Abstract
OBJECTIVE
Among patients with type 2 diabetes uncontrolled with metformin, exenatide once weekly (QW) plus dapagliflozin combination produced greater reductions in glycemia, weight, and systolic blood pressure (SBP) at 28 weeks than exenatide QW or dapagliflozin alone (DURATION-8). Here, we investigated the safety and maintenance of efficacy at 52 weeks, after a 24-week extension.
RESEARCH DESIGN AND METHODS
This phase 3, multicenter, double-blind study randomized adults with type 2 diabetes (with glycated hemoglobin [HbA1c] 8.0–12.0% [64–108 mmol/mol] and on metformin ≥1,500 mg/day) to exenatide QW (2-mg subcutaneous injection) plus once-daily dapagliflozin (10-mg oral tablet), exenatide QW plus oral placebo, or dapagliflozin plus injected placebo. Extension-period P values were nominal.
RESULTS
Of 1,375 patients screened, 695 were randomized (mean baseline HbA1c 9.3% [78 mmol/mol]); 81.2% completed the study, and 75.3% completed treatment. At 52 weeks, HbA1c reductions were greater with exenatide QW plus dapagliflozin (least squares mean change −1.75% [−19.1 mmol/mol]) versus exenatide QW (−1.38% [−15.1 mmol/mol]; P = 0.006) or dapagliflozin (−1.23% [−13.4 mmol/mol]; P < 0.001); mean HbA1c values were 6.9% (52 mmol/mol), 7.2% (55 mmol/mol), and 7.4% (57 mmol/mol), respectively. Weight and SBP reductions were greater with exenatide QW plus dapagliflozin (−3.31 kg and −4.5 mmHg) versus exenatide QW (−1.51 kg and −0.7 mmHg; both P < 0.001) but similar to those with dapagliflozin (−2.28 kg and −2.7 mmHg; P = 0.057 and P = 0.100, respectively). The exenatide QW plus dapagliflozin regimen was well tolerated with no unexpected safety findings; more patients treated with exenatide QW experienced gastrointestinal and injection site–related adverse events. No major hypoglycemia occurred.
CONCLUSIONS
Among patients with type 2 diabetes uncontrolled with metformin, exenatide QW plus dapagliflozin provided sustained improvements in glycemia, weight, and SBP over 52 weeks, with no unexpected safety findings.
Introduction
The choice of therapeutic combinations that maximize glycemic control and minimize adverse events (AEs), such as weight gain or hypoglycemia, is critical and important to both health care providers and patients when selecting therapy to achieve glycemic targets in patients with type 2 diabetes (1). In addition, it is essential to choose therapies shown to have durability of effect and safety over time. Professional guidelines (2–4) further support this aim by recommending the combination of glucose-lowering therapies with different modes of action to achieve glycemic targets.
Two major classes of glucose-lowering drugs, glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium–glucose cotransporter 2 (SGLT2) inhibitors, have been launched in the last decade for the treatment of type 2 diabetes. GLP-1RAs increase insulin secretion, decrease glucagon secretion, slow gastric emptying, and increase satiety (5), whereas SGLT2 inhibitors increase caloric loss via urinary glucose excretion (6). Despite their different effects on glucose metabolism, these two classes of glucose-lowering drugs both reduce blood glucose levels with a minimal risk of hypoglycemia, produce sustained reductions in weight, and reduce systolic blood pressure (SBP), which may contribute to reduced cardiovascular risk (7,8). In addition, SGLT2 inhibition appears to improve incretin sensitivity of pancreatic β-cells, providing a further rationale for the glycemic efficacy of the GLP-1RA/SGLT2 inhibitor combination (9).
Despite the potential benefits of combining a GLP-1RA and an SGLT2 inhibitor, the combination was not formally investigated in patients with type 2 diabetes until the DURATION-8 study. DURATION-8 was a multicenter, double-blind, randomized, active-controlled phase 3 trial that evaluated the effects of adding a combination of once-weekly exenatide (an extended-release formulation of the GLP-1RA exenatide that is encapsulated in biodegradable microspheres and administered by subcutaneous injection) and dapagliflozin (an SGLT2 inhibitor administered orally daily) in 695 patients with type 2 diabetes and poor glycemic control who are receiving metformin monotherapy (10). The results of the initial 28-week treatment period, which have been published previously, demonstrated that concomitant use of exenatide once weekly (QW) and dapagliflozin resulted in significantly superior clinical improvements in glycemic control, weight, and SBP compared with exenatide QW or dapagliflozin alone, with no unexpected safety findings (10). Here, we present results from the first 24-week extension of DURATION-8, which investigated the durability of the response for the efficacy and safety of this combination over 52 weeks (a further 52-week extension, period 2, is ongoing).
Research Design and Methods
Study Design and Participants
The design of the DURATION-8 study (clinical trial reg. no. NCT02229396, ClinicalTrials.gov) has been previously published (10). Briefly, DURATION-8 enrolled adults (≥18 years of age) with type 2 diabetes and inadequate glycemic control (glycated hemoglobin [HbA1c] 8.0–12.0% [64–108 mmol/mol]) despite stable metformin monotherapy (≥1,500 mg/day). Patients were randomized to receive exenatide 2 mg QW by subcutaneous injection plus dapagliflozin 10-mg oral tablets daily, exenatide QW with dapagliflozin-matched oral placebo daily, or dapagliflozin daily with exenatide QW–matched placebo injections. After week 28, patients continued into a 24-week double-blind extension period where they continued to receive their randomized treatment (extension period 1 [Supplementary Fig. 1]). From weeks 8 to 36, patients with inadequate glycemic control based on progressively stricter fasting plasma glucose (FPG) criteria (>15.0 mmol/L from weeks 8 to 12, >13.2 mmol/L from weeks 12 to 20, and >11.1 mmol/L from weeks 20 to 36) remained in the study and received open-label rescue therapy with basal insulin (Supplementary Table 1). From weeks 36 to 52, patients received rescue therapy if HbA1c level was >8.0% (>64 mmol/mol). The study protocol was approved at each study site by the appropriate institutional review board, and the study was conducted in accordance with the principles described in the Declaration of Helsinki and a common clinical protocol. Patients provided written informed consent before any study procedure.
