Abstract
BACKGROUND
Cervical intervertebral disc herniation can lead to myelopathy. Aging is an established variable related to spondylotic myelopathy. Studying this association will help in controlling the increase in spondylotic myelopathy with age.
OBJECTIVES
To study the association between cervical disc level, its direction, and the frequency of myelopathy with age, and to assess the epidemiology of age-related cervical disc herniation and myelopathy.
DESIGN
Retrospective review of magnetic resonance (MR) images.
SETTING
Tertiary referral hospital.
PATIENTS AND METHODS
We studied the MR images of adults patients (>18 years of age) referred to our department between 2001 and 2012 for suspected cervical spondylopathy. The direction and severity of herniation and the presence of myelopathy was determined for spinal levels C2 to C7.
MAIN OUTCOME MEASURE(S)
Relationship between age-related cervical disc herniation and myelopathy.
RESULTS
We studied 6584 patient MR images, which included 2402 males (39.1%) and 3737 females (60.9%). The frequency of myelopathy increased with age from 0.6% in patients <20 years of age, reaching 9.1% in patients >70 years of age. The most common level affected by myelopathy was C5–C6. In elderly patients (>60 years), the C3–C4 level became the predominant level affected by myelopathy. Likewise, the frequency of central disc herniation increased significantly (P<.001) with age at all cervical levels. Furthermore, upper cervical levels showed a higher frequency of central disc herniation than lower cervical levels in the elderly.
CONCLUSION
The increased frequency of central disc herniation with age suggest an important, and probably a cause-effect relationship, between herniation and myelopathy.
LIMITATIONS
We were unable to access clinical data or electrophysiological studies to correlate with MR image findings.
Cervical spondylosis constitutes the age-related degenerative changes affecting the vertebrae, intervertebral discs, true joints of the cervical spine and associated ligaments,1 each of which can be affected by a wide spectrum of degenerative changes.2 The wide spectrum of spondylotic changes in the cervical spine may have variable manifestations, ranging from an incidental finding on a magnetic resonance (MR) image to severe morbidity in the form of myelopathy.
Extensive work has attempted to correlate the neurological complaints associated with spondylotic changes with the major degenerative changes leading to narrowing of the spinal canal.3 The main three spondylotic changes resulting in canal narrowing are intervertebral disc (IVD) herniation, the appearance of osteophytes and ossification of joint ligaments.1 These changes are the most common known causes of nontraumatic myelopathy, which in severe form is known as cervical spondylotic myelopathy (CSM).4
Cervical IVD herniation associated with spondylotic changes is one of the most common causes of myelopathy, and the only process significantly associated with clinical manifestations.5 The IVD is composed of a central nucleus pulposus and surrounding annulus fibrosus. The basic structural unit of the nucleus pulposus is glycosaminoglycan, which gradually diminishes in size and number with age, increasing the vulnerability of IVD material to herniation.6
Aging is the only independent variable that is significantly related to the development of spondylotic changes.7 Aging also changes the pattern of myelopathy (in terms of severity and level of affection), as elderly patients have more severe manifestations and worse surgical outcomes as compared with younger patients. In addition, myelopathy in the elderly affects upper cervical levels as compared with lower levels in younger age groups.8–10
We conducted a retrospective review of the cervical MR images taken in our tertiary referral hospital for cervical spondylosis. The MR images were analyzed according to the location and severity of IVD herniation and its relationship to age, gender and the presence or absence of cervical myelopathy. We mainly focused on the effects of aging on both cervical IVD herniation and myelopathy. We also provide an epidemiological overview of cervical IVD herniation and myelopathy in Jordan.
PATIENTS AND METHODS
This retrospective study was approved by our institutional review board who waived informed consent. Patients referred to the department of radiology and nuclear medicine at our tertiary, referral university hospital between 2001 and 2012 for suspected cervical spondylopathy (due to a wide range of complaints from simple neck pain to motor weakness) were included in this study.
Exclusion criteria included incidental findings of malignant, Inflammatory, traumatic or demyelinating processes. Only subjects older than 18 years of age were included.
