Abstract
BACKGROUND:
Attributing causes of postoperative mortality is challenging, as death may be multifactorial. A better understanding of complications that occur in patients who die is important, as it allows clinicians to focus on the most impactful complications. We sought to determine the postoperative complications with the strongest independent association with 30-day mortality.
METHODS:
Data were obtained from the 2012–2013 National Surgical Quality Improvement Program Participant Use Data Files. All inpatient or admit day of surgery cases were eligible for inclusion in this study. A multivariable least absolute shrinkage and selection operator regression analysis was used to adjust for patient pre- and intraoperative risk factors for mortality. Attributable mortality was calculated using the population attributable fraction method: the ratio between the odds ratio for mortality and a given complication in the population. Patients were separated into 10 age groups to facilitate analysis of age-related differences in mortality.
RESULTS:
A total of 1,195,825 patients were analyzed, and 9255 deceased within 30 days (0.77%). A complication independently associated with attributable mortality was found in 1887 cases (20%). The most common causes of attributable mortality (attributable deaths per million patients) were bleeding (n = 368), respiratory failure (n = 358), septic shock (n = 170), and renal failure (n = 88). Some complications, such as urinary tract infection and pneumonia, were associated with attributable mortality only in older patients.
DISCUSSION:
Additional resources should be focused on complications associated with the largest attributable mortality, such as respiratory failure and infections. This is particularly important for complications disproportionately impacting younger patients, given their longer life expectancy.
Mortality is commonly chosen as a primary outcome in perioperative studies. It is easily measured, externally verifiable, and clinically important. Depending on the surgical population being studied, it can even be considered a relatively common occurrence.
The postoperative mortality rate is poorly defined and varies widely among studies depending on the exact population being examined, whether intraoperative mortality is included, and the duration of follow-up. Despite extensive efforts, the crude perioperative mortality rate may not have changed significantly in the past 60 years. In their landmark 1954 study, Beecher and Todd1 found an all-cause peri-operative mortality rate of approximately 1.3%. A recent Dutch study examined 30-day postoperative mortality and found an incidence of 1.85%.2 Current postoperative mortality rates in the United States appear to be similar, with an estimated 30-day mortality rate of 2.1% after inpatient procedures.3 Death rates may vary widely between hospitals and across countries, with a range of 3.5%–6.9% after high-risk procedures in the United States and 1.2%–21.5% in Europe.4–6 Death after surgery usually follows a complication, such as infection, myocardial infarction, kidney injury, and thromboembolism. Determining the chronology of these events may be difficult, particularly in a retrospective fashion. Some patients have >1 complication, and the risk of death is increased with the number of complications. Patients who have complications are also more likely to have preoperative comorbidities.
Attributing causes of postoperative mortality may be difficult, as the death may be related to complications, comorbidities, or their combinations and may involve pathology in numerous organ systems. Furthermore, mortality may be related to both the occurrence of a postoperative complication and the effectiveness of the therapy used to address the complication, “failure to rescue.”7–11
Attributable mortality, the mortality that would be prevented if that complication had not occurred, is used by epidemiologists to estimate the public health impact of a wide variety of diseases and complications and provides a statistically validated method of estimating the number of deaths that can be attributed to each comorbidity or complication, adjusting for all the others.12–16 It is defined as the ratio of the odds ratio increase of mortality and a given complication. Attributable mortality estimates may further be used to estimate years of potential life lost due to a disease or complication, allowing clinicians to better understand which complications should be identified as targets for intervention and allocation of resources.
We used a large, heterogeneous dataset of surgical cases to determine the attributable mortality of 21 common postoperative complications. We hypothesized that the attributable mortality of postoperative complications varies in a postoperative population by complication and age groups.
METHODS
As the study utilized publicly available, deidentified data, it was conducted under the Federal Policy for the Protection of Human Subjects or the “Common Rule” and HHS regulations 45 CFR part 46 and did not require Institutional Review Board approval. It was deemed “not regulated” by the University of Michigan Institutional Review Board, and a waiver of informed consent was granted (HUM00091751).