Outcomes
The primary end point of the DURATION-8 study was the change in HbA1c from baseline to week 28. All end points at 52 weeks were considered exploratory and included the change from baseline in glycemic parameters (HbA1c, FPG, 2-h postprandial glucose [PPG] during a standardized liquid meal test (10), and change in six-point self-monitored blood glucose), the proportion of patients achieving glycemic targets (HbA1c <7.0% or ≤6.5% [<53 or ≤48 mmol/mol]), the change from baseline in selected cardiovascular risk factors (including weight, SBP, diastolic blood pressure [DBP], waist circumference, and fasting lipids [total cholesterol, LDL cholesterol, HDL cholesterol, non-HDL cholesterol, and triglycerides]), and the proportion of patients with weight loss of ≥5%.
Safety and tolerability over the 52-week study were also assessed. As previously described (10), safety was assessed using spontaneously reported AEs, laboratory tests, and vital signs. Hypoglycemic episodes were also recorded and were classified as major, minor, or other. Major hypoglycemia was defined as loss of consciousness, seizure, or coma resolving after glucagon or glucose administration, or any event requiring third-party assistance to resolve because of severe impairment in consciousness or behavior with a glucose concentration of <3.0 mmol/L. Minor hypoglycemia was defined as a nonmajor hypoglycemia event with symptoms consistent with hypoglycemia and a glucose concentration of <3.0 mmol/L before treatment of the episode. If a hypoglycemia event did not meet the criteria for a major or minor event, as described above, it was classified as “other” hypoglycemia.
Statistical Analysis
All efficacy variables were assessed in the intention-to-treat population, which was defined as all randomly assigned patients who received at least one dose of study drug with at least one postbaseline HbA1c assessment. All safety variables were analyzed in the safety analysis set, defined as all randomly assigned patients who received at least one dose of study drug.
Because all end points at 52 weeks were exploratory, only nominal P values were calculated. Changes in continuous variables were analyzed with a mixed-effects model for repeated-measures analyses (HbA1c, FPG, weight, SBP, DBP, waist circumference, and self-monitored blood glucose level) or an ANCOVA model (2-h PPG and fasting lipids). Categorical response variables (proportions achieving glycemic or weight targets) were analyzed using stratified Cochran-Mantel-Haenszel tests; missing 52-week data were imputed by the last observation carried forward (LOCF) method.
Data collected after the initiation of glycemic rescue therapy, or at the post-treatment follow-up visits after a premature treatment discontinuation, were excluded from the analyses of glycemic variables and weight. However, supportive analyses were also conducted including data after the initiation of rescue therapy for changes in HbA1c, FPG, and weight over time and are shown in Supplementary Fig. 2.
All analyses were conducted using SAS version 9.2 or higher (SAS Institute, Inc., Cary, NC).
Results
Patients
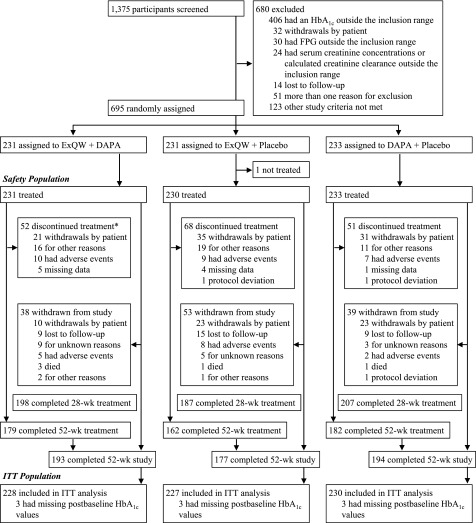
Of the 695 patients enrolled and randomized, 564 (81.2%) completed the 52-week study period and 523 (75.3%) completed 52 weeks of treatment (Fig. 1). The most common reasons for treatment discontinuation or study withdrawal were withdrawals by patients, AEs, or lost to follow-up. Over the 52 weeks of treatment, adherence to treatment was high (Supplementary Table 2).
Figure 1.
Patient disposition. *Indicates those participants discontinuing therapy with exanatide QW (ExQW); numbers discontinuing dapagliflozin (DAPA) within the ExQW plus DAPA arm were 23 (withdrawals by patient), 15 (for other reasons), 9 (had AEs), and 5 (missing data). ITT, intention-to-treat; wk, week.
Baseline characteristics and demographics of patients included in the DURATION-8 study have been reported previously (10). Briefly, patients (mean age 54.2 years; 47.9% male) had a mean baseline HbA1c of 9.3% (78 mmol/mol) and a mean diabetes duration of 7.4 years, and were mostly obese (mean BMI 32.7 kg/m2), with a low incidence of moderate renal dysfunction (3.6% with an estimated glomerular filtration rate [eGFR] of ≥30 to <60 mL/min/1.73 m2 and 96.4% with an eGFR of ≥60 mL/min/1.73 m2). Demographic and baseline characteristics were similar across treatment groups, with the exception of fewer women in the exenatide QW group and fewer Hispanic patients in the dapagliflozin group (Supplementary Table 3).
