Abstract
Depression is associated with deficits in executive functions (EFs)—cognitive control abilities that regulate goal-directed thoughts and actions—but the etiology of these associations is unclear. We examined the relations between depressive symptoms and multiple EF latent variables in a population-based sample of 439 twin pairs assessed at mean ages 12, 17, and 23 years. Greater depressive symptoms negatively related to a Common EF factor capturing shared variance across response inhibition, working memory updating, and mental set shifting tasks, and also negatively related to an Updating-Specific factor, but not a Shifting-Specific factor. Cross-lagged panel models suggested that the Common EF correlations reflected within-wave associations rather than prospective effects, whereas the Updating-Specific correlations reflected associations of earlier depression levels with later Updating-Specific ability. Twin models were consistent with a model in which Common EF and Updating-Specific abilities relate to depression through correlated genetic risk, with no significant environmental correlations.
Keywords: executive control, endophenotype, psychopathology, internalizing
A large body of research suggests that major depressive disorder (MDD) is associated with deficits in executive functions (EFs), higher-level cognitive control abilities that regulate goal-directed thoughts and behavior (e.g., Snyder, 2013). Indeed, EFs have been implicated by cognitive theories of depression, proposed as risk factors, and identified as potential intermediate phenotypes/endophenotypes for depression and psychopathology more generally (Nolen-Hoeksema & Watkins, 2011). However, the nature of EF deficits associated with MDD and depressive symptoms is still unclear. First, is depression associated only with common variance across multiple EFs, or perhaps also associated with specific EF components? Second, what is the direction of the relationship? Does depression impair EFs, do low EFs lead to depression, or is the association more likely due to common causes? And third, to what extent are the associations genetic and environmental in origin? We address these questions with a longitudinal analysis of the relations between depressive symptoms and multiple EFs in a population-based sample of 439 twin pairs. Our design realizes a number of Snyder, Miyake, and Hankin's (2015) suggestions for "bridging the gap" between clinical and cognitive approaches to understanding EF impairments in psychopathology: latent variable measures of multiple EF components, longitudinal assessments, and a genetically informative sample that can be useful for examining causal hypotheses.
Unity and Diversity of EFs and Relations to Depression
EFs is an umbrella term used to describe a family of domain-general cognitive control processes that are typically assessed with laboratory cognitive or neuropsychological tasks. The most commonly studied EFs are response inhibition (inhibiting), working memory updating (updating), and mental set shifting (shifting; Friedman & Miyake, 2017). Inhibiting tasks involve stopping a prepotent or dominant response, sometimes to enact a less dominant response (e.g., the antisaccade task assesses how often one can avoid the reflexive response of looking at a cue to instead look in the opposite direction). Updating tasks require maintaining information in working memory and updating it as new information becomes relevant (e.g., the 2-back task assesses how often one can identify whether the current stimulus matches the one two trials back, which requires continuously maintaining and updating the last two items encountered); and shifting tasks typically require rapidly switching between two or more subtasks (e.g., the color–shape task assesses how quickly one can switch from categorizing stimuli on one dimension [color or shape] to another). Latent variables models suggest that these three EFs are correlated but separable (Miyake et al., 2000). Their correlations (unity) suggest that they share some processes in common, such as maintaining a task goal and using it to bias attention toward relevant stimuli and away from distractions. The fact that these correlations are significantly less than 1.0 (diversity) suggest that there are also some processes unique to particular EF constructs, such as gating information in working memory (updating) and clearing no-longer relevant goals (shifting; Friedman & Miyake, 2017).
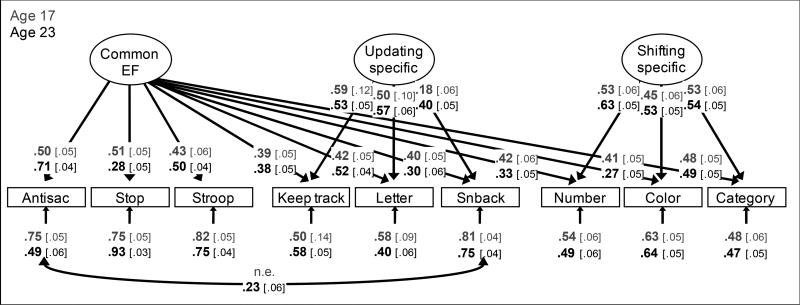
This unity and diversity was captured directly in a recent study focusing on the same EF data used in the current study (Friedman et al., 2016), which included nine EF tasks (three each tapping inhibiting, updating and shifting) assessed at two waves. This bifactor model (including parameter estimates for the current study) is shown in Figure 1. The unity of EFs was captured by a "Common EF" latent variable, which predicted performance on all nine tasks. The diversity of EFs was captured by the "Updating-Specific" and "Shifting-Specific" latent variables, respectively, which predicted additional variance in performance of updating and shifting tasks, respectively. As discussed by Friedman and Miyake (2017), multiple independent studies using this same set of nine tasks have found that the Common EF factor captures all the variance in response inhibition abilities, so there was no "Inhibiting-Specific" factor. That is, Common EF can be considered isomorphic with inhibiting at the level of individual differences.
Figure 1.
Unity/diversity executive function (EF) model estimated (separately) for age 17 (top, gray font) and age 23 (bottom, black font). These EF models are identical to those presented by Friedman et al. (2016), except that they were conducted with age- and sex-residualized scores for each task. Numbers on arrows are standardized factor loadings, those under the smaller arrows are residual variances, and the one on the curved double-headed arrow is a residual correlation estimated at age 23 (this residual correlation improved model fit for the age 23 models, but does not change results). Numbers in brackets are standard errors. All parameters were statistically significant (p < .05). Antisac=antisaccade, Stop=stop-signal, Letter=letter memory, Snback=spatial n-back, Number=number–letter, Color=color–shape, Category=category-switch; n.e.=not estimated at Wave 1.
Prior work examining EF deficits in depression have implicated inhibition, updating, and shifting as mechanisms of cognitive vulnerability to depression. As described by Disner, Beevers, Haigh, and Beck (2011), neurobiological research suggests that attenuated inhibition of negative stimuli combined with increased activation of subcortical processing regions may explain the biased attention and distorted cognitions central to Beck's (1967) cognitive model of depression. Joormann and Tanovic (2015) similarly discussed biased attention to negative information as central to emotion dysregulation, though they suggested it might result from a shifting deficit (an inability to shift attention away from negative emotional material toward positive information). Deficits in inhibition or updating may also make it difficult to disengage from negative material and remove previously relevant material from working memory (De Raedt & Koster, 2010; Joormann & Tanovic, 2015), which may result in rumination, the central risk and maintenance factor of Nolen-Hoeksema's (1991) response styles theory. Joormann and Tanovic (2015) also suggested that deficits in inhibition might lead to emotional dysregulation through an inability to reappraise negative interpretations. Taken together, this literature suggests broad EF deficits in depression, encompassing EF components of inhibiting, updating, and shifting, although it is not clear whether deficits in correlated components might reflect common processes (i.e., the Common EF factor).
Pervasive EF deficits in depression have also been supported by recent meta-analytic work. Snyder (2013) grouped 113 case-control studies according to EF constructs and found significant deficits across EF tasks, with similar effect sizes (ds ranging from 0.45 to 0.58) across EF composites. Snyder suggested that this pattern was consistent with a depression-related deficit on the unity (Common EF) component. Although associations with inhibiting, updating, and shifting abilities (e.g., Snyder, 2013) does suggest that depression is associated with the unity component, it is possible that depression is related to a specific component (e.g., Updating-Specific) in addition to the common factor. To test that possibility, one would need to partition variance in each EF factor into the common and specific components and examine correlations with each, which would require multiple components to be included in the same study. Such a test would help to further characterize the nature of cognitive impairments associated with depression.
This question is important to answer, because different components of EF have been shown to be correlated but separable behaviorally (Miyake et al., 2000), neurally (e.g., Collette, Hogge, Salmon, & Van der Linden, 2006), and genetically (Friedman et al., 2008) in late adolescence/adulthood. Clarifying the nature of EF deficits associated with depression and depressive symptoms will provide insight into the mechanisms of current cognitive theories of depression, and elucidate the possible cognitive, neural, and genetic mechanisms underlying these associations. Understanding the nature of the association between EF deficits and depression will also inform current research into the biological basis of, and potential treatments for, depression and other mental disorders (e.g., the Research Domain Criteria or RDoC initiative; e.g., Sanislow et al., 2010).