Data were obtained from the 2012–2013 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) participant use data file. The ACS-NSQIP methodology has been described in detail elsewhere.17,18 Briefly, the first 40 surgical procedures at a participating institution are sampled every 8-day cycle. High-volume procedures are limited to avoid oversampling common procedures. A trained reviewer at each site collects data on all sampled patients and is responsible for documentation of postoperative complications (Supplemental Digital Content, Appendix 1, http://links.lww.com/AA/C209). Participants are followed for a 30-day period postoperatively. The ACS-NSQIP and participating hospitals are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Statistical Analysis
All surgery cases present in the 2012–2013 ACS-NSQIP participant use data file were eligible for inclusion in this study (https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx). A complete data dictionary can be found at this link. There were no exclusion criteria. Because the risks of developing a complication and subsequently dying from that complication may vary by age, we chose to stratify our population into commonly used age ranges: <31, 31–40, 41–50, 51–55, 56–60, 61–65, 66–70, 71–75, 76–80, and >80 years. A multivariable, least absolute shrinkage and selection operator regression analysis was conducted to determine the independent variables associated with mortality. This regression was used to adjust for patient pre- and intraoperative risk factors that might be associated with mortality but also for multiple complications that each patient could develop. After variable selection by least absolute shrinkage and selection operator, the selected variables were then reentered in a logistic regression to calculate the odds ratios and confidence intervals. We used 20 of 21 complications that NSQIP identifies and records (Supplemental Digital Content, Appendix 1, http://links.lww.com/AA/C209). We prospectively excluded cardiac arrest as a possible predictor of mortality as it preceded most instances of mortality, as defined by NSQIP. We used the Tripod statement (www.tripod-statement.org) for guidance in describing and presenting our models.
Attributable mortality was calculated using the population attributable fraction method: attributable mortality = number of complications × ((P(D|C+) − P(D|C−)), where P(D|C+) is the probability of death given a complication and P(D|C+) is the probability of death without a complication.12,19,20 For example, if 110 of 1000 patients die, 150 patients have complication X, and the adjusted odds ratio of dying associated with X is 1.8, then we can calculate as 85 of 850 patients without X die and 25 of 150 with X die, so the odds of dying without X is 9:1 [P(D|C−) is 10% or (0.10)] and with X the odds are 5:1 (odds ratio = 1.8) [P(D|C+) is 16.7% or (0.167)]. Without X, only 15 of the 150 would die, and these deaths would be attributable to other things—perhaps their comorbidities or other complications. Attributable mortality from X is 150 • (0.167 − 0.10) = 10.
These data were then combined with actuarial mortality data from the 2011 United States Life Tables, published by the Division of Vital Statistics.21 The 2011 tables were the most recent available at the time the study was conducted. In these tables, age is listed as an ordinal category. The life expectancy for an age group was defined as the average life expectancy (“expectation of life at age”) for the median age of that group and calculated separately for each sex. This life expectancy was used in calculating years of life lost attributable to complications.
For a power analysis, we assumed that mortality was 1% in patients without complications and 1.98% in patients with complications. To detect an odds ratio = 2 with 90% power and an α = .05, it would require 64,000 patients.22
All data processing and statistical analyses were completed using SPSS Version 22 (IBM, Chicago, IL) and R 3.2.2 for Windows (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 1,195,825 patients underwent analysis. A majority of the patients were women (n = 686,812; 57%) and Caucasian (n = 887,869; 74%). The most common comorbidities were hypertension (n = 548,332; 46%) and diabetes mellitus (n = 113,885; 16%; Table 1). Mortality occurred within 30 days of surgery in 9255 patients (0.77%). The overall mortality rate was higher for men (n = 4863; 0.96%) than for women (n = 4392; 0.64%; P < .001). Emergency cases, comprising 9.9% of the population (n = 118,709), had nearly a 10-fold higher risk (4.2% vs 0.40%) of death compared to nonemergency cases (P < .001). Similarly, patients with comorbidities had an increased risk of perioperative mortality (Table 1). Mortality rate increased with increasing age, rising from 0.09% in patients <31 years of age to 2.70% in patients >80 years of age (Table 2).
Table 1.
