Abstract
BACKGROUND
Primary hyperparathyroidism (PHPT) has a variable clinical expression. Symptomatic PHPT is still the predominant form of the disease in many parts of the world, especially developing countries. Because the clinical profile of the disease has changed from that described in the past, we sought to improve our understanding of the disease in patients in north India.
METHODS
We summarized the clinical presentation, biochemical and radiological features, and operative findings from the case records from the last 13 years of 52 patients at a tertiary care centre in north India who had documented PHPT.
RESULTS
The male: female ratio was 1: 3.3 with ages ranging from 6 to 60 years (mean±SD, 36.38±12.73). Bone disease (46%), recurrent renal stones (21%) and body aches and pains (21%) were the most common modes of presentation. The lag time varied ranged from 1 month to 16 years. Common clinical manifestations included bone pain (67%), weakness/fatigue (56%), fracture of the long bones (48%), abdominal pain (39%), polyuria (37%) and psychiatric manifestations (23.1%). Hypertension was observed in 42% and a palpable nodule in the neck in 19%. Biochemical features included hypercalcemia (86.5%), hypophosphatemia (65.4%) and hyperphosphatasia (67.3%). Mean intact PTH (±SD) was 809.0±696.3 ng/L with levels significantly lower in patients who had only kidney stone disease as compared with those with bone disease (P=0.017). A single parathyroid adenoma was localized in 50 (98%) patients. Hungry bone disease was seen in 59% patients.
CONCLUSION
PHPT in India continues to be a symptomatic disorder with skeletal and renal manifestations at a much younger age.
Primary hyperparathyroidism (PHPT) is a syndrome characterized by inappropriately excessive production of parathormone (parathyroid hormone) with subsequent hypercalcemia, hypophosphatemia, and normal to high serum alkaline phosphatase. Since the classic descriptions of the disease by Fuller Albright about eighty years ago,1 changes have taken place in the expression of PHPT. First, there has been a rise and a fall in the apparent prevalence and incidence of the disease in the West,2 and second, the clinical profile of PHPT in the Western countries had shifted from a symptomatic disorder, with hypercalcemic symptoms, kidney stones, overt bone disease, or a specific neuromuscular dysfunction, toward a more asymptomatic state.3,4 However, PHPT has a variable clinical expression, and symptomatic PHPT is still the predominant form of disease in many developing countries, with skeletal manifestations (osteitis fibrosa cystica) being very common.5 The pathogenesis of PHPT is poorly understood. Consistent somatic mutations in the parathyroid glands and irradiation of the neck are demonstrable in only a fraction of patients.6,7 Vitamin D deficiency has been considered a contributory cause but it may be clinically occult.8 However, the presence of a severe form of PHPT in some vitamin D-sufficient populations,8 and the lack of skeletal disease in other vitamin D-deficient populations5 raise the possibility of additional pathogenetic factors. In this study, we describe data on 52 patients with PHPT seen over the last 13 years with the aim of furthering our understanding of PHPT in North India.
Patients and Methods
Over the last 13 years (since records were available), 52 cases (12 male, 40 female) of documented primary hyperparathyroidism were identified from the Central Medical Records of Nehru Hospital at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. This large tertiary care hospital in North India caters to patients mainly from the Indian states of Punjab and Haryana where consumption of dairy products is in plenty and exposure to sunlight is good. The medical records of these 52 documented PHPT patients were retrospectively reviewed for age, sex, marital status, previous medical history, presenting signs and symptoms, routine biochemical investigations, radiological findings and histopathological diagnosis. The diagnosis of PHPT was based on one or more of the following criteria: 1) persistent elevation of serum calcium above the upper limit of normal range of 2.74 mmol/L (11 mg/dL), excluding other demonstrable causes of hypercalcemia; 2) increased circulatory immunoreactive intact parathyroid hormone (PTH) above the upper limit of normal range of 72 ng/L; 3) characteristic radiographic features of PHPT; and 4) histological evidence (after parathyroidectomy) of a parathyroid adenoma. Patients with a suggestion of multiple endocrine neoplasia (MEN) were excluded from the study.