Etiology of the Link Between Depression and EFs
It is well established from family studies that depression is 30% to 40% heritable, whether measured as a liability for the disorder (Sullivan, Neale, and Kendler, 2000) or with symptom scales such as the Center for Epidemiological Studies-Depression (CES-D) scale used in the current study (e.g., Johnson, Whisman, Corley, Hewitt, & Friedman, 2014). Twin studies of EFs suggest that, when measured with latent variables that reduce the influences of measurement error and task impurity, EFs are highly heritable from childhood to adulthood (Engelhardt, Briley, Mann, Harden, & Tucker-Drob, 2015; Friedman et al., 2008, 2016). Moreover, using the same dataset used in the current study, we (Friedman et al., 2016) found evidence for unity and diversity of genetic influences in late adolescence and early adulthood, such that there were unique genetic influences on the Updating-Specific and Shifting-Specific factors (i.e., separate from the genetic influences on the Common EF factor).
Thus, it appears that both depression and EFs have substantial genetic components. A key question, then, is the extent to which the genetic influences on depression are shared with those on EFs. This question is important to answer, because EFs are often proposed as potential endophenotypes for depression and psychopathology more generally (Nolen-Hoeksema & Watkins, 2011), and the hypothesis that EFs are endophenotypes for psychopathology requires a genetic relationship (Kendler & Neale, 2010). This question can be answered with multivariate twin models, which partition the phenotypic correlation between two traits into genetic and environmental correlations.
Longitudinal and Environmental Relations Between Depression and EFs
Typical studies of the relation between depression and EFs are cross-sectional (see meta-analysis by Snyder, 2013). These studies have demonstrated that individuals who are depressed or exhibit more symptoms of depression also show worse concurrent EF performance. That relation could be consistent with multiple causal models. EF deficits may cause or exacerbate depressive symptoms, as is implied by the hypothesis that EF deficits are a risk factor or intermediate phenotype for depression. However, it is also possible that depression could cause EF deficits, that depression and EF have a bidirectional relationship, or that a third factor could cause both depressive symptoms and EF deficits. Randomized experimental designs are considered the gold standard for evaluating causal hypotheses, but they are neither feasible nor ethical in this context. Although no observational design can prove a causal model, some designs can contribute evidence against causal hypotheses (Rutter, 2007).
One such design is a longitudinal one, which allows for tests of whether earlier levels of one variable predict later levels of the other, or whether changes in one variable accompany changes in the other. Such tests provide a more dynamic view of the relationship. Twin designs can also be useful for examining causal models (Rutter, 2007). The association between EFs and depression may reflect shared genetic influences, but holding those constant, the twin who is more depressed should have lower EF if depression causes EF deficits. Such a hypothesis can be examined with a discordant twin design, but can also be examined with twins that vary in discordance, using a full multivariate twin model (Plomin & Haworth, 2010). Specifically, a causal association would manifest as a negative nonshared environmental correlation: Controlling for genetic and shared environmental confounds, differences in depression are related differences in EFs. Finding such an environmental correlation would be consistent with causation, but not prove it. Conversely, finding no nonshared environmental contribution to the relationship between EFs and depression would be inconsistent with a simple causal model.
The Current Study
We present a longitudinal analysis of the relations between depressive symptoms at mean ages 12, 17, and 23 years, and EFs at ages 17 and 23 years, in a sample of 439 twin pairs. EFs were assessed with latent variables (see Figure 1), which reduces the influence of task impurity and measurement error that are particularly problematic for EF tasks (Miyake et al., 2000). Depressive symptoms were assessed with a diagnostic interview (the Diagnostic Interview Schedule – IV [DIS]; Robins et al., 2000) and a self-report questionnaire (CES-D; Radloff, 1977). The latter enabled us to consider continuous levels of depressive symptomology. Subclinical levels may be important to consider in longitudinal models given increasing evidence that levels of depressive symptoms that do not reach clinical significance may fall on the same continuum in terms of impairment and genetic influences (e.g., Kessler, Zhao, Blazer, & Swartz, 1997; Lewinsohn, Klein, Durbin, Seeley, & Rohde, 2003), and predict future diagnoses of depression (e.g., Fergusson, Horwood, Ridder, & Beautrais, 2005). Moreover, severity levels below and above clinical thresholds show relations to EF tasks or composites in prior studies (Snyder, 2013). Thus, considering depressive symptoms as a continuum may capture important variation related to EFs.
Based on Snyder's (2013) meta-analysis, we predicted that the Common EF component would be related to depression levels at each time point. No studies have examined relations with the Updating- and Shifting-Specific components (i.e., regressing out Common EF), but there is some reason to believe that there might be an association with the Updating-Specific factor. Prior studies suggest that interference from irrelevant material may be a key mechanism underlying rumination in depression (Levens & Gotlib, 2010; Yoon, Lemoult, & Joormann, 2014). Additionally, Snyder (2013) found similar meta-analytic effect sizes for inhibition (d=0.58) and updating (d=0.57), which is inconsistent with the lower factor loading of Updating on Common EF in a hierarchical model (Friedman et al., 2008) and suggests that depression may show a relation with Updating-Specific in addition to its relation with Common EF.
We examined whether earlier levels of depressive symptoms predict later levels of EF (or vice versa) with a longitudinal cross-lagged panel model. Finally, we examined the genetic and environmental correlations between EFs and depression measures to evaluate the genetic and environmental etiology of the relationships. Given the substantial genetic variance in Common EF (which is stable from late adolescence to early adulthood; Friedman et al., 2016), we expected to find a significant genetic correlation at each time point. Although there is little environmental variance in Common EF at age 17, there is at age 23 (Friedman et al., 2016). Finding a nonshared environmental correlation at this age would provide evidence consistent with a causal relationship between depression and Common EF, whereas the absence of that correlation would be inconsistent with a simple causal model.
Method
Participants
Participants were 877 individuals (450 female, 427 male) from 439 same-sex twin pairs (235 monozygotic, MZ; 205 dizygotic, DZ) with data for at least one measure used in the study (see supplemental Table S1 for Ns). The sample was 92% Caucasian, 1.1% American Indian, 0.2% Native Hawaiian/Pacific Islander, 0.2% Asian, 0.0% Black/African American, 5% multiracial, and 1.1% unknown/not reported. Nine percent of the sample was Hispanic.
All twins were part of the ongoing Colorado Longitudinal Twin Study (LTS), which includes families identified by the Colorado Department of Health’s Division of Vital Statistics as having same-sex twins born between 1986 and 1990 (Rhea, Gross, Haberstick, & Corley, 2006). The LTS twins have been included in a number of studies of emotional and cognitive development, beginning when they were age 14 months and continuing through the present day (as they are entering their 30's). The data for the current study include depressive symptom measures collected during a three-wave study conducted by the Center for Antisocial Drug Dependence (CADD) at the University of Colorado, and EF measures collected during separate studies run concurrently with CADD waves 2 and 3.
At each wave of data collection, participants could have completed the CES-D, DIS, and EF battery (at waves 2 and 3) on different days, though the majority completed all measures within the same week. At wave 1, participants completed the CES-D and DIS when they were age 12.4 years (SD=0.4, range=11.3 to 14.0). At wave 2, participants completed the CES-D, DIS, and EF assessments when they were age 17.3 years (SD=0.6, range=16.1 to 20.1). At wave 3, participants completed the CES-D, DIS, and EF assessments when they were age 22.8 years (SD=1.3, range=21.1 to 28.0). Henceforth, we refer to waves 1-3 with their mean ages (i.e., age 12, age 17, and age 23).
All research protocols were reviewed and approved by the University of Colorado’s Institutional Review Board. Parental permission and informed consent or assent was obtained from each participant or parent, as appropriate, at each assessment.
Measures
EF tasks
Participants completed nine tasks tapping response inhibition, working memory updating, or mental set shifting. These tasks were modeled with the Common EF, Updating-Specific, and Shifting-Specific latent variables depicted in Figure 1 (data are identical to those reported by Friedman et al. 2016, except that each EF task was residualized on sex and age of assessment prior to analysis in the current study). To avoid ceiling effects, the age 23 versions of these tasks were modified to be more difficult, and some changes were made to improve reliability when possible; thus, the EF latent variables are not modeled as invariant. However, because the latent variables extract common variance within time, they mitigate bias that might be caused by changes to task-specific variance. Indeed, results reported by Friedman et al. (2016) suggest that they tapped the same EFs: The EF latent variables were highly stable across this six-year period (phenotypic rs = .86, 1.0, and .91 for the Common EF, Updating-Specific, and Shifting-Specific factors, respectively; genetic rs = .99 to 1.0). Full methods for each task and results are provided in Friedman et al., (2016). Here, we briefly describe the key requirements.