Demographics, Operative Factors, and Comorbidities
| Attribute | Total Number | Percent of Population | Number Expired | 30-Day Mortality (%) |
|---|---|---|---|---|
| Overall | 1,195,825 | 100 | 9255 | 0.77 |
| Female sex | 686,812 | 57 | 4392 | 0.64 |
| Male sex | 509,013 | 43 | 4863 | 0.96 |
| Emergency surgery | 118,709 | 10 | 4943 | 4.2 |
| Surgery | ||||
| Cardiac | 7121 | 1.0 | 185 | 2.6 |
| Vascular | 90,371 | 8.0 | 1841 | 2.0 |
| Thoracic | 15,772 | 1.0 | 287 | 1.8 |
| Noncardiovascular/thoracic | 1,082,561 | 91 | 6942 | 0.64 |
| Primary anesthesia technique: general | ||||
| General | 1,078,475 | 90 | 8799 | 0.82 |
| Neuraxial | 53,422 | 4.0 | 237 | 0.44 |
| Othera | 63,928 | 5.0 | 219 | 0.34 |
| ASA physical status l-lll | 1,118,486 | 94 | 3602 | 0.32 |
| ASA physical status IV-V | 3499 | <1.0 | 33 | 0.94 |
| Current smoker | 218,055 | 18 | 1948 | 0.89 |
| Dyspnea at rest | 5975 | 1.0 | 477 | 8.0 |
| Caucasian race | 887,869 | 74 | 6678 | 0.75 |
| Insulin-dependent diabetes mellitus | 68,537 | 6.0 | 1316 | 1.9 |
| Noninsulin-dependent diabetes mellitus | 113,885 | 10 | 1108 | 0.97 |
| Ventilator dependent | 4581 | <1.0 | 1553 | 34 |
| Chronic obstructive pulmonary disease | 55,309 | 5.0 | 1752 | 3.2 |
| Presence of ascites | 5114 | <1.0 | 627 | 12 |
| Congestive heart failure | 9548 | 1.0 | 848 | 8.9 |
| Hypertension | 548,332 | 46 | 6507 | 1.2 |
| Acute renal failure | 4642 | <1.0 | 563 | 12 |
| Chronic dialysis | 16,710 | 1.0 | 873 | 5.2 |
| Disseminated cancer | 26,134 | 2.0 | 947 | 3.6 |
All P values are <.001 and compare patients with each condition to those without that condition.
Abbreviation: ASA, American Society of Anesthesiologists.
Other—anesthetic technique other than general or neuraxial (ie, local, monitored anesthesia care, etc).
Table 2.
Mortality Rate by Age Group
| Age (y) | Number of Patients | Percentage of Population | Number Deceased | Mortality Rate (%) | Life Expectancy at Median Age (y) | A | B |
|---|---|---|---|---|---|---|---|
| <31 | 92,554 | 8 | 83 | 0.1 | 56.2 | 2050 | 2215 |
| 31–40 | 122,075 | 10 | 144 | 0.1 | 45.3 | 602 | 493 |
| 41–50 | 192,764 | 16 | 420 | 0.2 | 36.0 | 1120 | 581 |
| 51–55 | 123,572 | 10 | 460 | 0.4 | 29.4 | 1253 | 1014 |
| 56–60 | 130,825 | 11 | 681 | 0.5 | 25.0 | 1823 | 1394 |
| 61–65 | 135,237 | 11 | 974 | 0.7 | 21.1 | 2830 | 2093 |
| 66–70 | 129,636 | 11 | 1182 | 0.9 | 17.3 | 3927 | 3030 |
| 71–75 | 101,341 | 8 | 1273 | 1.3 | 13.8 | 2513 | 2480 |
| 76–80 | 76,255 | 6 | 1255 | 1.7 | 10.5 | 3312 | 4343 |
| >81 | 76.732 | 6 | 2074 | 2.7 | 4.6 | 2952 | 3847 |
Life expectancy data derived from the Centers for Disease Control and Prevention Life Tables, using median age for each age group.21 A—Years of life lost among patients in that age group. B—Years of life lost among patients in that age group, per 100,000 patients.
