Abstract
OBJECTIVE
There is uncertainty about the importance of glycemic variability in cardiovascular complications in patients with type 2 diabetes. Using the Veterans Affairs Diabetes Trial (VADT), we investigated the association between variation in fasting glucose and glycated hemoglobin (HbA1c) over time and the incidence of cardiovascular disease (CVD) and assessed whether this is influenced by intensive or standard glycemic control.
RESEARCH DESIGN AND METHODS
During the VADT, fasting glucose and HbA1c were measured every 3 months for up to 84 months in 1,791 individuals. Variability measures included coefficient of variation (CV) and average real variability (ARV) for fasting glucose and HbA1c. Overall mean glucose and HbA1c measures as well as their maximum and the most recent measurement were also examined.
RESULTS
Variability measures (CV and ARV) of fasting glucose were significantly associated with CVD even after adjusting for other risk factors, including mean fasting glucose. When considering separately groups receiving intensive and standard glycemic control, this relationship was evident in the intensive treatment group but not in the standard group. Additional adjustment for severe hypoglycemic episodes did not alter the relationship between fasting glucose variability and CVD. Interestingly, no HbA1c measures were associated with CVD after adjusting for multiple baseline risk factors.
CONCLUSIONS
Our analysis indicates that in the VADT, variability of fasting glucose plays a role in the development of CVD complications beyond the influence of standard fasting glucose measures. The adverse consequences of fasting glucose variability on CVD appear greatest in those receiving intensive glucose control.
Introduction
Current management of type 2 diabetes (T2D) uses periodic glucose measures and average glycated hemoglobin (HbA1c) to monitor glycemic control and inform decisions regarding glucose-lowering therapy. The rationale is based on their ease of measurement and on observational and trial evidence that demonstrates that these measures are relatively good predictors of micro- and macrovascular complications of diabetes (1–6). However, there is less consensus about the relative importance of glycemic variation over time as a contributor to diabetes complications.
Recent evidence has raised the possibility that visit-to-visit fasting glucose or/and HbA1c variability may add to standard glycemic measures for prediction of cardiovascular complications in patients with diabetes (7,8). For example, one analysis of the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial indicated in the intensive glycemic control group an increase in variability of HbA1c and blood fasting glucose in the first 2 years was associated with an increased risk of subsequent vascular events and mortality for patients with T2D (9). These results were even more impressive given the relatively good overall glycemic control in this cohort. However, in ADVANCE, only three measurements of HbA1c were taken during the first 2 years of follow-up. Moreover, as no glucose levels were recorded in the standard group over this time frame, no comparison with the control group could be conducted. Although not all studies have supported the additional predictive value of visit-to-visit variability in glucose control in predicting diabetes complications (10,11), a recent review and meta-analysis by Gorst et al. (12) reported positive contributions of HbA1c variability to adverse outcomes including renal disease, diabetic retinopathy, diabetic neuropathy, cardiovascular macrovascular events, and death for patients with T2D. Similar results were shown for patients with type 1 diabetes (4,13). Therefore, glycemic variability is emerging as a possible additional measure of glycemic control that predicts vascular complications (14–16). However, few studies have examined the role of glycemic variability in the context of a glucose-lowering trial, and no studies have determined whether these effects differ by treatment group.
Using data from the Veterans Affairs Diabetes Trial (VADT) study, we performed a comprehensive examination of the relationship between visit-to-visit fasting glucose and HbA1c variability and incident cardiovascular disease (CVD). Using the VADT cohort allowed us to investigate whether the relationship between fluctuation in glucose control and incident CVD differed according to intensity of glycemic treatment. Indeed, although intensive treatment provided mild protection from CVD events in the VADT cohort, the harmful effects of glucose variability were much greater in this group than in the patients with T2D receiving standard care.
Research Design and Methods
Study Design and Analysis Cohort
The VADT was a randomized trial that enrolled 1,791 military veterans (mean age 60.4 years) who had a suboptimal response to therapy for T2D (HbA1c >7.5%) to receive either intensive or standard glucose control. The design and principal results have been described previously (17,18). Following an established algorithm, the two groups were treated with similar medications (but different doses) with a goal of achieving an absolute difference of 1.5% HbA1c difference between treatment groups (17). HbA1c and fasting glucose were measured every 3 months up to a maximum of 84 months. At 3 months into the trial, median HbA1c levels had decreased in both groups and had stabilized by 6 months, with a level of 8.4% in the standard therapy group and 6.9% in the intensive therapy group (Supplementary Fig. 1).