Interpretation of images
All cervical MR images for our population were retrieved and reviewed by four experienced neuroradiologists (the authors) during the years 2012 to 2015. IVD herniation at each cervical level from C2–C7 was examined for the direction and severity of herniation and the presence of myelopathy. As no consensus on the nomenclature or classification of cervical IVD herniation for MR imaging exists, we adopted the classification used for lumbar disc herniation, which was applied to all cervical levels on each MR image.11
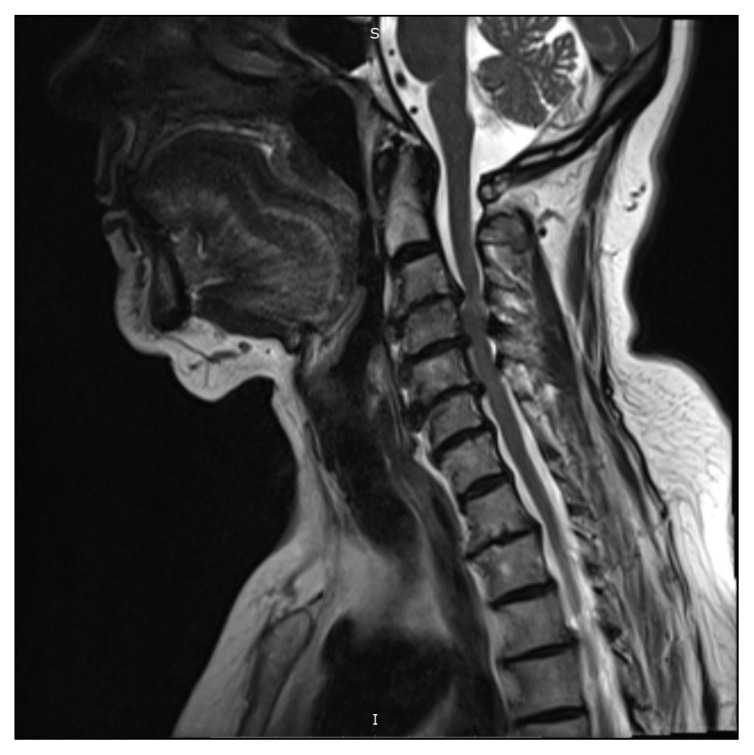
The direction of herniation was classified as central (between the medial parts of the facets), right and left posterolateral (between the medial parts of facets and the lateral parts of the pedicles), foraminal (lateral to the lateral part of the pedicle on both sides), and as a diffuse bulge (more than 50% of disc circumference beyond the disc space, arbitrarily included as a disc herniation to study its role in myelopathy with the other types of herniation). Severity was classified according to proximity to the spinal cord as normal (0), indenting the thecal sac (1), abutting the spinal cord (2), and compressing the spinal cord (4). Myelopathy was diagnosed based on the presence of intramedullary high-signal intensity on T2-weighted MR image in patients with a spondylotic lesion of the cervical spinal cord (Figure 1).12
Figure 1.
Sagittal T2 weighted image of the cervical spine showing cord compression and myelopathy manifested by focal hyperintensity in the cervical cord.
MR Imaging protocol
The cervical MR images done in our department from 2001 to 2006 were performed using Siemens Vision Plus 1.5 Tesla MRI Scanner while from 2006 to 2012 a Siemens Magnetom Verio 3T MRI scanner was used. With both scanners, a similar protocol for the cervical spine was used: T1-weighted sagittal (3 mm thickness, TR/TE 700/10, matrix 384*384), T2-weighted sagittal (3 mm thickness, TR/TE 3000/107, FOV 220mm, matrix 384*384), T2-weighted axial (3 mm thickness, TR/TE 4500/102, FOV 160mm, matrix 320*320) and STIR-weighted sagittal (3 mm thickness, TR/TE 4000/57, FOV 220mm, Ti 220, matrix 320*320) sequences.