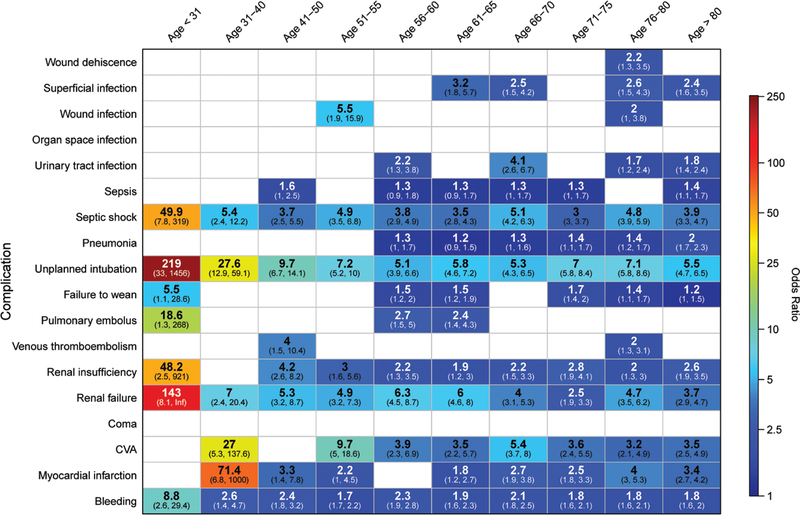
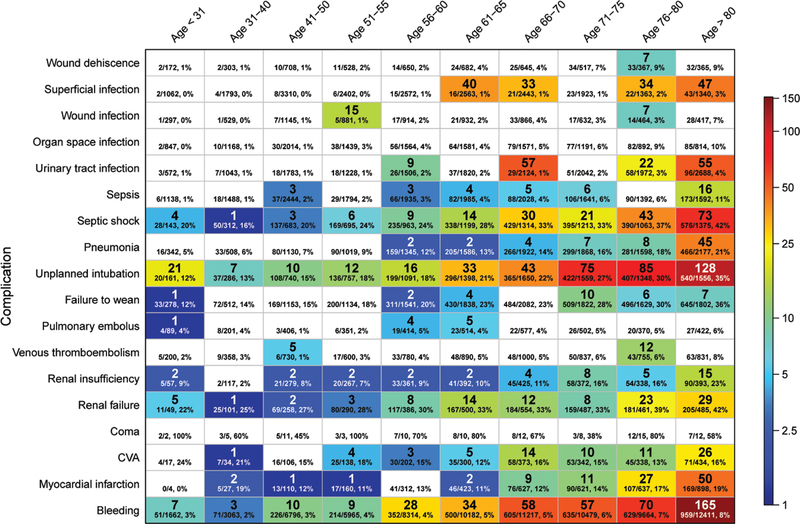
After adjustment for comorbidities (Table 1), sex, race, types of surgery, and emergency status, 20% of deaths (n = 1887) were attributed to a postoperative complication (Figure 1), with the 3 most common being bleeding (n = 441; 4.8%), unplanned intubation (n = 430; 4.6%), and septic shock (n = 204; 2.2%; Table 3). While complications were frequently present in the remaining patients who died (n = 7735; 83.6%), their mortality was attributed to other factors, such as comorbidities or type of surgery. Some complications, such as unplanned intubation, bleeding, septic shock, renal failure, and stroke, were associated with increased attributable mortality in all or almost all age groups, while others, such as pneumonia and urinary tract infections, were only increased in the elderly (Figures 1–2). Figure 1 displays the adjusted odds ratio for mortality in varying age groups but, importantly, does not account for the number of patients in each group. Figure 2 displays these same data but uses the attributable mortality instead of the adjusted odds ratio, thereby showing the effect of both the odds ratio and the frequency of the occurrence.
Figure 1.
Heat map of a complication’s adjusted odds ratio of mortality, by age group. Each box shows adjusted odds ratio. CVA: stroke. Blank cells did not achieve statistical significance or were not included after variable selection by least absolute shrinkage and selection operator. CVA indicates cerebrovascular accident.
Table 3.