For this analysis, we excluded observations from the first 6 months of the trial to eliminate the effect of rapid reduction (per protocol) in fasting glucose and HbA1c on glycemic variation measures in this early period of the trial. We additionally excluded individuals with two or fewer measurements of fasting glucose or HbA1c. This left 1,659 individuals who had at least two measurements of fasting glucose or HbA1c for analysis after the first 6 months of the study.
Primary and Secondary Outcomes
The primary outcome for the VADT, and this analysis, was the time to the first occurrence of any one of a composite of CVD events, adjudicated by an end point committee that was blinded to the assignments of study groups. The composite CVD events were documented myocardial infarction, stroke, death from cardiovascular causes, new or worsening congestive heart failure, surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease, inoperable coronary artery disease, and amputation for ischemic gangrene (17). A sensitivity analysis was conducted using major adverse cardiac events (MACEs; i.e., cardiovascular death, myocardial infarction, and stroke).
Glycemic Variables
We compared risks of cumulative mean, maximum, and most recent fasting glucose or HbA1c values prior to the CVD event with measures of variability for both fasting glucose and HbA1c. Many different definitions of glycemic variation have been adopted in prior studies. Most commonly used are SD, coefficient of variation (CV), variability independent of mean (VIM), and average real variability (ARV) (7–9,14,15). Definitions of variability measures are included in Supplementary Table 1. We selected CV and ARV for this analysis, as VIM needs extra estimation, which can generate bias, and SD does not consider mean levels of glycemic control that are particularly relevant in a glucose-lowering trial. CV and ARV for both fasting glucose and HbA1c were defined over quarterly visits during the trial and capture long-term glycemic variation. Our measures are distinct from short-term glycemic variability, usually captured by repeated blood or capillary glucose measures using glucometers or implanted sensors that focus on glycemic fluctuation within a day or between multiple days in an individual. Glycemic risk variables were calculated as continuous and time-dependent covariates in Cox proportional hazard models (16,19). We also categorized variability variables into quintiles and compared the risks of CVD between high versus low variability groups.
Statistical Analysis
Data are expressed as means (SD) for continuous variables or as numbers and percentages for categorical variables. Differences between patients who did and did not develop an event were analyzed using the Wilcoxon test for continuous variables and the χ2 test or Fisher exact test, as appropriate, for categorical variables shown in Table 1.
Table 1.
Baseline characteristics by incident CVD event status
| Primary outcome |
P value | MACE |
P value | |||
|---|---|---|---|---|---|---|
| No (n = 1,181) | Yes (n = 478) | No (n = 1,430) | Yes (n = 229) | |||
| Age (years) | 59.4 (8.4) | 62.7 (8.6) | 0.0001 | 60.0 (8.5) | 63.0 (8.5) | <0.0001 |
| Treatment, n (%) | ||||||
| Standard | 581 (49.2) | 252 (52.7) | 0.21 | 714 (49.9) | 119 (52.0) | 0.57 |
| Intensive | 600 (50.8) | 226 (47.3) | 716 (50.1) | 110 (48.0) | ||
| Sex, n (%) | ||||||
| Male | 1,141 (96.6) | 469 (98.1) | 0.11 | 1,385 (96.9) | 225 (98.3) | 0.30 |
| Female | 40 (3.4) | 9 (1.9) | 45 (3.1) | 4 (1.7) | ||
| NHW, n (%) | ||||||
| No | 491 (41.6) | 136 (28.5) | 0.0001 | 556 (38.9) | 71 (31.0) | 0.023 |
| Yes | 690 (58.4) | 342 (71.5) | 874 (61.1) | 158 (69.0) | ||
| Smoking status, n (%) | ||||||
| No | 986 (83.5) | 402 (84.1) | 0.83 | 1,196 (83.6) | 190 (83.0) | 0.92 |