Statistical analysis
We used IBM SPSS Statistics for Windows, Version 19.0 (Armonk, NY: IBM Corp.). Descriptive statistics were used to describe our sample population, including patient age and gender. One-way ANOVA was used to study the relation between age and direction of IVD herniation and age and presence of myelopathy. After that we grouped our sample population by age to study changes in patterns of both myelopathy and IVD herniation in each age group (Table 1). Chi-square and the z-test for proportions were used to analyze the relation of age with level of myelopathy, age with herniation, and direction of herniation with severity of herniation. These tests were also used to study the relationships between gender and both myelopathy and IVD direction (Tables 2, 3 and 4), with P value of less than .05 as significant.
Table 1.
Frequency of direction of herniation by age group.
| Age group | Cervical spine level | Number of patients | ||||
|---|---|---|---|---|---|---|
| C2–C3 | C3–C4 | C4–C5 | C5–C6 | C6–C7 | ||
|
| ||||||
| Central herniation | ||||||
| 18–30 | 0.0 | 7 | 13 | 12.0 | 5 | 584 |
| 31–40 | 1 | 21 | 27 | 23 | 12 | 1085 |
| 41–50 | 2 | 35 | 40 | 34 | 20 | 1799 |
| 51–60 | 2 | 43 | 47 | 34 | 24 | 1448 |
| 61–70 | 3 | 47 | 49 | 37 | 28 | 862 |
| >70 | 5.0 | 54 | 55 | 43 | 29 | 361 |
| Overall | 1.7 | 34.7 | 38.9 | 31.1 | 19.9 | 6139 |
| Left posterolateral | ||||||
| 18–30 | 0.3 | 0.7 | 1.5 | 3.8 | 2.1 | 584 |
| 31–40 | 0 | 1.1 | 4.3 | 9.2 | 5.1 | 1085 |
| 41–50 | 0.2 | 2.7 | 4.9 | 10.2 | 7.4 | 1799 |
| 51–60 | 0 | 3.1 | 5.2 | 11.8 | 9.4 | 1448 |
| 61–70 | 0.2 | 2.9 | 5.5 | 11.8 | 10.1 | 862 |
| >70 | 0.8 | 5.5 | 4.7 | 11.6 | 11.6 | 361 |
| Overall | 0.2 | 2.5 | 4.6 | 10.1 | 7.6 | 6139 |
| Right posterolateral herniation | ||||||
| 18–30 | 0 | 0.7 | 1.5 | 2.4 | 1.5 | 584 |
| 31–40 | 0.1 | 1.9 | 2 | 7.7 | 4.2 | 1085 |
| 41–50 | 0.2 | 2.1 | 4.8 | 9.8 | 7.1 | 1799 |
| 51–60 | 0.1 | 3 | 5.2 | 10 | 8.2 | 1448 |
| 61–70 | 0.2 | 3.2 | 6 | 10 | 7.4 | 862 |
| >70 | 0.6 | 3 | 4.4 | 8.9 | 6.4 | 361 |
| Overall | 0.2 | 2.4 | 4.3 | 8.7 | 6.3 | 6139 |
| Foraminal disc herniation | ||||||
| 18–30 | 0 | 0.2 | 0.3 | 0 | 0 | 584 |
| 31–40 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 1085 |
| 41–50 | 0 | 0.2 | 0.3 | 0.2 | 0.3 | 1799 |
| 51–60 | 0.1 | 0.2 | 0.2 | 0.3 | 0.1 | 1448 |
| 61–70 | 0 | 0.6 | 0.5 | 0.5 | 0.6 | 862 |
| >70 | 0 | 0.3 | 0 | 0 | 0 | 361 |
| Overall | 0.01 | 0.2 | 0.3 | 0.2 | 0.2 | 6139 |
| Diffuse bulge herniation | ||||||
| 18–30 | 0 | 7.4 | 9.6 | 11 | 8.4 | 584 |
| 31–40 | 1.2 | 17.1 | 18.2 | 18.8 | 18.8 | 1085 |
| 41–50 | 1.2 | 21.9 | 20.8 | 23.2 | 26.7 | 1799 |
| 51–60 | 1.2 | 22.1 | 22.1 | 26 | 29.5 | 1448 |
| 61–70 | 1.2 | 21.1 | 21.6 | 26.2 | 30.5 | 862 |
| >70 | 1.2 | 17.7 | 23.3 | 23.5 | 29.9 | 361 |
| Overall | 1.2 | 19.4 | 19.8 | 22.4 | 24.9 | 6139 |
Values are percentages.
