 0.284 | −0.055 | 0.033 | 2.79 | 0.096 | −0.020 | 0.012 | 2.51 | 0.113 |
| Tanner stage 2 (reference = 1) | 0.128 | 0.077 | 1.66 | 0.097 | 0.057 | 0.037 | 1.57 | 0.117 | 0.020 | 0.014 | 1.48 | 0.140 |
| Tanner stage 3 (reference = 1) | 0.254 | 0.102 | 2.49 | 0.013 | 0.066 | 0.048 | 1.36 | 0.175 | 0.023 | 0.019 | 1.23 | 0.221 |
| Tanner stage 4 (reference = 1) | 0.240 | 0.1557 | 1.54 | 0.124 | 0.071 | 0.074 | 0.95 | 0.341 | 0.017 | 0.025 | 0.67 | 0.504 |
| Total fat mass (kg) | 0.019 | 0.004 | 25.16 | <0.0001 | 0.010 | 0.002 | 28.73 | <0.0001 | 0.001 | 0.001 | 2.61 | 0.107 |
| Fasting concentration3 | 0.004 | 0.001 | 17.96 | <0.001 | 0.052 | 0.009 | 29.63 | <0.0001 | 0.003 | 0.001 | 14.59 | <0.001 |
| Randomization order (reference = SIT) | 0.034 | 0.059 | 0.34 | 0.562 | 0.007 | 0.028 | 0.060 | 0.803 | 0.027 | 0.010 | 6.60 | 0.011 |
| Time | 135.08 | 0.001 | 186.32 | <0.0001 | 80.86 | <0.0001 | ||||||
| Visit type (reference = SIT) | −0.146 | 0.053 | 18.60 | <0.001 | −0.066 | 0.028 | 16.79 | <0.0001 | −0.024 | 0.014 | 2.100 | 0.148 |
| Time × visit type | 2.80 | 0.007 | 2.26 | 0.029 | 0.750 | 0.632 | ||||||
| 0 min | −0.066 | 0.053 | −1.24 | 0.215 | −0.016 | 0.029 | −0.55 | 0.5830 | 0.002 | 0.014 | 0.16 | 0.873 |
| 20 min | −0.013 | 0.055 | −0.24 | 0.812 | −0.024 | 0.030 | −0.78 | 0.438 | −0.003 | 0.015 | −0.22 | 0.823 |
| 30 min | 0.068 | 0.053 | 1.27 | 0.205 | 0.068 | 0.028 | 2.39 | 0.017 | 0.022 | 0.014 | 1.55 | 0.121 |
| 60 min | 0.146 | 0.054 | 2.69 | 0.007 | 0.061 | 0.029 | 2.09 | 0.037 | 0.008 | 0.014 | 0.58 | 0.560 |
| 90 min | 0.161 | 0.054 | 3.00 | 0.003 | 0.085 | 0.029 | 2.95 | 0.003 | 0.006 | 0.014 | 0.43 | 0.665 |
| 120 min | 0.044 | 0.053 | 0.83 | 0.408 | 0.015 | 0.029 | 0.53 | 0.595 | −0.013 | 0.014 | −0.91 | 0.365 |
| 150 min | 0.177 | 0.053 | 3.36 | <0.001 | 0.083 | 0.029 | 2.89 | 0.004 | 0.013 | 0.014 | 0.89 | 0.373 |
| 180 min | 0.146 | 0.053 | 2.72 | 0.007 | 0.066 | 0.028 | 2.32 | 0.021 | 0.024 | 0.014 | 1.66 | 0.097 |
| FFAs1 | Triglycerides1 | Cortisol1 | ||||||||||
| Estimate |
SE |
F2 |
P |
Estimate |
SE |
F2 |
P |
Estimate |
SE |
F2 |
P |
|
| Intercept | −1.35 | 0.165 | −8.20 | <0.001 | 1.485 | 0.140 | 10.65 | <0.001 | 0.842 | 0.130 | 6.47 | <0.001 |
| Age (years) | −0.012 | 0.017 | 0.52 | 0.470 | 0.008 | 0.015 | 0.30 | 0.5860 | −0.026 | 0.014 | 3.80 | 0.052 |
| Sex (reference = male) | 0.033 | 0.041 | 0.65 | 0.421 | −0.035 | 0.043 | 0.66 | 0.4164 | 0.011 | 0.032 | 0.12 | 0.730 |
| Tanner stage 2 (reference = 1) | −0.019 | 0.044 | −0.44 | 0.663 | −0.035 | 0.049 | −0.72 | 0.474 | −0.022 | 0.036 | −0.62 | 0.536 |
| Tanner stage 3 (reference = 1) | 0.002 | 0.060 | 0.03 | 0.987 | −0.067 | 0.063 | −1.07 | 0.287 | 0.027 | 0.048 | 0.57 | 0.567 |
| Tanner stage 4 (reference = 1) | 0.064 | 0.082 | 0.78 | 0.435 | 0.035 | 0.088 | 0.40 | 0.689 | 0.015 | 0.065 | 0.24 | 0.813 |
| Total fat mass (kg) | <0.001 | 0.003 | 0.12 | 0.734 | 0.002 | 0.002 | 0.84 | 0.3587 | <0.001 | 0.002 | 0.02 | 0.901 |