| Yes | 195 (16.5) | 76 (15.9) | 233 (16.3) | 38 (16.6) | ||
| BMI (kg/m2) | 31.2 (4.4) | 31.4 (4.6) | 0.41 | 31.3 (4.4) | 31.3 (4.6) | 0.87 |
| Diabetes duration (years) | 10.9 (7.3) | 13.1 (7.8) | 0.0001 | 11.4 (7.5) | 12.5 (7.6) | 0.035 |
| Prior event, n (%) | ||||||
| No | 822 (69.6) | 162 (33.9) | 0.0001 | 889 (62.2) | 95 (41.5) | <0.0001 |
| Yes | 359 (30.4) | 316 (66.1) | 541 (37.8) | 134 (58.5) | ||
| History of hypertension, n (%) | ||||||
| No | 353 (29.9) | 101 (21.1) | 0.0003 | 401 (28.0) | 53 (23.1) | 0.15 |
| Yes | 826 (69.9) | 376 (78.7) | 1,027 (71.8) | 175 (76.4) | ||
| History of TZD, n (%) | ||||||
| No | 954 (80.8) | 386 (80.8) | 1.0 | 1,155 (80.8) | 185 (80.8) | 1.0 |
| Yes | 227 (19.2) | 92 (19.2) | 275 (19.2) | 44 (19.2) | ||
| Fasting glucose (mg/dL) | 201.9 (66.1) | 207.7 (71.9) | 0.12 | 202.7 (66.6) | 209.0 (75.1) | 0.19 |
| HbA1c level (%) | 9.4 (1.5) | 9.4 (1.5) | 0.64 | 9.4 (1.5) | 9.4 (1.5) | 0.68 |
| DBP (mmHg) | 76.2 (9.8) | 75.5 (11.1) | 0.34 | 76.0 (10.0) | 75.6 (11.3) | 0.50 |
| SBP (mmHg) | 130.7 (15.5) | 133.5 (18.9) | 0.052 | 131.2 (16.1) | 133.8 (19.7) | 0.16 |
| HDL cholesterol (mg/dL) | 36.6 (10.4) | 34.1 (9.3) | 0.0001 | 36.3 (10.2) | 33.4 (9.7) | <0.0001 |
| LDL cholesterol (mg/dL) | 110.0 (63.0) | 111.5 (69.2) | 0.41 | 110.0 (58.7) | 118.5 (95.3) | 0.50 |
| Total cholesterol (mg/dL) | 183.0 (47.7) | 184.0 (47.9) | 0.82 | 182.0 (46.2) | 191.4 (55.8) | 0.037 |
| Triglycerides (mg/dL) | 210.1 (304.0) | 225.1 (224.2) | 0.006 | 208.0 (283.5) | 254.4 (279.7) | 0.0001 |
Data are means (SD) unless otherwise noted. DBP, diastolic blood pressure; NHW, non-Hispanic white; SBP, systolic blood pressure; TZD, thiazolidinediones.
Multivariable analyses were performed using Cox proportional hazard models to evaluate the time-dependent effects of fasting glucose and HbA1c. To ease interpretation of statistical models, hazard ratios (HRs) for all variables of glycemic control were standardized to a change of one SD. SD of time-dependent measures of variation were calculated at every time point over all samples and then averaged over time points. Analyses were performed after adjusting for 1) age only and 2) age and covariates, reflecting significant baseline differences in characteristics between those who did and did not develop CVD or MACEs during the study (Table 1). When estimating risk of variability measures, cumulative mean of fasting glucose and HbA1c were also separately included in the models to clarify whether variability measures provided information above standard glucose measures. Differential risks of glycemic measures between treatment groups were examined by adding an interaction term between treatment group and glycemic measures in the Cox proportional hazard models. Stratified analysis within different treatment groups was also performed.
To further assess the relationship between fasting glucose variation and CVD events, we examined HRs for CVD events across quintiles of log(CV)-glucose and ARV-glucose. Quintiles of log(CV)-glucose and ARV-glucose were each generated in the whole group, and then stratified analyses were conducted separately by treatment arms across these quintiles. We used the distribution of variability measures from all time points to define the ranges of quintile categories. The quintile category for each individual’s specific variability measure was time dependent and calculated using the measures from earlier time points. Trend tests in the Cox proportional hazard model were conducted by assuming a linear trend between the increase of quintiles and the increase of CVD risk (i.e., treating them as time-dependent continuous variables). In addition, the risk of the four upper quintiles was compared with the first quintile assuming no trend (treating them as time-dependent categorical variables).