Fasting blood samples were collected on three consecutive days for estimation of serum total calcium, inorganic phosphorus, total serum alkaline phosphatase, albumin and creatinine. All calcium values were corrected for respective serum albumin concentrations. Twenty-four hour renal excretion of calcium and phosphorus were used as additional helpful tests to complete the biochemical profile. In addition to an abbreviated skeletal survey (radiograph of hands, skull, lumbar spine, pelvis and any suspected or known sites of fractures and brown tumors) in all patients, a bone scan was done in some patients to look for the extent of bone involvement. Imaging of the neck included one or more of the following: ultrasonography, contrast enhanced computerized tomography (CECT), and parathyroid scan, either a thallium-technetium subtraction study or a sestamibi scan, whenever indicated.
The Statistical Program for the Social Sciences (Release 10.0.1, PC Windows; SPSS Inc., Chicago, IL) was used for data analysis. Data are expressed as mean±SD. In addition to descriptive statistics, the chi-square test was used for comparison between categorical variables and student’s t test was used in comparing continuous variables. When the data was not normally distributed, an appropriate non-parametric test was used for comparison. Other tests used included one-way analysis of variance (ANOVA). All t-values were calculated as two-tailed, and P<0.05 was considered statistically significant.
Results
Age of the patients ranged from 6 to 60 years (mean±SD, 36.38±12.73 years) with no significant difference between males and females (36.79±14.61 vs. 36.25±12.31 years). The male: female ratio was 1: 3.3. The majority (67%) of patients were less than 40 years old, including six patients (2 males) less than 20 years old. The single most cited reason for referral was recurrent renal stones and body aches (21% each). However, if different manifestations of bone disease are combined into a single entity then bone disease was the commonest mode of presentation (46%). The lag time from first reported symptom to the time of evaluation for PHPT varied, ranging from 1 month to 16 years with a median of 3 years (mean±SD, 48.9±47.9 months). Clinical characteristics of the 52 patients are shown in Table 1. There was no statistically significant difference in the frequency of clinical manifestations between males and females except that bone pain was more often reported by females than males (75% vs. 42%; P=0.031). Psychiatric abnormalities were seen in 12 (23.1%) patients with no sex preponderance. Mean blood pressure was 133±21 mm Hg (systolic) and 85±12 mm Hg (diastolic). Hypertension (BP(140/90 mm Hg) was more often seen in males than in females (83% vs. 30%, P=0.001). A palpable nodule in the neck was found in 10 (19%) patients.
Table 1.
Clinical characteristics of 52 patients with primary hyperparathyroidism.
| Clinical manifestation | Frequency | % |
|---|---|---|
| Bone pain | 35 | 67 |
| Fracture | 25 | 48 |
| Weight loss | 14 | 27 |
| Kyphosis | 4 | 8 |
| Weakness/fatigue | 29 | 56 |
| Polyuria | 19 | 37 |
| Anorexia | 12 | 23 |
| Nausea/vomiting | 9 | 17 |
| Constipation | 10 | 19 |
| Abdominal pain | 20 | 39 |
| Ulcer dyspepsia | 8 | 15 |
| Dysuria | 5 | 10 |
| Graveluria | 10 | 19 |
| Hematuria | 3 | 6 |
| Depression | 8 | 15 |
| Irritability | 6 | 12 |
| Memory loss | 1 | 2 |
| Emotional lability | 5 | 10 |
| Others | 7 | 14 |
| Hypertension (≥140/90 mmHg) | 22 | 42 |
Mean values of biochemical parameters for the 52 patients are shown in Table 2. Hypercalcemia (>2.74 mmol/L) was documented in 45 (86.5%) patients though hypercalcemic symptoms were seen only in 40 (77%). Mean serum calcium in our patients was 0.3 mmol/L (1.2 mg/dL), higher than the upper limit of normal value of 2.74 mmol/L. All seven patients who had normocalcemia were females. On average, corrected serum calcium was 0.27 mmol/L higher in females than in males (P=0.002). Hypophosphatemia (P<1 mmol/L) was seen in 34 (65.4%) patients. Of 18 patients who did not have hypophosphatemia, 14 had had hypercalcemia while 4 had normocalcemia. Serum phosphorus had a significant correlation with serum calcium (r=−0.3; P=0.039) and serum iPTH (intact parathyroid hormone) (r=0.4; P=0.014). Serum iPTH was elevated in all 47 patients in whom it was available, with 29% having PTH ≥1000 ng/L. The mean serum alkaline phosphatase (SAP) was higher than lab normal (28–92 IU/L) in only 35 (67.3%) patients.
