Abstract
The role of cutaneous viral infections in the development of non-melanoma skin cancer (NMSC), including cutaneous squamous cell carcinoma (SCC), among chronic lymphocytic leukemia (CLL) and blood and marrow transplant (BMT) patients is not established. CLL (n=977) and BMT (n=3,587) patients treated at the Moffitt Cancer Center were included in a retrospective cohort study. Human papillomavirus (HPV) and human polyomavirus (HPyV) DNA was examined in a subset of incident SCC tumors. Five year cumulative incidence of NMSC was 1.42% in both BMT (n=31 NMSCs) and CLL (n=18 NMSCs) cohorts. Of the 9 SCC tumors examined from each cohort, 22.2% and 33.3% were positive for viral DNA in the transplant (HPV 65, MCV) and CLL (HPV 38, HPV 15, HPyV6) cohort, respectively. Enhanced skin cancer screening of BMT/CLL patients should be conducted to better capture incident NMSCs and examine the role of viral infections in these tumors.
Keywords: chronic lymphocytic leukemia, bone marrow transplant, squamous cell carcinoma, HPV, polyomavirus
Introduction
Chronic lymphocytic leukemia (CLL), a common malignancy of lymphocytes among adults, accounts for an estimated 18,960 new cases and 4,660 deaths in the United States[1]. Currently, the only curative treatment available for CLL is allogeneic BMT. However BMT is associated with both short and long term complications. While many CLL patients remain asymptomatic for several years [2–4], they are at significantly higher risk of developing second primary malignancies (SPMs) [2,5–8] compared to the general population, with the most common SPM being non-melanoma skin cancer (NMSC)[5].
It is well known that compared to the general population, solid organ transplant patients have 65- to 200-fold higher risk of NMSC [9–12]. Among recipients of BMT, myeloablative conditioning, including high dose chemotherapy and total body irradiation, leads to compromised host immune response [13,14]. Similar to solid organ transplant patients, immunocompromised BMT patients, with conventional myeloablative conditioning, have been found to have higher risk of skin cancer than the general population [14]. A prospective study reported a 3.7-fold increased risk of NMSC among Danish CLL patients compared to the general population [4]. In addition, CLL patients experience a 17-fold significantly increased mortality from NMSC than the general population [2], suggesting an aggressive nature of NMSCs in the presence of CLL.
Immunosuppression is thought to play an important role in the development of NMSC post organ transplant [15] as well as post CLL (reviewed in [2]). As for other cancers that are more common among immunosuppressed individuals, a viral etiology has been proposed for the development of NMSC in this setting. In a study by Myer and colleagues, 75% SCCs detected among organ transplant patients were positive for cutaneous human papillomavirus (HPV) DNA, including HPV types 5 and 8, compared to 47% HPV DNA positive SCCs detected among immunocompetent patients [16]. In a meta-analysis of 12 studies, SCC tumors detected in immunocompromised subjects were three times more likely to be positive for HPV (mucosal and/or cutaneous HPVs) than SCC tumors detected in immunocompetent subjects [17]. Thus, viral infections could play a role in the etiology of NMSC among immunosuppressed patients. We hypothesized that SCCs detected in BMT and CLL patients would harbor cutaneous HPV and human polyomavirus (HPyV).
Using a retrospective cohort design, incidence of NMSC was assessed in two separate cohorts of CLL and BMT patients, and presence of cutaneous HPV and HPyV DNA, including Merkel cell polyomavirus (MCV), was examined in a subset of incident SCCs, as described below.