A severe hypoglycemia episode was defined as “incomplete loss of consciousness that requires assistance” or “complete loss of consciousness” occurring since the last visit. The cumulative severe hypoglycemia episodes were included in multivariable analysis using Cox proportional hazard models as time-dependent covariates. The association between glucose variation measures and severe hypoglycemic episodes over time was evaluated using a marginal logistic regression model. Effects sizes and SE were estimated by generalized estimation equations. Time-dependent variability measures were calculated and included in the model in a similar fashion as in the Cox model (i.e., they were only calculated using HbA1c and fasting glucose measures up to the severe hypoglycemia episode). We then evaluated whether severe hypoglycemia affects the risk of variability measures predicting CVD.
All statistical analyses were performed using R version 3.4.0 (https://www.r-project.org). A two-sided P < 0.05 was considered statistically significant.
Results
A total of 1,659 individuals who had at least two measurements of fasting glucose or HbA1c after the first 6 months were included in the analysis. Primary composite CVD events occurred in 478 individuals. The mean and median follow-up times for the cohort were 64.6 and 67.3 months, respectively. Three-quarters of the cohort had >60 months of follow-up. There were on average 18.5 visit fasting glucose and HbA1c measures for individuals within the cohort and a maximum of 26 measures. Baseline characteristics are shown for those having or not having a primary event during the study (Table 1). As fasting glucose and HbA1c observations were missing <2% of the time, analyses were performed without imputation. Baseline age, ethnicity, diabetes duration, history of hypertension, prior CVD event, and HDL and triglycerides differed significantly between those with and without CVD events. Mean fasting glucose and HbA1c values over 60 visits during the study are shown in Supplementary Fig. 1 and reveal substantial treatment group separation developing over the initial 6 months of the trial that persists during the remaining duration of the trial.
Risk of Glycemic Measures to CVD
We evaluated risk of glycemic measures, including mean and maximum glucose and glucose prior to the CVD event, and measures of glucose variation [log(CV) and ARV] as predictors of CVD. In the left section of Table 2, we show estimated HRs for the primary CVD outcome for the multiple glucose measures adjusted for age in the whole cohort. Cumulative mean fasting glucose, cumulative maximum fasting glucose, log(CV) and ARV of fasting glucose, cumulative mean HbA1c, cumulative maximum HbA1c, and HbA1c prior to CVD were significant risk factors (P < 0.05) of CVD events. Interestingly, whereas cumulative mean or maximum HbA1c or HbA1c measures prior to a CVD event were significant predictors of CVD, variability measures of HbA1c were not.
Table 2.
HR (95% CI) and P values estimated by Cox proportional hazards model for primary cardiovascular outcome
| Variables | Age adjusted |
Multivariate adjustment |
||
|---|---|---|---|---|
| Whole group (n = 1,659) | Whole group (n = 1,608)§ | Standard (n = 807) | Intensive (n = 801) | |
| Blood glucose* | ||||
| Cum-mean glucose | 1.088 (1.003, 1.179), 0.041 | 1.059 (0.974, 1.151), 0.180 | 1.049 (0.922, 1.194), 0.467 | 1.059 (0.905, 1.239), 0.473 |
| Cum-max glucose | 1.133 (1.036, 1.239), 0.006 | 1.077 (0.982, 1.181), 0.117 | 1.087 (0.937, 1.260), 0.272 | 1.053 (0.914, 1.213), 0.476 |
| Prior glucose | 1.024 (0.931, 1.127), 0.623 | |||
| Log(CV)-glucose | 1.162 (1.054, 1.281), 0.003 | 1.111 (1.005, 1.228), 0.041 | 1.030 (0.899, 1.180),0.674 | 1.219 (1.042, 1.425), 0.014 |
| ARV-glucose | 1.168 (1.069, 1.275), 0.0006 | 1.138 (1.038, 1.247), 0.006 | 1.093 (0.959, 1.246),0.181 | 1.197 (1.044, 1.372), 0.010 |
| HbA1c* | ||||
| Cum-mean HbA1c | 1.117 (1.032, 1.210), 0.007 | 1.093 (1.005, 1.189), 0.038 | 1.077 (0.944, 1.229), 0.270 | 1.154 (0.975, 1.367), 0.096 |
| Cum-max HbA1c | 1.109 (1.016, 1.209), 0.020 | 1.085 (0.989, 1.191), 0.084 | 1.091 (0.940, 1.265), 0.253 | 1.080 (0.913, 1.278), 0.370 |
| Prior HbA1c | 1.132 (1.038, 1.233), 0.005 | 1.114 (1.018, 1.219), 0.019 | 1.098 (0.962, 1.252), 0.165 | 1.156 (0.980, 1.363), 0.085 |
| Log(CV)-HbA1c | 1.052 (0.956, 1.158), 0.303 | |||
| ARV-HbA1c | 1.056 (0.964, 1.157), 0.241 | |||
Glucose control variables that were significant in age-adjusted models were further adjusted for ethnicity (non-Hispanic white or not), diabetes duration, prior CVD event, history of hypertension, baseline systolic blood pressure, baseline HDL cholesterol, and baseline triglycerides; variability measures (CV and ARV) were additionally adjusted for the cumulative mean of glucose or HbA1c, respectively. Top portion shows results for fasting glucose measures and the bottom portion for blood HbA1c measures. P values in boldface show significant (P < 0.05) risk for the primary outcome. Cum, cumulative; max, maximum.