Efficacy
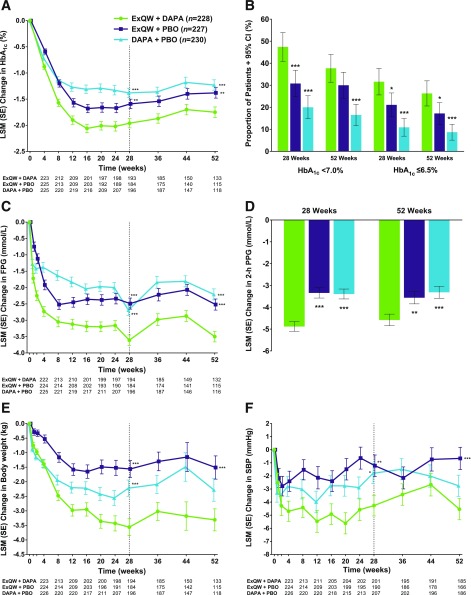
Treatment with exenatide QW plus dapagliflozin resulted in significantly greater mean reductions in HbA1c from baseline to week 28 (Fig. 2A), which were maintained through week 52 (least squares mean [LSM] change from baseline −1.75% [−19.1 mmol/mol]) compared with exenatide QW plus placebo (−1.38% [−15.1 mmol/mol]; P = 0.006) or dapagliflozin plus placebo (−1.23% [−13.4 mmol/mol]; P < 0.001) (Table 1). At week 52, mean HbA1c was 6.87% (52 mmol/mol) with exenatide QW plus dapagliflozin, 7.21% (55 mmol/mol) with exenatide QW plus placebo, and 7.36% (57 mmol/mol) with dapagliflozin plus placebo. The proportions of patients who achieved glycemic goals with exenatide QW plus dapagliflozin were generally similar at 28 and 52 weeks (Fig. 2B). At 52 weeks, more patients achieved an HbA1c level of <7.0% or ≤6.5% (<53 or ≤48 mmol/mol), respectively, with exenatide QW plus dapagliflozin (37.7% and 26.3%) than with exenatide QW plus placebo (30.0% and 17.2%) or dapagliflozin plus placebo (16.5% and 8.7%) (Fig. 2B and Table 1).
Figure 2.
A: LSM (SE) change in HbA1c over time. B: Proportion of patients achieving an HbA1c <7.0 or ≤6.5% (<53 or ≤48 mmol/mol) at weeks 28 and 52. C: LSM (SE) changes in FPG over time. D: LSM (SE) change in 2-h PPG at weeks 28 and 52. E: LSM (SE) changes in weight over time. F: LSM (SE) change in SBP over time. Error bars show SEs or 95% CIs. To convert FPG or 2-h PPG from mmol/L to mg/dL, divide by 0.0555. DAPA, dapagliflozin; ExQW, exenatide QW; PBO, placebo. *P < 0.05, **P < 0.01, ***P < 0.001 vs. ExQW + DAPA (P values at week 52 are nominal).
Table 1.
Primary and secondary efficacy end points at weeks 28 and 52 in the intention-to-treat patient population
| | Exenatide QW + dapagliflozin (n = 228) | Exenatide QW + placebo (n = 227) | Dapagliflozin + placebo (n = 230) | Between-group difference (95% CI) | |
|---|---|---|---|---|---|
| Exenatide QW + dapagliflozin vs. exenatide QW + placebo | Exenatide QW + dapagliflozin vs. dapagliflozin + placebo | ||||
| HbA1c, % | |||||
| Baseline | 9.34 (1.07) | 9.30 (1.06) | 9.30 (1.03) | ||
| Week 28 | 7.24 (1.28) | 7.58 (1.30) | 7.74 (1.13) | ||
| Change at week 28 | −1.98 (0.09) | −1.60 (0.10) | −1.39 (0.09) | −0.38 (−0.63 to −0.13); P = 0.003 | −0.59 (−0.84 to −0.34); P < 0.001 |
| Week 52 | 6.87 (0.78) | 7.21 (1.04) | 7.36 (0.86) | ||
| Change at week 52 | −1.75 (0.10) | −1.38 (0.10) | −1.23 (0.10) | −0.37 (−0.64 to −0.11); P = 0.006 | −0.52 (−0.79 to −0.26); P < 0.001 |
| HbA1c, mmol/mol | |||||
| Baseline | 79 (11.7) | 78 (11.6) | 78 (11.3) | ||
| Week 28 | 56 (14.0) | 59 (14.2) | 61 (12.4) | ||
| Change at week 28 | −21.6 (1.0) | −17.5 (1.1) | −15.2 (1.0) | −4.2 (−6.9 to −1.4); P = 0.003 | −6.4 (−9.2 to −3.7); P < 0.001 |
| Week 52 | 52 (8.5) | 55 (11.4) | 57 (9.4) | ||
| Change at week 52 | −19.1 (1.1) | −15.1 (1.1) | −13.4 (1.1) | −4.0 (−7.0 to −1.2); P = 0.006 | −5.7 (−8.6 to −2.8); P < 0.001 |
| HbA1c <7.0%* | |||||
| Week 28 | 108 (47.4; 40.9–53.9) | 70 (30.8; 24.8–36.8) | 46 (20.0; 14.8–25.2) | 16.5; P < 0.001 | 27.4; P < 0.001 |
| Week 52 | 86 (37.7; 31.4–44.0) | 68 (30.0; 24.0–35.9) | 38 (16.5; 11.7–21.3) | 7.8; P = 0.079 | 21.2; P < 0.001 |
| HbA1c ≤6.5%† | |||||
| Week 28 | 72 (31.6; 25.6–37.6) | 48 (21.1; 15.8–26.5) | 25 (10.9; 6.9–14.9) | 10.4; P = 0.011 | 20.7; P < 0.001 |
| Week 52 | 60 (26.3; 20.6–32.0) | 39 (17.2; 12.3–22.1) | 20 (8.7; 5.1–12.3) | 9.1; P = 0.018 | 17.6; P < 0.001 |
| FPG, mmol/L | |||||
| Baseline | 11.01 (3.01) | 10.66 (2.80) | 10.58 (2.63) | ||
| Week 28 | 7.17 (1.90) | 8.21 (2.75) | 7.89 (1.93) | ||
| Change at week 28 | −3.66 (0.16) | −2.54 (0.17) | −2.73 (0.16) | −1.12 (−1.55 to −0.68); P < 0.001 | −0.92 (−1.36 to −0.49); P < 0.001 |
| Week 52 | 6.66 (1.55) | 7.48 (1.93) | 7.78 (1.92) | ||
| Change at week 52 | −3.50 (0.16) | −2.52 (0.17) | −2.21 (0.17) | −0.98 (−1.42 to −0.54); P < 0.001 | −1.29 (−1.73 to −0.85); P < 0.001 |
| 2-h PPG, mmol/L | |||||
| Baseline | 15.04 (3.69) | 14.96 (3.70) | 14.58 (3.38) | ||
| Week 28 | 9.84 (2.65) | 11.38 (3.38) | 11.23 (3.02) | ||
| Change at week 28 | −4.88 (0.23) | −3.34 (0.24) | −3.39 (0.23) | −1.54 (−2.10 to −0.98); P < 0.001 | −1.49 (−2.04 to −0.93); P < 0.001 |
| Week 52 | 9.98 (2.84) | 11.03 (3.39) | 11.14 (3.02) | ||
| Change at week 52 | −4.58 (0.27) | −3.56 (0.28) | −3.31 (0.28) | −1.02 (−1.70 to −0.34); P = 0.004 | −1.26 (−1.94 to −0.59); P < 0.001 |
| Weight, kg | |||||
| Baseline | 91.79 (22.24) | 89.77 (20.22) | 91.06 (19.71) | ||
