Abstract
Biofilms on implanted medical devices cause thousands of patients each year to undergo multiple surgeries to remove and replace the implant, driving billions of dollars in increased health care costs due to the lack of viable treatment options for in situ biofilm eradication. Remotely activated localized heating is under investigation to mitigate these biofilms; however, little is known about the temperatures required to kill the biofilms. To better understand the required parameters this study investigated the thermal susceptibility of biofilms as a function of their fluidic and chemical environment during growth, as well as their propensity for regrowth following thermal shock. Pseudomonas aeruginosa biofilms were cultured in shaker plate fluidic conditions in four different growth media, then thermally shocked at various temperatures and exposure times. Biofilms were re-incubated to determine their regrowth potential following thermal shocks of various intensity. Results indicate that growth media has little impact on thermal susceptibility, while fluidic conditions strongly influence susceptibility to modest thermal shocks. This effect disappears, however, with increasingly aggressive shocks, reducing biofilm populations by up to 5 orders of magnitude. Regrowth studies indicate a critical post-shock bacterial loading (~103 CFU/cm2) below which the biofilms were no longer viable, while biofilms above that loading slowly regrew to their previous population density.
Keywords: heat shock response, non-oncologic applications of hyperthermia
Introduction
Roughly 45% of all nosocomial, or hospital acquired, infections are device related [1]. Over 100,000 of the 3.6 million medical devices implanted in the United States each year develop a nosocomial infection, typically manifesting as a biofilm on the device surface [2]. These biofilms can lead to systemic infections causing the mortality of otherwise relatively healthy patients. Of the total episodes of systemic infections, 51.4% are nosocomial [3] with crude mortalities ranging from 20 to 40% depending on the pathogen [4]. The most common treatment for these implant infections is high doses of antibiotics and removal of the implant until the infection subsides. Once the patient is clear of infection a third surgery may be performed to place a new implant, though the infection risk is higher for the replacement device [1], [5]. Incidence of nosocomial prosthetic joint infections increase from 1.5–2.5% upon initial surgery to 3.2–5.6% in revision surgeries demonstrating that additional surgeries increase the risk of having an infection [6]. In addition to the long recovery time and decreased quality of life, these biofilm infections are expensive with an estimated cost of $6 billion annually in the United States alone [7].
Biofilms are formed when planktonic, or free swimming, bacteria adhere to a surface and begin to form an extracellular polymeric substance (EPS). The EPS is comprised of glycolipids, glycoproteins, polysaccharides, proteins, and DNA. From the biofilm, bacteria replicate and disperse, causing a possibly life-threatening systemic infection in the patient [8–10]. One reason for the increasing interest in biofilms is their inherently heightened resistance to antibiotics. Both antibiotics and the host’s immune system are less effective against biofilms than they are against planktonic bacteria [11–14]. While planktonic bacteria typically show inhibition at antibiotic concentrations on the order of 1 mg/L [15–18], their biofilm counterparts require antibiotic concentrations up to 128 times higher [18, 19]. However, such high antibiotic doses lead to host cell toxicity long before eradication of the biofilm can occur [20]. This increased resistance to antimicrobial agents in biofilms is due to the protection provided by the EPS, the limited transport through the EPS [12, 13, 21, 22], changes in gene regulation [14, 22–26], and the presence of persister cells [9, 21]. On fully implanted devices, these biofilms are also physically inaccessible, requiring explantation surgery in order to remove the biofilm, followed by re-implantation surgery once the infection has cleared [2, 27].
Many approaches to prevent biofilm formation on medical implants have been investigated; most fall into one of three main categories: drug-eluting surfaces, antimicrobial surfaces, and antiadhesion surfaces. Drug elution is in practice clinically for joint implants, but often results in an increase in the bacteria’s adaptive antibiotic resistance since the antibiotics often elute late and in lower amounts than the minimum inhibitory concentration [7, 28]. Moreover, multiple antibiotics would be needed to address the full spectrum of possible pathogens. Similarly, antimicrobials immobilized to the implant surface would need to be broad spectrum and would therefore lack some of the antimicrobial specialization needed to mitigate all possible pathogenic complications. The greater challenge to this approach, however, is surface fouling by non-specific proteins simply covering the antimicrobial agents [8]. Many surfaces that slow down bacterial adhesion to the implant also decrease the implant’s capability to integrate well with the patient’s body, prolonging recovery [29]. Once a biofilm has formed on an implant it becomes more difficult to eradicate the bacteria. Many approaches have been pursued, ranging from application of DC current [30–32] or ultrasound for dislodging bacteria [33, 34] to enzymes [35, 36] and particles to break up the EPS [8, 37, 38]. Each approach faces implementation or efficacy issues with no clear path to the clinic.
Elevated temperature has proven to be a reliable approach for eliminating bacterial populations in both the planktonic and biofilm phenotype. Heating protocols for planktonic bacteria have long been established at a variety of temperatures and exposure times, enabling a careful balance between reduction of bacterial population and damage to the rest of the suspension (i.e. food pasteurization). To sterilize surfaces with biofilms, pressurized autoclaves at ≥ 120 °C are typically used, however, few efforts have been made to quantify biofilm reduction from less damaging exposures. To thermally eliminate biofilm infections while minimizing the amount of adjacent tissue damage, the relationship between the degree of bacterial population reduction and degree of thermal exposure must be well characterized across the entire range of potential exposures [39–42]. Recently, the decrease in colony forming units (CFU) of Pseudomonas aeruginosa within a bacterial biofilm has been quantitatively correlated to the degree and duration of thermal shock, according to Equation 1:
| (Equation 1) |
where is the temperature in degrees Celsius, is the exposure time at that temperature in minutes, and is the original population density of the bacteria in the biofilm [43]. The bacterial cell death described by Equation 1 covered a temperature range from 37 °C to 80 °C with exposure times ranging from 1 to 30 minutes. In order to quantify the dramatic CFU decrease within this range (up to six orders of magnitude) the biofilms used in that study were grown in tryptic soy broth (TSB) for 1 day in a drip flow reactor to obtain initial CFU loads of nearly 109 CFU/cm2 [43], believed to be far beyond the CFU density typically observed on an infected medical implant. The literature to-date offers little guidance on the applicability of these findings to biofilms grown in vivo, and unfortunately less on the magnitude of reduction necessary to eliminate an infection.