Table 2.
Biochemical parameters for patients with primary hyperparathyroidism.
| Biochemical Parameter | Value | |
|---|---|---|
| Mean±SD | Range | |
|
| ||
| Serum calcium (mmol/L) | 3.04 ± 0.27 | 2.45–3.79 |
|
| ||
| Serum phosphorus (mmol/L) | 0.9 ± 0.19 | 0.39–1.39 |
|
| ||
| Serum PTH (ng/L) | 809.0 ± 696.3 | 88–2720 |
|
| ||
| Serum alkaline phosphatase (IU/L) | 361 ± 360 | 22–1578 |
|
| ||
| Serum creatinine (μmol/lL) | 106.1 ± 88.4 | 26–380 |
|
| ||
| 24 hr. urinary calcium (mmol/d) | 83.1 ± 51.3 | 20–266 |
|
| ||
| 24 hr. urinary phosphorus (mmol/d) | 642.5 ± 350.2 | 49–565 |
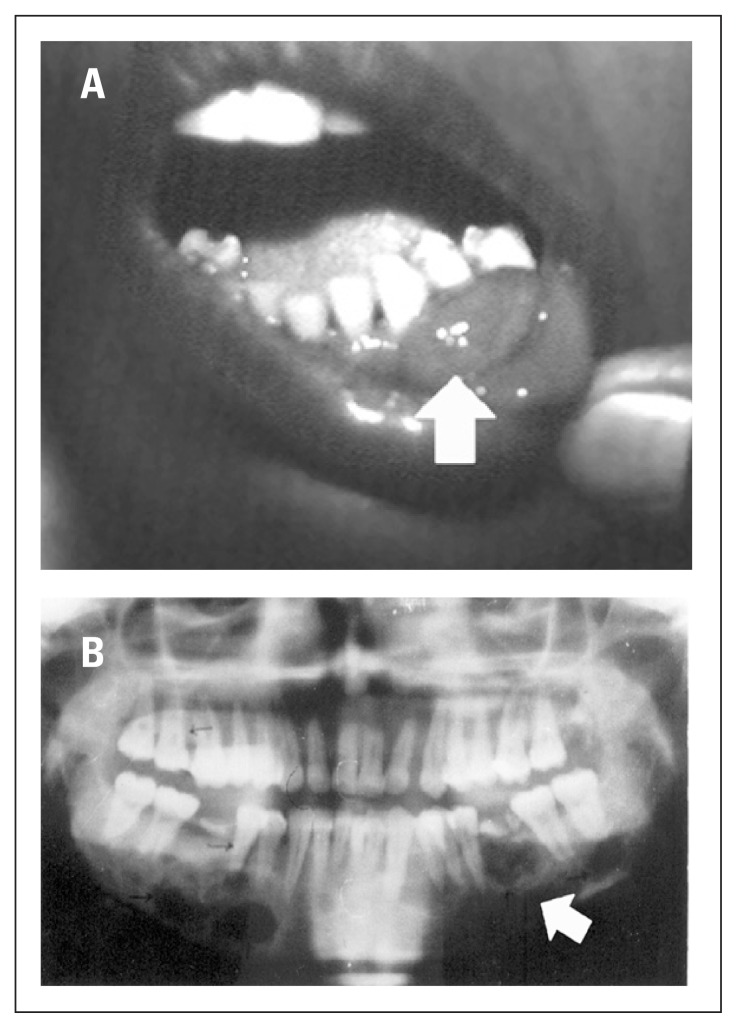
Patients with high SAP activity were younger, had a shorter lag time, and more frequent neuromuscular symptoms than patients with normal SAP activity (Table 3). Interestingly, all 35 patients with high SAP activity had confirmed bone disease on radiology though an additional 10 patients with bone disease had normal SAP activity. SAP activity had a significant positive correlation with serum iPTH (r= 0.5; P=0.001) but no significant correlation with serum calcium or phosphorus. Serum vitamin D levels were available in only a few patients and were within the normal range. Radiological findings included subperiosteal resorption (81%), osteitis fibrosa cystica (58%), pathological fractures of long bones (48%), nephrocalcinosis (18%), nephro-urolithias (60%), and both nephrocalcinosis plus nephrourolithias (24%). Bone disease (osteopenia, brown tumor, subperiosteal resorption, etc.) was ultimately found in 45 (86.5%) patients of whom 29 (56.8%) also had evidence of kidney disease. The remaining 7 (13.5%) patients had kidney disease only. Patients with kidney disease only had a significantly lower PTH as compared with those with bone disease (226±65 vs. 988±175, P=0.017). A few of our patients had some unusual features. The youngest patient, a 6-year old male child, who presented with recurrent pneumonitis and multiple rib fractures had metastatic calcification in the lung. One 14-year old girl presented with rickets with a single parathyroid adenoma. One 28-year old female who presented with a bad obstetric history had successful pregnancies after surgery. Other interesting observations included paraganglioma masquerading as parathyroid adenoma, combination single kidney and papillary thyroid carcinoma, and epulis (Figure 1).
Table 3.
Characteristics of patients with normal serum alkaline phosphatase (SAP) activity vs. those with high SAP activity.
| Characteristic | Normal SAP (n=17) | High SAP (n=35) | P value |
|---|---|---|---|
| Age (years) | |||
