ABSTRACT
Physical exercise can improve gait, balance, tremor, flexibility, grip strength and motor coordination in Parkinson’s disease (PD) patients. Several lines of evidence have also shown the therapeutic potential of dietary management and supplementation in halting the progression of PD. However, there is a lack of research on the combined effects of physical activity and nutrition in the progression of PD. We test the effects exercise and dietary modification in a Drosophila model of PD. In this study, we fed Drosophila parkin mutants high protein and high carbohydrate diets without and with stearic acid (4 treatments in total). In parallel, we subjected mutants to a regimen of exercise using a purpose-built ‘Power tower’ exercise machine. We then measured climbing ability, aconitase activity, and basal mitochondrial ROS levels. We observed that exercising parkin mutants fed the high protein diet improved their climbing ability and increased aconitase activity. There was an additional improvement in climbing and aconitase activity in exercised parkin mutants fed the high protein diet supplemented with stearic acid. No benefits of exercise were seen in parkin mutants fed the high carbohydrate diet. Combined, these results suggest that dietary management along with physical activty has potential to improve mitochondrial biogenesis and delay the progression of PD in Drosophila parkin mutants.
KEYWORDS: Drosophila parkin mutants, protein, carbohydrate, stearic acid, exercise
Introduction
The primary goal of this study is to investigate whether dietary management with physical exercise, is more effective than either alone, in delaying the onset of Parkinson’s disease (PD) like symptoms in a Drosophila model of PD. Most generally, there are at least four approaches that can delay the onset of PD and improve health span. We define health span as the length of time that a person is healthy – not just alive. First, overall well-being may be improved by a general improvement in health and enhanced immune function. Second, improving the strength and vigor of individuals with any neurological disease is expected to slow the development of PD. Third, strategies targeted towards the molecular mechanisms underpinning PD are a highly specific approach to improve the health of people with the disease. Fourth, interactions between both environmental and genetic variables may provide novel treatment opportunities.
A general improvement of diet is expected to increase well-being and immune function. Seidl et al. [1] outlined the foods that are linked to a reducing risk or progression of PD in humans. These include fruits and vegetables, and what has been referred to as a ‘Mediterranean diet,’ although this relationship is not straightforward since pomegranate juice exacerbated oxidative stress in a rotenone model of PD [2]. A high carbohydrate diet has been shown to increase longevity in mice and flies, and in a fly model of PD, it delays the onset of PD like symptoms [3–5]. Here, we follow our previous study and include 1:2 Protein: Carbohydrate (P:C) and 1:16 P:C diets [5]. Protein and carbohydrate are the two-major energy-yielding macronutrients in the fly diet and these ratio’s span range naturally fed upon by flies in nature.
Developing novel methods of stimulating neuroplasticity is a promising treatment approach to counterbalance maladaptive influences and alleviate symptomologies. One non-pharmacological approach with significant and direct impacts on neuroplasticity is aerobic exercise [6], however, there is debate about the benefit of aerobic exercise training as a specific treatment for PD [7–9]. Here, Drosophila flies fed four diets were exercised by employing their negative geotaxis behavior [10]. Prior to this approach, experiments using insect models to examine the relationship of exercise on aging and disease utilized the continuous increase in flying activity as an exercise stimulus. Under these highly challenging conditions, exercise was shown to increase oxidative damage and to decrease lifespan [11,12].
Targeted strategies are the most specific approach to improve the health of people with PD. In humans, delaying the onset and severity of motor symptoms is beneficial for patients with PD as it can delay the onset of levodopa therapy and the subsequent onset of motor complications [13]. In this study, we add stearic acid to the high protein and high carbohydrate diets, conduct an exercise program over 11 days, and then assess aspects of physiology and mitochondrial health. We included stearic acid because it has been shown to act as a signaling molecule to improve survival and mitochondrial function in Drosophila parkin mutants [14].
Here, we test male Drosophila parkin mutant flies as a model for PD. Drosophila parkin mutants show a phenotype that highly resembles PD, including diminished climbing and flight ability, reduced mitochondrial functions, reduced longevity, and muscle degeneration [15,16]. Drosophila parkin is the homolog of human PARK2 and mutation of the PARK2 gene is a major cause of autosomal recessive juvenile PD [17,18].
Climbing ability, mitochondrial aconitase activity and basal mitochondrial reactive oxygen species (ROS) were quantified as measures of the nutritional and exercise regimes. We have previously shown improvement in the climbing ability of parkin- males fed 1:16 P:C food as compared to a 1:2 P:C diet [5]. Mitochondrial aconitase is an enzyme in the tricarboxylic acid (TCA) cycle, and the catalytic loss of activity is an indicator of oxidative damage [19]. Elevated levels of ROS produced by mitochondria as a by-product of energy production causes oxidative stress [20], which is important in the progression of PD [reviewed in, [21]].