*HRs for all glycemic exposures were standardized according to one SD of the exposures. For cum-mean glucose, it is per 35.05 mg/dL, for cum-max glucose it is per 0.16 mg/dL. For prior glucose, it is 59.63 mg/dL. For log(CV)-glucose, one SD is 0.53, and for ARV-glucose, one SD is 0.12. For cum-mean HbA1c, it is per 1%. For cum-max HbA1c, it is per 1.54%, and for prior HbA1c, it is per 1.31%. For log(CV)-HbA1c, one SD is 0.50, and for ARV-HbA1c, one SD is 0.03. Variability measures are unit free.
§In the multivariate adjusted models, missing covariates lead to reduced sample sizes.
Measures of fasting glucose control or variability measures that were significant in the age-adjusted models were evaluated further by adjusting for multiple additional risk factors (Table 2, right section). To clarify whether variability measures provided information above standard glucose measures, all estimates of fasting glucose variability were further adjusted in these models for cumulative mean fasting glucose during the study. We note that because HbA1c represents both fasting and postprandial glucose control, we also in sensitivity analyses reestimated the risk of variability measures (fasting glucose and HbA1c) by adjusting for the cumulative mean of HbA1c. Differences of results were negligible. As interactions between fasting glucose variability measures and treatment groups were significant (P < 0.05) or borderline significant (P = 0.06), stratified analyses were conducted. Both log(CV)-glucose and ARV-glucose remained significant predictors of CVD events in the whole group, and this was largely explained by their strong and significant effects in the intensive treatment group (Table 2). Neither maximum fasting glucose nor measures of fasting glucose variability measures were significant with CVD outcomes in the standard treatment group. In contrast, cumulative mean HbA1c (P = 0.03) and prior HbA1c (P = 0.019) were significant predictors for the whole group but were not in either the standard or intensive group separately. As the VADT cohort was enriched with male veterans, we also performed an analysis excluding the small number of women. This did not change the results (Supplementary Table 4).
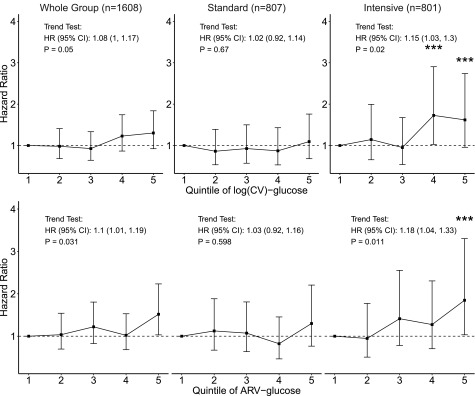
To further assess the relationship between fasting glucose variation and CVD events, we examined HRs for CVD events across quintiles of log(CV)-glucose and ARV-glucose. The percentage of individuals from each treatment group within each quintile was similar when averaged over the study duration, which indicated that variation within quintiles is comparable between groups. In the whole cohort, there was a significant but weak trend for increasing risk of CVD with higher quintiles of glucose variability [log(CV) and ARV-glucose] (Fig. 1). These trends were stronger in the intensive group (both P < 0.02) and not apparent in the standard group. In the intensive group, the highest quintile of each of these measures of glucose variation was associated with ∼80% higher CVD risk than the lowest quintile.