Table 2.
Frequency of compressed spinal cord (grade 3 severity) in each direction of herniation in all age groups.
| C2–C3 | C3–C4 | C4–C5 | C5–C6 | C6–C7 | |
|---|---|---|---|---|---|
|
| |||||
| Central | 67 | 85 | 77 | 57 | 57 |
| Left posterolateral | 3 | 4 | 7 | 15 | 16 |
| Right posterolateral | 12 | 5 | 7 | 13 | 14 |
| Foraminal | 3 | 0 | 1 | 1 | 1 |
| Diffuse Bulge | 15 | 5 | 8 | 13 | 12 |
Values are percentages.
Table 3.
Locations of IVD herniation at each cervical level by gender.
| Central | Left posterolateral | Right posterolateral | Foraminal | Diffuse bulge | Number of patients | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| C2–C3 | Male | 2.1 | 0.3 | 0.2 | 0.0 | 1.7 | 2402 |
| Female | 1.4 | 0.1 | 0.1 | 0.0 | 1.3 | 3737 | |
| C3–C4 | Male | 37.4 | 3.7 | 3.3 | 0.3 | 19.4 | 2402 |
| Female | 33.0 | 1.7 | 1.8 | 0.2 | 19.3 | 3737 | |
| C4–C5 | Male | 43.5 | 5.2 | 4.3 | 0.3 | 18.9 | 2402 |
| Female | 36.0 | 4.3 | 4.2 | 0.2 | 20.4 | 3737 | |
| C5–C6 | Male | 32.3 | 10.9 | 10.7 | 0.3 | 22.1 | 2402 |
| Female | 30.4 | 9.6 | 7.5 | 0.2 | 22.5 | 3737 | |
| C6–C7 | Male | 20.0 | 9.7 | 8.3 | 0.2 | 25.1 | 2402 |
| Female | 19.8 | 6.2 | 5.1 | 0.2 | 24.8 | 3737 | |
Values are percentages. (P<.001).
Table 4.
Frequency of myelopathy in each gender.
| C2–C3 | C3–C4 | C4–C5 | C5–C6 | C6–C7 | Total number | |
|---|---|---|---|---|---|---|
|
| ||||||
| Male | 0 | 33 | 30 | 29 | 9 | 117 |
| Female | 2 | 25 | 23 | 47 | 4 | 57 |
Values are percentages. (P<.001).
RESULTS
MR images for 6139 patients were included in the study. There were 2402 males (39.1%) with a mean (SD) age of 49.6 (14.5) (range, 18–95) years and 3737 females (60.9%) with a mean (SD) age of 48.1 (12.8) (range 18–98) years. MR imaging records of 445 were excluded due to a traumatic process (n=224), intramedullary tumors (n=41), and transverse myelitis (n=17). Also excluded were 163 patients 18 years of age or younger.
