Abstract
BACKGROUND
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder caused by defective DNA repair that results in extreme sensitivity to ultraviolet (UV) rays. Depending on the type of XP, the disease may affect the skin, eyes and nervous system.
OBJECTIVES
Describe the dermatologic manifestations in patients suffering from XP.
DESIGN
Retrospective, descriptive review of medical records.
SETTING
Dermatology clinic at tertiary care center in Riyadh.
PATIENTS AND METHODS
This study included Saudi patients with clinically confirmed XP.
MAIN OUTCOME MEASURE(S)
Demographic and clinical data including pathology and associated conditions and outcomes.
RESULTS
Of 21 patients with XP, the most common manifestation was lentigines, affecting 18 patients (86%). The most common skin cancer was basal cell carcinoma followed by squamous cell carcinoma (SCC) affecting 15 (71.4%) and 9 (42.8%), respectively. Other skin findings included neurofibroma, trichilemmoma and seborrheic keratosis. Ocular involvement included photophobia, which was the most common finding followed by dryness and ocular malignancies. Two patients showed neurological involvement, which correlated with the type of mutation.
CONCLUSION
Considering that XP is a rare genetic disease, this description of our patient population will aid in early recognition and diagnosis.
LIMITATIONS
Retrospective and small number of patients. Genetic analyses were done for only 5 of the 21 patients.
Xeroderma pigmentosum (XP) is a rare autosomal recessive disease. It was first described in Vienna 1874,1 but its link to defective DNA repair was reported nearly a century later by James Cleaver.2 The specific defect lies within the nucleotide excision repair (NER) pathway and is characterized by hypersensitivity to ultraviolet (UV) light, specifically UVB and UVC. As of now, there are seven known complementation groups, XPA through XPG, with an additional variant type known as XPV.
The clinical manifestations of this disease are predominantly cutaneous, presenting as pigmentary changes and early cutaneous malignancies. Other manifestations include ocular and neurological symptoms such as photophobia and cognitive impairment. There is an established association with consanguinity, and thus, the prevalence of this disease is highest in areas where consanguinity is common, such as in North Africa and the Middle East.
PATIENTS AND METHODS
We conducted a retrospective examination of the medical records of Saudi patients who had been diagnosed clinically or genetically with XP at the Dermatology Clinic, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. This study was conducted after obtaining approval from the Office of Research Affairs. Images are reproduced on consent of the parent or guardian. Data was collected between the months of February and June 2016 from medical records. The prevalence estimate was based on a report of the General Authority for Statistics, Saudi Arabia, 2016.
RESULTS
The earliest and most common manifestation in our review was lentigines, affecting 18 of 21 patients (86%) on sun-exposed areas (Table 1). Nineteen patients (90%) exhibited non-melanoma skin cancer (NMSC) (Table 2). BCC was the most common NMSC finding, which affected 15 patients (71%). Nine patients (42%) had SCC; 7 patients were male and 2 female. The most common location of NMSC was the face, mainly over the nose. Moreover, the two patients without NMSC at the time of writing were aged 1 year and 18 months, respectively. Four patients (19%) had malignant melanoma; two on the scalp, with invasive superficial spreading of malignant melanoma and invasive nodular melanoma. The other two had lentigo maligna melanoma over the cheeks. Other skin findings observed in 7 patients (33%) included seborrheic keratosis, affecting 3 of the 21 patients (14%), neurofibromas in 3 (14%), trichilemmomas in 3 patients (14%) and actinic cheilitis in 1 (4.7%) (Figure 1).
Table 1.