| Fasting concentration3 | 0.397 | 0.055 | 51.63 | <0.001 | 0.002 | <0.001 | 266.79 | <0.0001 | 0.010 | 0.003 | 13.72 | <0.001 |
| Randomization order (reference = SIT) | 0.023 | 0.034 | 0.47 | 0.492 | 0.040 | 0.037 | 1.16 | 0.2810 | 0.014 | 0.027 | 0.28 | 0.597 |
| Time | 366.71 | <0.001 | 18.70 | <0.0001 | 31.55 | <0.001 | ||||||
| Visit type (reference = SIT) | 0.085 | 0.040 | 0.07 | 0.7892 | 0.027 | 0.0201 | 14.94 | 0.0001 | 0.013 | 0.036 | 0.63 | 0.429 |
| Time × visit type | 1.30 | 0.246 | 0.94 | 0.475 | 0.54 | 0.803 | ||||||
| 0 min | 0.001 | 0.040 | 0.24 | 0.814 | 0.006 | 0.020 | 0.31 | 0.759 | 0.042 | 0.036 | 1.19 | 0.236 |
| 20 min | −0.007 | 0.042 | −0.16 | 0.875 | −0.003 | 0.021 | −0.16 | 0.872 | −0.024 | 0.037 | −0.64 | 0.525 |
| 30 min | 0.065 | 0.040 | 1.62 | 0.106 | −0.053 | 0.020 | −2.59 | 0.010 | −0.008 | 0.036 | −0.22 | 0.825 |
| 60 min | 0.048 | 0.040 | 1.20 | 0.230 | −0.037 | 0.020 | −1.84 | 0.066 | −0.049 | 0.036 | −1.39 | 0.165 |
| 90 min | 0.025 | 0.040 | 0.61 | 0.539 | −0.036 | 0.020 | −1.79 | 0.075 | −0.020 | 0.036 | −0.55 | 0.581 |
| 120 min | −0.009 | 0.040 | −0.23 | 0.821 | −0.040 | 0.020 | −1.99 | 0.047 | −0.014 | 0.036 | −0.40 | 0.693 |
| 150 min | −0.015 | 0.040 | −0.38 | 0.705 | −0.035 | 0.020 | −1.75 | 0.081 | 0.004 | 0.036 | 0.11 | 0.912 |
| 180 min | −0.085 | 0.040 | −2.11 | 0.035 | −0.027 | 0.020 | −1.34 | 0.180 | −0.012 | 0.036 | −0.35 | 0.724 |
Boldface type indicates significant P values.
1Log-transformed AUC.
2The t value for intercept and time × visit type interactions (random effects).
3Fasting concentration for each respective hormone.
Figure 1.
The effect of sitting interrupted with 3 min of moderate-intensity walking every half hour (SIT + WALK; black circles and bars) vs. uninterrupted sitting (SIT; white squares and bars) on serum insulin concentrations (A), 3-h insulin AUC (B), serum C-peptide concentrations (C), 3-h C-peptide AUC (D), plasma glucose concentrations (E), and 3-h glucose AUC (F). Unadjusted mean ± SE are shown in A, C, and F. AUC results (B, D, and F) are mean ± SE adjusted for randomization order. *Significantly different, post hoc paired t test, SIT + WALK vs. SIT, P < 0.05.
For plasma glucose, no main effect by condition was identified, and the condition × time interaction was not significant in the mixed model (Table 3) (F = 0.75; P = 0.63) (Fig. 1E). The glucose AUC was not significantly different between condition types (Table 2 and Fig. 1F).
Insulin sensitivity, as calculated by the Matsuda index, was greater during the SIT + WALK compared with SIT condition (4.2 ± 2.5 vs. 3.64 ± 2.1; P = 0.013), but there was no detected difference in calculated GE between conditions (Table 2) (P = 0.12). There were no significant main effects, time × condition interactions, or differences in AUC for FFAs, cortisol, or triglycerides (Tables 2 and 3).