The objective of this study was to determine whether a biofilm’s growth conditions do in fact impact the biofilm’s thermal susceptibility, to quantify this impact, if any, and to determine the regrowth potential of these thermally shocked biofilms. The biofilms used to determine Equation 1 were cultured in a drip flow reactors which provided unlimited nutrient and oxygen access, prompt waste removal, and a virtually shear-free environment. While these conditions promote tremendous population density sizes, they are generally not present physiologically. For this study, biofilms were cultured using a shaker table protocol, with a finite source of nutrients, a finite sink for waste, and a constantly oscillating shear. Moreover, these biofilms matured for four days rather than one, more closely matching the time for a clinical infection to manifest and be addressed. Finally, while the chemical environment in vivo is clearly different from TSB, it is unclear how this would affect the biofilms’ subsequent thermal susceptibility, so biofilms were cultured in a variety of media, including one used primarily for mammalian cell culture: TSB, Mueller Hinton broth (MHB), a minimum glucose medium (GM), and a medium more commonly used for mammalian cell culture (MEM-α). To determine the regrowth potential of thermally shocked biofilms, TSB shaker table biofilms subject to thermal shocks of various intensity were re-incubated and their population density over time was compared with that of freshly inoculated biofilms. Consistent with prior studies, these trials used biofilms of P. aeruginosa, a well-studied, model organism. P. aeruginosa is the third most common bacterium to cause etiologic infections of orthopedic implants, making up 9.2% of all the medical implant infections [44], and its systemic infection mortality (38.7%) is at the top of the range for nosocomial infections [4]. While the absolute degree of population reduction may be different for other bacterial species and remains to be investigated, we anticipate that the impact of growth conditions on thermal susceptibility will be common across most biofilms.
Materials and Methods
Inoculum
Pseudomonsa aeruginosa
PAO1 (15692, American Type Culture Collection, Manassas, VA) stored in glycerol were thawed and streaked onto agar plates (Difco Nutrient Agar, Sparks, MD, USA) and incubated while inverted for 24 hours at 37 °C. Two colonies were then removed and placed into 5 mL of sterilized (autoclaved at 121 °C and allowed to cool prior to use) tryptic soy broth (TSB, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and grown for 24 hours at 37 °C, obtaining an average of 2.12 × 109 ± 0.07 × 109 CFU/mL.
Biofilm Growth and Medium Preparation
Glass microscope slides (75 mm × 25 mm × 1 mm), fully frosted on one side, were placed individually in polystyrene 4-well dishes (Thermo Fisher Scientific, Waltham, MA, USA) along with 333 μL of the inoculum and 5 mL of media per well, and the dishes were sealed with parafilm. These dishes were placed on an orbital shaker table (VWR 1000, 15 mm orbit, Radnor, PA, USA) set at 160 rpm in an incubator at 37 °C for 96 hours. Four different media were used: tryptic soy broth, Mueller Hinton broth (MHB, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), a minimum glucose medium (GM), and a mixture containing 90% by volume minimum essential medium α without nucleosides (MEM-α, Thermo Fisher Scientific, Waltham, MA, USA) mixed with 10% by volume fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA) to better simulate the growth anticipated in a mammal.
Thirty grams of TSB powder were dissolved into a liter of de-ionized water and heated for 10 minutes in a 700 W microwave. Powder MHB was dissolved at a concentration of 21 g per liter of de-ionized water and similarly heated for 10 minutes in a 700 W microwave. Both TSB and MHB were then autoclaved at 121 °C to ensure sterility. The minimum glucose medium (GM) was made by mixing 1.44 mg ferrous sulfate heptahydrate, 24 mg magnesium sulfate, 2.7 g potassium phosphate dibasic, 2.7042 g glucose, 4.3 g potassium monophosphate, and 5.232 g 3-(N-morpholino)propanesulfonic acid into 500 mL of de-ionized water (all chemical components purchased from Fisher Scientific, Waltham, MA, USA) and filter sterilized in a 0.2 μm pore nylon vacuum filter (VWR, Radnor, PA, USA). The 90% MEM-α and 10% FBS (MEM-α/FBS) mixture was made by combining the two components (both liquids) in a 9:1 ratio by volume, MEM-α to FBS, and then filter sterilizing.
Thermal Shock Procedure
After the 96 hour growth period the biofilms and their underlying glass substrates were transferred to a preheated 4-well dish containing 5 mL water per well whose temperature was maintained by a water bath (Isotemp 3013P, Fisher Scientific, Hampton, NH, USA) at the target temperature. To guard against thermal inertia [45] concerns control trials with thermistor arrays confirmed maintenance of the target temperature in the wells. After the target exposure time the substrate and biofilm were swiftly transferred to the recovery plate, another 4-well dish containing 5 mL water in each well at room temperature. Biofilms were shocked at 50, 60, or 80 °C (plus controls at 37 °C), with exposure times of 1, 5, or 30 minutes. Each condition had at least 12 samples, four parallel replicates of three different plates.