Attributable Mortality by Complication
| Complication | Attributable Deaths in Study Population | Attributable Deaths per 1 Million Surgeries |
|---|---|---|
| Bleeding | 441 | 368 |
| Unplanned intubation | 430 | 358 |
| Septic shock | 204 | 170 |
| Superficial infection | 154 | 128 |
| Urinary tract infection | 143 | 119 |
| Renal failure | 105 | 88 |
| Myocardial infarction | 103 | 86 |
| Stroke | 74 | 62 |
| Pneumonia | 68 | 57 |
| Renal insufficiency | 42 | 35 |
| Failure to wean | 30 | 25 |
| Sepsis | 37 | 31 |
| Wound infection | 22 | 18 |
| Venous thromboembolism | 17 | 14 |
| Pulmonary embolus | 10 | 8 |
| Wound dehiscence | 7 | 6 |
| Organ space infection | 0 | 0 |
| Coma | 0 | 0 |
| Total infectious mortalitya | 635 | 529 |
| Total respiratory mortalityb | 528 | 440 |
| Total cardiovascular mortalityc | 204 | 170 |
| Total renal mortalityd | 147 | 123 |
| Total other mortalitye | 448 | 374 |
| Total attributable mortality from listed complications | 1887 | 1573 |
| Total overall mortality | 9255 | 7739 |
Septic shock, pneumonia, sepsis, urinary tract infection, superficial infection, wound infection.
Unplanned intubation, failure to wean, pneumonia.
Myocardial infarction, stroke, venous thromboembolism, pulmonary embolus.
Renal failure, renal insufficiency.
Bleeding, wound dehiscence.
Figure 2.
Heat map of a complication’s attributable mortality, by age group. Each box shows the attributable mortality (top number, bolded) followed by the overall (unadjusted) mortality ratio and percentage for patients in that age group who experienced that complication (bottom line in each box). White cells did not achieve statistical significance or were not included after variable selection by least absolute shrinkage and selection operator. CVA indicates cerebrovascular accident.
When grouped by organ system, infectious complications, including pneumonia, accounted for the largest share of attributable mortality in the study population of 9255 deaths (n = 635; 6.9%) followed respiratory (n = 528; 5.7%) and bleeding (n = 441; 4.8%; Table 3).
When taking age and life expectancy into account using Centers for Disease Control and Prevention life table data, unplanned intubation (5397 years), bleeding (4876 years), and septic shock (2202 years) were associated with the greatest number of years of life lost (Table 4).
Table 4.
Years of Life Lost Associated With Each Complication, per 1 Million Patients
| In Study Population | Per Million Surgeries | |||||
|---|---|---|---|---|---|---|
| Complication | Women | Men | Total | Women | Men | Total |
| Bleeding | 3474 | 2378 | 5852 | 2895 | 1981 | 4876 |
| Unplanned intubation | 3110 | 3367 | 6477 | 2592 | 2806 | 5397 |
| Septic shock | 1357 | 1284 | 2641 | 1131 | 1070 | 2202 |
| Superficial infection | 1171 | 816 | 1987 | 976 | 680 | 1656 |
| Urinary tract infection | 1094 | 613 | 1707 | 911 | 511 | 1422 |
| Renal failure | 679 | 961 | 1640 | 566 | 801 | 1367 |
| Myocardial infarction | 465 | 532 | 997 | 387 | 443 | 830 |
| Stroke | 510 | 441 | 951 | 425 | 368 | 792 |
| Pneumonia | 265 | 272 | 537 | 221 | 226 | 447 |
| Renal insufficiency | 266 | 357 | 623 | 222 | 297 | 519 |
| Failure to wean | 192 | 224 | 416 | 160 | 187 | 347 |
| Sepsis | 255 | 250 | 505 | 212 | 208 | 420 |
| Wound infection | 314 | 203 | 517 | 261 | 169 | 430 |
| Venous thromboembolism | 183 | 124 | 307 | 153 | 103 | 256 |
| Pulmonary embolus | 146 | 116 | 262 | 122 | 97 | 219 |
| Wound dehiscence | 45 | 29 | 74 | 38 | 24 | 62 |
| Organ space infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Come | 0 | 0 | 0 | 0 | 0 | 0 |
DISCUSSION
Using the ACS-NSQIP database, a large, heterogeneous national dataset of 1.2 million surgical patients, we have defined the 30-day postoperative mortality rate associated with multiple postoperative complications, grouped by age. We found that many deaths can be attributed to an individual complication, and the complications that cause the most attributable mortality vary with age. The remaining deaths were attributable to other factors, such as preexisting comorbidities and type of surgery.