Methods
Study population
A retrospective cohort study was conducted at Moffitt Cancer Center, Tampa, Florida. CLL patients who received clinical care at Moffitt Cancer Center between 2002 and 2013 (n=1,449) were identified from the patient database maintained by the Department of Malignant Hematology. Patients who received BMT (n=3,836) for a variety of hematological malignancies in 2004–2013, were identified from a separate database maintained by the Department of Blood and Marrow Transplant and Cellular Immunotherapy at Moffitt Cancer Center. A total of 3,587 BMT patients and 977 CLL patients with complete follow up information were included in the final sample, including 59 cases that were common to both cohorts. Dates of CLL diagnosis, BMT, incident NMSC, and last contact or death, as well as type of BMT were available from the departmental databases. Data on demographics, smoking, alcohol consumption, past history of cancer, vital status, and family history of cancer, were available through Moffitt’s enterprise wide data warehouse, including information from Moffitt’s Cancer Registry and patients’ medical records. Data on NMSCs diagnosed outside Moffitt were not available. The study protocol was approved by the institutional review board at Moffitt Cancer Center.
Viral DNA assay
Recuts of formalin-fixed paraffin embedded (FFPE) tumor tissues from 18 incident SCCs, diagnosed after the date of CLL diagnosis or date of BMT, were obtained from Moffitt’s Pathology Department and shipped to the laboratory at the International Agency for Research on Cancer for viral DNA genotyping. Viral DNA corresponding to 44 beta HPV types (5, 8, 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, 36, 37, 38, 47, 49, 75, 76, 80, 92, 93, 96, 98, 99, 100, 104, 105, 107, 110, 111, 113, 115, 115, 118, 120, 122, 124, 143, 145, 150, 151), 29 gamma HPV types (4, 48, 50, 60, 65, 88, 95, 101, 103, 108, 109, 112, 116, 119, 121, 123, 126, 127, 128, 129, 130, 131, 132, 133, 134, 148, 149, 156, SD2) and 11 human polyomaviruses (BKV, KIV, JCV, WUV, MCV, HPyV6, HPyV7, TSV, HPyV9, HPyV10, HPyV12) were measured in the SCC tumors, using a bead-based multiplex PCR assay, as described in detail previously [18,19].
Statistical analyses
Analyses were conducted separately for the CLL and BMT cohorts. Among those who developed NMSC, follow-up time was defined as years between date of CLL diagnosis (or date of BMT) and date of NMSC diagnosis. Among those who did not develop NMSC during the study follow up period, follow up time was defined as years between date of CLL (or BMT) and date of last contact or death. Incidence rates of NMSC were calculated based on person-years of follow up. Kaplan-Meier curves of incident NMSC were derived. Cox proportional hazards ratios (HRs) and 95% confidence intervals (CIs) were estimated to examine the associations of demographic, clinical and lifestyle factors with incident NMSC. Factors associated with time to incident NMSC diagnosis were examined using Chi Square and Fisher’s exact test, as appropriate. All analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) or R software version 3.0.2 [20].
Results
BMT cohort
Characteristics of the BMT cohort are described in Table 1. The cohort included 85.3% Whites, 57.3% males and 84.7% never-smoking patients (Table 1). Mean age at BMT was 53.5 years, and BMT patients were followed for a median of 2.0 years. During follow up, 31 incident cases of NMSC were diagnosed, including 15 SCCs, 5 BCCs and 11 NMSCs with an unknown subtype (Fig 1), and a median time to NMSC diagnosis of 1.5 years (Fig 2). Five year cumulative incidence of NMSC was 1.42% (Figure 2). BMT patients who underwent allogeneic transplant had a significant 2.8 fold increased risk of NMSC compared to BMT patients who underwent autologous transplant (HR=2.85, 95% CI= 1.36–5.96), after adjusting for age at BMT (Table 1).
Table 1.