The overall goal of the research is to determine an optimum set of modifications that delays the onset of a Parkinsonism like phenotype in parkin- males. Here, we hypothesized that moderate exercise and the dietary addition of stearic acid would improve physiological and mitochondrial functions in parkin- males. We have previously shown that parkin mutants have a longer lifespan when fed the 1:16 P:C compared to those fed the 1:2 P:C diet. Parkin mutants fed the high carbohydrate diet also have delayed climbing deficit, increased resistance to starvation, and improved mitochondrial functions [5]. We had also shown that parkin- flies had significant improvement in all physiological and mitochondrial functions assayed when stearic acid was added to high protein 1:2 P:C but not high carbohydrate 1:16 diet [22].
Results
Exercise training with climbing
To test our purpose-built Power tower, yw flies were exercised following the ramping protocol of Piazza and colleagues [10] and climbing assays conducted every second day from 3 d to 28 d of age. Relative to unexercised flies, the climbing ability of exercised flies declined more slowly, and there was a clear difference in this physiological trait until the cessation of exercising at 24 d of age (Figure 1). Student’s t-test showed a significant difference between the slopes of exercised and unexercised flies during the exercise period (t116 = 2.25, P = 0.026). Following the cessation of exercise, its benefits were quickly lost (Figure 1). These data are similar to that reported by Piazza and colleagues [10], though we observed a slight extension in the benefit of exercise.
Figure 1.

Climbing index of yw males. Flies were exercised from day 3 until day 24, five days a week. The left side of the dashed line is the exercise period, and right side of the dashed line is the post-exercise period. A total of 90 exercised and 90 unexercised yw flies were used. Each treatment had 6 replicates with 15 flies per replicate. During week one they were exercised for 2 hours/session, during week two for 2.5 hours/session and during week three for 3 hours/session. The lines indicate the regression for exercise and unexercised flies (for exercised flies y = − 0.0094x + 0.95 and for unexercised flies y = − 0.0172x + 0.97). Symbols indicate mean, and bars indicate s.e.m.
The parkin- males are known to have motor disorders [16], so we optimized the exercise program for 11 d old flies fed 1:2 P:C ratio diet. The most noticeable improvement came from Regimen 4 where parkin- males were subjected to 30 mins of exercise every alternate day (Figure 2). ANOVA showed a significant effect of regimen (F5,30 = 8.11, P < 0.001). Dunnett’s multiple comparison tests demonstrated significantly low climbing index of regimen 3 (Q = 2.65, P = 0.015) and an improved climbing index on regimen 4 (Q = 2.65, P = 0.002) compared to the unexercised parkin-.
Figure 2.
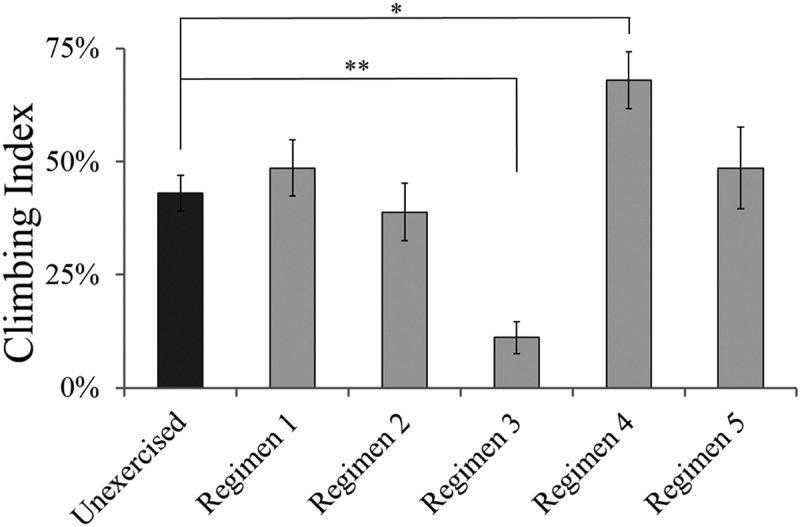
Optimization of exercise regimen for parkin- males using the climbing assay. Parkin- males had highest climbing index when exercised for 30 mins every alternate day (Regimen 4). Regimens 1, 2 and 3 exercised parkin- for 30, 60 and 120 mins every day, respectively. Regimens 4 and 5 exercised parkin- males for 30 and 60 mins, respectively on alternate days. Each regimen had 6 replicates of 12 parkin- males for 72 parkin- males/regimen (a total of 432 flies were included in the study). The flies were fed 1:2 P:C ratio diet throughout the exercise period. Bars indicate means and error bars indicate standard error. Significance determined by Dunnett’s test at *P < 0.05, **P < 0.01 (see text for details).