Existing research on mortality after a postoperative complication, also known as failure to rescue, has mostly focused on studying sources of variation between centers and individual surgeons.23–25 Only a few studies have attempted to determine the amount of postoperative mortality attributed to individual complications. A recent single-center study of 117 deaths in 7763 patients in Rhode Island found that only respiratory failure, sepsis, and renal failure were significantly associated with failure to rescue and mortality.26 Our study agrees with that study in finding that those complications are among the most common complications associated with death and extends their work by using a much larger database that permitted us the statistical power to find more mortality-associated complications and to calculate the age-related years of life lost for each complication.
Several complications were associated with attributable mortality in all age groups. Unplanned intubation was associated with the largest attributable mortality and the greatest number of years of life lost. We stress that unplanned intubation is largely a marker of underlying conditions or complications, such as hypoxia, deconditioning, multisystem organ dysfunction, and pneumonia. While it is probably the underlying conditions that cause death, 2 recent prospective studies found that, while noninvasive ventilation or high-flow nasal cannula oxygen decreased intubation rates, they failed to lower the mortality rate.27,28 However, given that unplanned intubation is associated with the greatest number of years of life lost after surgery, further studies on preoperative optimization, intraoperative lung protective ventilation and fluid management, and postoperative respiratory physiotherapy are urgently needed to determine how best to prevent postoperative respiratory failure. While studies have shown that prehabilitation decreases the occurrence of postoperative complications, its effect on mortality has not been studied.29
Similarly, improved perioperative management may decrease attributable mortality from sepsis and renal failure. The risk of perioperative infections can be lowered by correct antibiotic usage and timing, hand hygiene, use of sterile technique for line insertions and administering medications, and, perhaps, regional anesthesia.30–33
We found that renal failure was a large contributor to years of life lost. Recent studies have suggested that anesthetic management, in particular, hypotension, even mild, is associated with kidney injury, and that the choice of vasopressor to treat the hypotension is associated with more or less kidney injury.34,35
Myocardial infarction was significantly associated with mortality in all age groups except <31 years and 56–60 years. Recent studies have shown that nonclinically apparent myocardial infarction, diagnosed by troponin elevations, is associated with increased mortality.36,37 A significant limitation of this study is that it is unknown how frequently patients are screened postoperatively for troponin levels. As this was not common clinical practice in 2012–2013, the years of the data collection, we suspect that routine troponin screening would lead to even more deaths attributable to myocardial infarction. Despite recent guidelines on the preoperative management of patients undergoing noncardiac surgery, controversy remains about how best to treat patients to minimize cardiovascular morbidity and mortality.38
Interestingly, we only found 22 deaths from either deep vein thrombosis or pulmonary embolism. This may represent the success of perioperative prophylactic anticoagulation in preventing these disorders. Alternatively, this may be due to the failure to recognize pulmonary embolism in otherwise ill patients.39 Further study is needed on this topic.40
Certain strengths of this study should be highlighted. We used a large, national database containing data from a wide variety of patient populations and practice settings. Consistent data definitions are used throughout the database, and rigorous standards of data quality are enforced. The ACS-NSQIP database takes steps to limit oversampling of high-volume procedures. Patients are closely followed for 30 postoperative days by trained reviewers to ensure low rates of missing or incomplete data. Our sample size of almost 1.2 million patients allowed for more robust statistical analysis than may have been conducted on single-center data, thus improving its generalizability. However, despite this sample size, we may have type II errors and missed rarely occurring complications that were truly associated with increased mortality. Only 2 years of data were used to minimize temporal bias. Analyses were conducted by complication and age group. As increasing age is one of the strongest predictors of mortality, this allowed us to better account for age-related variation in mortality.