| Mean±SD | 42.3±12.4 | 33.5 ± 12.01 | 0.018* |
| > 30 years | 88 % | 60 % | 0.036* |
| Lag time < 3 years | 24 % | 60 % | 0.014* |
| Bone pain | 35 % | 83 % | 0.001* |
| Fracture | 35 % | 54 % | 0.161 |
| Creatinine ≥133μmol/L | 50 % | 19 % | 0.030* |
| Hypercalcemia (>2.74mmo/L) | 88 % | 86 % | 0.587 |
| Hypophosphatemia (<1mmol/L) | 77 % | 60 % | 0.390 |
| PTH (ng/L), | |||
| Mean±SD | 338±256 | 1044 ± 729 | 0.001* |
| >500 ng/L | 18 % | 82 % | 0.007* |
| Osteitis fibrosa cystica | 41 % | 66 % | 0.093 |
| Bone disease | 60 % | 100 % | 0.001* |
Significant
Figure 1.
A 35-year old woman with PHPT who presented with epulis (A); jaw radiograph of same patient (B).
Of 52 patients with PHPT, pre-op localization was ultimately achieved in 50, whereas in one patient MRI showed an artifact in the parathyroid area. In pre-operative localization, ultrasound was positive in 41/52 (78.8%), CT scan in 29/39 (74.4%), MRI in 3/4 (75%) and thallium-technetium subtraction/sestamibi scan in 15/16 (93.8%). All patients were operated on with the exception of two, as both refused surgery. The majority (70%) had adenoma on the left side with left inferior parathyroid adenoma observed in 28 (56%) patients. Twenty-three patients (48%) developed features of hungry bone disease with most (59%) developing hypocalcemia on day 2 of surgery. All patients with hungry bone disease were treated with oral calcium (3–6 g/d) and calcitriol (0.5–1.0 μg QD) though some cases needed intravenous calcium initially. Parathyroid gland weight (available in 32 patients only) ranged from 1.2 g to 15 g (mean±SD, 6.35±4.4, median 4.8). Adenoma was found in 48 (96%) and carcinoma was found in three (6%) patients. The diagnosis of parathyroid carcinoma was based on the presence of gross capsular and vascular invasion, cellular and nuclear pleomorphism and a high mitotic index with or without lymph node metastasis. Their clinical presentation was almost similar to that of PHPT patients with adenoma except for a shorter lag time. Of three patients with parathyroid carcinoma, two had a palpable nodule in the neck with one of these two patients also having distant metastasis. Follow up ranged from 6 months to 13 years with a mean (±SD) of 3.82±2.96 years. In patients with parathyroid adenoma, healing of bone lesions and normalization of serum calcium was observed in all except in two patients who had recurrence. Residual disease was observed in two of three patients with parathyroid carcinoma on follow up. Five patients died during follow up; two with end-stage renal disease, one with sepsis, one because of metastatic disease and one due to an unrelated cause.