To further test whether the exercise regimen influenced the number of inactive flies we recorded the flies that climbed less than 20 mm. Regimen 4 had lowest numbers of inactive flies while regimen 3 had the highest (Supplementary Figure 2). ANOVA showed a significant effect of regimen (F5,30 = 5.57, P < 0.001) and Dunnett’s multiple comparison test showed significantly higher inactive flies on regimen 3 (Q = 2.65, P = 0.0008). As a result of these climbing results, Regimen 4 was followed for the remainder of the assays. A limitation of this optimization protocol was that we did not test flies fed the 1:16 P:C food.
Climbing ability with exercise and dietary modification
PD is characterized by a battery of physiological symptoms including slowness of movement [23]. We measured the climbing ability of unexercised and exercised parkin- flies fed four isocaloric diets. Both exercise and stearic acid improved the climbing ability of parkin- flies fed 1:2 P:C ratio diet but not the 1:16 P:C diet (Figure 3A). For parkin- fed 1:2 P:C diet, ANOVA showed significant main effects of stearic acid supplementation and exercise (F1,28 = 23.9, P < 0.001; F1,28 = 8.02, P = 0.01, respectively) with no significant interaction (F1,28 = 0.30, P = 0.58). For the 1:2 P:C diet, Student’s t-tests showed a significant effect of stearic acid (t28 = 4.88, P < 0.0001) and exercise (t14 = 3.44, P = 0.003). For parkin- flies fed the 1:16 P:C diet, ANOVA reported no significant effects of stearic acid supplementation, exercise or their interaction (F1,28 = 0.12, P = 0.73; F1,28 = 1.4, P = 0.25 and F1,28 = 1.65, P = 0.21, respectively).
Figure 3.
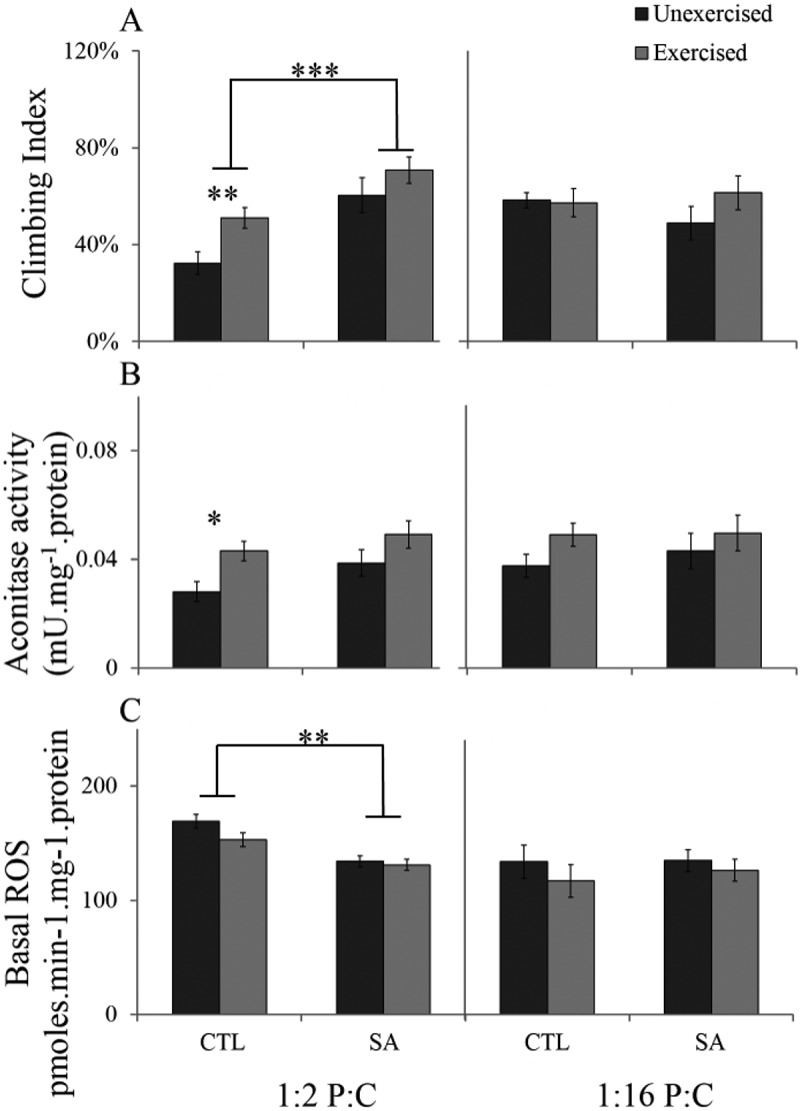
Climbing index, aconitase activity and Basal ROS of unexercised and exercised parkin- males fed high Protein (1:2 P:C) and high Carbohydrate (1:16 P:C) ratio diet without (CTL) or with stearic acid (SA). A. Climbing. B. Mitochondrial aconitase activity. C Basal ROS. Bars indicate means and bars indicate s.e.m. Significance determined by Student’s t-test at *P < 0.05, **P < 0.01 and ***P < 0.001 (see text for details).