Inhibiting
The three response inhibition tasks required participants to avoid executing a dominant response. In the antisaccade task, participants had to avoid the reflex to look toward a cue that flashed on one side of the screen and instead look in the opposite direction to see a target number before it was masked (dependent measure [DM]: accuracy of target identification). In the stop-signal task, participants had to withhold a well-practiced categorization response on 25% of trials during which a signal occurred (DM: stop-signal reaction time). In the Stroop task, participants had to name the font colors of color words or asterisks rather than reading the words (DM: mean response time [RT] on incongruent word trials minus mean RT on asterisk trials).
Updating
The updating tasks required participants to continuously add and delete information in working memory. In each trial of the keep-track task, participants were shown a series of words from six categories and had to remember the most recently presented words belonging to two to five target categories (DM: accuracy). In the letter memory task, participants had to continuously say aloud the last three (age 17) to four (age 23) letters in a series of unpredictable length (DM: accuracy of recall of the last three letters of the sequence at age 17, and accuracy of rehearsal at age 23). In the spatial n-back task, participants saw squares on the screen that flashed one at a time and had to indicate whether the square that flashed was the same one that flashed n-trials (either 2 or 3 trials) before (DM: accuracy of both yes and no responses).
Shifting
The set shifting tasks required participants to switch between two subtasks according to a cue that appeared just before the stimulus and remained on the screen with the stimulus until they responded with one of two buttons. The tasks were mixed such that half the trials required repeating the task from the prior trial, and half required switching tasks (DM for all three tasks was the local switch cost: the average RT for switch trials minus the average RT for repeat trials within the mixed blocks). In the number–letter task, participants saw a number-letter or letter-number pair in one half (age 17) or quadrant (age 23) of a box and categorized either the number (as even or odd) or the letter (as a consonant or vowel) depending on the location (top or bottom); the cue was a darkening of the box surrounding the location. In the color–shape task, participants categorized a stimulus based on color (red or green) or shape (circle or triangle) depending on a cue (C or S) that appeared above the stimulus. In the category-switch task, participants categorized words as describing something that is smaller or bigger than a soccer ball, or living or nonliving, depending on a cue (crossed arrows or heart) that appeared above the stimulus.
Depressive symptoms
The CES-D (Radloff, 1977) is a 20-item self-report measure of depressive symptom severity. Participants indicate “how often they felt this way in the past week” on a scale from 0 (rarely or none of the time) to 3 (most or all of the time). Items included “I felt sad” and “I thought my life had been a failure.” The DIS (Robins et al., 2000) is a structured clinical interview based on the diagnostic criteria found in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994). Participants under the age of 18 completed the child version (Shaffer, Fisher, Lucas Dulcan, & Schwab-Stone, 2000). We used lifetime symptom endorsement at our earliest time point (approximately age 12), and past-year symptom endorsement at ages 17 and 23.
Statistical Analysis
Data transformation and trimming
To improve reliability, obtain the best measures of central tendency, and reduce the influence of extreme scores, the EF data were trimmed and transformed, as described by Friedman et al. (2016). Specifically, mean RTs for each subject and condition were calculated after within-subject trimming robust to nonnormality (Wilcox & Keselman, 2003) to enable the best measures of central tendency. Only RTs for correct trials were examined, and for the three shifting tasks, RTs for trials following errors were also eliminated (because the correct set might not have been achieved on the previous trial, making it ambiguous whether the current trial was a switch or repeat trial); average accuracy was greater than 92% in all RT tasks in both waves. Accuracy scores were arcsine-transformed to improve normality when there was evidence of ceiling effects (age 17 accuracy measures and age 23 spatial n-back). Finally, to reduce the influence of extreme scores at the between-subjects level, we replaced observations farther than 3 SDs from the group mean with values 3 SDs from the mean. This Winsorization procedure affected no more than 2.0% of the observations for any measure at either wave of assessment. Descriptive statistics are presented in supplemental Table S1. Note that in all models, directionality of RT measures was reversed so that higher scores indicate better performance.
Table S1 also presents total scores for the CES-D, but we analyzed it as a latent variable based on three parcels at each age to remove unreliability, which can inflate nonshared environmental variance estimates and attenuate nonshared environmental correlations1. Each parcel consisted of 6 or 7 randomly selected items, with the at least 1 of the 4 reverse-coded items in each parcel. Parcel 1 contained items 1, 5, 8, 9, 14, 16, and 17; parcel 2 contained items 3, 7, 10, 12, 15, 18, and 19; and parcel 3 contained items 2, 4, 6, 11, 13, and 20. We averaged the items in each parcel if at least 80% were answered. We then multiplied the mean by the number of items and took the square root to improve normality.
Given that symptom count variables were highly skewed, we created an ordinal depressive symptoms variable (MDDsx), in which a score of 0 indicated no criteria met, 1 indicated at least one criterion met but no diagnosis, and 2 indicated diagnosis (see supplemental Table S2 for frequencies). We used this ordinal variable to estimate the underlying liability based on the frequencies within each category (i.e., a threshold model with two thresholds), which decreased the potential for biased parameter estimates compared to other potential transformations of such skewed symptom count data (Derks, Dolan, & Boomsma, 2004).
Model estimation
Structural equation modeling was conducted using Mplus 7.4 (Muthen & Muthen, 1998–2012). For models that included the ordinal MDDsx variable, we used the means and variance adjusted weighted least squares (WLSMV) estimation method, for which the only missing data option in Mplus is pairwise deletion. For all other models, we used maximum likelihood estimation. This estimation method uses full-information maximum likelihood for missing data, which treats missing data as missing at random and uses all available data to compute parameter estimates. In phenotypic analyses, we used the TYPE=COMPLEX option to cluster data by family, which uses a weighted likelihood function and a sandwich estimator to obtain a scaled chi-square (χ2) and standard errors corrected for non-independence; prior studies demonstrate that it adequately corrects for nonindependence of twin data (Rebollo, de Moor, Dolan, & Boomsma, 2006).
We supplemented the χ2 with the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). A CFI > .95 and RMSEA < .06 indicate good fit (Hu & Bentler, 1998). Parameter significance was determined with chi-square difference (Δχ2) tests, incorporating scaling factors (Satorra & Bentler, 2001) to adjust for nonindependence in phenotypic analyses. We used an alpha of .05 for all analyses.
Controlling for age and sex
Sex and age differences for each measure were mostly nonsignificant except for effects on MDDsx (see supplement for details). However, to remove any small effects of sex and age of assessment, we regressed all continuous variables (EF tasks and CES-D parcels) on sex and age, and used the resulting residuals as the input for the models. We could not obtain such residuals for the ordinal MDDsx variables, so MDDsx was regressed on sex and age of assessment within the models (except for age of assessment at wave 1, which was unrelated to MDDsx).
Phenotypic model parameterization
The primary phenotypic EF models examined are shown in Figure 1, age 17 EF model χ2 (21)=58.48, p<.001, CFI=.960, RMSEA=.048; age 23 EF model χ2 (20)=39.55, p=.006, CFI=.982, RMSEA=.036. These models are very similar to those reported by Friedman et al. (2016), except that the Friedman et al. study did not use age- and sex-regressed residuals for the EF tasks. Models that included both waves of EF data allowed residual correlations for the same task across time. We used this bifactor parameterization, rather than a correlated factors parameterization, because it allows for correlations with the common and specific components directly, thus allowing tests of whether constructs of interest are related to the unity and/or diversity EF components; correlated factors models are ambiguous in this respect. Some have examined EF unity and diversity with a hierarchical model (e.g., Engelhardt et al., 2015), including Friedman et al. (2008). At an empirical level, the results with, and interpretation of, these bifactor latent variables is not much different than those of a hierarchical common factor and its residuals. However, our notion that EF tasks tap multiple EF processes, some of which are needed for all tasks and some of which are unique to particular tasks, is more consistent with a common factor and specific factors directly predicting EF tasks than with a hierarchical model in which inhibiting, updating, and shifting are subfactors of Common EF.
