Abstract
BACKGROUND
Academic stress is a good model of psychological stress in humans and is thus useful for studying psychoneurohormonal changes. The aim of the current study was to examine the effect of academic examination stress on activation of the hypothalamus-autonomic nervous system (HANS) and the hypothalamic-pituitary-adrenocortical (HPA) axis, through the measurements of changes in neuro-hormones during final exams as compared to the pre-exam baseline.
MATERIALS AND METHODS
Forty-eight first- and second-year female medical students participated. Plasma leptin, neuropeptide Y (NPY), nitrite, nitrate, andrenomedullin, cortisol and adrenocorticotropic hormone (ACTH) were measured at baseline and during final examinations.
RESULTS
Plasma levels of cortisol, ACTH, NPY, adrenomedullin, nitrite and nitrate increased during times of academic stress as compared to baseline levels. However, only plasma leptin level was decreased during the academic stress as compared to baseline, probably through a negative feedback mechanism resulting from sympathetic stimulation. The results indicate that both the HANS and HPA are involved in this type of stress and both are activated at the same time.
CONCLUSION
: Academic stress induced significant neurohormonal changes. Leptin, NPY, nitrite, nitrate, adrenomedullin, cortisol and ACTH can be considered part of a complex mosaic model of the neuroendocrine system during academic stress.
The hypothalamic-pituitary-adrenocortical (HPA) axis is activated by a wide variety of stresses. The anterior pituitary secretes adrenocorticotropin (ACTH), which in turn stimulates the synthesis and secretion of glucocorticoids.1 Stressful experiences may influence neuroendocrine, immune and cytokine function, as well as physical and psychological well being. Deinze et al demonstrated a reduction in salivary IgA secretion during exam periods, which lasted for at least two weeks post-exam.2 Plasma concentrations of norepinephrine (NE), epinephrine (E), adrenocorticotropic hormone (ACTH), cortisol (Cor) and prolactin (Prol) are proven to reflect stress level. Exposure of healthy subjects to a psychological stressor such as the Stroop color word interference task, public speaking or mental arithmetic in front of an audience resulted in a significant elevation of NE, E, ACTH, and Cor plasma levels. Prolactin levels on the other hand were significantly decreased. These results indicated the involvement of hypothalamopituitary axis and catecholamine in response to psychological stress.3
Adrenomedullin (AM), which was initially isolated from human pheochromocytoma, is a 52-amino acid peptide.4 AM apparently has a number of functions, such as hypotensive activity by inducing vasodilation,5 natriuretic and diuretic activity,6 and a bronchodilator function7. A high level of AM is found in mammalian central nervous systems.8
Leptin (OB protein) is an important factor in the regulation of energy balance. The brain is an established critical site of leptin function yet little is known about its function in the brain.9,10 Leptin may be involved in the acute stress response, regulating inflammatory parameters. Leptin modifies the activity of the hypothalamopituitary axis in the adult rodent and inhibits the production of glucocorticoids from human and rat adrenals in vitro.
Neuropeptide Y (NPY) is a 36-amino-acid neuropeptide present in peripheral sympathetic nerves. It is released during sympathetic activation and potentiates catecholamine effects. NPY exerts an inhibitory effect on human lymphocytes (in vitro) and an inhibitory effect on antibody production when exogenously administered to rats in vivo.11,12
Academic stress (examination stress) is a good example of naturally occurring psychological stress and is proven to alter human immune function. Until now there is no single work on the effect of such stress through the hypothalamo-pitutary-adrenal pathway on leptin or adrenomedullin plasma levels. Our aim was to study the effect of academic stress in first and second year medical students, on plasma levels of NPY, AM, nitrite, nitrate, cortisol and ACTH.