Enumeration
Biofilm population density was quantified via resuspension and plating. After the thermal shock, each 4-well dish of recovered biofilm was wrapped in parafilm and sonicated for 10 minutes at 45 kHz (VWR Symphony, 9.5 L, Radnor, PA, USA). The homogenized suspension was then serially diluted in tenfold increments and spot plated using 10 μL samples on agar plates (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). After five minutes to let the samples adsorb, the plates were inverted and incubated at 37 °C for 20–24 hours before counting the colony forming units (CFU). The CFUs were then converted into a more relevant logarithmic population density, via Equation 2:
| (Equation 2) |
where plate count is the number of CFUs in a sample, dilution factor is the number of tenfold dilutions to make that sample, (5 mL / 0.01 mL) is the ratio of total biofilm suspension to the volume sampled, and 18.75 cm2 is the surface area of the biofilm’s substrate [43]. Plates with counts from 3 CFU to 30 CFU were used for calculations. In the case of two dilution sets landing within this range the lower dilution was used. The upper limit, 30 CFU, was chosen based on the ability to reliably count the individual units without overlap issues and the lower limit, 3 CFU, was established to limit the effect of a single CFU skewing the result by more than log(1.5). In the case when the undiluted sample count was lower than the 3 CFU, the count was used but was below the quantification limit.
Confocal Imaging
Biofilm architecture was observed via confocal fluorescent microscopy. Both enumeration and fluorescent microscopy are destructive techniques, so separate biofilms must be used for each. Bacteria were selectively dyed using a Filmtracer LIVE/DEAD Biofilm Viability Kit (Molecular Probes, Inc., Eugene, OR, USA). In this membrane permeability assay Syto9 (excitation wavelength 488 nm, emission wavelength 500 nm, green) enters all cells and fluoresces when bound to nucleic acid, while propidium iodide (excitation wavelength 568 nm, emission wavelength 635 nm, red) can only access cells with damaged membranes, displacing Syto9 in those cells. With non-overlapping emission and excitation peaks, these dyes allow clear differentiation between the dead and live bacteria via confocal microscopy. Thirty microliters each of Syto9 and propidium iodide were added to the recovery well of each biofilm imaged. Biofilms grown in the drip flow reactor (DFR) were imaged using an upright Bio-Rad Radiance 2100 multiphoton/confocal microscope (Hemel-Hempstead, United Kingdom) with a 40x dip lens, while biofilms grown on the shaker table were inspected using a Zeiss LSM 710 confocal microscope (Oberkochen, Germany) with a 63x dip lens. Both microscopes used confocal settings with an argon laser to excite the propidium iodide and a helium-neon laser to excite the Syto9 dye. Biofilms were scanned by horizontal rastering with 1 μm vertical increments from bottom to top. Each row was scanned separately by each laser before advancing to the next row to decrease any overlap in the resulting excitations and emissions. Images were collected in a 1024 × 1024 pixel array and the resulting images were post-processed in the Java-based ImageJ processor (freely available from the NIH website at http://imagej.nih.gov/ij).
Regrowth Trials
An initial growth curve of the biofilms was determined by enumerating biofilms incubated for 1, 2, 4, 24, or 96 hours, then rinsed for 1 minute in sterile, de-ionized water and resuspended by sonication. Post-shock regrowth was investigated by reincubating thermally shocked biofilms in fresh TSB for 2, 4, 12, 24, or 96 hours, then rinsing for 1 minute in sterile, de-ionized water and resuspending for enumeration. The regrowth of biofilms was investigated after heat shocks at 60 °C for 5, 7.5, and 30 minutes, and 80 °C for 1, 5, and 30 minutes. At least three replicates were performed for each heat shock and regrowth time point.
Statistical Analysis
Statistical analysis of the enumeration results was performed in GraphPad Prism 6. Averages and standard deviations were obtained via arithmetic calculations of log (CFU/cm2) values. A two way ANOVA with a 95% confidence interval set was used to compare the means. The graphs were produced in GraphPad Prism 6 based on the calculated arithmetic mean and standard deviation.
Results
Biofilm Architecture and Population
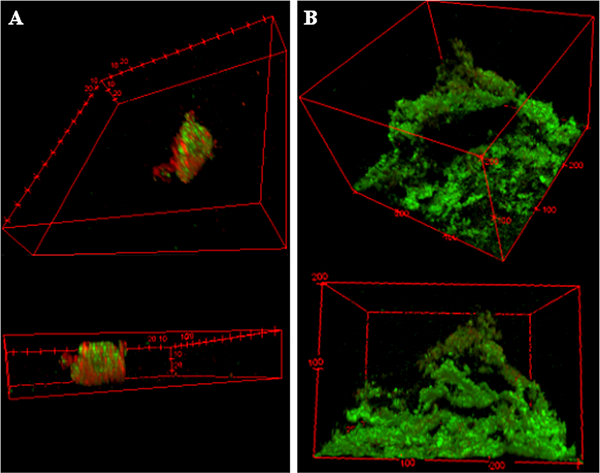
P. aeruginosa biofilms grown on a shaker table for 96 hours were starkly different from the biofilms grown in a drip flow reactor (DFR) for 24 hours in both morphology and number of colony forming units (CFU) even when using the same growth medium, TSB. The DFR biofilms had bacteria more densely covering the microscope slides’ surface area, typically 100 μm thick with plumes up to 200 μm (Figure 1B). The shaker table biofilms were less contiguous across the microscope slides with fewer adhered bacteria in between biofilm plumes ranging only up to 50 μm in thickness (Figure 1A). Figure 1 has the 3-D confocal image renderings of each biofilm with the live cells fluorescing green with Syto9 dye, while the dead cells contain red-fluorescing propidium iodide due to poor membrane integrity. Quantitatively, the CFU population density in the shaker table-grown biofilms was one hundred fold smaller (106.64 ± 0.53 CFU/cm2) than the DFR-grown biofilms (108.55 ± 0.32 CFU/cm2).