| Week 28 | 88.35 (20.57) | 87.62 (18.05) | 88.64 (18.89) | ||
| Change at week 28 | −3.55 (0.29) | −1.56 (0.29) | −2.22 (0.28) | −2.00 (−2.79 to −1.20); P < 0.001 | −1.33 (−2.12 to −0.55); P < 0.001 |
| Week 52 | 89.44 (20.99) | 89.69 (18.00) | 89.49 (16.85) | ||
| Change at week 52 | −3.31 (0.38) | −1.51 (0.40) | −2.28 (0.39) | −1.80 (−2.87 to −0.73); P < 0.001 | −1.02 (−2.08 to 0.03); P = 0.057 |
| Weight loss ≥5% | |||||
| Week 28 | 77 (33.8; 27.6–39.9) | 36 (15.9; 11.1–20.6) | 49 (21.3; 16.0–26.6) | 17.9; P < 0.001 | 12.5; P = 0.003 |
| Week 52 | 70 (30.7; 24.7–36.7) | 32 (14.1; 9.6–18.6) | 49 (21.3; 16.0–26.6) | 16.6; P < 0.001 | 9.4; P = 0.022 |
| SBP, mmHg | |||||
| Baseline | 130.1 (12.7) | 129.1 (13.1) | 130.0 (12.9) | ||
| Week 28 | 126.5 (13.0) | 129.2 (12.6) | 128.4 (13.7) | ||
| Change at week 28 | −4.3 (0.8) | −1.2 (0.8) | −1.8 (0.8) | −3.0 (−5.2 to −0.9); P = 0.005 | −2.4 (−4.5 to −0.4); P = 0.022 |
| Week 52 | 126.5 (12.6) | 130.2 (12.9) | 127.8 (14.0) | ||
| Change at week 52 | −4.5 (0.8) | −0.7 (0.9) | −2.7 (0.8) | −3.9 (−6.1 to −1.7); P < 0.001 | −1.8 (−3.9 to 0.3); P = 0.100 |
Data exclude measurements after the initiation of rescue therapy with the exception of SBP. For HbA1c (%), HbA1c (mmol/mol), FPG (mmol/L), 2-h PPG (mmol/L), weight (kg), and SBP (mmHg), baseline, week 28, and week 52 data are mean (SD), change at week 28 and week 52 data are LSM (SE), and difference data are LSM (95% CI). For HbA1c <7%, HbA1c ≤6.5%, and weight loss ≥5%, week 28 and week 52 data are n (%; binomial CIs), and difference data are percent. To convert FPG or 2-h PPG from mmol/L to mg/dL, divide by 0.0555.
*<53 mmol/mol.
†<48 mmol/mol.
Patients who received exenatide QW plus dapagliflozin achieved significantly greater mean reductions in FPG from baseline to week 28 (Fig. 2C), which were maintained through week 52 (LSM change from baseline −3.50 mmol/L) compared with patients who received exenatide QW plus placebo (−2.52 mmol/L; P < 0.001) or dapagliflozin plus placebo (−2.21 mmol/L; P < 0.001) (Table 1). Furthermore, mean reductions in 2-h PPG at 28 weeks were significantly greater with exenatide QW plus dapagliflozin (Fig. 2D), which were maintained through week 52 (LSM change from baseline −4.58 mmol/L) compared with exenatide QW plus placebo (−3.56 mmol/L; P = 0.004) or dapagliflozin plus placebo (−3.31 mmol/L; P < 0.001) (Fig. 2D and Table 1). Similar results were also observed for mean average six-point self-monitored blood glucose concentrations: reductions at 28 weeks were significantly greater with exenatide QW plus dapagliflozin (Supplementary Table 4), which were maintained through week 52 (LSM change from baseline −2.85 mmol/L) compared with exenatide QW plus placebo (−2.36 mmol/L; P = 0.011) or dapagliflozin plus placebo (−2.18 mmol/L; P < 0.001) (Supplementary Table 4).
Treatment with exenatide QW plus dapagliflozin resulted in significantly greater mean reductions in weight from baseline to week 28 compared with exenatide QW plus placebo or dapagliflozin plus placebo (Fig. 2E), and these differences between treatment groups were maintained at 52 weeks (Fig. 2E and Table 1). A significantly greater proportion of patients who received exenatide QW plus dapagliflozin achieved a weight loss of ≥5% at 28 weeks (Table 1), which was maintained through week 52 (30.7%) compared with exenatide QW plus placebo (14.1%; P < 0.001) or dapagliflozin plus placebo (21.3%; P = 0.022) (Table 1).
Patients who received exenatide QW plus dapagliflozin achieved significantly greater mean reductions in SBP from baseline to week 28 compared with patients who received exenatide QW plus placebo or dapagliflozin plus placebo (Fig. 2F), which were maintained through week 52 (LSM change from baseline −4.5 mmHg), compared with patients who received exenatide QW plus placebo (−0.7 mmHg; P < 0.001) or dapagliflozin plus placebo (−2.7 mmHg; P = 0.100) (Fig. 2F and Table 1). As observed in the 28-week analysis, no intergroup differences were noted for the changes in waist circumference, DBP, or fasting cholesterol measures at week 52 (Supplementary Table 4). Although nominal P values for treatment differences were ≥0.05, the magnitude of reduction in mean triglyceride concentrations was numerically greater with exenatide QW plus dapagliflozin (−0.22 mmol/L, an ∼10% reduction from baseline) compared with that observed with exenatide QW plus placebo (−0.06 mmol/L) or dapagliflozin plus placebo (0.01 mmol/L).