Discussion
The clinical picture of PHPT in India today is similar to that recognized previously in the West. The disease is seemingly rare, patients are comparatively young, and they suffer from overt skeletal and renal manifestations.9 In this series of patients with PHPT, we have demonstrated that PHPT in India remains a severe symptomatic disorder with variable clinical presentation at a younger age. We do not screen for asymptomatic PHPT and hence have no data on the prevalence of this entity in our region. With 65% of patients below the age of 40 years, we found that mean age at presentation of PHPT was two decades younger than that described in reports from developed countries where mean age at presentation of patients with PHPT is reported from 55 years to 62 years.10,11 In the series reported by Norris12 and Dent,13 62.8% and 68.8% of patients with PHPT were above the age of 40 years, respectively. Our observations are in agreement with other Indian studies where a mean age of less than 40 years has been reported.9,14,15
It is not clear why our patients present at a younger age. As rightly pointed out by some authors, if the reasons for more prevalent osteitis fibrosa cystica in developed countries were mainly due to the delay in diagnosis or late presentation, one would have expected the age at presentation to be higher rather than lower than the cohort in the West.16 One obvious reason for early presentation could be associated vitamin D deficiency, which has been shown in a few previous reports from India.9,15 However, in our series, vitamin D levels in a small number of patients were normal, possibly because most of our patients were from the adjoining states of Punjab and Haryana where consumption of dairy products and exposure to sunlight is good.
Skeletal manifestations of primary hyperparathyroidism (PHPT) account for most of the morbidity associated with this disease. In our series, 46% of patients were referred because of symptoms related to bone disease and at the end bone disease was confirmed in 86.5% patients. Calcium and vitamin D nutrition determine the severity of skeletal disease in PHPT, but additional pathogenetic factors also contribute.17,18 In nutritionally sufficient populations where biochemical screening for hypercalcemia is used routinely, overtly symptomatic PHPT and osteitis fibrosa cystica (OFC), the classic form of skeletal disease in PHPT, have all but disappeared. The frequency of specific radiological manifestations of PHPT has fallen from 23% in the Cope Series19 to less than 2% in the series of Silverberg et al.20 In fact, overt skeletal disease in PHPT is so infrequent in the West that skeletal X-rays are rarely indicated. In such populations, subtle skeletal manifestations can be demonstrated using sensitive techniques such as bone densitometry.21 However, overt manifestations and OFC still dominate the presentation of PHPT in India.9 We found OFC in 58% of our patients. Similar clinical and pathological features of this disease have been reported from other developing countries.22–24
Hypercalcemia was found in about 87% of our patients, which is more like the West where the vast majority of patients with PHPT have hypercalcemia. However, this finding is in sharp contrast to the belief that PHPT in India is more often accompanied by normocalcemia. A previous Indian study reported normocalcemia in about 50% of patients.15 Whether our cohort reflects a different subset of patients with PHPT or is merely a reflection of a possible improvement in vitamin D status is not possible to know since vitamin D levels were not available in all patients. Symptoms suggestive of hypercalcemia were seen in about 77% in our series, whereas these symptoms were conspicuous by their absence in a previous Indian study.15 However, that study had low sensitivity/specificity for detection of hypercalcemia, despite having a good positive predictive value. The majority (99%) of PHPT patients with hypercalcemia in the West have symptoms. Hypophosphatemia (<1 mmol/L) was seen in about 65% of our patients with PHPT (<0.8 mmol/L in 17%). This finding is in agreement with data from the West where hypophosphatemia is reported invariably in patients with PHPT,25 but in sharp contrast to results of previous study on PHPT in India, where mean serum phosphorus was reported as normal.15
Total alkaline phosphatase activity in patients with PHPT is in the high normal range as is the case also for more specific markers of bone turnover–bone-specific alkaline phosphatase activity, osteocalcin, or collagen breakdown products (N-telopeptide, deoxypyridinoline). Surprisingly, serum alkaline phosphate was elevated in only two-thirds of our patients. In other series from India high SAP has been consistently reported, perhaps due to the high level of bone disease in the population. Interestingly, all our patients who had SAP higher than the normal lab value had bone disease. We tried to characterize those who had normal SAP, and found that they were relatively older, had a longer duration of symptoms, less bone pain, less neuromuscular symptoms and a lower iPTH level.