In a model with three CES-D latent variables, one for each age, residual correlations between the same CES-D parcels across time significantly improved model fit, Δχ2(9)=29.70, p<.001, and thus were included in all models, χ2(15)=18.54, p=.236, CFI=.999, RMSEA=.016 for a model with just the three CES-D latent variables. In this model, the standardized loadings for parcels 1, 2, and 3, respectively, were as follows: age 12 λs=.76, .68, and .83; age 17 λs=.87, .76, and .80; and age 23 λs=.89, .85, and .83. The unstandardized loadings could not be constrained to equality across age, Δχ2(4)=15.05, p=.005, reflecting the slightly different reliabilities for the full scales reported in Table S1. Modeling the scale as a latent variable with freed loadings across time adjusts for these different reliabilities, ensuring that they do not lead to differences across time in CES-D's relations to EFs.
Genetic model parameterization
Standard ACE twin models decompose a measure's phenotypic variance into three components: additive genetic (A) influences, which represent the sum of additive effects of large number of genetic variants; shared environmental (C) influences, which lead individuals raised together to be similar; and nonshared environmental (E) influences, which lead individuals raised together to be uncorrelated. The A factors correlate 1.0 in MZ twins, because they share 100% of their alleles, but 0.5 for DZ twins because on average they share 50% of their alleles identical by descent. The C factors correlate 1.0 for both MZ and DZ twins, because both types are reared together. The E factors do not correlate across twins, by definition. When estimated for individual tasks, the E estimate can include random measurement error, as unreliability will lead twins to be uncorrelated. However, E estimates for latent variables are free from random error, which is captured with the indicator-specific residuals.
We computed genetic (rA) and environmental (rE) correlations between each CES-D latent variable and each wave of EF latent variables from a series of genetic Cholesky decompositions (i.e., six models total). In these models, we freely estimated A, C, and E variance components for all the latent variables. Genetic and environmental relations between EFs and CES-D were modeled by allowing the A and E factors for the Common EF, Updating-Specific, and Shifting-Specific factors (which were orthogonal to each other) to predict the CES-D latent variable. We did not allow for correlations between variance components that were close to zero (i.e., the C components for all EF latent variables and the E component for Updating-Specific), as they could not account for much covariance and removing them increased power for the remaining variance components.2 Each rA was then computed as the standardized cross-path from the EF A component to CES-D, divided by the square root of the total genetic variance for CES-D; each rE was computed with an analogous formula for the E parameters.
In all models, the EF tasks also had specific A and E components (i.e., capturing residual variance not explained by the latent variables). To aid model convergence, we did not include task-specific C components for the EF tasks, which were close to zero and did not reduce model fit when dropped, Δχ2(9) = 2.54, p = .980 for age 17 EFs; Δχ2(9) = 0.64, p = .999 for age 23 EFs. The CES-D parcels had specific E components to capture variance unexplained by the latent variable; we did not model parcel-specific As and Cs, as there was no reason to expect that there would be parcel-specific genetic or shared environmental variance, and dropping these components did not harm fit for age 12 CES-D, Δχ2(6)=11.79, p=.067; age 17 CES-D, Δχ2(6)=6.69, p=.351; or age 23 CES-D, Δχ2(6)=2.91, p=.820.
Results
How Do Depressive Symptoms Relate to the Unity and Diversity EF Factors?
We obtained correlations between the two waves of EF data and three waves of depressive symptoms with two confirmatory factor analysis models, one for the CES-D data and the other for the DIS data. Latent variable correlations are provided in Table 1; supplemental Table S3 provides the zero-order correlations between all measures (including correlations with the CES-D sum scores instead of parcels). For comparison to prior studies that do not separate EF measures into common and specific components (as in the bifactor parameterization used here), Table S4 provides correlations from a model with three correlated Inhibiting, Updating, and Shifting factors.
Table 1.
Phenotypic Correlations [Standard Errors] of Depression Measures with EF Latent Variables
| Common EF | Updating-Specific | Shifting-Specific | ||||
|---|---|---|---|---|---|---|
| Depression Measure | Age 17 | Age 23 | Age 17 | Age 23 | Age 17 | Age 23 |
| CESD Latent | ||||||
| Age 12 | −.19* [.07] | −.12* [.06] | −.17* [.08] | −.28* [.07] | .11 [.08] | .06 [.06] |
| Age 17 | −.30* [.06] | −.26* [.06] | −.04 [.07] | −.14* [.07] | .05 [.07] | .06 [.05] |
| Age 23 | −.13* [.06] | −.13* [.05] | −.03 [.07] | −.15* [.06] | .06 [.07] | −.02 [.05] |
| MDDsx | ||||||
| Age 12 Lifetime | −.26* [.12] | −.20 [.12] | .16 [.11] | −.03 [.12] | −.02 [.13] | .04 [.13] |
| Age 17 Past Year | −.14 [.08] | −.10 [.09] | −.06 [.11] | −.13 [.08] | .09 [.09] | .04 [.09] |
| Age 23 Past Year | −.09 [.07] | −.08 [.07] | −.07 [.09] | −.11 [.07] | .09 [.07] | .00 [.07] |
Note. CES-D=Center for Epidemiological Studies–Depresssion latent variable; MDDsx=Major depressive disorder symptoms ordinal variable, coded as 0 for no criteria met, 1 for at least one criterion met but no diagnosis, and 2 for diagnosis, analyzed with a threshold model; EF=executive function.
p<.05, determined with chi-square difference tests.
CES-D
The model with CES-D latent variables fit well, χ2(269)=368.66, p<.001, CFI=.985, RMSEA=.021. As predicted, depressive symptoms negatively related to the Common EF factor, and this relation held within each age and across ages (rs= −.12 to −.30). The strongest correlations occurred with the age 17 CES-D latent variable, which was significantly related to both age 17 Common EF (r= −.30, Δχ2[1]=29.24, p<.001) and age 23 Common EF (r= −.26, Δχ2[1]=19.29, p<.001); these correlations could be constrained to be equal without harming fit, Δχ2(1)=0.82, p=.366, suggesting age 17 depressive symptoms were not more related to concurrent Common EF than later Common EF. Interestingly, age 23 depressive symptoms were somewhat less related to Common EF than age 17 depressive symptoms. Specifically, the age 23 CES-D correlation with age 23 Common EF (r= −.13, Δχ2[1]=5.37, p=.021), was significantly weaker than the −.30 correlation of age 17 CES-D with age 17 Common EF, Δχ2(1)=6.55, p=.010, and the −.26 correlation between age 17 CES-D and age 23 Common EF, Δχ2(1)=3.92, p=.048. Finally, even age 12 CES-D scores were related to both age 17 Common EF (r= −.19, Δχ2[1]=7.32, p=.007) and age 23 Common EF (r= −.12, Δχ2[1]=4.28, p=.038), and these correlations were not significantly different from each other, Δχ2(1)=1.29, p=.257, nor were they different from the correlations with later CES-D variables, all Δχ2(1)>3.28, p>.069, except that the −.12 correlation of age 12 CES-D with age 23 Common EF was significantly lower than the − .30 within-wave correlation at age 17, Δχ2(1)=5.40, p=.020.
The CES-D latent variables were also negatively related to the Updating-Specific factor. Specifically, age 23 Updating-Specific abilities were significantly related to age 12 CES-D (r= − .28, Δχ2[1]=10.58, p=.001), age 17 CES-D (r= −.14, Δχ2[1]=4.03, p=.045), and age 23 CES-D (r= −.15, Δχ2[1]=4.88, p=.027). Age 17 Updating-Specific abilities were also related to age 12 CES-D (r= −.17, Δχ2[1]=4.73, p=.029). Although each age of CES-D seemed to be more strongly correlated with age 23 Updating-Specific than age 17 Updating-Specific, the correlations were only significantly different for age 23 CES-D, Δχ2(1)=4.51, p=.034; they were not significantly different for age 12 CES-D, Δχ2(1)=2.03, p=.155, nor age 17 CES-D, Δχ2(1)=2.91, p=.088. There were no significant correlations of CES-D with Shifting-Specific abilities (rs = −.02 to .11, all Δχ2[1]<1.64, p>.200).
DIS
The model with the DIS MDDsx variables also fit well, χ2(206)=258.98, p=.007, CFI=.979, RMSEA=.017. There were generally fewer relations with this measure of depression; the only correlation that reached significance was age 12 lifetime MDDsx with age 17 Common EF (r= −.26, Δχ2[1]=4.678, p=.031).