Figure 1. Biofilm Architectural Differences Due to Growth Method.
Confocal fluorescent microscopy images of biofilms grown in A) on a shaker table for 72 hours; and B) a drip flow reactor where the media is applied and drained dripwise for 20 hours following four hours of static incubation. Both biofilms use the same strain of P. aeruginosa and the same tryptic soy broth supply. 3-D images at 1 μm depth increments are overlaid to form these figures, with green indicating viable bacteria and red indicating dead bacteria.
Thermal Susceptibility
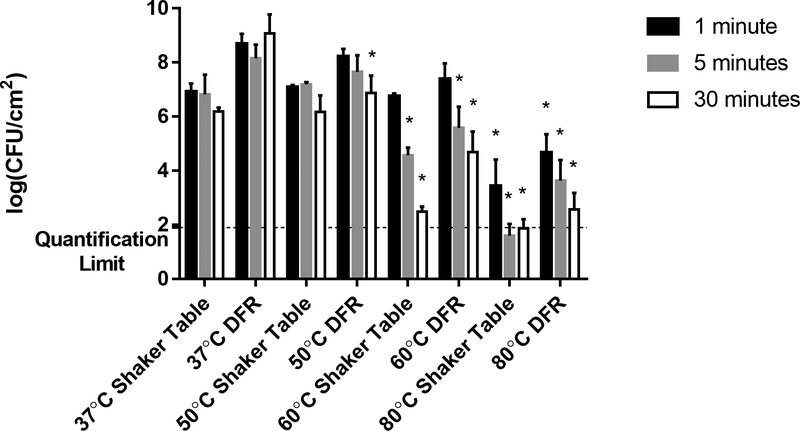
The shaker table biofilms also demonstrated significantly different susceptibilities to the thermal shock than seen in the DFR biofilms, as shown in Figure 2. While the CFU viability count in DFR biofilms decreased by 0.3–1.7 orders of magnitude depending on exposure time at 50 °C, the shaker table biofilms showed no susceptibility, maintaining the same CFU/cm2 values even after 30 minutes of exposure. At 60 °C, however, the shaker table biofilms showed a sharp dependence on exposure time, with no discernable effect at 1 minute of exposure and increased cell death when exposed for longer periods of time. At five minutes of exposure to the 60 °C thermal shock the viable bacterial population dropped by two orders of magnitude and at 30 minutes of exposure time the CFU/cm2 count dropped by four orders of magnitude. These decreases are comparable in magnitude and more time dependent than those exhibited by DFR-grown biofilms subjected to the same conditions. Similarly, the 80 °C thermal shock on the shaker table biofilms had 3.2 to at least 5 orders of magnitude decrease in the viable cell counts, below the quantification limit of the experiments, in some cases yielding no CFU at all.
Figure 2. Effect of Growth Method on Thermal Susceptibility.
Surviving CFU/cm2 following thermal shock are compared for biofilms grown on a shaker table versus grown in a drip flow reactor (DFR). All trials used the same tryptic soy broth supply, incubation conditions, and thermal shock protocols, with trials at 37, 50, 60, and 80 °C for 1, 5, or 30 minutes as indicated. The asterisk indicates values that are statistically different (p < 0.05) from their corresponding control thermal shock at 37 °C.
Growth Media Effects
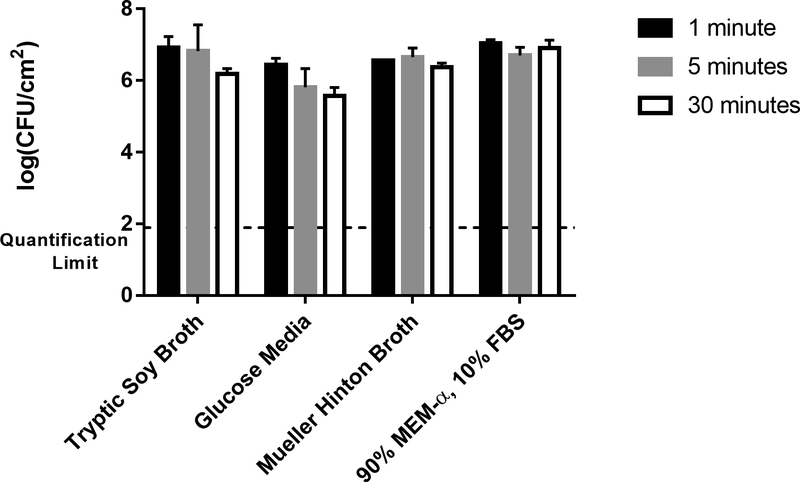
Investigating the effect of various growth media, the MHB and MEM-α/FBS produced shaker table biofilms comparable to the shaker table biofilms grown in TSB, as determined by two-way ANOVA, with only the GM biofilms differing significantly (for a p value of 0.05). These biofilms had a lower bacterial load (105.94±0.49 CFU/cm2) than the other control biofilms by over half an order of magnitude, as shown in Figure 3. This figure also indicates the effect of exposure time. For the control biofilms, the thermal shock temperature was the same as the incubation temperature (37 °C), so the duration of the thermal shock (1 to 30 minutes) was not anticipated to have an effect. This is confirmed by the results in Figure 3, where biofilms from any given medium show no statistical difference (p > 0.05) in CFU/cm2 regardless of exposure time to the control temperature. The quantification limit indicated in Figure 3 (101.9 CFU/cm2) is based on the criterion that plate counts of undiluted biofilm suspension below 3 CFU are not reliably quantified.
Figure 3. Effect of Growth Media on Biofilm Population Density.