The proportions of patients who initiated rescue therapy by week 52 were 26.8%, 32.2%, and 37.8% in the exenatide QW plus dapagliflozin, exenatide QW plus placebo, and dapagliflozin plus placebo groups, respectively. These proportions were greater than those observed during the 28-week treatment period (3.9%, 4.4%, and 7.4%, respectively), mainly as a result of the change in rescue criteria from confirmed FPG thresholds to a single HbA1c measurement of >8.0% (>64 mmol/mol) that commenced at 36 weeks (Supplementary Table 1). Between baseline and week 36, low numbers of patients were rescued for lack of glycemic control; after week 36, the proportion of patients rescued increased (Supplementary Fig. 3). This inflection point follows the change in rescue criteria to HbA1c >8.0% (>64 mmol/mol).
The results of treatment efficacy assessed at 52 weeks in a patient population that included data after the initiation of rescue therapy (Supplementary Fig. 2) were very similar to those in which data obtained after the initiation of rescue therapy were excluded (Fig. 2 and Table 1).
Safety and Tolerability
Exenatide QW plus dapagliflozin was well tolerated; similar proportions of patients experienced an AE over 52 weeks across all treatment groups (Table 2). The most common AEs reported with exenatide QW plus dapagliflozin were injection-site nodule, urinary tract infection, headache, and nausea. Most AEs were mild or moderate in intensity. Patients who received exenatide QW plus dapagliflozin and exenatide QW plus placebo experienced more gastrointestinal or injection site–related AEs than those who received dapagliflozin plus placebo (Table 2).
Table 2.
AEs in the safety population
| Exenatide QW + dapagliflozin(n = 231) | Exenatide QW + placebo(n = 230) | Dapagliflozin + placebo(n = 233) | |
|---|---|---|---|
| Any AE | 153 (66.2) | 143 (62.2) | 144 (61.8) |
| Any SAE | 11 (4.8) | 12 (5.2) | 12 (5.2) |
| Deaths | 3 (1.3) | 1 (0.4) | 1 (0.4) |
| AE leading to discontinuation | 10 (4.3) | 12 (5.2) | 8 (3.4) |
| AEs occurring in ≥5% of patients | |||
| Injection site nodule | 20 (8.7) | 14 (6.1) | 13 (5.6) |
| Nausea | 13 (5.6) | 22 (9.6) | 9 (3.9) |
| Urinary tract infection | 16 (6.9) | 13 (5.7) | 14 (6.0) |
| Diarrhea | 11 (4.8) | 15 (6.5) | 9 (3.9) |
| Upper respiratory tract infection | 8 (3.5) | 14 (6.1) | 13 (5.6) |
| Headache | 14 (6.1) | 8 (3.5) | 10 (4.3) |
| AEs of special interest | |||
| Volume depletion–related AEs | 2 (0.9) | 1 (0.4) | 4 (1.7) |
| Dehydration | 2 (0.9) | 0 | 1 (0.4) |
| Hypotension | 0 | 1 (0.4) | 2 (0.9) |
| Syncope | 0 | 0 | 1 (0.4) |
| Hematocrit >55% | 5 (2.2) | 0 | 5 (2.1) |
| Pancreatitis | 2 (0.9) | 1 (0.4) | 0 |
| Acute renal disorders | 0 | 2 (0.9) | 2 (0.9) |
| Acute kidney injury | 0 | 1 (0.4) | 1 (0.4) |
| Renal failure | 0 | 1 (0.4) | 1 (0.4) |
| Gastrointestinal AEs | 41 (17.7) | 45 (19.6) | 33 (14.2) |
| Genital infection AEs | 11 (4.8) | 4 (1.7) | 12 (5.2) |
| Injection site–related AEs | 32 (13.9) | 27 (11.7) | 17 (7.3) |
| Nodule | 20 (8.7) | 14 (6.1) | 13 (5.6) |
| Induration | 5 (2.2) | 6 (2.6) | 2 (0.9) |
| Bruising | 4 (1.7) | 4 (1.7) | 2 (0.9) |
| Pruritus | 2 (0.9) | 2 (0.9) | 2 (0.9) |
| Injection site mass | 0 | 2 (0.9) | 2 (0.9) |
| Injection site reaction | 3 (1.3) | 0 | 0 |
| Erythema | 0 | 3 (1.3) | 1 (0.4) |
| Inflammation | 2 (0.9) | 0 | 0 |
| Dermatitis | 0 | 0 | 1 (0.4) |
| Hemorrhage | 1 (0.4) | 0 | 0 |
| Hypersensitivity | 0 | 1 (0.4) | 0 |
| Adjudicated cardiovascular AEs | 1 (0.4) | 3 (1.3) | 3 (1.3) |
| Adjudicated hepatic AEs | 0 | 1 (0.4) | 1 (0.4) |
| Hypoglycemia | 12 (5.2) | 7 (3.0) | 5 (2.1) |
| Major | 0 | 0 | 0 |
| Minor | 3 (1.3) | 0 | 1 (0.4) |
| Other | 12 (5.2) | 7 (3.0) | 4 (1.7) |
| Highest anti-exenatide antibody levels over study period | |||
| Negative | 55 (24.6) | 53 (23.6) | |
| High positive (≥625) | 94 (42.0) | 64 (28.4) | |
| Low positive (<625) | 75 (33.5) | 108 (48.0) | |
| Any positive | 169 (75.4) | 172 (76.4) | |
| Injection site–related AEs by anti-exenatide antibody levels | |||
| Negative | 3 (1.3) | 3 (1.3) | |
| High positive (≥625) | 18 (7.8) | 10 (4.3) | |
| Low positive (<625) | 11 (4.8) | 14 (6.1) | |
| Any positive | 29 (12.6) | 24 (10.4) |
Data are n (%). SAE, serious adverse event.
Five patients died during the study; all deaths occurred in the first 28-week treatment period and were discussed in detail previously (10). Serious AEs were reported in 4.8% of patients who received exenatide QW plus dapagliflozin and in 5.2% who received exenatide QW plus placebo or dapagliflozin plus placebo. Most of these events occurred during the first 28-week treatment period. During the 24-week extension period, serious AEs were reported by seven patients who did not report serious AEs during the primary 28-week treatment period (exenatide QW plus dapagliflozin n = 1, exenatide QW plus placebo n = 4, and dapagliflozin plus placebo n = 2). A similar proportion of patients discontinued treatment because of an AE across all groups (Table 2).