What is the Direction of the Relationship Between Depressive Symptoms and EFs?
We estimated two longitudinal cross-lagged panel models, one for the CES-D data and the other for the DIS data. Variables at each wave were only allowed to correlate with variables within the same wave and to predict variables at the next wave. These models examine the same information as the correlational models presented in the previous section, but reveal whether cross-age correlations reflect new prediction (e.g., earlier depressive symptoms predict later Common EF, controlling for earlier levels of depressive symptoms and Common EF), or reflect stable covariance from earlier ages. Note that although we had EF data only for ages 17 and 23, we included the age 12 depressive symptom data in the models, as it allowed us to control for early levels of depression when examining relations between later depression and EFs.
CES-D
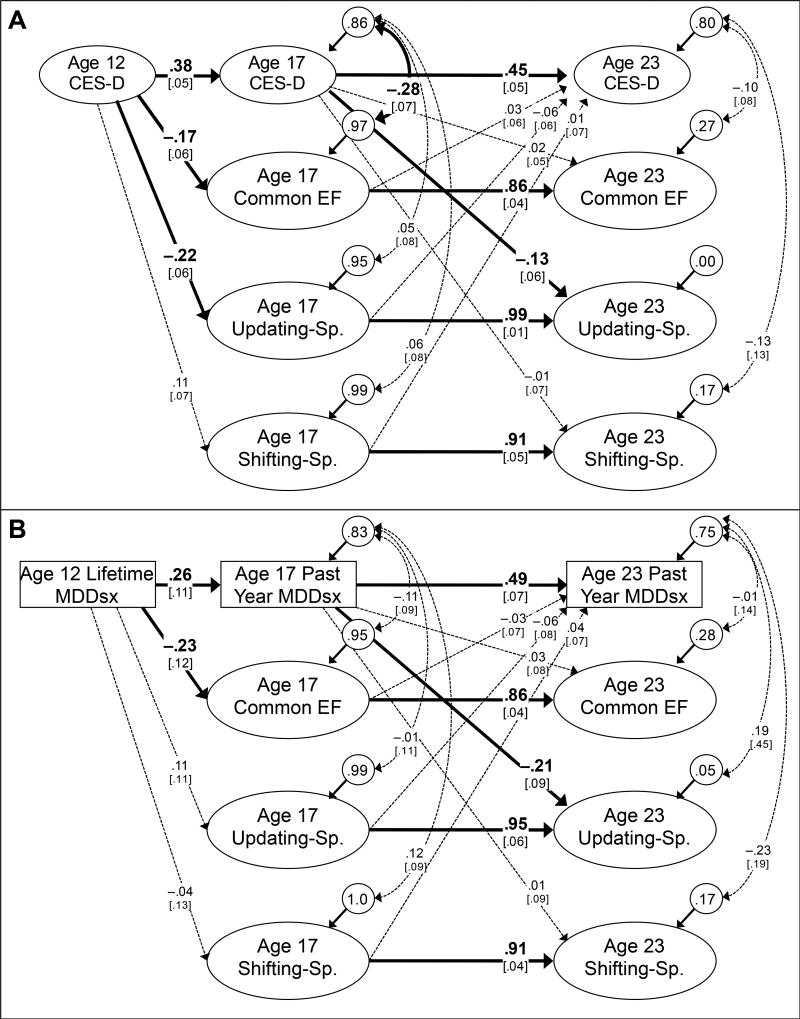
The model with CES-D latent variables fit well, χ2(275)=370.29, p<.001, CFI=.985, RMSEA=.0203. As shown in Figure 2A, the autoregressive paths for each construct were significant, indicating some stability for CES-D scores (β= .38 from age 12 to age 17, and β= .45 from age 17 to age 23), and considerable stability for the Common EF (β= .86), Updating-Specific (β= .99), and Shifting-Specific (β= .91) factors, consistent with the stability correlations reported by Friedman et al. (2016).
Figure 2.
Cross-lagged panel analysis of the executive function (EF) data and the Center for Epidemiological Studies-Depression (CES-D; Panel A) and major depression disorder symptoms (MDDsx, Panel B) data. Ellipses indicate latent variables estimated with age and sex-regressed residuals (indicators not shown for simplicity). MDDsx was an ordinal variable, with zero for no criteria met, 1 for at least one criterion met but no diagnosis, and 2 for diagnosis, and was regressed on sex and age of assessment (paths not shown). Numbers on arrows are standardized regression coefficients, numbers inside circles are residual variances, numbers on curved double-headed arrows are residual correlations, and numbers in brackets are standard errors. The residual variance of age 23 Updating-Specific (Sp.) was fixed to zero in Panel A; thus its correlation with age 23 CES-D was not estimated. Boldface type and solid lines indicate statistical significance (p<.05), and dashed lines indicate non-significance (p<.05), determined with chi-square difference tests. Total N=877.
Of primary interest for this analysis are the cross-lagged paths and the within-wave residual correlations. The significant paths from age 12 CES-D to age 17 Common EF (β= −.17, Δχ2[1]=7.61, p=.006) and Updating-Specific (β= −.22, Δχ2[1]=12.17, p<.001) reflect the same information as the correlations in Table 1, as those paths do not control for earlier levels of Common EF and Updating-Specific. However, the paths from age 17 CES-D to the three age 23 EF factors and vice versa do control for prior relations. These cross-lagged paths suggest that age 17 Common EF, Updating-Specific, and Shifting-Specific did not prospectively predict age 23 CES-D, controlling for age 17 CES-D (all β > −.06 and < .03). Age 17 CES-D also did not prospectively predict age 23 Common EF, controlling for age 17 Common EF (β=.02), but it did significantly predict age 23 Updating-Specific ability, controlling for age 17 Updating-Specific ability (β= −.13, Δχ2[1]=3.93, p=.047).
There was also a significant residual correlation between age 17 CES-D and age 17 Common EF (r= −.28, Δχ2[1]=19.39, p<.001). This residual correlation suggests that the within-wave relation at age 17 was not completely explained by age 17 CES-D's and age 17 Common EF's relation to age 12 CES-D. That is, within-person changes in CES-D from age 12 to 17 were associated with variance in Common EF that was unrelated to age 12 CES-D; however, this residual correlation is not informative as to the direction of the association. There were no significant residual correlations at age 23.
DIS
The model with the ordinal MDDsx variables fit well, χ2 (210)=268.96, p=.004, CFI=.977, RMSEA=.018. As shown in Figure 2B, the autoregressive paths for the MDDsx liability variable were significant, indicating stability (β= .26 from age 12 lifetime MDDsx to age 17 past year MDDsx, and β= .49 from age 17 to age 23).
As in the CES-D cross-lag model, the significant path from age 12 MDDsx to age 17 Common EF (β= −.23, Δχ2[1]=5.16, p=.023) reflects the same information as the correlation in Table 1, as that path does not control for earlier levels of Common EF. As in the CES-D model, the other cross-lagged paths suggest that age 17 Common EF, Updating-Specific, and Shifting-Specific did not prospectively predict age 23 MDDsx, controlling for age 17 MDDsx (all β > − .06 and < .04). Also consistent with the CES-D model, age 17 MDDsx did not prospectively predict age 23 Common EF, controlling for age 17 Common EF (β=.03), but it did significantly predict age 23 Updating-Specific ability, controlling for age 17 Updating-Specific ability (β= − .21, Δχ2[1]=4.35, p=.037). This significant cross-lagged path emerged even though the corresponding correlation between age 17 MDDsx and age 23 Updating-Specific ability shown in Table 1 (r= −.13, Δχ2[1]=2.52, p=.112) was not significant, because this path controls for the substantial stability of the Updating-Specific construct. Unlike the model with CES-D, no residual correlations were significant, consistent with the lack of correlations of ages 17 and 23 MDDsx with Common EF.
To What Extent are the Associations Genetic and Environmental in Origin?
ACE results for the EF factors and CES-D latent variables at each age are presented in the supplement and summarized in Table 2. Because there were few phenotypic associations with the MDDsx variables, and the low frequencies of affected individuals for some of these variables when the sample was split into twin1 and twin2 for MZ and DZ groups led to reduced power and other problems in the univariate twin models (see supplement), we focused our multivariate genetic analyses on the CES-D latent variables. The rAs and rEs of each wave of EF with each CES-D latent variable are presented in Table 2. The models fit acceptably, all models with age 17 EFs χ2(584)<706.75, p>.0003, CFI>.947, RMSEA<.032; all models with age 23 EFs χ2(583)<700.28, p>.0005, CFI>.953, RMSEA<.032.