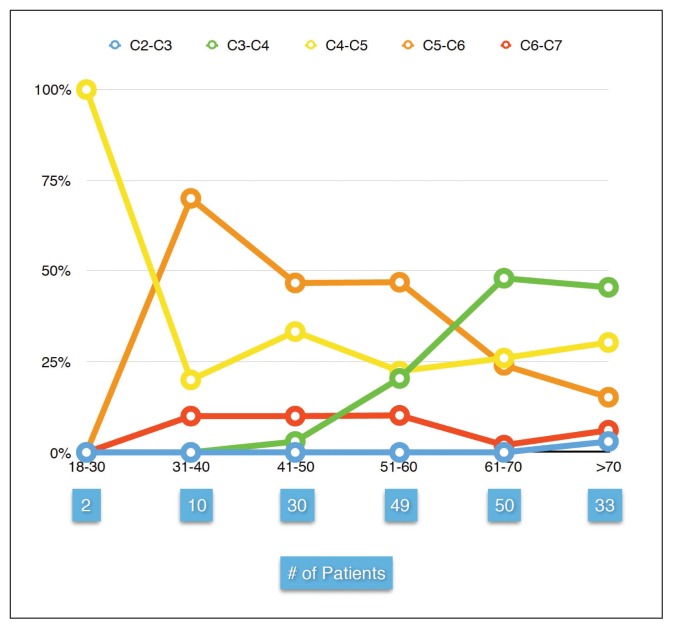
From our study population of 6139 patients, 174 patients (2.8%) with a mean (SD) age of 60 years had myelopathy. The frequency of myelopathy increased with age from 0.6% in patients less than 20 years, reaching 9.1% in patients over 70 years of age. The mean age of myelopathy between each cervical level was significantly different (P<.001), especially between C3–C4 (mean [SD] age of 66 (12.3) years) and C5–C6 (mean [[SD]] age of 55 (11.9) years) so that C5–C6 level was predominantly affected by myelopathy in patients younger than the age of 60 years. After the age of 60 years, the C3–C4 level became predominantly affected (Figure 2).
Figure 2.
Percentage of patients with cervical myelopathy (Y axis) in each age group (X axis), total number of patients in each age group are shown below the corresponding age group. Note that C5–C6 level is the most common level affected by myelopathy in patients below 66 years of age, after that age C3–C4 become the most common level affected.
The frequency of central disc herniation was significantly increased with age at all cervical levels (P<.001). This increase occurred more in older age groups (Table 1). Upper cervical levels (C3–C4, C4–C5) had significantly higher frequency of herniation in a central direction compared with lower levels (C5–C6, C6–C7) (P<.001).
Herniation in the central direction was the most common IVD herniation associated with spinal cord compression (grade 3 severity) (Table 2). At C3–C4 level, discs herniating in the central direction that were compressing or abutting the spinal cord had a significantly higher frequency of myelopathy compared with other levels (P<.001, chi-square or z test). The highest frequency of myelopathy was found with disc herniation in the central direction at the C3–C4 level in patients over 70 (Figure 2).
The higher frequency of disc herniation in the central direction in males at C3–C4 (37.4% male, 33.0% female) and C4–C5 (43.5% male, 36.0% female) levels was statistically significant (P<.001, z test for proportions) (Table 3). The overall frequency of myelopathy was 5% in males and 1.5% in females. The level most commonly affected by myelopathy in males was C3–C4 (33%), while in females the level most commonly affected was C5–C6 (47%) (Table 4).
Analysis of each cervical level
An age-related increase in the frequency of disc herniation was noted for herniation in the central direction and for the left posterolateral direction. Other locations (right posterolateral and foraminal) tended to stabilize or even decrease in frequency around the age of 60. The age-related increase in the frequency of central disc herniation was more pronounced at upper cervical levels (C4–C5 and C3–C4). There was a constant male-to-female ratio in the frequency of IVD herniation of 1.9 to 1 at all levels except for C2–C3, which had a ratio of 1.5 to 1, while the male-to-female ratio for myelopathy was 3.3 to 1. The mean (SD) age for developing IVD herniation was 52 ( 0.5) years except for C2–C3 which was 55 (13.5) years. Diffuse bulge disc was most common at the C6–C7 level with a frequency of 24%. The frequency of diffuse disc bulge tended to decrease after the age of 60 at all levels except for C4–C5. The frequency of diffuse bulge was slightly higher in females at the C4–C5 and C5–C6 levels. C5–C6 was the most commonly affected level for IVD herniation (71.7%) and myelopathy (35.1%).
