Abstract
Previously, we have synthesized a diverse range of 2,5-furandicarboxylic acid (FDCA)-based semiaromatic polyamides via enzymatic polymerization. This novel class of polymers are biobased alternatives to polyphthalamides, which are petrol-based semiaromatic polyamides. From a commercial perspective, they have interesting properties as high-performance materials and engineering thermoplastics. It is even more appealing to explore novel FDCA-based polyamides with added functionality, for the development of sustainable functional materials. Here, a set of FDCA-based heteroatom polyamides have been successfully produced via Novozyme 435 (N435)-catalyzed polymerization of biobased dimethyl 2,5-furandicarboxylate with (potentially)heteroatom diamines, namely, 4,9-dioxa-1,12-dodecanediamine (DODA), diethylenetriamine, and 3,3-ethylenediiminopropylamine. We performed the enzymatic polymerization in solution and bulk. The latter approach is more sustainable and results in higher molecular weight products. Among the tested heteroatom diamines, N435 shows the highest catalytic activity toward DODA. Furthermore, we find that all obtained FDCA-based heteroatom polyamides are amorphous materials with a relatively high thermal stability. These heteroatom polyamides display a glass-transition temperature ranging from 41 to 107 °C.
Introduction
Most commonly, semiaromatic polyamides are used as high-performance materials and engineering thermoplastics. This is owned to their good mechanical properties, excellent chemical resistance, and other interesting features.1,2 These polymers have many applications in the automobile industry, electronic and electrical appliances, packaging, photovoltaic parts and panels, medical devices, and also for materials that are used for oil and gas extraction.
Currently, semiaromatic polyamides are mainly produced from fossil fuels. However, these resources are limited and are expected to be depleted within a few centuries.3−5 Generally, semiaromatic polyamides are obtained by polycondensation of aliphatic diamines with petrol-based terephthalic acid (TPA) and isophthalic acid,6,7 although the prevalent combination of aliphatic diamines and aromatic diacids gives access to a large range of semiaromatic polyamides with diverse properties. Using these compounds in polycondensation to obtain high-molecular-weight products at high conversion requires extreme condition and is energy-intensive.8−11
Recent research shows that 2,5-furandicarboxylic acid (FDCA) has been put forward as an alternative renewable building block to replace TPA. FDCA is a rigid difunctional furan compound resembling TPA in structure, which is likely to play an important role in the construction of biobased polymer materials,12−15 for example, via polycondensation.14 FDCA can be directly generated by oxidation of 5-(hydroxymethyl)furfural, which is easily prepared from widely available renewable C6 sugars or polysaccharides.16,17 In addition, the production of other heteroatom-containing chemicals from renewable resources (including heteroatom amines) is steadily under development.18 Previously, the polymerization of diverse furanic monomers has been reported, for example, the synthesis of furanic-aromatic polyamides from difuranic acid chloride and various difuranic diamines.19,20 FDCA-based polymers reportedly have better or similar thermal and mechanical properties compared to TPA-based polymers.15,21−27 Consequently, polyphthalamides (semiaromatic polyamides) can potentially be replaced by sustainable FDCA-based polyamides.
In general, living organisms synthesize macromolecules by in vivo enzyme-catalyzed polymerization. Mimicking such behavior in nature has let to in vitro enzymatic polymerization to be a well-known field, leading to a notable interest in the production of novel and commodity polymeric materials in a sustainable manner.28−31 Side reactions can be significantly inhibited by using enzymatic polymerization because of the high specificity of biocatalysts and mild reaction conditions.32 In the past decade, a vast array of polymer classes have been produced via enzymatic polymerization, such as polysaccharides, vinyl polymers, polyester, and polyamides. Hydrolase is among the most widely used biocatalyst for polymer synthesis. Hydrolases such as esterases, proteases, and lipases are popularly used in polyester synthesis. In addition, hydrolase can also catalyze the amide bond formation, making them good biocatalysis for polyamide synthesis. Currently, the most extensively studied enzymes in polyamide synthesis are lipases and protease. In our laboratory, various polyesters and polyamides are successfully synthesized via enzymatic polymerization, including furan-based polyesters and furan-based polyamides.7,33−36
Although the use of enzymatic polymerization is being extensively studied, there is still a need for the further exploration of this method to be applied in the synthesis of various novel biobased polymers that are still not accessible via conventional methods, for example, functional FDCA polymers. Because of the additional functionality, new materials can be developed. Moreover, FDCA-based polymers, especially polyamides, have not been well explored up until now, and the knowledge of such polymers is barely based on limited studies.