Table 2.
Genetic and Nonshared Environmental Correlations [Standard Errors] of CES-D and EF Latent Variables
| Common EF | Updating-Specific | Shifting-Specific | ||||
|---|---|---|---|---|---|---|
| Depression Measure | Age 17 | Age 23 | Age 17 | Age 23 | Age 17 | Age 23 |
| Additive Genetic (A) | 96%* [.04] | 80%* [.20] | 100%* [.00] | 98%* [.07] | 78%* [.09] | 79%* [.07] |
| Age 12 (18% [.22]) | −.48* [.30] | −.22 [.20] | −.43* [.27] | −.60* [.33] | .28 [.25] | −.03 [.18] |
| Age 17 (61*% [.06]) | −.38* [.09] | −.26* [.09] | −.05 [.09] | −.21* [.08] | .02 [.11] | −.11 [.10] |
| Age 23 (39*% [.06]) | −.13 [.11] | −.19 [.11] | .00 [.10] | −.27* [.09] | −.10 [.14] | −.01 [.11] |
| Shared Environmental (C) | 0% [.00] | 3% [.19] | 0% [.00] | 0% [.00] | 0% [.00] | 0% [.00] |
| Age 12 (29% [.18]) | -- | -- | -- | -- | -- | -- |
| Age 17 (0% [.00]) | -- | -- | -- | -- | -- | -- |
| Age 23 (0% [.00]) | -- | -- | -- | -- | -- | -- |
| Nonshared Environmental (E) | 4% [.04] | 16%* [.04] | 0% [.00] | 2% [.07] | 22%* [.09] | 21%* [.07] |
| Age 12 (53*% [.07]) | .21 [.30] | −.01 [.14] | -- | -- | .01 [.18] | .23 [.16] |
| Age 17 (39*% [.06]) | .04 [.28] | −.07 [.13] | -- | -- | .11 [.19] | .40* [.17] |
| Age 23 (61*% [.06]) | −.29 [.31] | −.01 [.12] | -- | -- | .34* [.17] | −.03 [.14] |
Note. Each CES-D and EF time point modeled separately (i.e., six models). Percentages are ACE variances taken from separate analyses for age 17 EFs, age 23 EFs, and each CES-D latent variable modeled alone; estimates changed negligibly in larger models. Dashes indicate that E correlations with Updating-Specific and C correlations were not modeled because of zero or near-zero variances for those EF factor variance components. CES-D=Center for Epidemiological Studies–Depresssion scale; EF=executive function.
p<.05, determined with chi-square difference tests. These tests may not agree with tests based on standard errors for ACE parameters, which can depend on model parameterization (Neale, Heath, Hewitt, Eaves, & Fulker, 1989).
The results shown in Table 2 suggest that the phenotypic correlations between CES-D latent variables and the Common EF factors presented in Table 1 are due almost entirely to genetic influences that are common to depressive symptoms and Common EF. Age 12 CES-D was significantly genetically correlated with age 17 Common EF (rA= −.48, Δχ2[1]=8.53, p=.003). Age 12 CES-D's genetic correlation with age 23 Common EF was not significant (rA= −.22, Δχ2[1]=1.85, p=.174), but the corresponding phenotypic correlation for this age, though significant, was only r= −.12. Age 17 CES-D was genetically correlated with age 17 Common EF (rA= −.38, Δχ2[1]=8.53, p=.003) and age 23 Common EF (rA= −.48, Δχ2[1]=8.53, p=.003). However, age 23 CES-D was not significantly genetically correlated with Common EF at either age (age 17 rA= −.13, Δχ2[1]=1.47, p=.225; age 23 rA= −.19, Δχ2[1]=3.38, p=.066), again reflecting small phenotypic correlations in Table 1 (both rs= −.13).
Because age 17 Common EF did not have significant nonshared environmental variance (4%), it is unsurprising that its E component did not significantly correlate with the Es for the CES-D factors (all Δχ2[1]<1.14, p>.287). Although the significant nonshared environmental variance for Common EF at age 23 (16%) raised the possibility that this new E variance might relate to environmental influences on depression, the rEs with age 23 Common EF were all small and non-significant (all rE > −.07, all Δχ2[1]<0.30, p>.584).
As Updating-Specific was nearly 100% heritable at both ages, environmental correlations with CES-D factors were not estimated; thus, the phenotypic correlations must be explained by genetic correlations. Indeed, the rAs with Updating-Specific were significant for each corresponding significant phenotypic correlation in Table 1. Specifically, age 12 CES-D was significantly genetically correlated with age 17 Updating-Specific (rA= −.43, Δχ2[1]=7.25, p=.007), and age 23 Updating-Specific (rA= −.60, Δχ2[1]=16.35, p<.001). Age 17 CES-D (rA= − .21, Δχ2[1]=6.47, p=.011) and age 23 CES-D (rA= −.27, Δχ2[1]=8.33, p=.004) also both significantly genetically correlated with age 23 Updating-Specific.
Finally, although there were no significant phenotypic correlations with Shifting-Specific abilities at either age, two nonshared environmental correlations did reach significance: between age 17 CES-D and age 23 Shifting-Specific (rE= .40, Δχ2[1]=5.86, p=.015), and between age 23 CES-D and age 17 Shifting-Specific (rE= .34, Δχ2[1]=3.91, p=.048). Both rEs were positive, indicating that the environmental influences related to higher depressive symptoms were also related to better Shifting-Specific abilities. Both of these rEs both reflect cross-age correlations, but are inconsistent in terms of direction of effect (i.e., earlier depression symptoms related to later Shifting-Specific in one case, but vice versa in the other case). Given that they are small and not accompanied by significant phenotypic correlations, we do not discuss them further.
Discussion
We found that higher levels of depressive symptoms were associated with lower Common EF ability, particularly in late adolescence (mean age 17), and with lower Updating-Specific abilities in late adolescence and young adulthood (mean age 23). Depressive symptoms were not associated with poorer Shifting-Specific ability. The longitudinal models suggested that the relations with Common EF reflected within-time covariances rather than prospective effects, but the Updating-Specific relations reflected effects of earlier depression levels on later Updating-Specific ability. Twin models suggested that these relations were almost entirely explained by genetic correlations; there were no significant nonshared environmental correlations between depressive symptoms at any age and Common EF nor Updating-Specific at any age. Taken together, these results suggest that depressive symptoms are associated with deficits in Common EF and Updating-Specific abilities, and that these relations most likely reflect shared genetic etiology, rather than a causal relationship.
Relations of Depressive Symptoms to Unity and Diversity EF Components
Our finding of persistent associations between Common EF and depressive symptoms is consistent with Snyder's (2013) meta-analysis, which indicated that individuals with depression were impaired on EF tasks tapping response inhibition, working memory maintenance/updating, mental set shifting, planning, and verbal fluency. The link between Common EF and depressive symptoms is also consistent with existing cognitive models of depression. As reviewed earlier, these models typically posit that deficits in particular EF abilities, such as inhibiting, updating, and shifting, contribute to and maintain depression by making it difficult to inhibit or shift away from negative information (Disner et al., 2011). Understanding which EF abilities are associated with depression allows for further integration of cognitive science and clinical theories of psychopathology. Our finding that depressive symptoms are related to the common variance across these correlated EF abilities extends prior research and suggests that it is the processes shared across EF abilities, rather than those that distinguish them, that are important for understanding cognitive aspects of depression. That said, our results also suggest that these associations reflect shared genetic influences but not environmental influences, which may be important to consider when interpreting such associations from a causal perspective. In particular, it may be that EF deficits do not cause increased depressive symptoms; nevertheless, understanding the genetic and neurobiological mechanisms of individual differences in Common EF may provide insight into the mechanisms of depressive symptoms.
The Common EF factor is thought to tap individual differences in the ability to implement and maintain goals and use those goals to influence ongoing processing (Friedman & Miyake, 2017; Herd et al., 2014). This ability is necessary for all EF tasks but may be particularly important for tasks that require attending to relevant cues or information to stop a dominant response, which is why Common EF may be isomorphic with a response inhibition factor (Friedman & Miyake, 2017). In the context of depression, this ability may be related to the ability to resist the automatic tendency to focus on negative information and appraisals, and the “habit” of rumination that can be automatically evoked by particular contexts (Watkins & Nolen-Hoeskema, 2014).