Aconitase activity with exercise and dietary modification
Aconitase activity is an indicator of mitochondrial efficiency and loss of activity in samples treated with pro-oxidants has been interpreted as a measure of oxidative damage [19]. Exercise tended to increase aconitase activity of parkin- males fed both diets but the effect was more marked in flies fed the high protein 1:2 P:C diet (Figure 3B). For flies fed 1:2 P:C diet, ANOVA demonstrated a significant effect of exercise (F1,20 = 6.62, P = 0.02) but no significant effect of stearic acid or exercise by stearic acid interaction (F1,20 = 2.75, P = 0.11 and F1,20 = 0.20, P = 0.65, respectively). Student’s t-test showed a significant difference of exercise in parkin- fed 1:2 diet without stearic acid (t10 = 2.44, P = 0.034). For flies fed the 1:16 P:C diet, there was no significant effect of exercise, stearic acid supplementation, or their interaction (F1,20 = 1.40, P = 0.25; F1,20 = 0.12, P = 0.73 and F1,20 = 0.67, P = 0.74, respectively).
Basal mitochondrial ROS with exercise and dietary modification
Basal mitochondrial ROS gives the levels produced at the resting state and are an indicator of mitochondrial coupling efficiency in respiration. Stearic acid reduced basal ROS levels in flies fed the 1:2 P:C diet (Figure 3C). For parkin- males fed 1:2 P:C ratio diet, ANOVA demonstrated a significant effect of stearic acid supplementation (F1,20 = 9.04, P = 0.01) and no significant effect of exercise or stearic acid by exercise interaction (F1,20 = 1.04, P = 0.32 and F1,20 = 0.48, P = 0.49, respectively). A Student’s t-test showed a significant effect of stearic acid in parkin- fed 1:2 diet (t20 = 3.00, P = 0.007). For parkin- fed 1:16 P:C diet, ANOVA showed no significant effects of exercise, stearic acid supplementation or their interaction (F1,20 = 0.11, P = 0.74; F1,20 = 0.72, P = 0.40 and F1,20 = 0.07, P = 0.79, respectively).
Discussion
In humans, exercise and nutritional management are non-invasive strategies to counteract neurological and cognitive disorders [24]. In this study, we test the influence of dietary intervention and exercise in delaying PD like phenotypes in parkin mutant flies. We include high protein and high carbohydrate diets and examine the impact of adding stearic acid and of exercise on climbing ability, aconitase activity, and basal mitochondrial ROS levels. We find that both stearic acid and physical activity improved aspects of physiological health and mitochondrial functions when flies were fed the high protein diet but not the high carbohydrate diet. Collectively, these results suggest that improving mitochondrial function may be one avenue to delay the progression of PD.
Addition of stearic acid to the high protein diet improved climbing ability and basal ROS levels, likely by decreasing mitochondrial proton and electron leak [25–28]. Stearic acid is known to be converted to oleic acid [25], which is incorporated into mitochondrial phospholipids in the rat [26]. Phospholipids make up the characteristic outer and inner membranes that give mitochondria their shape [27]. It is the phospholipids that also give rise to other characteristic mitochondrial structures such as cristae (formed from the invaginations of the inner mitochondrial membrane), the matrix (area within cristae) and the intermembrane space that separates the outer mitochondrial membrane and inner mitochondrial membrane [27]. The incorporation of oleic acid derived stearic acid likely results in increased mitochondrial membrane potential in Drosophila [28], which is a critical component of the proton motive force required for ATP. More recently it has also been shown that stearic acid stearoylates TFR1, thereby inhibiting its activation of JNK signaling. This leads to reduced ubiquitination of mitofusin, which promotes mitochondrial health through fusion [14].
Moderate exercise improved climbing ability and aconitase levels. Combined, this suggests a general improvement in mitochondrial functions, which may then stimulate antioxidants and decrease oxidative stress. To exercise the flies, we constructed a Power tower [10] and conducted an initial study to develop an optimal exercise program for parkin mutant flies. Human and animal studies have reported that regular physical activity can help recovery from brain injury and improve learning and memory in age-related neurodegenerative disorders [29–35]. For example, treadmill exercise is a promising non-pharmacological approach for reducing the risk of PD and has proven to reduce depression and produce neuroprotective effects in a mouse model of PD [31,34,36]. Aerobic exercise has shown to improve gait, mood, cognition, balance, tremor, flexibility, grip and motor coordination in PD patients [8,9]. More generally, physical activity can be neuroprotective to aging individuals and may help the patients with neurodegenerative disease [29,30,32,37,38]. In a rat model of PD, treadmill exercise improved gait speed and balance, reduced oxidative stress, improved mitochondrial fusion and fission, increased mitochondrial amounts, and potentially attenuated dopaminergic neuron degeneration [39].