Baseline catecholamine concentrations were not significantly different between conditions (Table 2) (P values >0.30). Time plus 30 min norepinephrine was greater during the SIT + WALK than SIT condition (401 ± 128 vs. 269 ± 62 pg/dL; P < 0.001). No significant differences were found for epinephrine or dopamine (Table 2) (P values >0.20).
Total energy intake and percentage of energy consumed from carbohydrates, fats, or proteins from the test meal did not differ by experimental condition (Table 2).
Conclusions
The data presented in this study demonstrate that children with overweight and obesity have improved insulin concentrations and insulin sensitivity measured during an OGTT when they engage in only 3 min of moderate-intensity walking every 30 min. This finding is consistent with results of studies in adults (13,27) and in studies with children of healthy weight (16,28). It is contrary to one study that identified no improvement in glucose metabolism in 19 children (generally healthy weight) who performed light-intensity walking breaks every 20 min for an 8-h day compared with a sedentary day (17). The current study found no change in circulating glucose or altered energy intake during interrupted behavior.
Moderate- and high-intensity sustained exercise is known to improve both non–insulin-mediated glucose uptake (GE) and insulin sensitivity (29–31), but the physiological processes through which sedentary behavior influences glucose homeostasis are less clear. Some data suggest that compared with continuous activity, prolonged sedentary behavior might affect both insulin sensitivity and GE in peripheral tissues such as muscle (32). One bout of continuous activity is conceptually different from interrupted activity; however, research on interrupted activity (including the current study) suggests that the effects on glucose homeostasis of interrupted activity may be physiologically similar to those of a single bout of continuous activity. It is well understood that continuous muscle contraction increases GE, and it is also likely that muscle contraction enhances muscle insulin sensitivity (33), both of which lead to decreased insulin demand. Thus, interrupted activity may improve glucose homeostasis through increased GE and/or increased insulin sensitivity associated with muscle contraction, although mechanistic data on interrupted activity are lacking. To explore the mechanisms through which interrupting sedentary time improved glucose homeostasis, we estimated GE using an approach that has recently been validated in youth with overweight and obesity (26), along with the Matsuda index as a marker for insulin sensitivity. The data identified no change in GE during the SIT + WALK condition but did find significantly improved estimated insulin sensitivity. Of note, the current study was underpowered to detect differences in calculated GE: a post hoc power analysis based on the current study’s GE data indicates 100 subjects would be required to find a significant difference in GE between SIT and SIT + WALK conditions. In regards to insulin sensitivity, the improved Matsuda index is contrary to the findings of a study of interrupted activity in 19 adults (34). It remains most likely that interrupted activity improves glucose metabolism as sustained physical activity does; however, given the extant mixed results, further studies using more rigorous methods to measure GE and insulin sensitivity are required before definitive conclusions can be reached.
Unlike some other studies of interruption of sedentary time (13,15,16,27), we did not find a difference in glucose AUC by condition. The lack of effect on glucose is at least partially explained by the difference in samples studied. Comparing (with unpaired t tests) the current study cohort with our previously published healthy-weight cohort that underwent an identical protocol (16), the overweight cohort had significantly increased insulin resistance assessed with HOMA of insulin resistance (5.3 ± 9.4 vs. 1.4 ± 0.7; P = 0.02) and decreased sensitivity via Matsuda index (3.6 ± 2.1 vs. 7.3 ± 3.4; P < 0.001). The current cohort was also significantly less fit, with reduced peak VO2 (26.2 ± 8.0 vs. the healthy-weight cohort, 42.9 ± 8.3 mg/kg/min; P < 0.001). In addition to these notable differences in study groups, we hypothesize that youth with overweight and obesity likely maintain somewhat increased GE compared with healthy-weight youth as part of their response to insulin resistance, such that their ability to improve glucose uptake with short, moderate-intensity walking might be reduced, potentially explaining why an improvement in glucose AUC was not obtained in the current study. Nonetheless, the significantly lower insulin AUC implies reduced endogenous insulin secretion demand that, if sustained in the long-term, might be anticipated to slow the development of type 2 diabetes (35).
There were no significant differences in energy intake by condition. This result is consistent with previous studies in children (16,28), which leads us to conclude that the dose of activity was insufficient to cause compensation for the energy used during walking by increasing immediate energy intake. If the small increase in energy expenditure from interrupted sitting can be carried out over longer duration (such as through multiple consecutive days) without affecting energy intake, this could potentially lead to improved energy balance in overweight children. For example, 18 cumulative minutes of moderate-intensity walking would be estimated to expend ∼70 kcal of energy/day, which would calculate to an ∼500 kcal energy deficit for the week if executed daily without compensatory energy intake. Data regarding the sustained effects, over longer intervention periods, of interrupting sedentary behaviors on energy intake and weight in children are needed.