Renal stone disease was present in about 70% of our patients, with 56% having both skeletal and renal disease and 13.5% having renal stone disease exclusively. In other reports from India, all patients with PHPT had bone disease with 53% having associated renal stone disease.14,15 In the West, the previously reported prevalence of 57% of renal stones has fallen to less than five percent in recent years.26 This is understandable because with the introduction of routine calcium estimations by autoanalyser in the West, more and more asymptomatic cases of PHPT are detected. However, the frequency of renal stone disease in our patients is even higher than that reported in other Indian studies. Whether this is related to PHPT per se or to a higher prevalence of renal disease in this region in general is difficult to say. Whether renal stone disease is related to a higher proportion of hypercalciuria seen in our patients is also not substantiated as similar levels of 24-hour urinary calcium were seen in patients without renal stone disease.
The parathyroid glands are well known to vary in anatomic location, requiring knowledge by the surgeon of typical ectopic sites such as intrathyroidal, retroesophageal, the lateral neck and the mediastinum. Because of potential difficulties in locating abnormal parathyroid tissue, preoperative approaches have been developed. Non-invasive imaging of the parathyroids includes use of ultrasound, computed tomography, magnetic resonance imaging, technetium-99m (Tc-99m) sestamibi, and thallium-technetium subtraction study. Although each of these approaches has advantages and disadvantages, the Tc-99m sestamibi procedure appears at this time to enjoy the greatest popularity. Tc-99m sestamibi has another advantage in that the entire mediastinal and cervical regions can be visualized. We used all these imaging techniques in our series depending on availability and affordable by the patient; ultrasound was however done in all patients. Lately we have been using the Tc-99m sestamibi scan only. Nonetheless, we found that ultrasonography was an effective imaging tool as it is cheap, easily available and had a sensitivity of about 79%. The sensitivities for parathyroid localization using Tc-99m sestamibi techniques have been reported in many series. 27–30 The success rate of Tc-99m sestamibi imaging in our series was about 94%.
Surgery is the only curative treatment for PHPT. Bilateral neck exploration with resection of enlarged parathyroid glands has emerged as the standard operation performed for PHPT. In our series, a single parathyroid surgeon with a success rate of 96% performed most of the parathyroid adenomectomies, and only two patients had a recurrence. Though hungry bone syndrome occurred in about half of our patients, it was not difficult to manage, especially after we started a policy of putting all PHPT patients with bone disease on calcium and vitamin D pre-operatively. Two of our patients who were too sick to withstand general anesthesia underwent unilateral neck exploration under local anesthesia and had successful removal of parathyroid adenoma. Recently, unilateral neck exploration with intra-operative PTH assessment has emerged as a valid surgical strategy in patients with solitary parathyroid adenoma with distinct advantages.31
In conclusion, we have shown that primary hyperparathyroidism in India continues to be distinct, with symptomatic skeletal and renal manifestations at a much younger age. We have, in addition, shown that a greater proportion of patients with PHPT now present with hypercalcemia and hypophosphatemia though serum alkaline phosphatase may not always be high especially in those with renal stone disease only. A high degree of suspicion is needed to diagnose such cases. Whether this presentation, which varies from that in earlier reports, represents a different subset of patients or a changing scenario for PHPT in India remains to be seen.