Bacterial biofilm population densities are shown for shaker table-grown biofilms cultured in four different media types. In these control trials, all “thermal shocks” were performed at the incubation temperature of 37 °C (i.e., no shock) for the indicated exposure time: 1, 5, or 30 minutes.
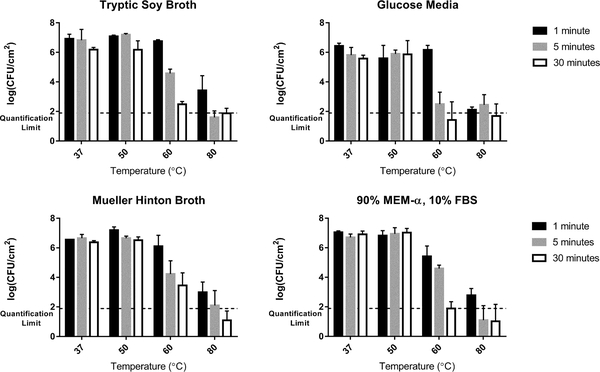
Similar observations were made for each of the different growth media types grown on a shaker table. For all media types there was no discernable decrease in bacterial viability after exposure to a 50 °C thermal shock regardless of exposure time. The viable cell count from the biofilms exposed to the 60 °C thermal shock for 1 minute also showed no statistical difference from the controls for all but the biofilms grown in MEM-α/FBS (p > 0.05). All biofilms grown on the shaker table showed decreases at 60 °C for exposure times above 1 minute regardless of growth media. Biofilms grown in TSB and GM showed more of a time dependence with the 60 °C thermal shock than others. Figure 4 summarizes these results for each growth medium, again demonstrating that exposure to 80 °C for more than 1 minute decreased the CFU/cm2 below the quantification limit, sometimes yielding no countable units in the undiluted plated samples.
Figure 4. Thermal Susceptibility of Shaker Table-Grown Biofilms.
At 50 °C, no population decrease is observed regardless of exposure time, while at 60 °C the population drops sharply with time. At 80 °C the decrease is typically too large to be quantified at exposure times of 5 minute and 30 minute, unlike the exposure time of 1 minute.
Post Thermal Shock Regrowth
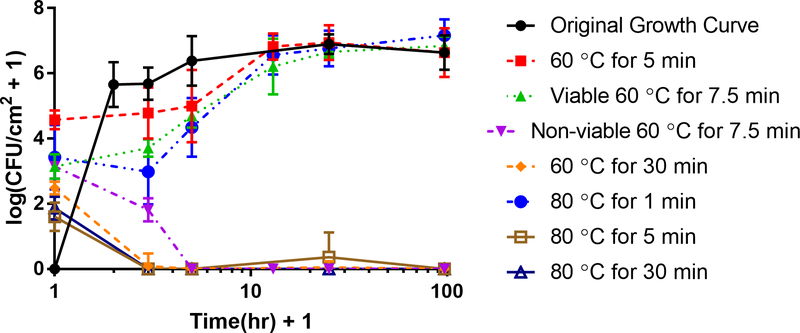
The original growth curve showed rapid attachment and proliferation within the first hour of inoculation, followed by a prompt climb to a plateau population density averaging 106.64 ± 0.53 CFU/cm2 within four hours, as seen in Figure 5. By comparison, thermally shocked biofilms showed no growth during their first four hours of reincubation, and required about a half day to reach their pre-thermal shock population density. This plateau density was unaffected by the thermal shock.
Figure 5. Biofilm Regrowth Post Thermal Shock.
Shaker plate-grown biofilms in TSB were thermally shocked and then re-incubated before enumeration to determine their regrowth rate. The horizontal axis indicates the hours after thermal shock (plus one hour in order to plot the data on a logarithmic scale). The original growth curve (time after inoculation, not heat shock) is included (black circles) for comparison. Note there are two lines for regrowth after a thermal shock at 60 °C for 7.5 minutes since three quarters of the biofilms died out while the other quarter regrew. Additionally, thermal shocks of 60 °C for 30 minutes, 80 °C for 5 minutes, and 80 °C for 30 minutes had little to no regrowth while the other thermal shocks resulted in fully recovered biofilms post heat shock.
Beyond a critical thermal shock intensity, however, the biofilms did not recover. Biofilms shocked at 60 °C for 30 minutes or 80 °C for at least 5 minutes initially showed an average of 102.48 CFU/cm2 and 101.87 CFU/cm2 of surviving bacteria, respectively, but two hours later no CFU were observed in almost all cases. Milder thermal shocks of 60 °C for 5 min or 80 °C for 1 min initially resulted in population densities of 104.58 CFU/cm2, and 103.43 CFU/cm2, respectively, and these biofilms recovered as described above. Biofilms shocked at 60 °C for 7.5 min initially showed 103.1 CFU/cm2; however, upon reincubation, three fourths of the biofilms experiencing this shock subsequently died off, while the remainder recovered as shown in Figure 5.
Discussion
Coatings which can be heated wirelessly via application of an alternating magnetic field are already under development [46], raising the possibility of a localized in situ thermal shock directly from the biofilm’s substrate. This approach, however, requires a clear understanding of the relationship between the thermal shock intensity and its subsequent bacterial population reduction, as well as the degree to which the population must be reduced to be non-viable. Previous studies have demonstrated that these thermal shocks can decrease the bacterial population of a biofilm by up to six orders of magnitude [43]. Even with a more labor-intensive enumeration protocol with a quantification limit of 101.1 CFU/cm2, such decreases require initial population densities well exceeding 107 CFU/cm2. Drip flow reactors, in which fresh media is slowly dripped onto a slanted substrate and allowed to drain off, generate biofilms with nearly 109 CFU/cm2, allowing quantification of population decreases over seven orders of magnitude, as correlated in Equation 1. It is however, difficult to determine whether biofilms cultured under such mild conditions would accurately represent in vivo biofilms, and if not, the degree to which their thermal susceptibility would be different.