We show that exercise improved climbing ability and increased aconitase activity in parkin- fed a high protein diet. Further, the addition of stearic acid to the high protein diet enhanced climbing ability. Neither dietary modification nor physical activity significantly improved physiological or mitochondrial functions in flies fed the high carbohydrate diet. One possible explanation for these results is that both nutritional modifications and exercise work, at least partially, by improving mitochondrial health. Mitochondrial dysfunction is emerging as a common feature of PD suggesting pathogenic overlap among the familial, environmental and sporadic disease pathways [40]. Future studies may add rapamycin in the diet to explore the potential for exercise to reduce TORC1 signaling and improve the climbing activity of parkin mutants. Rapamycin has shown to increase the survival, aconitase and antioxidant activity in Drosophila models [41]. Likely, however, the specific mechanisms will differ due to the complex and dynamic nature of the organelle and its interactions with its cellular environment [42].
Materials and methods
Drosophila strains and husbandry
Initial testing of the Power tower made by us was conducted on yw males. Experimentally, yw; park25/TM6B were maintained, and park25/park25 (parkin-) males were used in the study [22].
Flies were maintained in Carolina 424 Drosophila Instant Medium in an incubator set to 23 ± 1°C, 60 ± 5% relative humidity with a 12 h: 12 h light: dark cycle. The densities of flies in bottles were monitored to avoid larval competition. The 1 d old male flies were sorted on the ice and placed into a vial with one diet. Flies were transferred to new vials every 2–3 d to avoid fungal contamination.
Dietary modification
For the initial study with yw flies, males were fed on Instant Drosophila food (Carolina, Biological Supply). Instant food was used for this study as it is commercially available in multiple countries. For the initial optimizing experiment with parkin- males the 1:2 P:C diet was chosen. This diet was selected because the parkin- flies had the most diminished climbing ability when fed this diet [5].
For the experimental study with parkin- males four isocaloric diets were constructed [22]. Diets have high protein (1:2 P:C) and high carbohydrate (1:16 P:C) and presence/absence of stearic acid. The diets without stearic acid are the controls for those with stearic acid. We calculated and tested a range of stearic acid concentrations and optimized the dietary stearic acid level to 4% suitable for parkin- males [22]: a previous study added 10% stearic acid into the fly diet [12]. The amount of yeast (MP Biomedicals, catalog no. 103304), sucrose (MP Biomedicals) and stearic acid (Sigma Aldrich) is shown in (Supplementary Table 1). The total calorie content of the diets is 0.65 ± 0.04 kcal.ml−1. The mixture of yeast, sucrose, stearic acid and 1% agar was cooked in the microwave for 5 min per 100 ml of deionized water. After heating the mixture of food, 1 ml of 0.01% phosphoric acid, 0.1% propionic acid and 0.1% of nipagen was added as antifungals agents, 2 ml of food was then transferred into 30 ml vials. The food was prepared each week and stored at 4°C until used.
Table 1.
Exercise regimens for parkin- males. The time of exercise ranges from 30 to 120 min per day. Exercising was done every day and every alternate day. Climbing Index was performed 3 h after the exercise on 11 d. A total of 72 flies were assayed for each regimen.
| Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen 1 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Regimen 2 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Regimen 3 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
| Regimen 4 | 30 | Rest | 30 | Rest | 30 | Rest | 30 | Rest | 30 | Rest | 30 |
| Regimen 5 | 60 | Rest | 60 | Rest | 60 | Rest | 60 | Rest | 60 | Rest | 60 |
Exercise training
There is a need to explore the benefits and limitations of exercise in PD [7]. Here, the Power tower was constructed following Piazza and colleagues [10] (Supplementary Figure 1). The instrument consists of a motor attached to a rotating arm. As the arm rotates along the lever, it raises the platform. At the top, the platform is released. Rubber shock absorbers on the base of the platform reduced excessive shock and the flies are knocked to the base of the vial. As the platform is raised the flies walk upwards using their geotaxis behavioral response.
To confirm that the machine we built performed like that reported by Piazza and colleagues [10] we conducted an initial study following their protocol. We collected 1 d old yw flies and kept in them in groups of 15. Flies were exercised five days a week, utilizing a ramping schedule. In the first week, flies were exercised for 2 hours/session, week two for 2.5 hours/session, and week three for three hours/session. For exercising, flies were first transferred to a fresh vial containing 2 ml of 1% agar and then covered with sponge stopper before the exercise. The vials were inverted so that during the exercise they landed on a soft surface. For, non-exercised flies, the sponge stopper was inserted deep into the vial so that flies received the same stress but had reduced capacity for geotaxis induced exercise. No exercise was performed after 24 d which was termed the post-exercise period.