References
- 1.Albright F, Aub JC, Bauer W. Hyperparathyroidism: volume 2, a common clinical and polymorphic condition as illustrated by 17 proven cases from one clinic. J Am Med Assoc. 1934;102:1276–1287. [Google Scholar]
- 2.Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM, Melton LJ., 3rd The rise and fall of primary hyperparathyroidism: a populationbased study in Rochester, Minnesota, 1965–1992. Ann Intern Med. 1997;126:433–440. doi: 10.7326/0003-4819-126-6-199703150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Mundy GR, Cove DH, Fisken R. Primary hyperparathyroidism: changes in the pattern of clinical presentation. Lancet. 1980;1:1317–1320. doi: 10.1016/s0140-6736(80)91783-3. [DOI] [PubMed] [Google Scholar]
- 4.Rao DS. Primary hyperparathyroidism: changing patterns in presentation and treatment decisions in the eighties. Henry Ford Hospital. Med J. 1985;33:194–197. [PubMed] [Google Scholar]
- 5.Silverberg SJ, Sahne E, Demster DW, Bilezikian JP. The effect of Vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999;107:561–567. doi: 10.1016/s0002-9343(99)00294-6. [DOI] [PubMed] [Google Scholar]
- 6.Arnold A, Staunton CE, Kim HG, Gaz RD, Kronenberg HM. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med. 1988;318:658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- 7.Rao SD, Frame B, Miller MJ, Kleerekoper M, Block MA, Parfitt AM. Hyperparathyroidism following head and neck irradiation. Arch Intern Med. 1980;140:205–207. [PubMed] [Google Scholar]
- 8.Rao DS. Role of vitamin D and calcium nutrition in parathyroid growth and disease expression. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Lucknow: Indian Society of Bone Mineral Research; 1998. pp. 25–28. [Google Scholar]
- 9.Mishra SK, Agarwal G, Kar DK, Gupta SK, Mithal A, Rastad J. Unique clinical characteristics of primary hyperparathyroidism in India. Br J Surg. 2001;88:708–714. doi: 10.1046/j.0007-1323.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- 10.Rao DS, Wilson RJ, Kleerekoper M, Parfitt AM. Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: evidence for biphasic disease course. J Clin Endocrinol Metab. 1988;67:1294–1298. doi: 10.1210/jcem-67-6-1294. [DOI] [PubMed] [Google Scholar]
- 11.Parfitt AM, Rao DS, Kleerekoper M. Asymptomatic primary hyperparathyroidism discovered by multichannel biochemical screening: clinical course and considerations bearing on the need for surgical intervention. J Bone Miner Res. 1991;6(Suppl 2):S97–101. doi: 10.1002/jbmr.5650061421. discussion S121–4. [DOI] [PubMed] [Google Scholar]
- 12.Norris EH. The parathyroid adenoma - a study of 322 cases. Surg Gyne Obst. 1947;84:1–41. [PubMed] [Google Scholar]
- 13.Dent CE, Hartland BV, Hicks J, Sykes ED. Calcium intake in primary hyperparathyroidism. Lancet. 1961;12:336–338. doi: 10.1016/s0140-6736(61)90632-8. [DOI] [PubMed] [Google Scholar]
- 14.Kapur MM, Agarwal MS, Gupta A, Misra MC, Ahuja MMS. Clinical and biochemical features of primary hyperparathyroidism. Indian J Med Res. 1985;81:607–612. [PubMed] [Google Scholar]
- 15.Harinarayan CV, Gupta N, Kochupillai N. Vitamin D status in primary hyperparathyroidism in India. Clin Endocrinol (Oxf) 1995;43:351–358. doi: 10.1111/j.1365-2265.1995.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao DS. Primary hyperthyroidism in India: lessons from the past and directions for the future. Indian J Endocrinol Metab. 2000;2:42–49. [Google Scholar]