To determine the degree to which growth conditions influence thermal susceptibility, biofilms for this investigation were grown in a variety of chemical environments under starkly different conditions, with a limited supply of nutrients, increased oxygen transport resistance, no waste removal, and shear from the orbital shaker table. Moreover, the biofilms were allowed to mature for several days. Under these conditions, the same bacteria form markedly different biofilms, as demonstrated in Figure 1. Quantitatively, these biofilms contained only 1% as many bacteria as the previously reported drip flow biofilms. The chemical environmental composition, however, appeared to have little effect. Even the minimum glucose media produced largely comparable biofilms under the same control conditions.
The thermal susceptibility of these biofilms was also statistically different from the susceptibility quantified in Equation 1 for DFR biofilms. Most notably, the 50 °C thermal shock had little to no effect, regardless of exposure time or growth media type. The observed lack of susceptibility to the thermal shock was also seen for biofilms grown in all but the MEM-α/FBS media when exposed to 60 °C for one minute, indicating that most of the biofilms grown under the shaker table conditions were less susceptible to lower temperature heating than previously seen in the DFR-grown biofilms. This overall increased resistance to mild thermal shock may be attributed to the harsher conditions of the shaker table culture, precluding the growth of less robust bacteria. At longer exposure times, however, a 60 °C thermal shock decreased the shaker table-grown biofilms’ bacterial viability; in TSB it decreased by two orders of magnitude at five minutes (compared to three orders of magnitude for a DFR-grown biofilm) and four orders of magnitude at 30 minutes (matching a DFR-grown biofilm).
At higher temperatures the decrease in viable cells for the shaker table-grown biofilms more closely mimicked the decrease for DFR-grown biofilms, to the extent that this could be quantified. TSB shaker table-grown biofilms when exposed to 80 °C for one minute decreased the viable cell count by three orders of magnitude (compared to four orders of magnitude for a DFR-grown biofilm), then the viable cell population dropped below the quantification limit (> 4.7 orders of magnitude decrease) at longer exposures where the DFR-grown biofilms decreased by 5 and 6 orders of magnitude at 5 and 30 minutes of exposure time, respectively.
Unlike the large differences seen between the shaker table-grown biofilms and the DFR-grown biofilms, the overall thermal susceptibility trends were found to be the same irrespective of the growth media used for the shaker table-grown biofilms. The 50 °C thermal shock had no effect regardless of the chemical composition of the environment, and the 80 °C thermal shock nearly eliminated the bacteria in the biofilms in all but the shortest exposure times. Only at 60 °C was the bacterial reduction quantifiable across the entire exposure time range and the differences based on growth media evident. Biofilms grown in MHB and TSB had higher viable cell counts after 30 minutes at 60 °C, containing 103.5 CFU/cm2 and 102.5 CFU/cm2, respectively, while biofilms grown in GM and MEM-α/FBS only contained 101.4 CFU/cm2 and 101.9CFU/cm2, respectively, near the quantification limit. Even with a less strict quantification limit, allowing as few as 3 CFU in a quantified plate, only biofilms with a CFU density of 101.9 CFU/cm2 would be considered quantifiable. Many biofilms were below this threshold, however. For instance, the biofilms grown in GM were found to have no growth in about half of the experiments after being exposed to 60 °C for 30 minutes. These data points with a count of zero were included in calculating the overall averages, bringing the mean below the quantification limit.
Equation 1 indicates an equivalence between thermal shock temperature and exposure time, with the same degree of population reduction possible across a range of temperatures, each with a corresponding exposure time. Combined with thermal modeling [47] and an estimation of resulting tissue damage based on the local Cumulative Equivalent Minutes at 43 °C (CEM43) [48, 49], multiple optimum thermal shock combinations may be calculated to result in the same bacterial population reduction and the same depth of tissue damage above a given CEM43 threshold. For instance, while a 70 °C, 1 min shock in conjunction with antibiotics has been shown to eliminate biofilms [50], it also results in 2.5 mm of adjacent tissue experiencing at least 200 CEM43. Equation 1 suggests shocks at 60 °C for 2.72 min or 80 °C for 0.38 min should have comparable efficacy, but result in only 2.2 or 2.3 mm, respectively, of similarly heated tissue (assuming the nearest perfusive heat sink is 5 mm from the implant). This study indicates that higher temperature, shorter duration heat shocks are much more universal in their predicted population reduction, however, regardless of biofilm architecture. The resulting focus on a much narrower parameter space greatly increases the feasibility of preclinical investigations on biofilms cultured in vivo.
Interestingly, immediate destruction of all bacteria in these biofilms is not necessary for its elimination. Biofilms whose population reduced to ~103 CFU/cm2, by a variety of temperature and exposure time combinations, were not able to propagate and instead died off completely within a few hours. This may indicate that the most thermally resilient bacteria are not reliable biofilm formers, or that these temperatures interrupt or destroy necessary pathways for the bacteria to form a biofilm. Alternatively, the thermal shock may alter the EPS environment, rendering it lethal for the surviving bacteria. The high density of dying cells may create a mass release of autolysins into the EPS, destroying the walls of the remaining cells [51]. Whether shocked at 60 °C for 30 minutes or 80 °C for 5 minutes or more, these biofilms showed no CFU two hours after thermal shock, despite showing up to 103 CFU/cm2 immediately following thermal shock, indicating a threshold population density which a successful treatment must reach beneath. The 60 °C trials with 7.5 min exposure were performed explicitly because they yielded population densities of 103.1 CFU/cm2 and showed that this was a tipping point between elimination and regrowth.