To optimize the exercise program for parkin- flies, 1 d old flies were carefully sorted on ice, fed the 1:2 P:C ratio diet (without stearic acid) and climbing assay conducted at 11 d of age. We selected the 1:2 P:C diet because our previous study showed that parkin- fed 1:2 P:C ratio had significant climbing defects [5]. The 11 d age was selected because yw flies started to show exercise-induced differences at this age and a previous study including these diets, but not including exercise, measured climbing ability of parkin- males at this age [22].
To begin the exercise regimen, each vial included 12 parkin- males. The different exercise regimens are shown in Table 1. During the exercise period, no deaths were recorded in unexercised flies or regimens 1 and 4. However, 13 flies died in regimen 2, 19 in regimen 3 and 7 in regimen 5. At 11d of age, replacement exercised flies from the appropriate regimen were added to replace dead flies so that there was 12 parkin- males/vial. We employed this approach so that the 11 d climbing assays were not biased by small numbers. A preliminary study showed that there were no differences in food consumption between exercised and unexercised 11 d parkin- males (F1,49 = 0.03, P = 0.85).
Climbing ability
We tested the climbing ability of yw and parkin- males following Le Bourg and Lints [43] with slight modification. For each diet, flies were raised in groups of 12 per vial. A horizontal line was drawn 80 mm above the bottom of the 110 by 27 mm vial, and another same vial was used as a cover to provide additional space. We also recorded flies that were inactive (between 0–20 mm). The flies were acclimated for 10 min at room temperature. The flies were given 20 s to climb the vial, and the percentage of flies that crossed each mark each time was recorded.
A total of 90 exercised and 90 unexercised yw males were tested and 360 exercised, and 72 unexercised parkin- flies were assayed. Following each climbing assay, the yw males were returned to their vials to continue their exercise regime. For yw flies, the climbing assay was carried out on 3, 5, 7, 10, 12, 14, 17, 19, 21, 24, 26 and 28th day. For the parkin- flies, the climbing assay was conducted only at 11 d of age. After this assay flies were processed for aconitase activity and basal ROS.
Mitochondrial aconitase activity
Exercised wild-type flies have higher aconitase activity then unexercised ones [10], but the influence of exercise on parkin- males is unknown. Aconitase activity of parkin- flies was measured following the conversion of citrate into α-oxoglutarate coupled with the reduction of NADP, as previously described by [44]. We extracted mitochondria from thorax muscles following Melvin and Ballard [45]. The mitochondrial pellets were subject to four freeze-thaw cycles in liquid nitrogen in buffer containing 154 mM Tris HCl, pH 7.4, and 5 mM citrate. The reaction mixture contained 27 mM Tris-HCl, pH 7.4, 5 mM sodium citrate, 0.2 mM NADP, 0.6 mM MnCl2 and 1 unit of isocitrate dehydrogenase (where one unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 µmol of isocitrate from citrate per min at pH 7.4 at 30°C). The slope of NADPH formation over time indicated the activity of aconitase. Absorbance at a wavelength of 340 nm using a SpectraMax Plus spectrophotometer and SoftMax Pro software was followed over time at 30°C at (Molecular Devices Corp., Sunnyvale, CA, USA). A total of 48 mitochondrial extractions were assayed.
Basal mitochondrial ROS
We have previously shown basal mitochondrial ROS is higher in parkin- males fed the 1:2 P:C ratio diet than the 1:16 P:C food [5], but the influence of exercise is not known. ROS was assayed from mitochondria isolated from thorax muscles of flies raised in groups of 10. Mitochondria were extracted, and basal ROS was determined using Amplex Red (Thermofisher Scientific) as previously described [45,46]. Basal ROS levels were determined by quantifying levels before the addition of ADP. H2O2 reacts with Amplex Red in the presence of horseradish peroxidase to form the oxidative product resofurin, which has a maximum absorbance at 560 nm [47]. No substrate controls showed no treatment effects [48]. Results were recorded every 1 min for 15 min using the SpectraMax Plus spectrophotometer and SoftMax Pro software (Molecular Devices Corp., Sunnyvale, CA, USA). The amount of ROS production in each well was expressed as pmol of H2O2 production per minute per mg of protein. A total of 48 mitochondrial extractions were assayed from four experimental diets in 6 replicates.