Potential cardiovascular and hepatic AEs were adjudicated (Table 2). Overall, 0.4%, 1.3%, and 1.3% of patients who received exenatide QW plus dapagliflozin, exenatide QW plus placebo, and dapagliflozin plus placebo, respectively, experienced a cardiovascular event. Two of these events occurred in the 24-week extension period: one case of angina pectoris in a patient who received exenatide QW plus placebo and one case of acute myocardial infarction in a patient who received dapagliflozin plus placebo. Hepatic events were reported in one patient in the exenatide QW plus placebo group (raised levels of alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, and total bilirubin) and one patient in the dapagliflozin plus placebo group (levels of alanine aminotransferase increased). Both occurred during the original 28-week treatment period, and both were adjudicated as unlikely to be related to study drugs (Table 2). Of note, patients with clinically significant cardiovascular disease occurring within 3 months of screening or significant hepatic disease were excluded from the study.
No episodes of major hypoglycemia were reported (Table 2). Episodes of minor hypoglycemia occurred among three patients (1.3%) in the exenatide QW plus dapagliflozin group and one patient (0.4%) in the dapagliflozin plus placebo group; all of these events occurred during the extension period (none during the 28-week randomized treatment period). The patient in the dapagliflozin plus placebo group was receiving rescue therapy with basal insulin at the time of the event. More patients in the exenatide QW plus dapagliflozin group had episodes of “other” hypoglycemia during the study (5.2% vs. 3.0% and 1.7% in the exenatide QW plus placebo and dapagliflozin plus placebo groups, respectively) (Table 2). During the 24-week extension period, overall events of hypoglycemia were reported by 10 patients who did not report hypoglycemia episodes during the primary 28-week treatment period (exenatide QW plus dapagliflozin n = 4 [other hypoglycemia n = 1; other hypoglycemia and minor hypoglycemia n = 3], exenatide QW plus placebo n = 4 [other hypoglycemia], and dapagliflozin plus placebo n = 2 [other hypoglycemia n = 1; minor hypoglycemia n = 1]).
Incidences of pancreatitis, volume depletion–related AEs, marked abnormalities of hematocrit, and acute renal disorders were low and similar between groups (Table 2). None of the volume depletion–related or acute renal failure–related events were severe or considered as serious AEs; most events were transient and reversible. The mean eGFR initially decreased in the dapagliflozin-containing groups, recovering to near-baseline levels by 52 weeks (Supplementary Fig. 4A).
Patients in the dapagliflozin-containing groups had an increase in hematocrit from baseline to week 28, and this was maintained at week 52 (Supplementary Fig. 4B). Patients in the exenatide QW–containing groups had small increases in heart rate at 28 weeks, which were maintained at 52 weeks (1.9 and 0.8 beats per minute at 52 weeks vs. baseline in the exenatide QW plus dapagliflozin and exenatide QW plus placebo groups, respectively [Supplementary Fig. 4C]). In contrast, the heart rates among patients who received dapagliflozin plus placebo remained unchanged.
At week 52, 45.8% of patients (82 of 179 patients) and 49.1% of patients (78 of 159 patients) treated with exenatide QW plus dapagliflozin and exenatide QW plus placebo, respectively, had positive anti-exenatide antibodies. Over 52 weeks, 75.4% of patients (169 of 224 patients) and 76.4% of patients (172 of 225 patients) who received exenatide QW plus dapagliflozin and exenatide QW plus placebo, respectively, developed anti-exenatide antibodies at some point (Table 2), without affecting HbA1c response. The mean changes from baseline in HbA1c among patients who were antibody negative were −1.98% (−21.6 mmol/mol) and −2.03% (−22.2 mmol/mol) in the exenatide QW plus dapagliflozin and exenatide QW plus placebo groups, respectively. Corresponding mean changes in HbA1c among patients with any positive anti-exenatide antibodies were −2.35% (−25.7 mmol/mol) and −1.78% (−19.5 mmol/mol) in the exenatide QW plus dapagliflozin and exenatide QW plus placebo groups, respectively. Injection site–related AEs were more common among patients with any positive anti-exenatide antibodies (12.6% and 10.4% in the exenatide QW plus dapagliflozin and exenatide QW plus placebo groups, respectively) compared with patients who were antibody negative (1.3% in both the exenatide QW plus dapagliflozin and exenatide QW plus placebo groups) (Table 2).
Conclusions
Over the last decade, medications for the treatment of type 2 diabetes have been developed that improve glycemic control without significant risk of hypoglycemia, as well as provide additional benefits such as weight loss, reduced blood pressure, and an improved lipid profile. The DURATION-8 study aimed to investigate the metabolic effects of using the combination of a GLP-1RA (exenatide QW) and an SGLT2 inhibitor (dapagliflozin) compared with each individual drug in patients with type 2 diabetes inadequately controlled with metformin. Previously reported results of this combination showed significant improvements in glycemic control, weight, and SBP after 28 weeks, with no unexpected safety findings (10). This 24-week extension of DURATION-8, in which patients continued to receive their randomized therapy, demonstrated that the initial efficacy and safety of the combination of exenatide QW and dapagliflozin were maintained over a 52-week treatment period.
Glycemic responses with the combination of exenatide QW plus dapagliflozin were less than additive compared with either individual drug, as expected based on changes at 28 weeks (10) and as typically observed with combinations of glucose-lowering therapies. Less than additive glycemic responses with combination therapy may result from a dependency on baseline glycemic control. The reduction in weight at 52 weeks with treatment with exenatide QW plus dapagliflozin was slightly less than additive, whereas reductions in SBP and triglycerides with combination treatment were more than additive. These additive and near-additive results suggest the presence of independent mechanisms for changes in weight, SBP, and triglycerides that did not trigger compensatory counteracting responses.