Factors associated with incident non-melanoma skin cancer (NMSC) among patients receiving bone marrow transplant (BMT) at Moffitt Cancer Center
| Variable | Overall Cohort n=3,587 n(%) |
Incident NMSC n=31 n(%) |
HR1 (95% CI |
|---|---|---|---|
| Age at BMT | 53.5 (12.9) | 57.2 (11.9) | 1.03 (1.00–1.06) |
| Mean(std) | |||
| Gender | |||
| Male | 2054 (57.3) | 22 (71.0) | 1.00 |
| Female | 1533 (42.7) | 9 (29.0) | 0.56 (0.26–1.22) |
| Race | |||
| White | 3061(85.3) | 29 (93.5) | 1.00 |
| Non white | 526 (14.7) | 2 (6.4) | 0.42 (0.10–1.79) |
| BMT type | |||
| Auto | 2205 (61.5) | 16 (51.6) | 1.00 |
| Allo | 1382 (38.5) | 15 (48.4) | 2.85 (1.36–5.96) |
| Acute GVHD skin | |||
| None (grade 0) | 482 ( 43.8) | 5 (33.3) | 1.00 |
| Grades 1–4 | 619 ( 56.2) | 10 (66.7) | 1.43 (0.49–4.20) |
| Smoking | |||
| Never | 2913 (84.7) | 7 (43.7) | 1.00 |
| Ever | 524 (15.2) | 9 (56.2) | 1.58 (0.59–4.24) |
HR=hazards ratio, CI=confidence interval, GVHD=graft versus host disease.
Age adjusted for all variables examined as risk factors, unadjusted for age as the risk factor.
Figure 1.
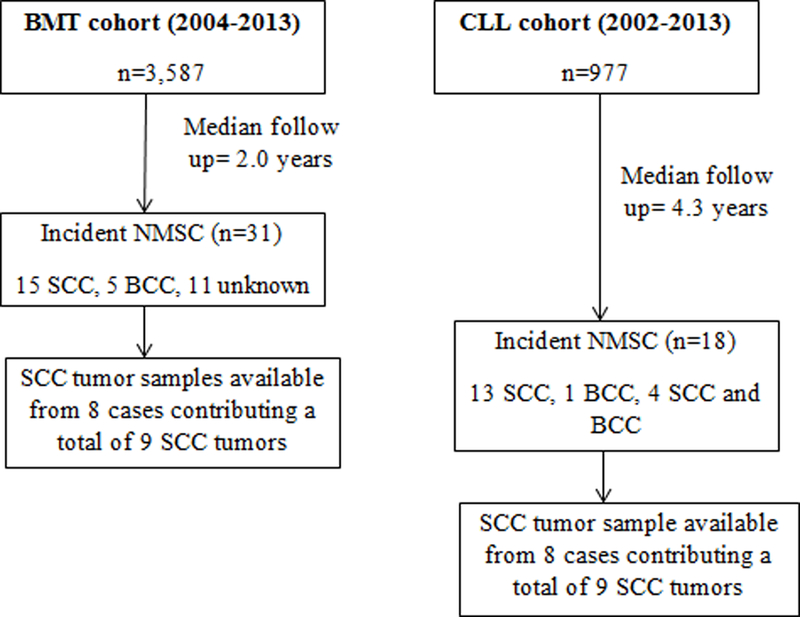
Incidence cases of non-melanoma skin cancer (NMSC) arising in cohorts of bone marrow transplant (BMT) patients and chronic lymphocytic leukemia (CLL) patients at Moffitt Cancer Center, Tampa, Florida
Figure 2.