The study has several limitations. The study format was retrospective in nature, and definitive causality cannot be established between any complication and mortality. All findings should be considered to represent correlation or association. While temporal trends likely exist and patterns or groupings of complications may be significant contributors to mortality and are worthy of further exploration, they were felt to be outside of the scope of this study. Future studies should build on our study and explore them in greater detail. Importantly, we were unable to differentiate between attributable mortality due to a complication and attributable mortality due to the treatment of that complication. It is possible that some of the attributable mortality of a given complication may represent suboptimal treatment, as treatment regimens are not standardized in the ACS-NSQIP database. While further study is needed to determine optimal treatment of these complications, our study identifies the complications that would most benefit from improved treatment. Another limitation is that, as mortality was only counted if it occurred within 30 days of surgery, later deaths were unknown, and therefore our estimates of mortality attributed to a complication may be low. Finally, while much of the failure to rescue literature has studied how mortality after complication varies by hospital, this was not available in our dataset, and we were unable to add it to our model.
Our use of actuarial life expectancy tables to calculate years of life lost among patients in different age groups allowed us to better assess the impact of complications on younger patients. These actuarial tables are derived from the US population as a whole and not a surgical population. The true life expectancy of a surgical population likely differs from the US population. Our experimental methodology allowed for some adjustment for this difference by basing years of life lost estimates on excess mortality in patients who experienced complications compared to a reference of patients who did not experience complications.
CONCLUSIONS
We found that 20% of all 30-day mortality is independently attributable to a postoperative complication. Unplanned intubation, septic shock, and bleeding are associated with the greatest number of years of life lost. Resources should be focused on preventing and better treating complications associated with the largest attributable mortality, particularly when they disproportionately impact younger patients, given their longer life expectancy.
Supplementary Material
KEY POINTS.
Question: Can we retrospectively determine which complications caused the largest share of attributable postoperative mortality using a large, national dataset?
Findings: A complication independently attributable to postoperative mortality was identified for 20% of all postoperative mortality.
Meaning: Additional resources should be focused on complications associated with the largest attributable mortality; this is particularly important for complications that disproportionately impact younger patients, given their longer life expectancy.
Acknowledgments
Funding: None.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
The authors declare no conflicts of interest.
REFERENCES
- 1.Beecher HK, Todd DP. A study of the deaths associated with anesthesia and surgery: based on a study of 599, 548 anesthesias in ten institutions 1948–1952, inclusive. Ann Surg. 1954;140:2–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in the Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112:1105–1115. [DOI] [PubMed] [Google Scholar]
- 3.Fecho K, Lunney AT, Boysen PG, Rock P, Norfleet EA. Postoperative mortality after inpatient surgery: incidence and risk factors. Ther Clin Risk Manag. 2008;4:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearse RM, Moreno RP, Bauer P, et al. ; European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology.Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. [DOI] [PubMed] [Google Scholar]
- 6.International Surgical Outcomes Study Group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silber JH, Rosenbaum PR. A spurious correlation between hospital mortality and complication rates: the importance of severity adjustment. Med Care. 1997;35:OS77–OS92. [DOI] [PubMed] [Google Scholar]
- 8.Silber JH, Rosenbaum PR, Schwartz JS, Ross RN, Williams SV. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA. 1995;274:317–323. [PubMed] [Google Scholar]
- 9.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. [DOI] [PubMed] [Google Scholar]
- 10.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. [DOI] [PubMed] [Google Scholar]
- 11.Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Med Care. 2007;45:918–925. [DOI] [PubMed] [Google Scholar]
- 12.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. [DOI] [PubMed] [Google Scholar]