Demographic and clinical characteristics of the 21 patients.
| Case | Gender | Age at diagnosis | Lentigines +/− | NMSC (SCC or BCC) | Cutaneous MM | Other skin findings | Ocular involvement | CNS |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | F | 11 y | + | BCC | − | − | Dryness | − |
| 2 | M | 9 mo | + | BCC | − | Neurofibroma | Photophobia | − |
| 3 | F | 10 y | + | BCC | − | Seborrheic keratosis | SCC, photophobia, dryness | − |
| 4 | M | 26 y | − | BCC+SCC | + | − | Photophobia, Pterygium | − |
| 5 | F | 14 y | + | BCC+SCC | + | − | Dryness, SCC | − |
| 6 | M | 1 y | + | − | − | − | − | − |
| 7 | M | 19 y | + | BCC+SCC | − | Actinic cheilitis | Photophobia, Pterygium | − |
| 8 | F | 23 | + | BCC | − | − | Pterygium | − |
| 9 | M | 6 mo | + | BCC | − | − | Photophobia | + |
| 10 | M | 6 mo | + | SCC | − | − | Photophobia | + |
| 11 | M | 1 y | + | BCC+SCC | + | Neurofibroma Trichilemmoma | Pterygium, photophobia, dryness | − |
| 12 | M | 2 mo | + | SCC | − | − | Photophobia | − |
| 13 | F | 2 y | + | BCC | − | Gingival involvement, gingivitis. | Photophobia | − |
| 14 | F | 3 y | + | BCC | − | Trichilemmoma | Photophobia | − |
| 15 | M | − | − | BCC | − | − | SCC | − |
| 16 | F | 15 y | + | BCC | − | Neurofibroma, seborrheic keratosis | Photophobia, dryness, SCC | − |
| 17 | M | − | − | SCC | − | − | − | − |
| 18 | F | 8 y | + | SCC+BCC | − | Seborrheic keratosis, trichilemmoma, | Limbal melanoma | − |
| 19 | M | Referred to KFSH at 30y | + | SCC | + | − | Photophobia | − |
| 20 | F | 2 y | + | BCC | − | Gingival involvement-pyogenic granuloma | − | − |
| 21 | F | 18 mo | + | − | − | − | Photophobia | − |
NMSC: non-melanoma skin cancer, BCC: basal cell carcinoma, SCC: squamous cell carcinoma
Table 2.
Frequency of skin cancers in the 21 patients.
| Number of patients | % | |
|---|---|---|
|
| ||
| No cancer | 2 | 9.5 |
| SCC only | 3 | 14.2 |
| BCC only | 10 | 47.6 |
| Concurrent SCC and BCC | 2 | 9.5 |
| Concurrent SCC and MM | 1 | 4.7 |
| Concurrent SC, BCC and MM | 3 | 14.2 |
| Total | 21 | |
Number of each type of cancer not shown. BCC: basal cell carcinoma, SCC: squamous cell carcinoma, MM: malignant melanoma.
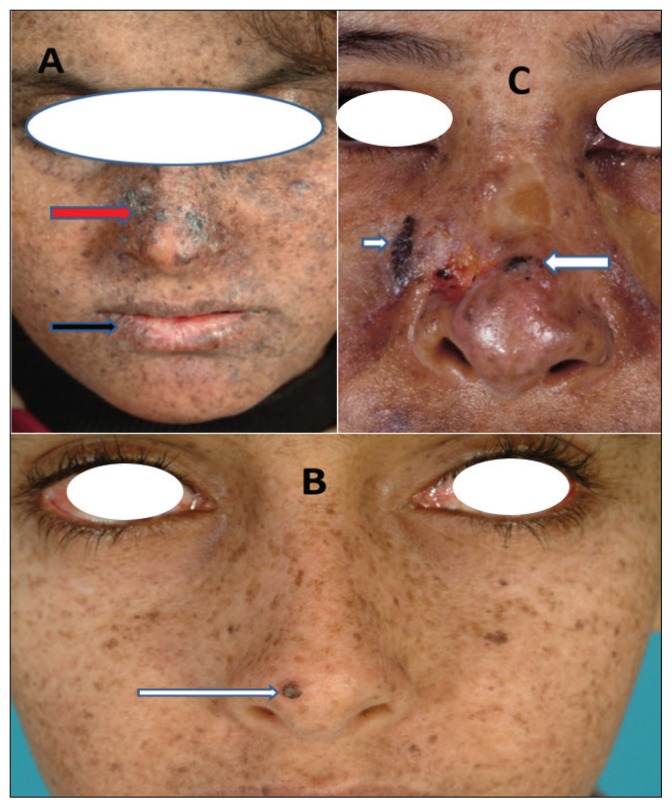
Figure 1.