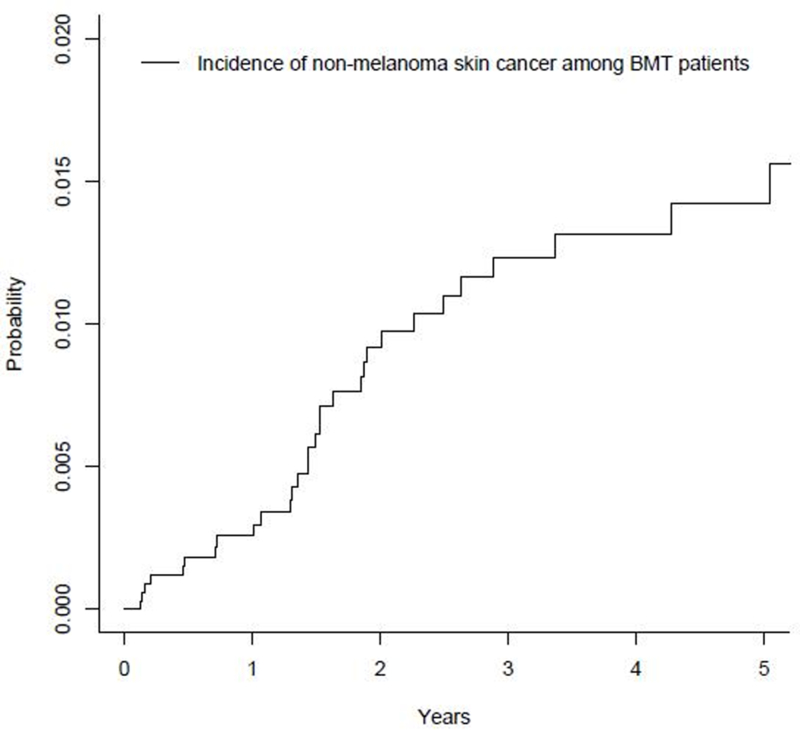
Cumulative incidence of non-melanoma skin cancer among bone marrow transplant patients (n=3,587)
CLL cohort
Characteristics of the CLL cohort are described in Table 2. The cohort included 63.1% males, 90% Whites and 55% never-smokers, and the mean age at CLL diagnosis was 60.1 years. Approximately 14% of CLL patients received BMT, including 60 patients who received autologous BMT and 5 received allogeneic BMT. CLL patients were followed for a median of 4.3 years (Fig 1). The longer median follow up of the CLL patients compared to that of the BMT patients could be a reflection of the indolent nature of CLL in many cases, who seldom require active treatment, whereas patients receiving BMT usually have an active and refractory disease, resulting in shorter median time to death (1.2 years in BMT patients versus 5.8 years in CLL patients).
Table 2.
Factors associated with incident non-melanoma skin cancer (NMSC) among chronic lymphocytic leukemia (CLL) patients
| Variables | Overall cohort n=977 n (%) |
Incident NMSC n=18 n (%) |
HR (95% CI)1 |
|---|---|---|---|
| Age* | 60.1 (11.1) | 63.3 (13.8) | 1.08 (1.02–1.13) |
| Gender | |||
| Male | 616 (63.1) | 16 (88.9) | 1.00 |
| Female | 360 (36.9) | 2 (11.1) | 0.16 (0.04–0.72) |
| Race | |||
| White | 875 (90.0) | 18 (100.0) | |
| Non-white | 97 (10.0) | 0 (0.0) | Could not be estimated |
| Smoking | |||
| Non smoker | 510 (55.0) | 5 (29.4) | 1.00 |
| Ever smoker | 417 (45.0) | 12 (70.6) | 2.68 (0.92–7.79) |
| Alcohol | |||
| Non drinker | 536 (58.8) | 9 (52.9) | 1.00 |
| Social or heavy abuse | 376 (41.2) | 8 (47.1) | 1.88 (0.695–5.10) |
| CLL family history | |||
| No | 899 (95.9) | 17 (100.0) | Could not be estimated |
| Yes | 38 (4.1) | 0 (0.0) | |
| Relapse | |||
| No | 238 (66.7) | 8 (72.7) | 1.00 |
| Yes | 119 (33.3) | 3 (27.3) | 0.30 (0.06–1.47) |
| Allogenic BMT | |||
| No | 407 (87.15) | 9 (90.0) | 1.00 |
| Yes | 60 (12.85) | 1 (10.0) | 2.05 (0.19–22.2) |
| Autologous BMT | |||
| No | 374 (98.68) | 6 (100.0) | Could not be estimated |
| Yes | 5 (1.32) | 0 (0.0) |
mean (standard deviation).
Age adjusted for all variables examined as risk factors, unadjusted for age as the risk factor.