Statistics
Student’s t-test on slopes tested whether our Power tower replicated the results of Piazza and colleagues [10]. Analysis of variance (ANOVA) with JMP© v12 (2007 SAS Institute, Cary, NC, USA) followed by Dunnett’s test was used to determine the significance in different exercise regimen tests. The high protein and high carbohydrate dietary studies were analyzed separately because visual inspection of the data suggested interesting high protein diet-specific effects. Climbing index data were arcsine square root transformed. ANOVA followed by Student’s t-tests were conducted to analyze the climbing, aconitase and basal ROS studies with parkin- males where diet and exercise were manipulated.
Funding Statement
The work was supported by Australian Research Council Discovery Project [DP160102575].
Acknowledgments
We wish to thank Prof. Richard Youle for providing the flies and motivating this study. We are grateful to Mr Pritipal Baweja from BABS workshop department for the construction of Power tower. Suggestions from Dr Rich Melvin, Dr Wen Aw, Sam Towarnicki and Joana Nunes were helpful and improved the manuscript. Gordon Smyth provided statistical advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Seidl SE, Santiago JA, Bilyk H, et al. The emerging role of nutrition in Parkinson’s disease. Front Aging Neurosci. 2014;6:36 PMID: 24639650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapias V, Cannon JR, Greenamyre JT.. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol Aging. 2014;35(5):1162–1176. PMID: 24315037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KP, Simpson SJ, Clissold FJ, et al. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105(7):2498–2503. PMID: 18268352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solon-Biet SM, McMahon AC, Ballard JWO, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. PMID: 24606899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajracharya R, Ballard JWO. Low protein to carbohydrate ratio diet delays onset of Parkinsonism like phenotype in Drosophila melanogaster parkin null mutants. Mech Ageing Dev. 2016;160:19–27. PMID: 27720857. [DOI] [PubMed] [Google Scholar]
- 6.Hendrikse J, Kandola A, Coxon J, et al. Combining aerobic exercise and repetitive transcranial magnetic stimulation to improve brain function in health and disease. Neurosci Biobehav Rev. 2017;83:11–20. PMID: 28951250. [DOI] [PubMed] [Google Scholar]
- 7.Lamotte G, Rafferty MR, Prodoehl J, et al. Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: a review. J Parkinson’s Dis. 2015;5(1):21–41. PMID: 25374272. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds GO, Otto MW, Ellis TD, et al. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson’s disease. Mov Disord. 2016;31(1):23–38. PMID: 26715466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu H-F, Yang T, Yu S-X, et al. Aerobic exercise for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(7):e100503 PMID: 24983753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piazza N, Gosangi B, Devilla S, et al. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One. 2009;4(6):e5886 PMID: 19517023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan LJ, Sohal RS. Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Rad Biol Med. 2000;29(11):1143–1150. PMID: 11121722. [DOI] [PubMed] [Google Scholar]
- 12.Magwere T, Pamplona R, Miwa S, et al. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61(2):136–145. PMID: 16510857. [DOI] [PubMed] [Google Scholar]
- 13.Jankovic J. Therapeutic strategies in Parkinson’s disease In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Philadelphia (PA): Lippincott Williams and Wilkins; 2002. p. 116–151. [Google Scholar]
- 14.Senyilmaz D, Virtue S, Xu X, et al. Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature. 2015;525(7567):124–128. PMID: 26214738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha G-H, Kim S, Park J, et al. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci USA. 2005;102(29):10345–10350. PMID: 16002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene JC, Whitworth AJ, Kuo I, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100(7):4078–4083. PMID: 12642658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbas N, Lucking CB, Ricard S, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s disease genetics study group and the European consortium on genetic susceptibility in Parkinson’s disease. Hum Mol Genet. 1999;8(4):567–574. PMID: 10072423. [DOI] [PubMed] [Google Scholar]
- 18.West AB, Maidment NT. Genetics of parkin-linked disease. Hum Genet. 2004;114(4):327–336. PMID: 14727181. [DOI] [PubMed] [Google Scholar]
- 19.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci USA. 1997;94(21):11168–11172. PMID: 9326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. PMID: 23434764. [DOI] [PubMed] [Google Scholar]