Figure 1.
HR estimates for quintiles of log(CV)-glucose and ARV-glucose for the primary CVD outcome adjusted for ethnicity (non-Hispanic white or not), diabetes duration, prior CVD event, history of hypertension, baseline systolic blood pressure, baseline HDL cholesterol, baseline triglycerides as well as the cumulative mean of glucose. Vertical bars shown are the 95% CIs associated with HR estimates. ***Indicates estimated HR in the related variability quintile is significantly higher than the HR of lowest variability quintile (quintile 1). Trend test results are presented as the text annotations in the figure.
In additional sensitivity analyses, we examined the effects of fasting glucose variability using the more easily adjudicated and traditional hard CVD outcome of MACEs. Although the absolute number of total events was reduced (n = 229 vs. 478), in age-adjusted models, all fasting glucose variables evaluated were significantly associated with MACEs. After multivariable adjustment, including accounting for cumulative mean fasting glucose levels, log(CV)-glucose and ARV-glucose again showed increased CVD risk that was most apparent in the intensive group (Supplementary Table 2). Interestingly, cumulative mean fasting glucose also showed increased MACE risk in the intensively treated group but not in the standard treatment group. The reverse pattern is shown for fasting glucose just prior to the MACE, in which there was a significant risk in the standard treatment group only. Maximum measure of glucose was shown to be significant in both treatment groups. For HbA1c measures, only the HbA1c value prior to the event was associated with risk for MACEs.
Glucose Variability and Severe Hypoglycemia
As previously reported (20–24), fasting glucose variability is a significant and relatively potent risk factor for severe hypoglycemia in the whole group as well as in each treatment arm (Supplementary Table 3). In fact, fasting glucose variability appeared a stronger predictor of severe hypoglycemia than standard measures of glucose control. All measures of HbA1c were significant predictors for severe hypoglycemia, although with less difference in strength between standard measures and measures of variability.
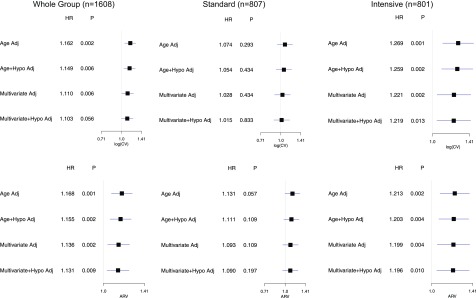
Therefore, to assess whether severe hypoglycemia may help account for the effects of fasting glucose variability on CVD, we evaluated the effect of additionally adjusting for severe hypoglycemia in multivariable adjusted models that examined the effect of fasting glucose variability on primary CVD outcomes (Fig. 2). Measures of fasting glucose variability remained important predictors of CVD events even after accounting for severe hypoglycemia.
Figure 2.
HRs of log(CV) and ARV and their 95% CI for the primary CVD outcome with and without adjustment for severe hypoglycemia (Hypo) events. Adj, adjusted.
Conclusions
Glycemic variability is emerging as a measure of glycemic control that may be an important predictor of complications in patients with diabetes. Many studies report adverse effects of glycemic variation, including on renal and cardiovascular complications and all-cause mortality (9,12,14,25,26). Our results provide further evidence that increased glucose variability leads to increased risk of CVD events. Importantly, this effect of visit-to-visit variability in fasting glucose persisted even after adjustment for standard baseline risk factors as well as average glucose (and cumulative HbA1c) during the same time frame. Moreover, this effect did not appear simply mediated by a relationship between fasting glucose variation and severe hypoglycemia. An additional novel finding was that the association between variability in fasting glucose and risk of CVD was most evident in the intensively treated group. As the extent (estimated by mean values) of log(CV)-glucose and ARV-glucose variation appeared generally similar between standard and intensive groups (at least after the first 6 months), this raises the possibility those receiving more intensive glucose lowering may be more sensitive to the harmful effects of fasting glucose variation. These intriguing latter results need further validation in other large studies of glucose lowering. We also note that in our analyses HbA1c variability measures did not appear to be a significant independent risk factor for CVD after accounting for multiple baseline covariates as well as cumulative mean. As HbA1c reflects long-term average glucose, whereas fasting glucose reflects real-time (e.g., day-to-day) variation, the latter may capture more fluctuation than HbA1c measures (Supplementary Fig. 1).