DISCUSSION
CSM is a devastating disease of the aging spine. As the size of the elderly population increases, more surgical techniques like cervical disc replacement have become available to manage the disease.13 Several studies have shown that elderly patients with CSM have a worse prognosis and surgical outcome than do younger patients.1,14–16 Possible reasons for this difference include age-related spinal cord changes in the form of decreased gamma motor neurons and the presence of comorbidities.17
MR imaging is currently one of the best techniques for diagnosing patients with cervical spine disorders. It has high sensitivity for detecting disc disease and showing the direction and severity of spondylopathy.18 MR imaging is also the most commonly used imaging method for the accurate evaluation of the spinal canal.19
Several studies have proposed that there is an “upward displacement” in the level at which myelopathy occurs from mostly lower levels (C5–C6, C6–C7) in younger patients to mostly upper levels (C3–C4, C4–C5) in the elderly.20,21 Factors relating to the higher frequency of pathology at C3–C4 in the elderly include greater C3–C4 angulation associated with age-related postural change and hypermobility at the C3–C4 segment compensating for decreased mobility at lower segments.22 Our findings on myelopathy were in accordance with this idea as there was a clear age-related upward shifting in our patients after the age of 40. At the age of 66, C3–C4 become the most common level affected by myelopathy compared with younger age groups in which C5–C6 was predominantly affected.
Electrophysiological studies can precisely determine the level of conduction block in CSM.12 Surprisingly, one study concluded: “A high frequency of focal conduction block at C3–C4 or C4–C5 with normal conduction at C5–C6 and C6–C7 characterizes cervical spondylotic myelopathy in elderly people.”23 This suggests that pathological processes resulting in CSM in the elderly occur at the C3–C4 and C4–C5 levels. A limitation of our study was the inability to get electrophysiological data on our patients. However, we made an important observation not yet clearly described in the literature: central disc herniation at the upper cervical levels (C3–C4 and C4–C5) increases remarkably with age (Table 1). Central disc herniation found in the elderly are mostly at the upper cervical levels (C3–C4 and C4–C5), which are mostly the same levels affected by myelopathy in the elderly.
At the same time, we also found that central disc herniation is the most common direction associated with cervical cord compression compared with other directions, which have milder severities. We also found that central discs at the C3–C4 level are associated with worse outcomes as more than 85% are compressing the spinal cord compared to other levels (Table 2). Similarly, Vyas et al showed maximal cord compression with spondylotic changes affecting the C3–C4 level.22 While another study showed a good correlation between severity of IVD herniation and myelopathy.19
A study on the course of disc herniation responsible for cervical myelopathy, which compared several types of disc herniation in patients with CSM, found that 87% of patients with myelopathy have a central disc “median” penetration compared to only 13% for paramedian penetration.23 A similar outcome (regarding myelopathy) was found between IVD herniation that compressed and abutted the spinal cord (which has a less severe compressive effect), a finding that can be explained by the pathophysiology of CSM, which includes the release of proinflammatory mediators from the herniated IVD material. Even touching the spinal cord may be sufficient to release these mediators from an IVD, thus resulting in myelopathy.24 Upon analyzing the frequency of each location and level of IVD herniation, we found a descending order: central, left posterolateral, right posterolateral and finally foraminal. A study by Modic et al also found central disc herniation to be most common.2
Previous studies on IVD herniation indicate that it increases with age.25,26 We found that this is only true for central disc herniation and partially true for left posterolateral herniation. We noted that herniation keeps increasing at all locations with age (including central) reaching a peak around the age of 60. The discs then stabilize or decrease except for herniations in central locations, which continue to increase in frequency increase in size even after the age of 70, becoming more pronounced at upper cervical levels.