Our results also suggest that processes specific to updating working memory are related to depressive symptoms. The Updating-Specific factor is thought to reflect the ability to selectively gate information into and out of working memory via the basal ganglia, as well as possibly controlled retrieval from episodic memory (Friedman & Miyake, 2017). This ability may be related to some aspects of rumination or selective retrieval of negative memories, consistent with prior work suggesting that updating deficits are associated with ruminative thought processes in depression (Yoon et al., 2014).
However, the longitudinal pattern with Updating-Specific was somewhat different than that for Common EF. In particular, the association manifested more in cross-wave correlations, with the strongest correlation obtained between age 12 CES-D and age 23 Updating-Specific ability. Correlations with later depressive symptoms were nominally weaker, and the within-wave correlation at age 17 was not significant. This pattern suggests that the mechanisms linking depressive symptoms to Updating-Specific abilities may unfold over a longer timeframe than those linking depressive symptoms to Common EF, and/or that depressive symptoms that occur earlier during EF development may be more associated with later Updating-Specific processes.
Deficits in Common EF do not appear to be unique to depression. In past studies with the current sample, we have found that Common EF is negatively associated with behaviors related to externalizing psychopathology, including teacher-rated attention problems (Friedman et al., 2007) and externalizing behavior (Hatoum, Rhee, Corley, Hewitt, & Friedman, in press), substance use (Gustavson et al., 2017), and a Behavioral Disinhibition latent factor (predicting attention problems, conduct disorder, substance use, and novelty seeking personality; Young et al., 2009). These results are consistent with a large literature suggesting general EF deficits across forms of psychopathology (see Snyder et al., 2015, for a review), and with the proposal that Common EF may be a common feature (in the terminology used by Weiss, Susser, & Catron, 1998) or a transdiagnostic feature of psychopathology (Nolen-Hoeksema & Watkins, 2011; Snyder et al., 2015). Consistent with this proposal, recent work has related general EF to a "P" factor capturing common variance across internalizing, externalizing, and thought disorder psychopathologies (Caspi et al., 2014).
In contrast, Updating-Specific may be more of a broad-band specific feature of internalizing, or even a narrow-band specific feature of depression (as suggested by Yoon et al., 2014). In the studies cited earlier, we have not found a negative association between the Updating-Specific factor and externalizing behaviors. Few other studies have separated updating or working memory measures into variance related to Common EF and variance specific to updating, so it is difficult to know how general of an effect this is. Moreover, the somewhat stronger relationship across time than within time means that it may be harder to detect this relationship in other samples without longitudinal designs. However, our results point to the intriguing possibility that depression may be differentiated from externalizing psychopathologies in its relation to Updating-Specific abilities. If true, clarifying the cognitive and neural processes specific to updating may provide insight into mechanisms specific to internalizing disorders or depression specifically.
We did not observe significant phenotypic or genetic associations between depressive symptoms and the Shifting-Specific factor, which is thought to capture the speed with which goal representations can be cleared from working memory (Friedman & Miyake, 2017; Herd et al., 2014). Although a null result, this lack of correlation is interesting because it contrasts with several of our prior studies with this sample in which we have found phenotypic, and sometimes genetic, correlations between Shifting-Specific and externalizing behaviors, such that individuals with more attention problems, higher behavioral disinhibition, and lower self-restraint have better Shifting-Specific abilities (see Herd et al., 2014, for a review). This pattern may reflect a stability-flexibility tradeoff, whereby individuals with these behavioral difficulties can clear goal representations more quickly (better Shifting-Specific) because they maintain these goals at a weaker level to start with (poorer Common EF). If this null finding is not a type II error, it would suggest that better Shifting-Specific ability may be a broad-band feature of externalizing psychopathology. However, future work examining multiple psychopathology measures within the same model would be needed to test this possibility.
Direction and Nature of the Associations Between Depressive Symptoms and EFs
The longitudinal and twin models provide more information about the possible direction of these effects. The lack of evidence for prospective effects of depressive symptoms on later Common EF, controlling for earlier Common EF (or vice versa) is more supportive of a correlated risk model than a causal model. A correlated risk model is also supported by the genetic analyses, which suggested that the negative phenotypic correlations between CES-D and Common EF at each age were almost entirely explained by shared genetic factors.
The absence of significant nonshared environmental correlations is also inconsistent with a causal relationship, as it suggests that when twins differ in their depression for environmental reasons, they do not also differ in Common EF. Even though we analyzed both Common EF and CES-D as latent variables, removing random measurement error, there were no significant nonshared environmental correlations. Although this lack of rEs is not surprising for age 17 Common EF, which did not have significant E variance (4%), it is informative for age 23 Common EF, which did have significant E variance (16%). Specifically, the nature of the new nonshared environmental influences on Common EF in early adulthood are unknown, and could reflect a number of environmental changes that individuals undergo from age 17 to 23 (Friedman et al., 2016); however, the results of this study provide no evidence that changes in depression levels explain this new Common EF E variance.
Results were more ambiguous for the Updating-Specific relationship. In the cross-lagged panel models, the significant cross-lagged path to age 23 Updating-Specific suggests that earlier depression levels led to later deficits in Updating-Specific, but that pattern is somewhat contradictory with two other aspects of the data. First, the Updating-Specific factor was highly stable, with autoregressive paths of .99 and .95 in the CES-D and MDDsx phenotypic models, respectively. That is, there was little to no change in rank orders on Updating-Specific abilities from age 17 to 23 to be related to depression scores. Second, the phenotypic relations between depressive symptoms and Updating-Specific abilities were entirely genetic in origin, which they had to be because there was no environmental variance in the Updating-Specific factor. Thus, the relationship of depressive symptoms with Updating-Specific ability, like that with Common EF, seems to be due to shared genetic risk.
The shared genetic risk for depression and EFs (significant rAs) satisfies a major criterion for endophenotypes: that they be genetically correlated with the psychiatric disorder (Kendler & Neale, 2010; Walters & Owen, 2007). Although it is commonly assumed that an endophenotype is on the causal path from genes to disorder, mediation is not necessary for a potential endophenotype to be informative (Kendler & Neale, 2010). Even if an endophenotype is a "liability-index" rather than "meditational" (in Kendler & Neale, 2010, terminology), it can be useful for investigating the biological function of relevant genes (Walters & Owen, 2007). It is also often hypothesized that endophenotypes are genetically simpler than the disorder of interest (Kendler & Neale, 2010), though that seems unlikely to be the case for EFs, for which there are as yet no genome-wide significant associations (Ibrahim-Verbaas et al., 2016). Nevertheless, Common EF’s and Updating-Specifics’s shared genetic risk with depressive symptoms suggests that, as we learn more about the neural and genetic mechanisms of individual differences in these EFs, we may gain insights into mechanisms of depression.
Limitations
Several strengths of the study, such as latent variables of unity and diversity EF component and the genetically informative sample, are a result of collecting these measures within the context of a large population-based twin study. However, the non-clinically ascertained sample also can be considered a limitation. We had few cases of MDD diagnosis, although we likely gained some power by considering an intermediate threshold for some symptoms. Although the lifetime prevalence of MDD diagnosis in our sample at age 23 (13%) is comparable to other large population-based samples of young adults (e.g., 15.4% in Kessler, Chiu, Demler, & Walters, 2005), we focused on past-year diagnosis and criteria met, as that was more informative for the longitudinal models. Because fewer individuals met criteria for past-year diagnosis, we had larger standard errors for the phenotypic models with MDDsx, and we were unable to incorporate the MDDsx variable into the genetic models. Fortunately, we also had a continuous measure of recent depressive symptom severity (CES-D), which showed similar patterns as the MDDsx measure based on the diagnostic interview. This measure enabled us to examine sub-clinical variation, which was related to EFs, but future studies should examine whether results differ with clinically ascertained samples.
The longitudinal assessments were another major strength of the study, but could also represent another limitation. The repeated assessments enabled us to examine how individual differences at the later times related to those at earlier assessments, but such effects would be underestimated if the time lag of 5–6 years was not optimal for examining causal effects of depression on EFs or vice versa (Cole & Maxwell, 2009). However, the twin analyses were also informative with respect to the causal relations, and both the longitudinal and twin models converged on the conclusion that the associations between depression and EFs is most consistent with a correlated genetic risk model.