During follow up, 1.8% (n=18) patients developed NMSC, corresponding to a five year cumulative incidence of 1.42% (Fig 3). The 18 incident cases of NMSCs included 13 SCCs, 1 BCC and 4 cases with both an SCC and BCC. Median time to NMSC development was 4.3 years. As seen in Table 2, each year increase in age at CLL diagnosis was associated with an 8% increased risk of NMSC (HR=1.08, 95% CI=1.02–1.13), although this association did not reach statistical significance. Females were significantly less likely to develop incident NMSC compared to males (HR=0.16, 95% CI=0.04–0.72), after adjusting for age. None of the other factors examined were associated with NMSC development in CLL patients (Table 2).
Figure 3.
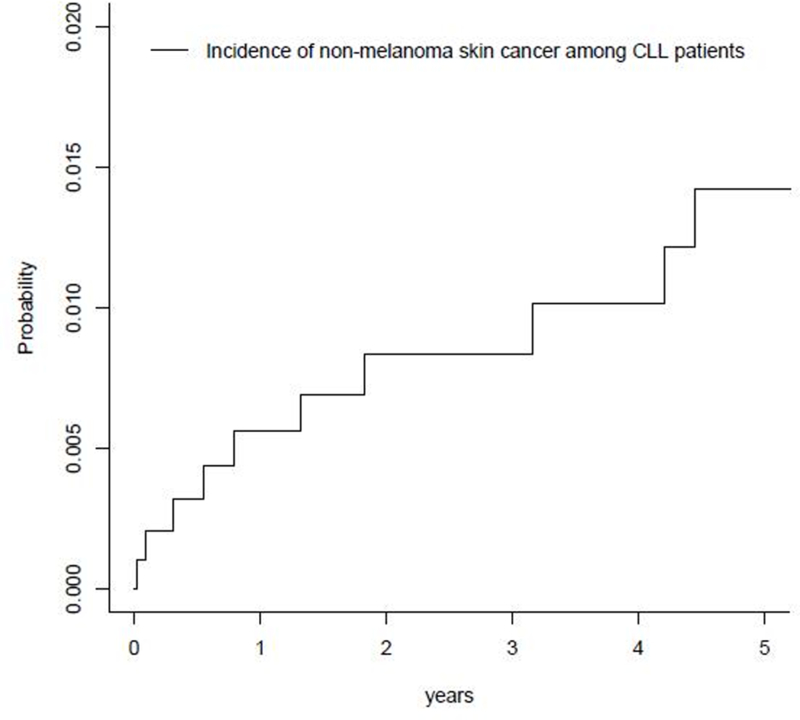
Cumulative incidence of non-melanoma skin cancer among chronic lymphocytic leukemia (CLL) patients (n=977)
Viral DNA in incident NMSC tumors
As shown in Fig 1, of the 31 incident diagnoses of NMSC (n= 15 SCC, n= 5 BCC, 11 unknown) detected in the BMT cohort, tumor samples from 8 patients with SCC (corresponding to 9 SCC tumors) were available for viral DNA genotyping. Similarly, of the 18 patients who developed NMSCs in the CLL cohort (13 SCC, 1 BCC, 4 SCC and BCC), tumor samples from 8 patients with SCC (corresponding to 9 tumors) were genotyped for viral DNA (Fig 1). Two out of nine (22.2%) SCC tumors detected in the BMT cohort were positive for DNA corresponding to a single virus (MCV or HPV65). Different virus types were detected in the CLL cohort, where 3 out of nine (33.3%) SCCs were positive for DNA corresponding to HPV 38, HPV 15 or HPyV6. Demographic characteristics of the five SCC cases with a virus positive tumor are shown in Table 3. The SCC case with an HPV 65-positive tumor was a 44-year-old, White male who was treated with allogenic transplant (Table 3), whereas the case with an MCV-positive SCC was a 70-year-old, White female who received an autologous transplant. In the CLL cohort, cases with virus positive SCC tumors were all White males, between 46 to 68 years of age (Table 3), with two cases treated with allogenic transplant.