The male-to-female ratio of myelopathy was 3.3 to 1, while for IVD herniation, the ratio was 1.9 to 1. The increased prevalence in males might be explained by the ratio between the cervical canal diameter to vertebral body height, which was larger in females, thus delaying the manifestations of degenerative changes.27
In a study of asymptomatic patients, the investigators found that the C5–C6 level was most commonly affected by diffuse disc bulge. We found that C6–C7 was the most common level.25 The descending order in our study was C6–C7, C5–C6, C4–C5, C3–C4 then C2–C3. The most common level affected by IVD herniation (regardless of direction) was C5–C6 (1), which was also the most commonly affected level affected in our middle eastern population. The mean age of development of IVD herniation in our population was at the upper limit (54 years) of the range suggested by European studies (45–54 years).25 We also found a constant male-to-female ratio (1.9:1) at all cervical levels (except C2–C3 where the ratio was lower), which is less than that found in American studies (2.2:1).28
The limitations of our study included the inability to access clinical data or electrophysiological studies for our study population in order to correlate them with MR imaging findings, and assess whether there was a difference in the severity of myelopathy between upper and lower cervical levels, because many of our patients were referred from other centers or clinics for MR imaging.
In summary, after analyzing cervical MR images for a large number of patients referred for cervical spondylosis, we noted that central disc herniation is the commonest direction for cervical disc herniation overall. In addition, the frequency of central disc herniation keeps increasing with age, especially for upper cervical levels including C3–C4 and C4–C5, unlike cervical herniation in other directions. Regarding myelopathy, its frequency increases with age from 0.6% below the age of 20, reaching 9.1% after the age of 70 years. This increase in frequency of myelopathy associated with an upward shifting in the most common level affected by myelopathy, from being at C5–C6 below the age of 70 to C3–C4 level after the age of 70 years. The increased frequency of central disc herniation with age suggest an important, and probably a cause-effect relationship, between herniation and myelopathy.
Footnotes
Conflict of interest
The authors report no conflict of interest.
REFERENCES
- 1.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010 May;16(3):176–187. doi: 10.1097/NRL.0b013e3181da3a29. [DOI] [PubMed] [Google Scholar]
- 2.Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology. 2007 Oct;245(1):43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 3.Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J. 2009 Jun;18(6):877–883. doi: 10.1007/s00586-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZQ, Zhao YP, Jia WY, et al. Surgical Treatment of Cervical Spondylotic Myelopathy Associated Hypertension--A Retrospective Study of 309 Patients. PLoS One. 2015;10(7):e0133828. doi: 10.1371/journal.pone.0133828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siivola SM, Levoska S, Tervonen O, Ilkko E, Vanharanta H, Keinanen-Kiukaanniemi S. MRI changes of cervical spine in asymptomatic and symptomatic young adults. Eur Spine J. 2002 Aug;11(4):358–363. doi: 10.1007/s00586-001-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbed KM, Coumans JV. Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery. 2007 Jan;60(1 Suppl 1):S28–34. doi: 10.1227/01.NEU.0000249223.51871.C2. [DOI] [PubMed] [Google Scholar]
- 7.Rudy IS, Poulos A, Owen L, et al. The correlation of radiographic findings and patient symptomatology in cervical degenerative joint disease: a cross-sectional study. Chiropr Man Therap. 2015;23:9. doi: 10.1186/s12998-015-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holly LT, Matz PG, Anderson PA, Groff MW, Heary RF, Kaiser MG, Mummaneni PV, Ryken TC, Choudhri TF, Vresilovic EJ, Resnick DK. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. Journal of Neurosurgery: Spine. 2009 Aug;11(2):112–8. doi: 10.3171/2009.1.SPINE08718. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N, Nakanishi K, Fujimoto Y, Sasaki H, Kamei N, Hamasaki T, Yamada K, Yamamoto R, Nakamae T, Ochi M. Clinical results of cervical myelopathy in patients older than 80 years of age: evaluation of spinal function with motor evoked potentials: Clinical article. Journal of Neurosurgery: Spine. 2009 Oct;11(4):421–6. doi: 10.3171/2009.4.SPINE08584. [DOI] [PubMed] [Google Scholar]