Materials and Methods
The study was conducted in the department of physiology, College of medicine, King Saud University, in the period between October 2002 to June 2003. An official ethics committee at the university approved the study. Forty-eight first-and second-year female medical students were recruited, with ages ranging between 18 to 21 years. Written consent was obtained before the beginning of the study. The participants were screened for health status by means of a questionnaire to rule out any pathological conditions that might interfere with the aim of the study. The students were evaluated twice: at the beginning of the academic year (baseline) and on the day of their final examination (stress). Two blood samples were collected between 8:00 and 9:00 AM by venipuncture. One sample was collected in a plain tube, and after gentle mixing was allowed to clot at room temperature, then centrifuged at 1500g for 10 minutes. The second sample was collected in an EDTA tube, and after gentle mixing, was centrifuged at 1500g for 10 minutes. Serum or plasma samples were then stored at −70°C until assay time. Samples were assayed in a single large batch, in duplicates.
Leptin was measured with a commercial radioimmunoassay RIA developed to sensitively measure low levels of human leptin (Linco Research, Inc., St. Charles, MO). The assay uses a polyclonal antibody raised in rabbits against highly purified recombinant human leptin. Calibrators and 125I-labeled tracer were prepared with recombinant human leptin.
Plasma NPY levels was analyzed by means of the RIA NPY kit (Peninsula Inc., Belmont, Calif., USA). The assay is based upon the competition of 125I NPY and unlabeled NPY (either standard or unknown) binding to the limited quality of the specific antibody in the reaction mixture. Results were expressed as nanograms/dL.
Since NO is a very labile molecule, its direct measurement in biological samples is very difficult. In aqueous solutions, NO reacts with molecular oxygen and accumulates in the plasma as nitrite and nitrate ions. Therefore, the stable oxidation end-products of NO, nitrite (NO-2) and nitrate (NO-3) can be readily measured in biological fluids and have been used in vitro and in vivo as indicators of NO production. Plasma nitrate levels were measured with the Griess reaction. Briefly, samples were initially deproteinized with the Somogyi reagent. 25 Total nitrite (nitite+nitrate) was measured after conversion of nitrate to nitrite by copperized cadmium granules using a spectrophotometer at 545 nm (Ultraspec Plus, Pharmacia LKB Biochrom Ltd, UK). A standard curve was established with a set of serial dilutions (10-8–10-3 mol 1-1) of sodium nitrite. Linear regression was done using the peak area from the nitrite standard. The resulting equation was then used to calculate the unknown sample concentrations. Results are expressed micromoles per liter plasma.
AM concentration was measured using commercially available ELISA kit from Phoenix Pharmaceuticals Ltd. This kit was designed to detect a specific peptide and its related peptides based on the principle of “competitive” enzyme immunoassay. Cortisol was measured with a commercial RIA Kit (INCASTAR Corp., Stillwater, MN). Results were expressed as RIA measured in nanograms/mL. ACTH immunoreactivity levels were assayed from unextracted plasma using an I251 kit (Allergo Nichols immunoassay: Nichols institute, San Juan, Capistrano, California, USA)
The data was analyzed by using EXCEL for windows. The results were expressed as mean±SD. Results were analyzed for significance between the groups, using the two-tailed Student’s t-test. Results were considered significant when P<0.05.
Results
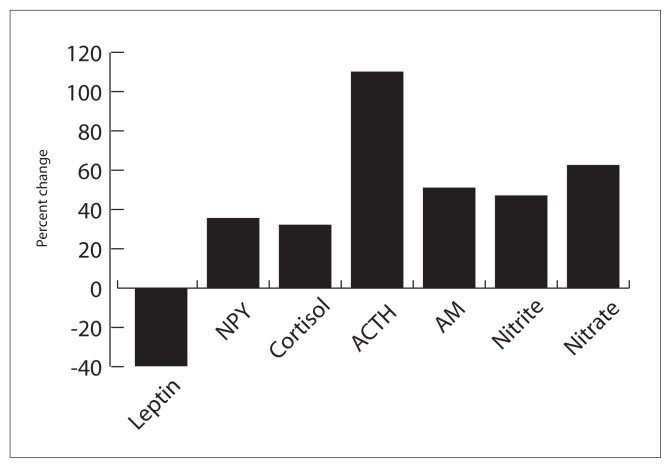
Forty-eight female students participated in the study (mean age of 20±1 years). None of the subjects were taking medication. Leptin plasma concentration significantly reduced during the exam period compared to the baseline period. In addition, ACTH, cortisol, NPY, adrenomedullin, nitrite and nitrate all showed a significant increase in the exam stress period as compared to the baseline time (Table 1 and Figure 1).