Thermal shocks resulting in viability counts above 103 CFU/cm2 (60 °C for 5 minutes and 80 °C for 1 minute), on the other hand, did not lead to the biofilm’s eventual demise. These biofilms eventually recovered to their previous population plateau, albeit much more slowly than with their original incubation. They demonstrated a longer lag phase followed by steady growth over the following 12 hours. By comparison, the biofilms initially reached their population plateau within four hours of inoculation. This sustained period of slower growth indicates a fundamental change in the bacteria themselves, either directly from the thermal shock or indirectly through selected killing, rather than from the presence of a chemical species generated during the thermal shock (akin to the previously speculated autolysins). Such a chemical would diffuse out of the biofilm in much less than 12 hours unless its diffusion coefficient were extraordinarily low (< 10−9 cm2/s), rendering it effectively immobile and unable to access cells.
These results provide confidence that brief, high-temperature thermal shock will effectively mitigate bacterial biofilm infections regardless of growth environment and provide an optimization window for in vivo animal trials. By equipping the implant with a magnetically susceptible coating, its surface can be directly heated by exposure to an externally applied alternating magnetic field if a biofilm infection is diagnosed [46]. Through careful optimization of the thermal shock, it may be possible to eliminate biofilm infections with less damage to adjacent tissue than from the current standard of care, explantation, and without the prolonged hospitalization and increased infection risk of multiple additional surgeries.
Conclusion
Biofilm infections are a daunting problem with limited options for treatment. Generating heat within a biofilm’s substrate to deliver a highly localized thermal shock may be an attractive approach, particularly for medical implants, where the current standard of care is explantation of the infected device and the surrounding tissue, followed by additional revision surgeries after the infection has cleared [2]. While the biofilm’s nutrient source does not appear to affect its thermal susceptibility significantly, the physical conditions of its growth do significantly impact its susceptibility to modest thermal shocks, such as at 50 °C. The efficacy of more aggressive thermal shocks, such as at 60 °C for 30 minutes or 80 °C for 1 minute, however, appears to be much more uniform, reliably decreasing biofilm population density by five orders of magnitude. That reduction in population density would bring even the most densely populated biofilms below 103 CFU/cm2, and apparently below their viability threshold, effectively killing them. Besides providing an alternative to multiple surgeries with increased infection risk and potentially months without a needed implant [52], this approach may also be applied to non-medical surfaces that struggle with biofilm fouling but which cannot be subjected to autoclave temperatures (121 °C), such as low glass-transition plastics.
Acknowledgement
This work was supported in part by the American Heart Association (11SDG7600044) and the National Science Foundation (CBET-1133297). E. Ricker was supported by the Predoctoral Training Program in Biotechnology from the National Institute for General Medical Sciences of the National Institutes of Health (T32 GM008365). The Zeiss LSM 710 confocal microscope was also obtained with support from the National Institutes of Health (1 S10 RR025439–01). The content of this material is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies.
Footnotes
Disclosure Statement
The authors declare no financial or commercial competing interests.
References
- [1].Tran N, Tran PA. Nanomaterial-based treatments for medical device-associated infections. ChemPhysChem. 2012;13:2481–2494. [DOI] [PubMed] [Google Scholar]
- [2].Darouiche RO. Treatment of infections associated with surgical implants. N. Engl. J. Med 2004;350:1422–1429. [DOI] [PubMed] [Google Scholar]
- [3].Ammerlaan HSM, Harbarth S, Buiting AGM, et al. Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clin. Infect. Dis 2013;56(6):798–805. [DOI] [PubMed] [Google Scholar]
- [4].Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- [5].Wisplinghoff H, Ewertz B, Wisplinghoff S, et al. Molecular evolution of methicillin-resistant Staphylococcus aureus in the metropolitan area of Cologne, Germany, from 1984 to 1998. J. Clin. Microbiol 2005;43(11):5445–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rohde H, Burandt EC, Siemssen N, et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007;28:1711–1720. [DOI] [PubMed] [Google Scholar]
- [7].Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015;86(2):147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol 2010;59:227–238. [DOI] [PubMed] [Google Scholar]
- [9].Zhang B, Powers R. Analysis of bacterial biofilms using NMR-based metabolomics. Future Med. Chem. 2012;4(10):1273–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haussler S, Fuqua C. Biofilms 2012: new discoveries and significant wrinkles in a dynamic field. J. Bacteriol 2013;195(13):2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Piddock LJV. Multidrug-resistance efflux pumps - not just for resistance. Nat. Rev. Microbiol 2006;4:629–636. [DOI] [PubMed] [Google Scholar]
- [12].Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. [DOI] [PubMed] [Google Scholar]
- [13].Anderl JN, Zahller J, Roe F, et al. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2003;47(4):1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anwar H, Strap JL, Costerton JW. Susceptibility of biofilm cells of Pseudomonas aeruginosa to bactericidal actions of whole blood and serum. FEMS Microbiol. Lett 1992;92:235–242. [DOI] [PubMed] [Google Scholar]
- [15].Andrews JM. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother 2001;48 Suppl S1:5–16. [DOI] [PubMed] [Google Scholar]
- [16].Bundtzen RW, Gerber AU, Cohn DL, et al. Postantibiotic suppression of bacterial growth. Rev. Infect. Dis 1981;3(1):28–37. [DOI] [PubMed] [Google Scholar]
- [17].Meng Z, Chongjin S, You X, et al. Characteristics of baicalin synergy with penicillin or Notopterygium ethanol extracts against Staphylococcus aureus. Tsinghua Sci. Technol 2006;11(4):459–461. [Google Scholar]