The aim of the research is, therefore, to demonstrate the use of a bioderived furan monomer in combination with heteroatom-containing diamines via enzymatic polymerization, to synthesize biobased polyamides with added functionality. The resulting products will hereinafter be referred to as FDCA-based heteroatom polyamides. We performed the enzymatic synthesis both in solution and in bulk, and the latter approach adds more sustainability aspects to the final products. Moreover, we studied the thermal properties and crystallinity of these heteroatom polyamides and investigated the difference compared to polymers synthesized from dimethyl 2,5-furandicarboxylate (DMFDCA) with linear aliphatic diamines.
Results and Discussion
N435-Catalyzed Polycondensation of DMFDCA and Various Heteroatom Diamines via Solution and Bulk Polymerization
In this work, a series of FDCA-based heteroatom polyamides, namely, PA DODAF, PA DETAF, and PA EDDAF, were successfully synthesized via enzymatic polymerization (see Scheme 1). The enzymatic polycondensation was carried out in bulk and in solution at 90 °C using the biocatalyst N435. Biobased DMFDCA and three heteroatom diamines were used as the monomer: 4,9-Dioxa-1,12-dodecanediamine (DODA) with ether groups and diethylenetriamine (DETA) and 3,3-ethylenediiminopropylamine (EDDA) having secondary amine groups. The enzymatic polymerization results are summarized in Table 1.
Scheme 1. Enzymatic Synthesis of FDCA-Based Heteroatom Polyamides via N435-Catalyzed Polycondensation of DMFDCA and Heteroatom Diamines in Solution or in Bulk.
Table 1. Molecular Weight and Thermal Properties of the FDCA-Based Heteroatom Polyamides.
| polymer | solvent | vaccum (mm Hg) |
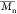 b (g mol–1) b (g mol–1) |
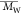 b (g mol–1) b (g mol–1) |
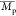 b (g mol–1) b (g mol–1) |
Đb (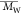 / /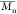 ) ) |
yieldd (%) | Tge (°C) | Td (°C) |
|---|---|---|---|---|---|---|---|---|---|
| PA DODAF | toluene | atm | 6360 | 14 930 | 14 200 | 2.35 | 26 | 58 | 264f; 351h |
| bulk | 30a | 8030 | 16 620 | 17 000 | 2.07 | 37 | 44 | 297f; 432h | |
| PA DETAF | toluene | atm | c | c | 3 700 | c | 71 | 107 | 204f; 292g; 361h |
| bulk | 30a | c | c | 5 300 | c | 93 | 93 | 202f; 288g; 358h | |
| PA EDDAF | toluene | atm | c | c | 4800 | c | 60 | 51 | 193f; 358h |
| bulk | 30a | c | c | 5300 | c | 79 | 41 | 186f; 366h |
The polymerization conditions used were stage-1: 80 °C, 2 h, atm and stage-2: 80 °C, 70 h, 30 mm Hg.
The number-average
molecular weight
(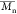 ), weight-average
molecular weight (
), weight-average
molecular weight (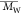 ), peak
molecular weight (
), peak
molecular weight (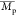 ), and
dispersity (Đ,
), and
dispersity (Đ, 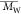 /
/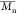 ) were
determined by SEC using DMF/LiBr
as the eluent.
) were
determined by SEC using DMF/LiBr
as the eluent.
Can not be corrected: the signal is partly outside the polystyrene standard range.
Isolated yield.
Tg (glass-transition temperature) was measured from the second DSC heating scan.
Decomposition temperature at 5% weight loss (Td-5%).
Decomposition temperature at 10% weight loss (Td-10%).
Temperature at the maximum rate of decomposition.
The obtained FDCA-based heteroatom polyamides chemical structures are confirmed by attenuated total reflectance–Fourier transform infrared (ATR–FTIR) and NMR (see Figures 1 and 2, respectively). The Experimental Section described detailed NMR and IR assignments.
Figure 1.
ATR–FTIR spectra of FDCA-based heteroatom polyamides produced via enzymatic polymerization in bulk.
Figure 2.
1H NMR spectra of FDCA-based heteroatom polyamides produced via enzymatic polymerization in bulk.
Influence of Diamines on Enzymatic Polymerization
FDCA-based heteroatom polyamides with relatively high molecular weight up to 14 900 g/mol were obtained by using DODA as a diamine monomer (Table 1). By changing to heteroatom diamines containing secondary amine groups (DETA and EDDA), the enzymatic polymerization resulted in lower molecular weight polyamides (see Figure S2). This indicated that N435 shows better catalytic activity toward DODA compared to that of DETA and EDDA. This result concur well with Schwab et al.,37 in which they also demonstrate that the amide formation by Candida Antarctica lipase B (CALB) is preferable with DODA compared to that with DETA. However, we have to take into account the fact that reactivity of the diamines has a strong influence on molecular weights. In general, the reactivity of amines depends on both their basicity and nucleophilicity. The basicity increases with the number of the electron-donating groups that are linked to the amine functionality. The nucleophilicity is determined by several factors such as its charge, the nature of the chemical group present in or near the amine substituents, and the nature of the solvent used in the reaction.38
The enzymatic polymerization with DODA resulted in PA DODAF with the lowest isolation yield compared to the other two. Upon changing to diamines having secondary amine groups, the isolation yield increases to more than ∼50%. This can be explained by the higher solubility of PA DODAF oligomers in the precipitant (THF). PA DODAF oligomers have a higher solubility in the THF compared to PA DETAF and PA EDDAF oligomers. During the purification steps, a higher amount of short chain oligomers were removed, thus resulting in lower yields. Another polymerization method or other suitable precipitants should be used to increase the reaction yield.short chain oligomers were removed, thus resulting in lower yields. Another polymerization method or other suitable precipitants should be used to increase the reaction yield.