The maintenance of effect observed in this analysis was not unexpected given the profile of the individual drugs. Long-term studies of treatment with exenatide QW have demonstrated sustained glycemic improvement, weight reduction, and improved markers of cardiovascular risk (11,12), even after 6 years of treatment (13). Similar durability has been observed in long-term studies (14–18) of dapagliflozin in patients with type 2 diabetes. In DURATION-8, the proportion of patients (33.8%) who achieved an HbA1c level of <7.0% (<53 mmol/mol) with exenatide QW plus dapagliflozin at 52 weeks was lower than in some of the aforementioned long-term studies of exenatide QW, whereas the proportion rescued as a result of inadequate glycemic control (26.8%) with exenatide QW plus dapagliflozin was higher than in some of the long-term studies of exenatide QW. These differences are likely due to the high baseline HbA1c inclusion criterion and differences in rescue criteria in DURATION-8; thus, direct comparisons cannot be made.
Safety and tolerability for the combination therapy of exenatide QW plus dapagliflozin were in line with expectations for the individual drugs. Treatments with exenatide QW plus dapagliflozin and exenatide QW plus placebo were associated with more gastrointestinal or injection site–related AEs than treatment with dapagliflozin plus placebo, as expected for the GLP-1RA class. At week 52, the frequency of anti-exenatide antibodies in the exenatide QW plus dapagliflozin (46%) and exenatide QW plus placebo (49%) groups was lower than previously reported in an analysis of four 24- to 30-week clinical trials of exenatide QW (57%) (19). In addition, the rates of antibody-positive patients at any time during 52 weeks in DURATION-8 did not change from those reported during the primary 28-week study period, in which the number of patients positive for anti-exenatide antibodies peaked at 12 weeks and subsequently declined (10).
Although the literature on the combination of a GLP-1RA and an SGLT2 inhibitor is limited (20), previous studies have established the feasibility of combining GLP-1RAs and SGLT2 inhibitors. A post hoc subgroup analysis of the Canagliflozin Cardiovascular Assessment Study (CANVAS) showed reductions in HbA1c, weight, and BP when the SGLT2 inhibitor canagliflozin was added to GLP-1RA therapy (21). A randomized, placebo-controlled study combining exenatide QW and dapagliflozin in participants who were obese without diabetes reported significant weight loss, BP reduction, and glucose normalization without unexpected AEs (22). Findings from real-world observational and retrospective analyses further support the use of these two drug classes in combination (23–28). Although having a different design to DURATION-8, the recent Assessment of Weekly Administration of LY2189265 (dulaglutide) in Diabetes-10 (AWARD-10) trial among patients with inadequately controlled type 2 diabetes receiving an SGLT2 inhibitor with or without metformin demonstrated improved glycemic control with add-on dulaglutide therapy versus placebo (29).
Dual therapy with exenatide QW plus dapagliflozin may have advantages over other combinations for patients with poor HbA1c control. Although dipeptidyl peptidase 4 inhibitors and SGLT2 inhibitors are orally administered, greater reductions in HbA1c, weight, and BP have generally been observed with a GLP-1RA than with a dipeptidyl peptidase 4 inhibitor (30,31). Combination therapies with GLP-1RAs and insulins are also available, but greater hypoglycemia risk and attenuated weight loss would be expected with this combination (32).
In 2018, the American Diabetes Association updated the Standards of Medical Care in Diabetes (4), indicating that a one-size-fits-all approach to treating uncontrolled hyperglycemia is not appropriate. Instead, they recommend balancing the benefits of glycemic control with its potential risks, particularly the risk of hypoglycemia associated with certain treatments in elderly patients or patients with comorbid conditions. This is further supported by a recent consensus statement from the American Association of Clinical Endocrinologists and American College of Endocrinology (3), which supports the individualization of glycemic goals and a nuanced approach to treating hyperglycemia that balances age, comorbidities, and hypoglycemia risk. The combination of GLP-1RAs and SGLT2 inhibitors, drug classes with complementary mechanisms of action that are associated with a low risk of hypoglycemia, positive effects on a number of cardiovascular risk markers (including weight and blood pressure), and some individual molecules with proven cardiovascular benefits (33–35) adds to the range of therapeutic options available in the era of individualized type 2 diabetes treatment.
There are some limitations to this analysis. First, all analyses at 52 weeks were exploratory, with only nominal P values calculated. Second, the study excluded patients with eGFR values of <60 mL/min/1.73 m2, so the results cannot be generalized to this population of patients with type 2 diabetes. The rate of study discontinuation represents another potential limitation, as ∼19% did not complete the 52-week study. Finally, this study was not designed to test the relative benefits of GLP-1RA/SGLT2 inhibitor dual therapy versus sequential add-on therapy, so this clinical question remains unanswered. Although the use of rescue therapy with insulin, which could affect weight and hypoglycemia results, could be viewed as a study limitation, we think that this may also be viewed as a strength, as the study design represents the real-life clinical scenario encountered by many patients with type 2 diabetes.
In conclusion, the metabolic effects of combined administration of exenatide QW plus dapagliflozin appear to be substantial and durable. Among patients with type 2 diabetes inadequately controlled with metformin, this combination provided sustained improvements in glycemic control, weight, and SBP over 52 weeks. Treatment with exenatide QW plus dapagliflozin was well tolerated, with no unexpected safety findings.