Short-term glycemic variability, usually captured by repeated blood or capillary glucose measures using glucometers or implanted sensors, refers to fluctuations within a day or between multiple days in an individual. Long-term glycemic variability refers to fluctuations over several weeks or months and is most commonly assessed by changes in HbA1c or repeated visit measures of glucose. However, there is currently no consensus on standard definitions of either short-term or long-term measures of glucose variation. In this paper, we defined long-term glycemic variation by estimating CV and ARV for both fasting glucose and HbA1c over serial visits during the trial. We also tested several other estimates of glycemic variation (SD, VIM, or glycemic residuals after accounting for the effects of trends over time), but found they either correlated extremely highly with our selected measures and provided similar results or were not appropriate to use in the time-dependent covariate models. In our analyses, we used measures of glucose control or variation as continuous and time-dependent covariates in Cox proportional hazard models that allowed us to consider their effects right up to the time of an outcome (16). In contrast, some long-term observational studies have used an initial period of time in which glucose variation was captured, “a landmark period,” and this was related to subsequent events during a set observation period (9). Although this is a reasonable alternative approach, it assumes that variation during the observational period remains similar to that during the landmark period or will not substantially influence the outcomes. Using time-dependent estimates of glucose control will presumably quantify the full extent of glucose variation more accurately.
There are several mechanisms that may explain the association between visit-to-visit glycemic variability and cardiovascular adverse events. It has been shown that glucose variability leads to activation of vascular oxidative stress, which may be a major contributor to development of atherosclerosis (23,24). Other potential mechanisms include activation of monocytes and macrophages and enhanced production of inflammatory cytokines from these and other vascular cells (27,28). One could also speculate that as glucose variability is associated with more frequent hypoglycemic events, this would lead to increased cardiovascular adverse events (29). However, this did not appear to account for the association of fasting glucose variability with CVD in our study.
Overall, these results may have several important clinical implications. First, these findings imply that the manner of glucose lowering may be as important as the degree of glucose lowering. Clinicians may need to consider how their therapy recommendations and medications used to achieve these changes affect day-to-day glucose variation (21,24,30,31). Second, the results indicate that the importance of reducing glucose variation is most relevant in patients with diabetes undergoing more aggressive glucose lowering. As these individuals are also at increased risk for episodes of severe hypoglycemia, already recognized as a major determinant of CVD events (29,32–34), the task of successfully lowering glucose toward more normal ranges may be more complicated than previously appreciated. Safely lowering glucose may require both avoiding marked glucose fluctuation as well as episodes of hypoglycemia. Although the number of individuals with both events occurring was too low in the VADT to allow careful analysis of the consequences of this co-occurrence, it is possible that these individuals might be at unique risk for future CVD events.
Our study has several limitations. The typical participant in the VADT was older and with known CVD or at high risk for subsequent CVD. Thus, we do not know if these findings may apply to younger and healthier patients with T2D. Glycemic measures were collected every 3 months over a 7-year period; thus, we could only evaluate cumulative effects of long-term visit-to-visit glucose variability on CVD. We were not able to estimate daily glucose variation, as that requires more extensive collection of daily glucose measures (with frequent fingersticks or continuous glucose monitoring) than was conducted within the VADT. Therefore, our analyses cannot adjust for daily mean glucose control or daily glucose fluctuation. Adjustment for hypoglycemic events was limited to those episodes that were severe, as these were the most reliably identified within the VADT. It remains possible that the association of glucose variation with CVD, particularly within the intensively treated group, could be accounted for through its relationship with other more frequent, less severe hypoglycemia episodes. However, this seems less likely as multiple studies, including the VADT, have reported that the consequences of hypoglycemia on mortality, and possibly on CVD events, appear most pronounced in participants receiving standard glucose-lowering therapy (29,35). We also cannot exclude the possibility that visit-to-visit glucose variation reflects noncompliance or other less healthy behaviors.