Clinical presentation of some patients with xeroderma pigmentosum. All showing multiple lentigines. White arrow: basal cell carcinoma; Black arrow: actinic cheilitis; Red arrow: hypertrophic actinic keratosis and squamous cell carcinoma
Two brothers (9%) suffered from neurological involvement in the form of mild mental retardation and speech impairment. Internal neoplasms were only found in one patient, a young female who suffered from a right breast phyllodes tumor. Two of the 21 patients (9%) had oral involvement; one in the form of gingivitis and inflammatory granulomas and the other with acute gingivitis, gingival hyperplasia and oral pyogenic granulomas. Many of our patients reported ocular involvement as well, mostly in the form of photophobia, which affected 13 patients (61%) and dryness, which affected 5 patients (23%). Pterygium was also noted in 4 patients (19%). Four patients (19%) had ocular squamous cell carcinoma, 3 of which affected the limbus, and 1 patient had melanoma in the limbus.
In the five patients who had genetic testing, two brothers with neurological symptoms had a homozygous missense mutation in the XPA gene, which results in c.682C>T (p.R228X). The patient with the breast tumor and two sisters in our review had homozygous missense mutations in the XPC gene which results in c.856A>T (p.R286X).
DISCUSSION
Under normal circumstances, DNA repair pathways are able to correct damage caused by endogenous or exogenous factors such as breaks, bulky adducts and oxidative lesions. However, in XP this process is deficient. The complementation groups affect various stages of the NER pathway resulting in mutations which affect the incision and repair processes. XP variant is caused by postreplication due to exposure to UV rays. The skin is the primary organ involved and cutaneous signs are often first to appear upon sun exposure. Initially they present as sunburns, which eventually turn into pigmentary changes and later into skin cancers.
XP prevalence is highest in areas with increased consanguinity rates such as in North Africa and the Middle East. In Japan, the prevalence is around 1:22 000,3 in the United States and Europe, it is estimated to be around 1:1 000 000.4 Higher rates were observed in Morocco.5 In Saudi Arabia, although it is difficult to determine an exact number, Alfawaz et al6 estimated the incidence to be around 15–20:1 000 000,6 which is about the same rate as in Libya.7 However, in our case series, we estimated an incidence of 1:1000 000, which is the same as in the United States and Europe. It affects men and women equally,4 which is consistent with our series. Consanguinity plays a major role in autosomal recessive disorders. As in our study, almost all parents are first-degree relatives.
The most common manifestation in our study was lentigines, which affects sun-exposed areas with a line of demarcation. The incidence of NMSC in XP patients is about 10 000 fold that of an unaffected individual. Ninety percent of our patients exhibited NMSC. In previous studies a rate of 60% has been reported.8 Taking into consideration the two unaffected individuals were less than two years of age, we expect that at the time of puberty the incidence of NMSC would be 100%. The risk of melanoma is increased by 2000 fold in XP patients. Four of our patients had melanoma. Contrary to that reported in previous studies, the distribution of melanoma in XP was more on the scalp and face rather than the extremities. Other skin manifestations included trichilemmoma, affecting three patients (14%). A potential correlation with XP was reported by Mane, et al in which neurological manifestations were estimated to affect 25% of XP patients.8 However, lower incidences have been reported, which can be explained by the predominance of the XPC type in regions including our region. Neurological manifestations occur more in XPA, XPB, XPD and XPG types.9 It has been reported that there is a 12-fold increase in internal malignancies younger than the age of 20 in sun-protected areas.10 In another study, the occurrence of internal malignancies were 10–20 times greater.11 In this study, internal neoplasms were only found in one patient, a young female who suffered from a right breast phyllodes tumor.
Ocular involvement in our study included photophobia, dryness and pterygium. Moreover, four patients (19%) had ocular SCC and one (4%) had limbal melanoma. Oral changes ranged from gingivitis to squamous cell carcinoma of the oral cavity.12,13 In our study, two patients (9%) had oral involvement; one in the form of gingivitis and inflammatory granulomas and the other with acute gingivitis, gingival hyperplasia and pyogenic granulomas.