- [18].Ceri H, Olson ME, Stremick C, et al. The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol 1999;37(6):1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abdi-Ali A, Mohammadi-Mehr M, Agha Alaei Y. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2006;27:196–200. [DOI] [PubMed] [Google Scholar]
- [20].Hengzhuang W, Wu H, Ciofu O, et al. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011;55(9):4469–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walters III MC, Roe F, Bugnicourt A, et al. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003;47(1):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Horswill AR, Stoodley P, Stewart PS, et al. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal. Bioanal. Chem 2007;387:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Majik MS, Naik D, Bhat C, et al. Synthesis of (R)-norbgugaine and its potential as quorum sensing inhibitor against Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett 2013;23:2353–2356. [DOI] [PubMed] [Google Scholar]
- [24].Parsek MR, Greenberg EP. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. PNAS. 2000;97(16):8789–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol. Adv 2013;31:224–245. [DOI] [PubMed] [Google Scholar]
- [26].Cady NC, McKean KA, Behnke J, et al. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One. 2012;7(6):e38492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fernandes A, Dias M. The microbiological profiles of infected prosthetic implants with an emphasis on the organisms which form biofilms. J. Clin. Diagnostic Res 2013;7(2):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Howlin RP, Brayford MJ, Webb JS, et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015;59(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vadillo-Rodríguez V, Pacha-Olivenza MA, Gónzalez-Martín ML, et al. Adsorption behavior of human plasma fibronectin on hydrophobic and hydrophilic Ti6Al4V substrata and its influence on bacterial adhesion and detachment. J. Biomed. Mater. Res. Part A 2013;101A:1397–1404. [DOI] [PubMed] [Google Scholar]
- [30].van der Borden AJ, van der Mei HC, Busscher HJ. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials. 2005;26:6731–6735. [DOI] [PubMed] [Google Scholar]
- [31].van der Borden AJ, Maathuis PGM, Engels E, et al. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials. 2007;28:2122–2126. [DOI] [PubMed] [Google Scholar]
- [32].van der Borden AJ, van der Werf H, van der Mei HC, et al. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl. Environ. Microbiol 2004;70(11):6871–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Carmen JC, Roeder BL, Nelson JL, et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 2005;33(2):78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Howlin RP, Fabbri S, Offin DG, et al. Removal of dental biofilms with an ultrasonically activated water stream. J. Dent. Res 2015;94(9):1303–1309. [DOI] [PubMed] [Google Scholar]
- [35].Kiran S, Sharma P, Harjai K, et al. Enzymatic quorum quenching increases antibiotic susceptibility of multidrug resistant Pseudomonas aeruginosa. Iran. J. Microbiol 2011;3(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- [36].Lauderdale KJ, Malone CL, Boles BR, et al. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res 2010;January:55–61. [DOI] [PubMed] [Google Scholar]
- [37].Kim J, Kwon S, Ostler E. Antimicrobial effect of silver-impregnated cellulose: potential for antimicrobial therapy. J. Biol. Eng 2009;3:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loo CY, Rohanizadeh R, Young PM, et al. Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J. Agric. Food Chem 2015;64(12):2513–2522. [DOI] [PubMed] [Google Scholar]
- [39].Chmielewski RAN, Frank JF. A predictive model for heat inactivation of Listeria monocytogenes biofilm on buna-N rubber. LWT. 2006;39:11–19. [DOI] [PubMed] [Google Scholar]
- [40].Wahlen L, Parker A, Walker D, et al. Predictive modeling for hot water inactivation of planktonic and biofilm-associated Sphingomonas parapaucimobilis to support hot water sanitization programs. Biofouling. 2016;32(7):751–761. [DOI] [PubMed] [Google Scholar]
- [41].Park H, Park HJ, Kim JA, et al. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011;84:41–45. [DOI] [PubMed] [Google Scholar]
- [42].Nguyen HTT, Corry JEL, Miles CA. Heat resistance and mechanism of heat inactivation in thermophilic campylobacters. 2006;72(1):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].O’Toole A, Ricker EB, Nuxoll E. Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling. 2015;31(8):665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Montanaro L, Speziale P, Campoccia D, et al. Scenery of Staphylococcus implant infections on orthopedics. Future Microbiol. 2011;6(11):1329–1349. [DOI] [PubMed] [Google Scholar]
- [45].Borrelli MJ, Thomas LL, Cain CA, et al. Time-temperature anaylsis of cell killing of BHK cells heated at temperatures in the range of 43.5°C to 57.0°C. Int. J. Radiat. Oncol. Biol. Phys 1990;19:389–399. [DOI] [PubMed] [Google Scholar]
- [46].Coffel J, Nuxoll E. Magnetic nanoparticle/polymer composites for medical implant infection control. J. Mater. Chem. B 2015;3:7538–7545. [DOI] [PubMed] [Google Scholar]
- [47].Coffel J, Nuxoll E. Poly(vinyl alcohol) tissue phantoms as a robust in vitro model for heat transfer. Int. J. Polym. Mater. Polym. Biomater 2016;65(15):797–806. [Google Scholar]
- [48].Yarmolenko PS, Moon EJ, Landon C et al. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth 2011;27(4):320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth 2003;19(3):267–294. [DOI] [PubMed] [Google Scholar]
- [50].Ricker EB, Nuxoll E. Synergistic effects of heat and antibiotics on Pseudomonas aeruginosa biofilms. Manuscript Submitted for Publication. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li Z, Clarke AJ, Beveridge TJ. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division, and secretion in surface membrane vesicles. J. Bacteriol 1996;178(9):2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moran E, Byren I, Atkins BL. The diagnosis and management of prosthetic joint infections. J. Antimicrob. Chemother 2010;65(Suppl 3):iii45–iii54. [DOI] [PubMed] [Google Scholar]