There are important strengths of this report. The VADT was a large, carefully conducted study that was specifically designed to address the impact of glucose lowering on CVD. Thus, the CVD events were substantial in number and carefully adjudicated in a blinded fashion, and the collection of information was performed in a careful and standardized fashion across sites. There were many visits over the ∼7 years of follow-up, providing many glucose measures for the estimates of visit-to-visit variation. As this was a randomized study of treatment intensity, it was possible to compare the association between glucose variation and CVD events between groups with different levels of treatment intensity independent of bias due to self-selection to treatment intensity.
In conclusion, our study finds associations between higher visit-to-visit fasting glucose variability and increased risks of CVD during the VADT. These associations persist even when accounting for more standard measures of glucose control and the increased risk for severe hypoglycemia that accompanies greater glucose variation. These results suggest that efforts to improve glucose control in patients may need to consider how these strategies influence long-term glucose fluctuation.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the contributions of the Hines VA Cooperative Studies Program Coordinating Center.
Funding. This work was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development. Additional support was received from the National Institutes of Health (grants R01-067690 and 5R01-094775 to P.R.) and the American Diabetes Association (to P.R.). J.J.Z. is supported by National Institutes of Health grant K01-DK-106116.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.J.Z. and P.R. conceived and designed the study, analyzed and interpreted the data, and wrote the manuscript. D.C.S. advised on statistical analysis and reviewed and edited the manuscript. G.B. advised on statistical analysis methods and acquired the data. P.R. was an executive committee member for the VADT. J.J.Z., D.C.S., G.B., and P.R. reviewed and edited the manuscript, approved the final version, and are accountable for all aspects of the work. J.J.Z. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0548/-/DC1.
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 5.Dormandy JA, Charbonnel B, Eckland DJ, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006;295:1707–1708 [DOI] [PubMed] [Google Scholar]
- 8.Bolli GB. Glucose variability and complications. Diabetes Care 2006;29:1707–1709 [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 2014;37:2359–2365 [DOI] [PubMed] [Google Scholar]
- 10.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006;29:1486–1490 [DOI] [PubMed] [Google Scholar]
- 12.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 2015;38:2354–2369 [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 2008;31:2198–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skriver MV, Sandbæk A, Kristensen JK, Støvring H. Relationship of HbA1c variability, absolute changes in HbA1c, and all-cause mortality in type 2 diabetes: a Danish population-based prospective observational study. BMJ Open Diabetes Res Care 2015;3:e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with type 2 diabetes. Diabet Med 2016;33:1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachin JM, Bebu I, Bergenstal RM, et al.; DCCT/EDIC Research Group . Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017;40:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 18.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 19.Lin CC, Chen CC, Chen FN, et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med 2013;126:1017.e1–1017.e10 [DOI] [PubMed] [Google Scholar]
- 20.Rutter MK. Devoting attention to glucose variability and hypoglycaemia in type 2 diabetes. Diabetologia 2018;61:43–47 [DOI] [PubMed] [Google Scholar]
- 21.Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia 2018;61:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CL, Sheu WH, Lee IT, et al. Trajectories of fasting plasma glucose variability and mortality in type 2 diabetes. Diabetes Metab 2018;44:121–128 [DOI] [PubMed] [Google Scholar]
- 23.Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: evidence from the PREDICTIVE study. Diabetes Res Clin Pract 2009;86:e15–e18 [DOI] [PubMed] [Google Scholar]
- 24.Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther 2011;13:813–818 [DOI] [PubMed] [Google Scholar]
- 25.Penno G, Solini A, Zoppini G, et al.; Renal Insufficiency and Cardiovascular Events (RIACE) Study Group . Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study. Cardiovasc Diabetol 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luk AO, Ma RC, Lau ES, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev 2013;29:384–390 [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Ihnat MA. ‘Glycaemic variability’: a new therapeutic challenge in diabetes and the critical care setting. Diabet Med 2010;27:862–867 [DOI] [PubMed] [Google Scholar]
- 28.Keating ST, van Diepen JA, Riksen NP, El-Osta A. Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia 2018;61:6–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT) . A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016;39:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller ME, Bonds DE, Gerstein HC, et al.; ACCORD Investigators . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR. Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care 1999;22:394–398 [DOI] [PubMed] [Google Scholar]
- 32.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 34.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pieber TR, Marso SP, McGuire DK, et al.; DEVOTE Study Group . DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia 2018;61:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.