- 17.Mithal A, Agarwal G, Singh AK, Mishra SK, Rao SD. Severe bone disease in primary hyperparathyroidism in Indians: a reflection of calcium and vitamin D nutritional status? J Bone Miner Res. 1997;12(Suppl):522–530. [Google Scholar]
- 18.Carling T, Rastad J, Akerstrom G, Westin G. Vitamin D receptor (VDR) and parathyroid hormone messenger ribonucleic acid levels correspond to polymorphic VDR alleles in human parathyroid tumors. J Clin Endocrinol Metab. 1998;83:2255–2259. doi: 10.1210/jcem.83.7.4862. [DOI] [PubMed] [Google Scholar]
- 19.Cope O. The study of hyperparathyroidism at the Massachusetts General Hospital. N Engl J Med. 1966;21(1):174–182. doi: 10.1056/NEJM196605262742105. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg SJ, Shane E, DeLaCruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Mineral Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg SJ, Gartenberg F, Jacobs TP, Shane E, Siris E, Staron RB, et al. Increased bone mineral density after parathyroidectomy in primary hyperparathyroidism. J Clin Endocrinol Metab. 1995;80:729–734. doi: 10.1210/jcem.80.3.7883824. [DOI] [PubMed] [Google Scholar]
- 22.Ingemansson SG, Hugosson CH, Woodhouse NJ. Vitamin D deficiency and hyperparathyroidism with severe bone disease. World J Surg. 1988;12:517–521. doi: 10.1007/BF01655437. [DOI] [PubMed] [Google Scholar]
- 23.Meng XW, Xing XP, Liu SQ, Zhan ZW. The diagnosis of primary hyperparathyroidism - analysis of 134 cases. Acta Academiae Medicinae Sinicae. 1994;16:13–19. [PubMed] [Google Scholar]
- 24.Syed Z, Khan A. Skeletal effects of primary hyperparathyroidism. Endocr Pract. 2000;6:385–388. doi: 10.4158/EP.6.5.385. [DOI] [PubMed] [Google Scholar]
- 25.Bilezikian JP, Silverberg SJ, Shane E, Parisien M, Dempster DW. Characterization and evaluation of asymptomatic primary hyperparathyroidism. J Bone Miner Res. 1991;6(Suppl 2):S85–89. doi: 10.1002/jbmr.5650061419. discussion S121–124. [DOI] [PubMed] [Google Scholar]
- 26.Heath H., 3rd Clinical spectrum of primary hyperparathyroidism: evolution with changes in medical practice and technology. J Bone Miner Res. 1991;6(Suppl 2):S63–70. doi: 10.1002/jbmr.5650061415. discussion S83–84. [DOI] [PubMed] [Google Scholar]
- 27.Chen CC, Skarulis MC, Fraker DL, Alexander HR, Marx SJ, Spiegel AM. Technetium-99m-sesta mibi imaging before reoperation for primary hyperparathyroidism. J Nucl Med. 1995;36:2186–2191. [PubMed] [Google Scholar]
- 28.Ishibashi M, Nishida H, Hiromatsu Y, Kojima K, Uchida M, Hayabuchi N. Localization of ectopic parathyroid glands using technetium-99m sestamibi imaging: comparison with magnetic resonance and computed tomographic imaging. Eur J Nucl Med. 1997;24:197–201. doi: 10.1007/BF02439553. [DOI] [PubMed] [Google Scholar]
- 29.MacFarlane MP, Fraker DL, Shawker TH, et al. Use of preoperative fine needle aspiration in patients undergoing reoperations for primary hyperparathyroidism. Surgery. 1994;116:959–965. [PubMed] [Google Scholar]
- 30.Neumann DR, Esselstyn CB, Macintyre WJ, Go RT, Obuchowski NA, Chen EQ, Licata AA. Comparison of FDG-PET and sestamibi-SPECT in primary hyperparathyroidism. J Nucl Med. 1996;37:1809–1815. [PubMed] [Google Scholar]
- 31.Bergenfeiz A, Lindblom P, Tibblin S, Westerdahl J. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: a prospective randomized controlled trial. Ann Surg. 2002;236:543–551. doi: 10.1097/00000658-200211000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]