In managing this disease, prevention is critical and early diagnosis and close follow up are key. Patients are instructed to adhere to photo-protective measures such as wearing sunscreen and protective gear (sunglasses, gloves, etc.). Regular visits to a dermatologist and ophthalmologist are vital for early detection and management. Due to the high likelihood of psychological stress and social withdrawal, a psychiatrist should also be involved as well as a genetic counselor.
Patients must also be given vitamin D supplements to compensate for strict sun avoidance. Vitamin A derivatives have been shown to reduce the frequency of skin cancers by about 63%.14 However, rebound phenomenon, which is the rapid appearance of malignancies after cessation of treatment, is usually encountered. Topical immunotherapy and chemotherapy haven been used to treat superficial types of skin cancers. In our center, these patients undergo chemical peelings at six-month intervals with 35% trichloroacetic acid under general anesthesia with or without curettage and electrodessication for any suspected superficial skin cancer. We use topical 5-fluorouracil 5% cream twice a week on the face as prophylaxis and twice a day on any suspected skin cancers in between peeling sessions. Trials in progress with gene therapy are showing promising results.15
REFERENCES
- 1.Hebra F, Kaposi M. On diseases of the skin including exanthemata. The New Sydenham Society. 1874;3(61):252–258. [Google Scholar]
- 2.Kraemer KH, DiGiovanna JJ. Shining a light on xeroderma pigmentosum. Journal of investigative dermatology. 2012;132:785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirai Y, Kodama Y, Moriwaki S, Noda A, Cullings HM, MacPhee DG, et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2006;601:171–8. doi: 10.1016/j.mrfmmm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–50. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Doubaj Y, Laarabi FZ, Elalaoui SC, Barakat A, Sefiani A. Carrier frequency of the recurrent mutation c.1643_1644delTG in the XPC gene and birth prevalence of the xeroderma pigmentosum in Morocco. The Journal of Dermatology. 2012;39(4):382–4. doi: 10.1111/j.1346-8138.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Alfawaz AM, Alhussain HM. Ocular Manifestations of Xeroderma Pigmentosum at a Tertiary Eye Care Center in Saudi Arabia. Ophthalmic Plastic and Reconstructive Surgery. 2011;10:1097–4. doi: 10.1097/IOP.0b013e31821c7323. [DOI] [PubMed] [Google Scholar]
- 7.Khatri ML, Bemghazil M, Shafi M, Machina A. Xeroderma Pigmentosum in Libya. International Journal of Dermatology. 1999;38:520–4. doi: 10.1046/j.1365-4362.1999.00751.x. [DOI] [PubMed] [Google Scholar]
- 8.Mane DR, Kale AD, Hallikerimath S, Angadi P, Kotrashetti V. Trichilemmal carcinoma associated with xeroderma pigmentosa: Report of a rare case. Journal of Oral Science. 2010;52:505–7. doi: 10.2334/josnusd.52.505. [DOI] [PubMed] [Google Scholar]
- 9.Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterizes the role of DNA repair. Journal of Medical Genetics. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5(4):511–4. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 11.Robbins JH. Xeroderma pigmentosum: defective DNA repair causes skin cancer and neurodegeneration. JAMA. 1988;260:384–8. doi: 10.1001/jama.260.3.384. [DOI] [PubMed] [Google Scholar]
- 12.Wayli HA. Xeroderma pigmentosum and its dental implications. European Journal of Dentistry. 2015;9:145–8. doi: 10.4103/1305-7456.149664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mareddy S, Reddy J, Babu S, Balan P. Xeroderma Pigmentosum: Man Deprived of His Right to Light. The Scientific World Journal. 2013;2013:534752. doi: 10.1155/2013/534752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. The New England Journal of Medicine. 1988;318(25):1633–1637. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 15.Bernerd F, Asselineau D, Vioux C, Chevallier-Lagente O, Bouadjar B, Sarasin A, et al. Clues to epidermal cancer proneness revealed by reconstruction of DNA repair-deficient xeroderma pigmentosum skin in vitro. Proceedings of the National Academy of Sciences of the USA. 2001;98:7817–22. doi: 10.1073/pnas.141221998. [DOI] [PMC free article] [PubMed] [Google Scholar]