Table 1.
Plasma neurohormonal changes during academic stress in 48 female medical students.
| Baseline* (mean±SD) | Under stress (mean±SD) | t (df=94) | |
|---|---|---|---|
| Leptin (μg/L) | 5.8±0.9 | 3.5±0.95 | 1.91 |
| NPY (ng/dL)) | 4.5±0.7 | 6.1±0.67 | 1.66 |
| Cortisol (ng/mL) | 158.9±35 | 210±19 | 5 |
| ACTH (pmol/L) | 10±2 | 21±4.5 | 2.77 |
| AM (pmol/L) | 4±2.4 | 24.4±7 | 4.11 |
| Nitrite (nmol/L) | 305±35 | 450±53 | 2.3 |
| Nitrate (μmol/L) | 24±5.7 | 45±4.4 | 2.1 |
P < 0.05 for baseline vs. under stress for all neurohormone changes.
Figure 1.
The effect of examination stress on plasma concentration of leptin, neuropeptide Y (NPY), cortisol, ACTH, adrenomedulline (AM), nitrite and nitrate in 48 female medical students. (P < 0.05 for baseline vs. under stress for all neurohormone changes.
Discussion
The behavioral and physiological effects of stressors on animals and humans have been widely studied. Most of those studies focused on physiological changes related to activation of the sympathetic system, and changes associated with the anterior pituitary. A wide range of situations, such as cold, heat, exercise, infections, surgery, hemorrhage, food restriction, physical restraint and academic examination have been considered stressful by scientists. Evidence suggests that the pituitary-adrenal (PA) and sympathomedulloadrenal (SMA) axes are common components of the response to stressful stimuli.13 Both the qualitative and quantitative features of the stressors as well as its duration markedly influence the final endocrine response. The role of ACTH in immune, neuroendocrine and behavioral responses in mammals has been well documented for some time. ACTH appears to reflect the intensity of the stressful experience.14 An increase in the circulating ACTH plasma levels is considered a typical initial response to stressful stimuli.14
Academic stress can be considered a good model of a natural stress situation in human. Several studies have focused on the hematological, immunological and hormonal outcome of academic stress. Jammot et al reported a reduction in salivary immunoglobulin A (IgA) levels before examination in a group of 64 dental students.15 Similar results were obtained by Dorian et al, who demonstrated impaired mitogen responsiveness in lymphocytes and a reduction in NK cell activity in students taking a final oral exam.16 The stress of the National Board Medical Examination produced a significant reduction in T lymphocyte polyclonal proliferation.17 In addition, Kiecolt-Glaser et al observed a reduction in the number and activity of NK cells, a decrease in T cell number and in the CD4/CD8 ratio, a reduced lymphocyte response to mitogens and diminished production of IFN-γ.18
In our study, both cortisol and NPY plasma levels increased during the academic stress time as compared to the baseline. This indicates that the two systems, that is the HANS and the HPA, are both responding in similar way. NPY has been coupled to other stress parameters such as norepinephrine, in response to sympathetic activation.19 Physiological and pathological conditions investigated by scientists indicate a largely neuronal source for plasma NPY, although there is a minor contribution from the adrenal gland, especially in hypoglycemia.20,21 Furthermore, elevated plasma levels ACTH, which is released from the anterior pituitary and acts on the adrenal cortex to release cortisol, further prove the involvement of hypothalamus-pituitary-adrenal axis in this type of stress.
Results from animal experimentation suggest a two-way interaction between leptin and the sympathetic nervous system, with leptin causing sympathetic activation and conversely, the sympathetic system imposing a feedback regulatory inhibitory mechanism over leptin release.22 Since leptin and cortisol show an inverse circadian rhythm, it has been suggested that a regulatory feedback mechanism exist between the two hormones.23 Results from our study showed a reduction in leptin plasma levels during academic stress as compared to baseline. During academic stress, the sympathetic nervous system activity increased, and according to the above hypothesis, this activity may exert a negative feedback mechanism over leptin production.