Table 3.
Characteristics of incident SCC cases (n=5) with virus positive tumors among bone marrow transplant (BMT) patients and chronic lymphocytic leukemia (CLL) patients
| Patient number | Gender | Race | Age at BMT (years) | Diagnosis | BMT type | Cutaneous virus type detected in the incident SCC tumors |
|---|---|---|---|---|---|---|
| BMT cohort | ||||||
| 1 | Male | White | 44 | Acute Monoblastic/Acute Monocytic Leukemia/(M5) | Allogeneic transplant | HPV 65 |
| 2 | Female | White | 70 | Multiple Myeloma IgG | Auto transplant | MCV |
| CLL cohort | Age at CLL diagnosis (years) | |||||
| 3 | Male | White | 68 | CLL | Unknown | HPV 15 |
| 4 | Male | White | 46 | CLL | Allogeneic transplant | HPyV6 |
| 5 | Male | White | 66 | CLL | Allogeneic transplant | HPV 38 |
Discussion
In the present study, five-year cumulative incidence of NMSC was 1.42 % in both the BMT and CLL cohorts. Allogenic transplant patients had a higher risk of developing NMSC than autologous transplant patients, and in the CLL cohort, females were less likely to develop incident NMSC compared to males. Of the incident SCC tumors available for viral DNA assay, a third were positive for viral DNA in CLL (HPV types 38, 15 or HPyV6) cohort, while 22.2% SCCs were positive for a virus in the BMT (MCV or HPV65) cohort.
Our findings are similar to other studies that have described allogeneic BMT [14,21] and male sex [22] as risk factors for NMSC, although the absolute NMSC incidence rates we observed are considerably lower than what has been previously reported for CLL [5,22] and BMT patients [21,23]. However, previous studies included long term survivors of CLL [5] and BMT [23] who were followed for a decade or longer, allowing more time for detection of new NMSC. In a recent systematic review of 18 studies reporting NMSCs detected among BMT patients, the median time to diagnosis of NMSC across the studies ranged from 7.3 to 8.4 years for BCC and 2.1 to 7.0 years for SCC [24]. The median follow up time for CLL and BMT patients in our study was 4.3 and 2.0 years, respectively. Since information on NMSC diagnosed outside of Moffitt Cancer Center was unavailable, it is likely that all NMSC cases were not captured in the present study. Incomplete NMSC case ascertainment more likely occurred among CLL patients than BMT patients, given the more extensive surveillance and active follow up required for BMT patients. Given that NMSC is not a reportable cancer to population-based registries, improvement of case ascertainment through linkage of patients to population-based cancer registry data was not possible.
As mentioned earlier, immunosuppression, either due to the underlying disease or treatment, may predispose to infections with viruses that can contribute to increased risk of NMSC among BMT and CLL patients. Three of the six viruses detected in SCC tumors in the present study (HPV types 38, 15 and MCV) have been reported to have carcinogenic potential. For example, MCV is an established cause of Merkel cell carcinoma [25–29], and MCV DNA has been previously detected in SCC [30–35]. HPV type 38 has been shown to have tumor transforming properties in an animal model, with transgenic mice expressing HPV type 38 oncoproteins developing SCC at a higher incidence when exposed to ultraviolet radiation than wild type mice [36,37]. In a recent meta-analysis of studies examining beta HPV using serology or viral DNA measurement in eyebrow hairs, HPV types 15 and 38 were found to be significantly associated with 25–37% increased risk of SCC among immunocompetent individuals [38]. Previously, we observed that eyebrow hair infection with HPV types 38 and 15 was associated with 6.3- and 9.3-fold increased risks of virus-positive SCCs, respectively [39].