- 21.Truban D, Hou X, Caulfield TR, et al. PINK1, Parkin, and mitochondrial quality control: what can we learn about Parkinson’s disease pathobiology? J Parkinson’s Dis. 2017;7(1):13–29. PMID: 27911343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajracharya R, Bustamante S, Ballard JWO. Steric acid supplementation in high protein to carbohydrate (P:C) ratio diet improves physiological and mitochondrial functions of Drosophila melanogaster parkin null mutants. J Gerontol B. 2017;glx246. PMID: 29236963. [DOI] [PubMed] [Google Scholar]
- 23.Tanner CM, Goldman SM. Epidemiology of movement disorders. Curr Opin Neurol. 1994;7(4):340–345. PMID: 7952243. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Pinilla F. The combined effects of exercise and foods in preventing neurological and cognitive disorders. Prev Med. 2011;52(Suppl 1):S75–S80. PMID: 21281667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonanome A, Bennett M, Grundy SM. Metabolic effects of dietary stearic acid in mice: changes in the fatty acid composition of triglycerides and phospholipids in various tissues. Atherosclerosis. 1992;94(2–3):119–127. PMID: 1632865. [DOI] [PubMed] [Google Scholar]
- 26.Nachbaur J, Colbeau A. Vignais. Incorporation of fatty acids into the outer and inner membranes of isolated rat liver mitochondria. FEBS Lett. 1969;3(2):121–124. [DOI] [PubMed] [Google Scholar]
- 27.Mejia EM, Hatch GM. Mitochondrial phospholipids: role in mitochondrial function. J Bioenerg Biomembr. 2016;48(2):99–112. PMID: 25627476. [DOI] [PubMed] [Google Scholar]
- 28.Holmbeck MA, Rand DM. Dietary fatty acids and temperature modulate mitochondrial function and longevity in Drosophila. J Gerontol A Biol Sci Med Sci. 2015;70(11):1343–1354. PMID: 25910846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14(24):2564–2571. PMID: 17979709. [DOI] [PubMed] [Google Scholar]
- 30.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson’s disease? Clin J Sport Med. 2006;16(5):422–425. PMID: 17016120. [DOI] [PubMed] [Google Scholar]
- 31.Lau Y-S, Patki G, Das-Panja K, et al. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci. 2011;33(7):1264–1274. PMID: 21375602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriat Soc. 2007;55(2):158–165. PMID: 17302650. [DOI] [PubMed] [Google Scholar]
- 33.Shin M-S, Kim T-W, Lee J-M, et al. Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J Exerc Rehabil. 2017;13(2):124–129. PMID: 28503522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22(4):529–539. PMID: 18813861. [PubMed] [Google Scholar]
- 35.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. PMID: 15548201. [DOI] [PubMed] [Google Scholar]
- 36.Koo J-H, Jang Y-C, Hwang D-J, et al. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-kappaB signaling pathway. Neurosci. 2017;356:102–113. PMID: 28527958. [DOI] [PubMed] [Google Scholar]
- 37.Dishman RK, Berthoud H-R, Booth FW, et al. Neurobiology of exercise. Obesity. 2006;14(3):345–356. PMID: 16648603. [DOI] [PubMed] [Google Scholar]
- 38.Scott SA, Crutcher KA. Nerve growth factor and Alzheimer’s disease. Rev Neurosci. 1994;5(3):179–211. PMID: 7889213. [DOI] [PubMed] [Google Scholar]
- 39.Chuang C-S, Chang J-C, Cheng F-C, et al. Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson’s disease. Life Sci. 2017;191:236–244. PMID: 28986095. [DOI] [PubMed] [Google Scholar]
- 40.Ryan BJ, Hoek S, Fon EA, et al. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200–210. PMID: 25757399. [DOI] [PubMed] [Google Scholar]
- 41.Calap-Quintana P, Soriano S, Llorens JV, et al. TORC1 Inhibition by rapamycin promotes antioxidant defences in a Drosophila model of Friedreich’s ataxia. PLoS One. 2015;10:e0132376 PMID: 26158631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballard JWO, Youngson NA. Review: can diet influence the selective advantage of mitochondrial DNA haplotypes? Biosci Rep. 2015;35(6):e00277 PMID: 26543031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Bourg E, Lints FA. Hypergravity and aging in Drosophila melanogaster. 6. Spontaneous locomotor activity. Gerontology. 1992;38(1–2):71–79. PMID: 1612464. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy MC, Emptage MH, Dreyer JL, et al. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983;258(18):11098–11105. PMID: 6309829. [PubMed] [Google Scholar]
- 45.Melvin RG, Ballard JWO. Intraspecific variation in survival and mitochondrial oxidative phosphorylation in wild-caught Drosophila simulans. Aging Cell. 2006;5(3):225–233. PMID: 16842495. [DOI] [PubMed] [Google Scholar]
- 46.Picard M, Ritchie D, Wright KJ, et al. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9(6):1032–1046. PMID: 20849523. [DOI] [PubMed] [Google Scholar]
- 47.Zhou M, Diwu Z, Panchuk-Voloshina N, et al. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Analyt Biochem. 1997;253(2):162–168. PMID: 9367498. [DOI] [PubMed] [Google Scholar]
- 48.Miwa S, Treumann A, Bell A, et al. Carboxylesterase converts Amplex red to resorufin: implications for mitochondrial H2O2 release assays. Free Rad Biol Med. 2016;90:173–183. PMID: 26577176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