The lack of EF assessment at age 12 was another limitation of the study. Adolescence is a particularly interesting time with respect to change in EFs (Best & Miller, 2010), and also may be an interesting time to study the development of depression, as MDD has a particularly wide age of onset range (Kessler et al., 2005). Although very few of our participants met diagnostic criteria for depression at age 12 (1.5%), we did observe significant relations of the age 12 MDDsx and CES-D variables with age 17 EFs. These correlations suggest that subclinical levels in adolescence may already be related to later EFs, and future cross-sectional studies of these relations in adolescence may be fruitful.
Conclusions and Future Directions
The current study advanced the literature on cognitive models of depression by incorporating an influential latent variable model of EFs into a longitudinal behavioral genetic design, consistent with several of Snyder et al.'s (2015) suggestions for "bridging the gap" between clinical and cognitive approaches to understanding EF impairments in psychopathology. We found that depressive symptoms were primarily associated with Common EF ability, with some evidence for prospective associations to Updating-Specific abilities in the longitudinal models. These results provide more detail about which EF processes are linked to depressive symptoms, which extends current cognitive models of depression and, potentially, treatment. In particular, it may be that multiple cognitive features of depression (e.g., attention biases, ruminative processes, poor concentration) are related to the same Common EF deficit.
The results of the longitudinal and genetic analyses were most consistent with a model in which these associations reflect correlated genetic influences, consistent with the idea that Common EF may be a “liability index” (Kendler & Neale, 2010) endophenotype for depression. Thus, Common EF and Updating-Specific deficits may not cause (or be caused by) depressive symptoms, but may indicate genetic risk factors for depression and perhaps psychopathology more generally. If so, then attempting to generally improve these EF abilities is unlikely to relieve symptoms, though targeting the specific cognitive habits that may maintain or exacerbate symptoms (such as rumination) may still be beneficial. It may also be important to consider how existing deficits may interact with treatment approaches that rely on EF, as some studies have found that some older individuals with general EF deficits have poorer psychotherapy treatment outcomes (e.g., of cognitive behavioral therapy; Mohlman & Gorman, 2005).
More work is needed to characterize the cognitive, neural, and genetic mechanisms underlying the associations we documented. For example, future work could investigate whether individual differences in EFs are phenotypically and genetically related more closely to particular depressive symptoms (e.g., attentional difficulties) and to particular neural correlates of depression (e.g., orbitofrontal cortex, anterior and posterior cingulate, insula, and temporal lobes; Schmaal et al., 2017). Given increasing evidence that Common EF deficits may be a common feature of psychopathology (Snyder et al., 2015), such work would benefit from incorporating a transdiagnostic perspective (Nolen-Hoeksema & Watkins, 2011).
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health: MH063207, AG046938, MH016880, and DA011015. The authors would like to thank Sally Ann Rhea for her assistance with data collection and study coordination.
Footnotes
Results were similar when phenotypic models were estimated with square-root transformed CES-D total scores instead of latent variables.
These correlations were never significant, all Δχ2(1)<0.67, p>.412, and including them did not change the patterns of results.
In this model, we constrained the residual variance of the age 23 Updating-Specific factor to zero and did not allow it to correlate with the residual variance for age 23 CES-D, because in a model without these constraints, the age 23 Updating-Specific residual variance was estimated at −.016. These constraints did not result in a significantly worse model fit, Δχ2(2)=1.55, p=.461. The same constraint was not necessary for the model with MDDsx.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: Author; 1994. [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. University of Pennsylvania Press; 1967. [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Development. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Moffitt TE. The p Factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE. Statistical methods for risk-outcome research: Being sensitive to longitudinal structure. Annual Review of Clinical Psychology. 2009;5:71–96. doi: 10.1146/annurev-clinpsy-060508-130357. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, Boomsma DI. Effects of censoring on parameter estimates and power in genetic modeling. Twin Research. 2004;7:659–669. doi: 10.1375/twin.7.6.659. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Engelhardt LE, Briley DA, Mann FD, Harden KP, Tucker-Drob EM. Genes unite executive functions in childhood. Psychological Science. 2015;26:1151–1163. doi: 10.1177/0956797615577209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais D. Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK. Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology. 2016;52:326–340. doi: 10.1037/dev0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR. Symptoms versus a diagnosis of depression: differences in psychosocial functioning. Journal of Consulting and Clinical Psychology. 1995;63:90–100. doi: 10.1037//0022-006x.63.1.90. [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Stallings MC, Corley RP, Miyake A, Hewitt JK, Friedman NP. Executive functions and substance use: Relations in late adolescence and early adulthood. Journal of Abnormal Psychology. 2017;126:257–270. doi: 10.1037/abn0000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum AS, Rhee SH, Corley RP, Hewitt JK, Friedman NP. Do executive functions explain covariance between internalizing and externalizing behaviors? Development and Psychopathology. doi: 10.1017/S0954579417001602. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, Friedman NP. A neural network model of individual differences in task switching abilities. Neuropsychologia. 2014;62:375–389. doi: 10.1016/j.neuropsychologia.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, Winslow AR. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nature Genetics. 2016;48:1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim-Verbaas C, Bressler J, Debette S, Schuur M, Smith A, Bis J, Coker L. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Molecular Psychiatry. 2016;21:189–197. doi: 10.1162/jocn.2008.20125.Left-lateralized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, Whisman MA, Corley RP, Hewitt JK, Friedman NP. Genetic and environmental influences on rumination and its covariation with depression. Cognition & Emotion. 2014;28:1270–1286. doi: 10.1080/02699931.2014.881325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Tanovic E. Cognitive vulnerability to depression: Examining cognitive control and emotion regulation. Current Opinion in Psychology. 2015;4:86–92. doi: 10.1016/j.copsyc.2014.12.006. [DOI] [Google Scholar]
- Judd LL, Paulus MP, Wells KB, Rapaport MH. Socioeconomic burden of subsyndromal depressive symptoms and major depression in a sample of the general population. Psychiatry: Interpersonal and Biological Processes. 1996;153:1411–1417. doi: 10.1176/ajp.153.11.1411. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Boundaries of major depression: An evaluation of DSMIV criteria. American Journal of Psychiatry. 1998;155:172–177. doi: 10.1176/ajp.155.2.172. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: A conceptual analysis. Molecular Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the national comorbidity survey. Journal of Affective Disorders. 1997;45:19–30. doi: 10.1016/S0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology: General. 2010;139:654–664. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Klein DN, Durbin EC, Seeley JR, Rohde P. Family study of subthreshold depressive symptoms: Risk factor for MDD? Journal of Affective Disorders. 2003;77:149–157. doi: 10.1016/S0165-0327(02)00106-4. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subtreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109:345–351. doi: 10.1037/0021-843X.109.2.345. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organisation of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: A pilot study with anxious older adults. Behaviour Research and Therapy. 2005;43:447–465. doi: 10.1016/j.brat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037/0021-843X.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CMA. Genetics and intervention research. Perspectives on Psychological Science. 2010;5:557–563. doi: 10.1177/1745691610383513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rebollo I, de Moor MHM, Dolan CV, Boomsma DI. Phenotypic factor analysis of family data: Correction of the bias due to dependency. Twin Research and Human Genetics. 2006;9:367–376. doi: 10.1375/twin.9.3.367. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Røysamb E, Ørstavik RE, Neale MC, Torgersen S, Kendler KS. Major depression and dimensional representations of DSM-IV personality disorders: A population-based twin study. Psychological Medicine. 2010;40:1475–1484. doi: 10.1017/S0033291709991954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado twin registry. Twin Research and Human Genetics. 2006;9:941–949. doi: 10.1375/twin.9.6.941. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 2000. [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/BF02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, Veltman DJ. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of evecutive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6(328) doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Molecular Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- Weiss B, Süsser K, Catron T. Common and specific features of childhood psychopathology. Journal of Abnormal Psychology. 1998;107:118–127. doi: 10.1037/0021-843X.107.1.118. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: Measures of central tendency. Psychological Methods. 2003;8:254–274. doi: 10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Lemoult J, Joormann J. Updating emotional content in working memory: A depression-specific deficit? Journal of Behavior Therapy and Experimental Psychiatry. 2014;45:368–374. doi: 10.1016/j.jbtep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.