Strength of the present study includes examination of the presence of viral DNA corresponding to a large number of cutaneous HPV types within genus beta and gamma, and several HPyVs, in incident SCC tumors detected among CLL and BMT patients. Limitations should also be noted, including small sample size for analyses stratified by NMSC subtype. NMSC ascertainment was incomplete, due to the practice of hematologic malignancy patients being treated for NMSC outside of Moffitt Cancer Center, a phenomenon not uncommon to tertiary referral centers. In a study conducted at the Mayo Clinic in Minnesota, less than 36% CLL patients underwent skin cancer screening exam within 6 months of CLL diagnosis [24], with the low screening compliance mainly attributed to lack of provider’s recommendation or performance [24]. Given the large number of patients who receive BMT every year and an increasing number of long term survivors of CLL, it is important to enhance skin cancer screening efforts in this high risk population to inform early diagnosis and treatment of NMSC. If a role for viral infections is established in NMSC development among these patients, novel preventive measures for second primary skin malignancies could be developed.
Acknowledgement
This study was funded by the Center for Infection Research in Cancer, Moffitt Cancer Center (Pilot study grant awarded to Dr. Dana Rollison). This work was also supported in part by the Tissue Core and the Collaborative Data Services Core at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292). DER designed and supervised the research study, reviewed the manuscript; SSH conducted the analyses and wrote the manuscript; FLL, JCC, NSP and ARG reviewed the manuscript; TG and MT conducted HPV DNA assays and reviewed manuscript; KM conducted preliminary analyses and reviewed the manuscript.
Footnotes
Disclosure of interest
Dr. Locke serves as a scientific advisor to Kite pharma and consultant to Cellular Biomedicine Group inc., unrelated to the work presented in this study. Dr. Rollison reports grant from National Cancer Institute, during the conduct of the study. None of the other authors have any conflict of interest to declare.
References:
- 1.American Cancer Society,Cancer Facts & Figures 2016. Atlanta, 2016 [Google Scholar]
- 2.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer 2011;105:1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon BM, Rabe KG, Slager SL, Brewer JD, Cerhan JR, Shanafelt TD. Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a SEER population-based study. J Clin Oncol 2013;31:930–937. [DOI] [PubMed] [Google Scholar]
- 4.Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer 2007;121:151–156. [DOI] [PubMed] [Google Scholar]
- 5.Falchi L, Vitale C, Keating MJ, et al. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann Oncol 2016;27:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer JD, Shanafelt TD, Call TG, et al. Increased incidence of malignant melanoma and other rare cutaneous cancers in the setting of chronic lymphocytic leukemia. Int J Dermatol 2015;54:e287–293. [DOI] [PubMed] [Google Scholar]
- 7.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol 2009;27:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna DB, Stockton D, Brewster DH, Doherty VR. Evidence for an association between cutaneous malignant melanoma and lymphoid malignancy: a population-based retrospective cohort study in Scotland. Br J Cancer 2003;88:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol 1999;40:177–186. [DOI] [PubMed] [Google Scholar]
- 10.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006;154:498–504. [DOI] [PubMed] [Google Scholar]
- 11.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000;143:513–519. [PubMed] [Google Scholar]
- 12.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348:1681–1691. [DOI] [PubMed] [Google Scholar]
- 13.Morecki S, Gelfand Y, Nagler A, et al. Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs myeloablative regimen. Bone Marrow Transplant 2001;28:243–249. [DOI] [PubMed] [Google Scholar]
- 14.Lishner M, Patterson B, Kandel R, et al. Cutaneous and mucosal neoplasms in bone marrow transplant recipients. Cancer 1990;65:473–476. [DOI] [PubMed] [Google Scholar]