Adrenomedullin is present through the body and its action is mainly mediated by c-AMP, but also by c-GMP elevation. Previous studies have shown that the effect of AM might be secondary to NO release.24 Results from the current study showed a large increase in adrenomedullin plasma levels during academic stress time as compared to the baseline. Furthermore, this was associated with a statistically significant increase in nitrite and nitrate levels. It is well documented that nitrite and nitrate are metabolites of NO in biological systems. Increased nitrite and nitrate reflects an increase in NO production, most likely secondary to adrenomedullin release. Elevated adrenomedullin can exert a cytoprotective effect against organ damage, as suggested by Nishimatsu and coworkers in 2002.25 Elevation of plasma adrenomedullin during academic stress and the increase in NO production might be beneficial biologically as a protective mechanism against other physiological changes in response to the stress situation.
Overall, the HANS and HPA axis are both involved in this type of stress. In addition, both systems work in a similar way. NPY elevation reflects the fact that HANS was activated, whereas ACTH and cortisol changes reflect the involvement of HPA in this type of stress. Furthermore, academic stress caused a reduction in leptin plasma level, mostly due to negative feedback through activation of the sympathetic nervous system. Moreover, the great increase in adrenomedullin plasma levels, and thus an increase in NO production reflect the important role played by adrenomedullin and NO in this type of stress situation, which is very likely a protective effect. Leptin, NPY, nitrite, nitrate, adrenomedullin, cortisol and ACTH can be considered part of a complex mosaic model of the neuroendocrine system during academic stress.
Acknowledgments
The author wishes to express great gratitude to all students who participated in the study, and without them this work will not be possible. Special thanks to Professor Ali Al-Tuwaijri for help and support and for the use of the laboratory facilities. Many thanks to Mr. Casmairo Victoria for technical help.
References
- 1.Chrousos GP. The role of stress and hypothalamic-pituitary adrenal axis in the pathogenesis of the metabolic syndrome: neuroendocrine and target tissues related causes. Int J Obst Relat Metab Disord. 2002;24:50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 2.Deinzer R, Kleineidam C, Stiller-Winkler R, Idel H, Bachg D. Prolonged reduction of salivary immunoglobulin A (sIgA) after a major academic exam. Int J Psychophysiol. 2000;37(3):219–232. doi: 10.1016/s0167-8760(99)00112-9. [DOI] [PubMed] [Google Scholar]
- 3.Gerra G, Zaimovic A, Mascetti GG, et al. Neuroendocrine response to experimentally induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26(1):91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: A novel hypotensive peptide isolated from isolated human pheochromocytoma. Bioch Biophy Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 5.Nuki C, Kawasaki H, Kitamura K, et al. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993 Oct;15(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- 6.Jougaski M, Wei CM, Arthus LL, Heublen DM, Sandberg SM, Burnett JC. Renal localization and action of adrenomedullin; a naturetic peptide. Amr J Physiol. 1995;268:F567–F663. doi: 10.1152/ajprenal.1995.268.4.F657. [DOI] [PubMed] [Google Scholar]
- 7.Kanazawa H, Kurihara N, Hirata K, Kudoh S, Kawaguchi T, Takeda T. Adrenomedullin, a newly discovered hypotensive peptide, is a potent bronchodilator. Boich Bioph Res Commun. 1994;205(1):251–254. doi: 10.1006/bbrc.1994.2657. [DOI] [PubMed] [Google Scholar]
- 8.Serrano J, Uttenthal LO, Martinez A, et al. Distribution of adrenomedullin-like immunoreactivity in the rat central nervous system by light and electron microscopy. Brain Res. 2000;853(2):245–268. doi: 10.1016/s0006-8993(99)02273-8. [DOI] [PubMed] [Google Scholar]