- 13.Nonnemaker J, Rostron B, Hall P, MacMonegle A, Apelberg B. Mortality and economic costs from regular cigar use in the United States, 2010. Am J Public Health. 2014;104:e86–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmet S, Rehm J, Gmel G, Frick H, Gmel G. Alcohol-attributable mortality in Switzerland in 2011–age-specific causes of death and impact of heavy versus non-heavy drinking. Swiss Med Wkly. 2014;144:w13947. [DOI] [PubMed] [Google Scholar]
- 15.Vaara ST, Pettilä V, Kaukonen KM, et al. ; Finnish Acute Kidney Injury Study Group. The attributable mortality of acute kidney injury: a sequentially matched analysis. Crit Care Med. 2014;42:878–885. [DOI] [PubMed] [Google Scholar]
- 16.Muscedere JG, Martin CM, Heyland DK. The impact of ventilator-associated pneumonia on the Canadian health care system. J Crit Care. 2008;23:5–10. [DOI] [PubMed] [Google Scholar]
- 17.Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119:522–532. [DOI] [PubMed] [Google Scholar]
- 18.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110:505–515. [DOI] [PubMed] [Google Scholar]
- 19.Melsen WG, Rovers MM, Bonten MJ. Attributable mortality of ventilator-associated pneumonia: authors’ reply. Lancet Infect Dis. 2013;13:1015. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher M, Wangler M, Wolkewitz M, Beyersmann J. Attributable mortality due to nosocomial infections: a simple and useful application of multistate models. Methods Inf Med. 2007;46:595–600. [PubMed] [Google Scholar]
- 21.Arias E United States life tables, 2011. Natl Vital Stat Rep. 2015;64:1–63. [PubMed] [Google Scholar]
- 22.van der Ploeg T, Austin PC, Steyerberg EW. Modern modelling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol. 2014;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buettner S, Gani F, Amini N, et al. The relative effect of hospital and surgeon volume on failure to rescue among patients undergoing liver resection for cancer. Surgery. 2016;159:1004–1012. [DOI] [PubMed] [Google Scholar]
- 24.Silber JH, Rosenbaum PR, Kelz RR, et al. Examining causes of racial disparities in general surgical mortality: hospital quality versus patient risk. Med Care. 2015;53:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh MD, Kelly LA, Smith HL, Wu ES, Vanak JM, Aiken LH. Lower mortality in magnet hospitals. Med Care. 2013;51:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiulli LC, Stephen AH, Heffernan DS, Miner TJ. Association of medical comorbidities, surgical outcomes, and failure to rescue: an analysis of the Rhode Island Hospital NSQIP database. J Am Coll Surg. 2015;221:1050–1056. [DOI] [PubMed] [Google Scholar]
- 27.Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315:1354–1361. [DOI] [PubMed] [Google Scholar]
- 28.Jaber S, Lescot T, Futier E, et al. ; NIVAS Study Group. Effect of noninvasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA. 2016;315:1345–1353. [DOI] [PubMed] [Google Scholar]
- 29.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160:1189–1201. [DOI] [PubMed] [Google Scholar]
- 30.Berenholtz SM, Lubomski LH, Weeks K, et al. ; On the CUSP: Stop BSI program. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35:56–62. [DOI] [PubMed] [Google Scholar]
- 31.Cole DC, Baslanti TO, Gravenstein NL, Gravenstein N. Leaving more than your fingerprint on the intravenous line: a prospective study on propofol anesthesia and implications of stopcock contamination. Anesth Analg. 2015;120:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrini JE, Toledo P, Soper DE, et al. Consensus bundle on prevention of surgical site infections after major gynecologic surgery. Anesth Analg. 2017;124:233–242. [DOI] [PubMed] [Google Scholar]
- 33.Hausman MS Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg. 2015;120:1405–1412. [DOI] [PubMed] [Google Scholar]
- 34.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. [DOI] [PubMed] [Google Scholar]
- 35.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology. 2017;126:85–93. [DOI] [PubMed] [Google Scholar]
- 36.Devereaux PJ, Chan MT, Alonso-Coello P, et al. ; Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Jimenez F, Goldman L, Sacks DB, et al. Prognostic value of cardiac troponin T after noncardiac surgery: 6-month follow-up data. J Am Coll Cardiol. 1997;29:1241–1245. [DOI] [PubMed] [Google Scholar]
- 38.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American College of Surgeons, American Society of Anesthesiologists, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Vascular Medicine Endorsed by the Society of Hospital Medicine. J Nucl Cardiol. 2015;22:162–215. [DOI] [PubMed] [Google Scholar]
- 39.Blosser SA, Zimmerman HE, Stauffer JL. Do autopsies of critically ill patients reveal important findings that were clinically undetected? Crit Care Med. 1998;26:1332–1336. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs JJ, Mont MA, Bozic KJ, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Bone Joint Surg Am. 2012;94:746–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.