- 10.Shigematsu H. Degenerative spondylolisthesis does not influence surgical results of laminoplasty in elderly cervical spondylotic myelopathy patients. European Spine Journal. 2010 May 1;19(5):720–5. doi: 10.1007/s00586-010-1338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fardon DF, Milette PC Combined Task Forces of the North American Spine Society ASoSR, American Society of N. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976) 2001 Mar 1;26(5):E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lyu RK, Tang LM, Chen CJ, Chen CM, Chang HS, Wu YR. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry. 2004 Feb;75(2):256–261. [PMC free article] [PubMed] [Google Scholar]
- 13.Vital JM, Boissiere L. Total disc replacement. Orthop Traumatol Surg Res Feb. 2014;100(1 Suppl):S1–14. doi: 10.1016/j.otsr.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Machino M, Yukawa Y, Hida T, et al. Can elderly patients recover adequately after laminoplasty?: a comparative study of 520 patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2012 Apr 15;37(8):667–671. doi: 10.1097/BRS.0b013e31823147c9. [DOI] [PubMed] [Google Scholar]
- 15.Nagashima H, Morio Y, Yamashita H, Yamane K, Teshima R. Clinical features and surgical outcomes of cervical myelopathy in the elderly. Clin Orthop Relat Res. 2006 Mar;444:140–145. doi: 10.1097/01.blo.0000201156.21701.86. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Toyama Y, Chiba K, et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2004 Sep;1(2):168–174. doi: 10.3171/spi.2004.1.2.0168. [DOI] [PubMed] [Google Scholar]
- 17.Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015 Apr;24(Suppl 2):236–251. doi: 10.1007/s00586-013-2658-z. [DOI] [PubMed] [Google Scholar]
- 18.Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013 Feb;31(1):287–305. doi: 10.1016/j.ncl.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Lee JW, Koh YH, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol. 2011 Jul;197(1):W134–140. doi: 10.2214/AJR.10.5560. [DOI] [PubMed] [Google Scholar]
- 20.Northover JR, Wild JB, Braybrooke J, Blanco J. The epidemiology of cervical spondylotic myelopathy. Skeletal radiology. 2012 Dec 1;41(12):1543–6. doi: 10.1007/s00256-012-1388-3. [DOI] [PubMed] [Google Scholar]
- 21.Nagashima H, Dokai T, Hashiguchi H, et al. Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients aged 80 years or older: a multi-center retrospective study. Eur Spine J. 2011;20:240–246. doi: 10.1007/s00586-010-1672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyas KH, Banerji D, Behari S, Jain S, Jain VK, Chhabra DK. C3–4 level cervical spondylotic myelopathy. Neurol India. 2004 Jun;52(2):215–219. [PubMed] [Google Scholar]
- 23.Yamazaki S, Kokubun S, Ishii Y, Tanaka Y. Courses of cervical disc herniation causing myelopathy or radiculopathy: an analysis based on computed tomographic discograms. Spine (Phila Pa 1976) 2003 Jun 1;28(11):1171–1175. doi: 10.1097/01.BRS.0000067262.69584.0A. [DOI] [PubMed] [Google Scholar]
- 24.Demircan MN, Asir A, Cetinkal A, et al. Is there any relationship between proinflammatory mediator levels in disc material and myelopathy with cervical disc herniation and spondylosis? A non-randomized, prospective clinical study. Eur Spine J. 2007 Jul;16(7):983–986. doi: 10.1007/s00586-007-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst CW, Stadnik TW, Peeters E, Breucq C, Osteaux MJ. Prevalence of annular tears and disc herniations on MR images of the cervical spine in symptom free volunteers. Eur J Radiol. 2005 Sep;55(3):409–414. doi: 10.1016/j.ejrad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine (Phila Pa 1976) 2015 Mar 15;40(6):392–398. doi: 10.1097/BRS.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 27.Hukuda S, Kojima Y. Sex discrepancy in the canal/body ratio of the cervical spine implicating the prevalence of cervical myelopathy in men. Spine (Phila Pa 1976) 2002 Feb 1;27(3):250–253. doi: 10.1097/00007632-200202010-00009. [DOI] [PubMed] [Google Scholar]
- 28.Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994 Apr;117(Pt 2):325–335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]