Strengths and Limitations
Strengths of the study include accounting for numerous potential confounders, including, most notably, prior physical activity (monitored by accelerometry), body composition (obtained through DEXA), and pubertal development. Furthermore, we retained 90% of our participants through completion (despite only 82% having adequate data for final analyses), which demonstrates great feasibility and applicability for similar studies. Finally, this study used a randomized crossover study design, which inherently minimized the between-individual variation from intervention-related differences. However, there are potential limitations to consider. First, we relied on dietary recall to control for dietary intake on the day prior to study interventions. Experiments should be carried out standardizing energy intake prospectively. Second, the study design collected data only within the 3 h of intervention despite the possibility of the intervention having potentially salutary or deleterious effects thereafter. Third, although the study was adequately powered for the primary aims, it is possible that a larger sample would allow other effects of such interventions to be observed. Finally, the study group was limited to youth who were overweight and obese but otherwise had relatively normal glucose metabolism; thus, generalizability is limited to metabolically healthy youth, and the study does not provide definitive information for youth who have already reached metabolic impairment such as diabetes.
Conclusion
Interrupting sedentary behavior in children with overweight and obesity improved their glucose metabolism acutely. Future research should focus on evaluating sustained outcomes of such interventions and exploring the underlying physiologic mechanisms. If this intervention provides sustained improvement in glucose metabolism, given the logistic feasibility and child acceptance experienced in this study, widespread implementation into school or after-school care centers could provide notable improvement in glucose homeostasis in the community setting and potentially slow the onset of type 2 diabetes in youth.
Supplementary Material
Article Information
Acknowledgments. The authors thank the children and families who participated in these studies. The authors also thank the NIH Clinical Center staff, Pediatric Day Hospital staff, and the Metabolic Clinical Research Unit staff.
Funding. This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (Z1AHD00641 to J.A.Y.) with supplemental funding from the NIH Office of Disease Prevention (to J.A.Y.) and the Thrasher Research Fund (to M.M.B.). All authors were federal employees and special volunteers supported by the Intramural Research Program at the NIH. R.J.B., J.D.H., and K.Y.C. are supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (grant Z1CDK071013). B.R.B. is supported by a postdoctoral training award from the National Cancer Institute Cancer Prevention Fellowship Program in the Division of Cancer Prevention and by a career development award from National Institute of Diabetes and Digestive and Kidney Diseases (1K01DK113062).
Duality of Interest. J.A.Y. has received grant support for pharmacotherapy trials for obesity from Zafgen and Rhythm Pharmaceuticals unrelated to this article and is a Commissioned Officer of the U.S. Public Health Service. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.M.B., B.R.B., E.K.D., and J.A.Y. performed statistical analyses. M.M.B., B.R.B., R.J.B., I.L.T., F.S., A.P., J.D.H., S.M.B., S.B.B., A.B.C., K.P.S., D.R.R., and J.A.Y. collected data and reviewed and edited the manuscript. M.M.B., E.K.D., and J.A.Y. wrote the first draft of the manuscript. B.R.B., D.A.B., B.E.D., D.R.R., P.L.W., K.Y.C., and J.A.Y. conceived the study and wrote the protocol. D.A.B., B.E.D., P.L.W., and K.Y.C. reviewed and edited the manuscript. J.A.Y. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at ObesityWeek, the annual meeting of The Obesity Society and the American Society for Metabolic & Bariatric Surgery, Washington, DC, 29 October–2 November 2017.
Footnotes
Clinical trial reg. no. NCT01888939, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0774/-/DC1.
The opinions and assertions expressed in this article are those of the authors and are not to be construed as reflecting the views of the U.S. Public Health Service, the National Institutes of Health, or the U.S. Department of Health and Human Services.