- 15.Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sorensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol 2010;90:474–479. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T, Arndt R, Nindl I, Ulrich C, Christophers E, Stockfleth E. Association of human papillomavirus infections with cutaneous tumors in immunosuppressed patients. Transpl Int 2003;16:146–153. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol 2014;70:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheit T, Billoud G, de Koning MN, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J Clin Microbiol 2007;45:2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheit T, Landi S, Gemignani F, et al. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol 2006;44:2025–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 21.Cavalier M, Shmalo JA, Yu M, Billings SD, Abonour R, Nelson RP, Jr. Skin cancer after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant 0000;37:1103–1108. [DOI] [PubMed] [Google Scholar]
- 22.Beiggi S, Johnston JB, Seftel MD, et al. Increased risk of second malignancies in chronic lymphocytic leukaemia patients as compared with follicular lymphoma patients: a Canadian population-based study. Br J Cancer 2013;109:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa W, Pond GR, Rifkind JT, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant 2005;35:51–55. [DOI] [PubMed] [Google Scholar]
- 24.DePry JL, Vyas R, Lazarus HM, Caimi PF, Gerstenblith MR, Bordeaux JS. Cutaneous Malignant Neoplasms in Hematopoietic Cell Transplant Recipients: A Systematic Review. JAMA Dermatol 2015;151:775–782. [DOI] [PubMed] [Google Scholar]
- 25.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun SM, Yun SJ, Lee SC, Won YH, Lee JB. Merkel cell polyomavirus is frequently detected in korean patients with merkel cell carcinoma. Ann Dermatol 2013;25:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattori T, Takeuchi Y, Takenouchi T, et al. The prevalence of Merkel cell polyomavirus in Japanese patients with Merkel cell carcinoma. J Dermatol Sci 2013;70:99–107. [DOI] [PubMed] [Google Scholar]
- 28.Paolini F, Donati P, Amantea A, Bucher S, Migliano E, Venuti A. Merkel cell polyomavirus in Merkel cell carcinoma of Italian patients. Virol J 2011;8:8–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Locke FL, Rollison DE, Sondak VK. Merkel cell carcinoma and immunosuppression: what we still need to know. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin AM, Tseng SY, Allain DC, Iwenofu OH, Peters SB, Toland AE. Merkel cell polyomavirus in cutaneous squamous cell carcinoma of immunocompetent individuals. J Invest Dermatol 2009;129:2868–2874. [DOI] [PubMed] [Google Scholar]
- 31.Mertz KD, Paasinen A, Arnold A, et al. Merkel cell polyomavirus large T antigen is detected in rare cases of nonmelanoma skin cancer. J Cutan Pathol 2013;40:543–549. [DOI] [PubMed] [Google Scholar]
- 32.Murakami M, Imajoh M, Ikawa T, et al. Presence of Merkel cell polyomavirus in Japanese cutaneous squamous cell carcinoma. J Clin Virol 2011;50:37–41. [DOI] [PubMed] [Google Scholar]
- 33.Scola N, Wieland U, Silling S, Altmeyer P, Stucker M, Kreuter A. Prevalence of human polyomaviruses in common and rare types of non-Merkel cell carcinoma skin cancer. Br J Dermatol 2012;167:1315–1320. [DOI] [PubMed] [Google Scholar]
- 34.Imajoh M, Hashida Y, Nakajima H, Sano S, Daibata M. Prevalence and viral DNA loads of three novel human polyomaviruses in skin cancers from Japanese patients. J Dermatol 2013;20:1346–8138. [DOI] [PubMed] [Google Scholar]
- 35.Rollison DE, Giuliano AR, Messina JL, et al. Case-control study of Merkel cell polyomavirus infection and cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2012;21:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viarisio D, Mueller-Decker K, Kloz U, et al. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 2011;7:e1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viarisio D, Mueller-Decker K, Kloz U, et al. Correction: E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice. PLoS Pathog 2016;12:e1006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chahoud J, Semaan A, Chen Y, et al. Association Between beta-Genus Human Papillomavirus and Cutaneous Squamous Cell Carcinoma in Immunocompetent Individuals-A Meta-analysis . JAMA Dermatol 2016;152:1354–1364. [DOI] [PubMed] [Google Scholar]
- 39.Iannacone MR, Gheit T, Pfister H, et al. Case-control study of genus-beta human papillomaviruses in plucked eyebrow hairs and cutaneous squamous cell carcinoma. Int J Cancer 2014;134:2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]