Supplementary Material
Article Information
Duality of Interest. The study was supported by AstraZeneca. Exenatide and dapagliflozin are proprietary compounds of AstraZeneca. All authors interpreted the data and wrote the manuscript together with the sponsor’s medical writing services; Simone Boniface and Julian Martins of inScience Communications, Springer Healthcare, provided medical writing support that was funded by AstraZeneca. AstraZeneca was involved in the study design and protocol development, provided logistical support, and obtained the data, which were evaluated jointly by the authors and the sponsor. S.A.J. has received honoraria for consultancy from AstraZeneca, Eli Lilly, and Janssen. J.P.F. has received research support from AbbVie, Allergan, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Elcelyx Therapeutics, Inc., Eli Lilly, Genentech, IONIS, Janssen, Johnson & Johnson, Lexicon, Ligand, Madrigal Pharmaceuticals, Inc., Merck, Mylan, Myovant Sciences, Novartis, Novo Nordisk, Ogeda, Pfizer, Sanofi, TaiwanJ Pharmaceuticals Co Ltd., Theracos, Inc., and Viking Therapeutics and has received honoraria for consultancy and participation in advisory boards from AstraZeneca, Bristol-Myers Squibb, Echosens, Elcelyx Therapeutics, Inc., Johnson & Johnson, Ligand, Novo Nordisk, and Sanofi. E.H., H.W., and P.Ö. are employees and shareholders of AstraZeneca. A.A. has received research grants from AbbVie, AstraZeneca, Kowa Pharmaceuticals, Novo Nordisk, and Sanofi. C.G. participated in scientific advisory boards for and received consulting/lecturing fees from Alfa Wasserman, AstraZeneca, Bayer AG, Boehringer Ingelheim, Eli Lilly, Merck KGaA, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.J. assisted in the design of the study, was the international coordinating investigator, oversaw the conduct of the study, contributed to the acquisition and interpretation of the data, and provided critical revisions to the manuscript. J.P.F. was a principal investigator in the study, contributed to the acquisition and interpretation of the data, and provided critical revisions to the manuscript. E.H. and P.Ö. participated in study design, data interpretation, and manuscript writing. A.A. contributed to the acquisition and interpretation of the data and provided critical revisions to the manuscript. H.W. was responsible for statistical analysis and interpretation of the data and provided critical revisions to the manuscript. C.G. was a principal investigator in the study, contributed to the acquisition and interpretation of the data, and provided critical revisions to the manuscript. S.A.J., J.P.F., E.H., A.A., H.W., P.Ö., and C.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017, and at the 53rd Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 11–15 September 2017.
Footnotes
Clinical trial reg no. NCT02229396, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0680/-/DC1.
References
- 1.Schwartz SS, Kohl BA. Glycemic control and weight reduction without causing hypoglycemia: the case for continued safe aggressive care of patients with type 2 diabetes mellitus and avoidance of therapeutic inertia. Mayo Clin Proc 2010;85(Suppl.):S15–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 3.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract 2017;23:207–238 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S73–S85 [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 2018;27:740–756 [DOI] [PubMed] [Google Scholar]
- 6.Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep 2016;16:92. [DOI] [PubMed] [Google Scholar]
- 7.Røder ME. Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: evidence and clinical potential. Ther Adv Chronic Dis 2018;9:33–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AK, Warren B, Lee CJ, et al. . The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018;41:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn CH, Oh TJ, Kwak SH, Cho YM. Sodium-glucose cotransporter-2 inhibition improves incretin sensitivity of pancreatic β-cells in people with type 2 diabetes. Diabetes Obes Metab 2018;20:370–377 [DOI] [PubMed] [Google Scholar]
- 10.Frías JP, Guja C, Hardy E, et al. . Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–1016 [DOI] [PubMed] [Google Scholar]
- 11.Wysham C, Bergenstal R, Malloy J, et al. . DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med 2011;28:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamant M, Van Gaal L, Guerci B, et al. . Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2014;2:464–473 [DOI] [PubMed] [Google Scholar]
- 13.Henry RR, Klein EJ, Han J, Iqbal N. Efficacy and tolerability of exenatide once weekly over 6 years in patients with type 2 diabetes: an uncontrolled open-label extension of the DURATION-1 study. Diabetes Technol Ther 2016;18:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med 2013;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck MA, Del Prato S, Durán-García S, et al. . Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab 2014;16:1111–1120 [DOI] [PubMed] [Google Scholar]
- 16.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med 2015;32:531–541 [DOI] [PubMed] [Google Scholar]
- 17.Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E; Study 05 Group . Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab 2015;17:1075–1084 [DOI] [PubMed] [Google Scholar]
- 18.Mathieu C, Herrera Marmolejo M, González González JG, et al. . Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab 2016;18:1134–1137 [DOI] [PubMed] [Google Scholar]
- 19.Fineman MS, Mace KF, Diamant M, et al. . Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 2012;14:546–554 [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab 2017;19:1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulcher G, Matthews DR, Perkovic V, et al.; CANVAS Trial Collaborative Group . Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016;18:82–91 [DOI] [PubMed] [Google Scholar]
- 22.Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017;19:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorgojo-Martínez JJ, Serrano-Moreno C, Sanz-Velasco A, Feo-Ortega G, Almodóvar-Ruiz F. Real-world effectiveness and safety of dapagliflozin therapy added to a GLP1 receptor agonist in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis 2017;27:129–137 [DOI] [PubMed] [Google Scholar]
- 24.Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract 2015;21:1315–1322 [DOI] [PubMed] [Google Scholar]
- 25.Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine 2017;55:173–178 [DOI] [PubMed] [Google Scholar]
- 26.Curtis L, Humayun MA, Walker J, Hampton K, Partridge H. Addition of SGLT2 inhibitor to GLP-1 agonist therapy in people with type 2 diabetes and suboptimal glycaemic control. Pract Diabetes 2016;33:129–132 [Google Scholar]
- 27.McGovern AP, Dutta N, Watters K, Munro N, Feher M. Additive weight loss effect with a combination of an oral sodium-glucose cotransporter 2 (SGLT2) inhibitor and a glucagon-like peptide 1 (GLP-1) agonist in type 2 diabetes (Abstract). Diabet Med 2015;32:2–3
- 28.García-Rubi E. Real life experience with the combination of exenatide LAR and dapagliflozin in obese type 2 diabetic patients in Mexico City. Late-breaking abstract presented at the 26th Annual Scientific & Clinical Congress of the American Association of Clinical Endocrinologists (AACE), 3–7 May 2017, at the Austin Convention Center, JW Marriott Austin, Austin, Texas [Google Scholar]
- 29.Ludvik B, Frías JP, Tinahones FJ, et al. . Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2018;6:370–381 [DOI] [PubMed] [Google Scholar]
- 30.Russell-Jones D, Cuddihy RM, Hanefeld M, et al.; DURATION-4 Study Group . Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care 2012;35:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergenstal RM, Wysham C, Macconell L, et al.; DURATION-2 Study Group . Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 32.Owens DR, Monnier L, Barnett AH. Future challenges and therapeutic opportunities in type 2 diabetes: changing the paradigm of current therapy. Diabetes Obes Metab 2017;19:1339–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 34.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 35.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.