- 9.Caro JF, Kolaczynski JW. Decreased cerebrospinal fluid/serum leptin ration in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 10.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effect of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 11.Nair MP, Schwartz SA, Wu K, Kronfol Z. Effect of Neuropepetide Y on natural killer activity of normal human lymphocytes. Brain Behav Immun. 1993;7:70–78. doi: 10.1006/brbi.1993.1007. [DOI] [PubMed] [Google Scholar]
- 12.Friedman EM, Irwin MR, Nonogaki K. Neuropepetide Y inhibits in vivo specific antibody production in rats. Brain Behav Immun. 1995;9:182–189. doi: 10.1006/brbi.1995.1017. [DOI] [PubMed] [Google Scholar]
- 13.Mason JW. Specificity in the organization of neuroendocrine response Profiles. Front Neurol Neurosci Res. 1974;1:68–80. [Google Scholar]
- 14.Xie J, Nagle GT, Ritchie AK, Collins TJ, Childs GV. Cold stress and corticotropine-releasing hormone induced changes in messenger ribonucleic acid for the L-type Ca channels in the rat anterior pituitary and enriched population of corticotrops. Neuroendocrinology. 1999;70(1):10–16. doi: 10.1159/000054455. [DOI] [PubMed] [Google Scholar]
- 15.Jammot JB, Borysenko JZ, McClelland DC, Chapman R, Mayor D, Benson H. Academic stress, power motivation and decreased in secretory rate of salivary secretory IgA. Lancet. 1983;1:1400–1402. doi: 10.1016/s0140-6736(83)92354-1. [DOI] [PubMed] [Google Scholar]
- 16.Dorian B, Garfinkel P, Brown G, Shore A, Gladman D, Keystone E. Aberration in lymphocyte subpopulation and function during psychological stress. Clin Exp Immunol. 1982;50:132–138. [PMC free article] [PubMed] [Google Scholar]
- 17.Workman EA, La Via MF. T-lymphocytes polyclonal proliferation: effect of stress and stress response style on medical students taking National Board Examination. Clin Immunopathol. 1987;43(3):308–313. doi: 10.1016/0090-1229(87)90140-1. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Glaser R. stress and immune function in human. In: Ader R, Felten DL, Cohen N, editors. Psychooneuroimmunology. San Diego: Academic Press; 1991. pp. 849–867. [Google Scholar]
- 19.Moremede P, Castagne V, Rivet JM, Gaillard R, Corder R. Involvement of neuropeptide Y in neuroendocrine stress. Central and peripheral studies. J Neurol Transm. 1990;29:65–75. doi: 10.1007/978-3-7091-9050-0_8. [DOI] [PubMed] [Google Scholar]
- 20.Morris MJ, Russell AE, Kapor V, et al. Increase in Plasma neuropeptide Y concentrations during sympathetic activation in man. J Auton Nerv Syst. 1986;17:143–149. doi: 10.1016/0165-1838(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 21.Russell AE, Cain MD, Kapoor V, Morris MJ, Chalmers JP. Neuropeptide Y-like immunoreactivity of plasma during hypoglycemia in man. J Auton Nerv Syst. 1989;26:85–88. doi: 10.1016/0165-1838(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 22.Elkelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interaction between leptin and the human sympathetic nervous system. Hypertension. 2003;41(5):1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 23.Casanova FF, Dieguez C. Neuroendocrine regulation and action of leptin. Front Neuroendocrinol. 1999;20(4):317–363. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- 24.Tsuruda T, Brunett C., Jr Arenomedullin: An autocrine/paracrine factor for cardiorenal protection. Circulation Research. 2002;90(6):625–629. doi: 10.1161/01.res.0000015462.11528.28. [DOI] [PubMed] [Google Scholar]
- 25.Nishimatsu H, Hirata Y, Shinodo T, Kurihara H, Kakoki M, Nagat D, et al. Role of adrenomedullin in the regulation of vascular tone and ischemic renal injury: studies on transgenic/knockout mice of adrenomedullin gene. Cir Res. 2002;90:657–663. doi: 10.1161/01.res.0000013697.55301.e7. [DOI] [PubMed] [Google Scholar]