References
- 1.Davis CL, Pollock NK, Waller JL, et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA 2012;308:1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson MA, Gutin B, Le NA, et al. Effects of exercise training and its cessation on components of the insulin resistance syndrome in obese children. Int J Obes Relat Metab Disord 1999;23:889–895 [DOI] [PubMed] [Google Scholar]
- 3.Belcher BR, Berrigan D, Dodd KW, Emken BA, Chou CP, Spruijt-Metz D. Physical activity in US youth: effect of race/ethnicity, age, gender, and weight status. Med Sci Sports Exerc 2010;42:2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths LJ, Cortina-Borja M, Sera F, et al. How active are our children? Findings from the Millennium Cohort Study. BMJ Open 2013;3:e002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 2007;27:2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkau B, Mhamdi L, Oppert JM, et al.; EGIR-RISC Study Group . Physical activity and insulin sensitivity: the RISC study. Diabetes 2008;57:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010;121:384–391 [DOI] [PubMed] [Google Scholar]
- 8.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone Gibbs B, Pettee Gabriel K, Reis JP, Jakicic JM, Carnethon MR, Sternfeld B. Cross-sectional and longitudinal associations between objectively measured sedentary time and metabolic disease: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes Care 2015;38:1835–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogerson MC, Le Grande MR, Dunstan DW, et al. Television viewing time and 13-year mortality in adults with cardiovascular disease: data from the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Heart Lung Circ 2016;25:829–836 [DOI] [PubMed] [Google Scholar]
- 11.Sardinha LB, Andersen LB, Anderssen SA, et al. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9- to 10-year-old Portuguese children. Diabetes Care 2008;31:569–575 [DOI] [PubMed] [Google Scholar]
- 12.Henderson M, Gray-Donald K, Mathieu ME, et al. How are physical activity, fitness, and sedentary behavior associated with insulin sensitivity in children? Diabetes Care 2012;35:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altenburg TM, Rotteveel J, Dunstan DW, Salmon J, Chinapaw MJ. The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J Appl Physiol (1985) 2013;115:1751–1756 [DOI] [PubMed] [Google Scholar]
- 15.Henson J, Davies MJ, Bodicoat DH, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care 2016;39:130–138 [DOI] [PubMed] [Google Scholar]
- 16.Belcher BR, Berrigan D, Papachristopoulou A, et al. Effects of interrupting children’s sedentary behaviors with activity on metabolic function: a randomized trial. J Clin Endocrinol Metab 2015;100:3735–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders TJ, Chaput JP, Goldfield GS, et al. Prolonged sitting and markers of cardiometabolic disease risk in children and youth: a randomized crossover study. Metabolism 2013;62:1423–1428 [DOI] [PubMed] [Google Scholar]
- 18.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988;254:E248–E259 [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;314:1–27 [PubMed] [Google Scholar]
- 20.National Institutes of Health toolbox cognition battery (NIH toolbox CB). Monogr Soc Res Child Dev 2013;78:1–172 [DOI] [PubMed] [Google Scholar]
- 21.Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics 2002;110:743–747 [DOI] [PubMed] [Google Scholar]
- 22.Skinner J. Exercise Testing and Exercise Prescription for Special Cases. Philadelphia, PA, Lea & Febiger, 1993 [Google Scholar]
- 23.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027 [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 25.Nagasaka S, Kusaka I, Yamashita K, et al. Index of glucose effectiveness derived from oral glucose tolerance test. Acta Diabetol 2012;49(Suppl. 1):S195–S204 [DOI] [PubMed] [Google Scholar]
- 26.Weiss R, Magge SN, Santoro N, et al. Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia. Diabetes Care 2015;38:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulsford RM, Blackwell J, Hillsdon M, Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: a randomised cross-over study in inactive middle-aged men. J Sci Med Sport 2017;20:278–283 [DOI] [PubMed] [Google Scholar]
- 28.Penning A, Okely AD, Trost SG, et al. Acute effects of reducing sitting time in adolescents: a randomized cross-over study. BMC Public Health 2017;17:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol 1984;247:E726–E731 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi Y, Nagasaka S, Takahashi N, et al. A single bout of exercise at higher intensity enhances glucose effectiveness in sedentary men. J Clin Endocrinol Metab 2005;90:4035–4040 [DOI] [PubMed] [Google Scholar]
- 31.Brun JF, Guintrand-Hugret R, Boegner C, Bouix O, Orsetti A. Influence of short-term submaximal exercise on parameters of glucose assimilation analyzed with the minimal model. Metabolism 1995;44:833–840 [DOI] [PubMed] [Google Scholar]
- 32.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 2011;60:941–949 [DOI] [PubMed] [Google Scholar]
- 33.Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol 1985;249:C226–C232 [DOI] [PubMed] [Google Scholar]
- 34.Larsen RN, Kingwell BA, Robinson C, et al. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci (Lond) 2015;129:117–127 [DOI] [PubMed] [Google Scholar]
- 35.Gupta D, Krueger CB, Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev 2012;8:76–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.