Previously in our laboratory, different FDCA-based aromatic polyamides (see Scheme 2a) were successfully synthesized by using N435 as a biocatalyst.33 FDCA-based aromatic polyamides with high weight-average molecular weight up to 48300 g mol–1 were successfully prepared. However, in this study, the enzymatic polymerization gave significantly lower molecular weight heteroatom counterparts. This may suggest that the tested heteroatom diamines (DODA, DETA, and EDDA) are less favored by CALB because of its ether or amine groups. This is in good agreement with our earlier findings, in which we also found that the enzymatic polymerization of polyester involving alkane-α,ω-aliphatic linear diols is more favored compared to diethylene glycol.35 Nevertheless, our results prove the substrate promiscuity of CALB as the biocatalyst.
Scheme 2. Chemical Structures of (a) FDCA-Based Aromatic Polyamides (PAXF) and (b) FDCA-Based Heteroatom Polyamides.
Influence of the Enzymatic Polymerization Method on the Molecular Weights and Isolation Yields
Both the enzymatic polymerization in toluene and in bulk give FDCA-based heteroatom polyamide with comparable molecular weights, but in bulk, the molecular weights are higher. From this, we can conclude that the enzymatic polymerization in bulk is preferred. The high molecular weights in bulk polymerization could be attributed to the lower enzyme catalytic activity in the organic solvent: toluene. Toluene possesses a log P value of 2.73, which is a suitable organic solvent for the lipase-catalyzed polymerization. However, the presence of toluene in the system changes the structure conformation of the enzyme and thus reduces its catalytic activity.39 On the other hand, in the solvent-free system, the enzyme retains its structure and thus shows a higher catalytic activity. Furthermore, we applied vacuum in the enzymatic polymerization in bulk where the elimination of the residual alcohol and water is facilitated.
The enzymatic polymerization in bulk resulted in FDCA-based heteroatom polyamides with higher isolation yields compared to that in toluene. This is quite reasonable due to the fact that the enzymatic polymerization in toluene gives lower molecular weight FDCA-based heteroatom polyamides.
Microstructures of the Obtained FDCA-Based Heteroatom Polyamides
The microstructures and end groups of the FDCA-based heteroatom polyamides were analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-ToF MS). Figure 3 shows the representative MALDI spectra.
Figure 3.
(a) MALDI-ToF MS spectrum of the obtained PA DODAF and (b) magnified part with detailed peak interpretation. A–H represent eight polyamide species ionized by K+. G′ represents the polyamides having the acid/acid end groups that are ionized by Na+. H″ represents the polyamide having ester/amide end groups that are ionized by H+. I–M represent five polyamide species fragment because of the fragmentation in the heteroatom bond. I′–K′ represent the polyamide species fragment that are ionized by Na+. PA DODAF was produced via enzymatic polymerization in bulk.
Similarly as in our previous study, when we used monoatomic aliphatic diamines, eight different polyamide species were identified (see Table 2). They were terminated by ester/ester, amine/amine, ester/amine, acid/amine, acid/acid, ester/amide, ester/acid, and cyclic polyamides (without end groups). However, in this work, additional end groups are identified. The heteroatom bond in the amine end group can easily be cleaved off during the ionization of the molecules in the MALDI-ToF measurement, resulting in new fragmentation patterns. Therefore, additional peaks are observed. For example, in the MALDI-ToF spectrum of PA DODAF, the peaks assigning to the additional end groups are marked as peaks I and J (see Figure 3 and Table S1–S3), indicating that the amine (DODA) end group of PA DODAF undergoes fragmentation in C-α of the ether bond during MALDI-ToF measurements.
Table 2. MALDI-ToF MS Analysis: End Groups of the Obtained FDCA-Based Heteroatom Polyamides.
As previously reported by our group, the acid end group is formed because during the polymerization, the esters were catalytically hydrolyzed by N435.7,11,33 The formation of amide end groups occurred because of the reaction between amine groups and formic acid that we use at the purification step.11
Crystallinity and Thermal Properties of the Obtained FDCA-Based Heteroatom Polyamides
The thermal behavior of the tested FDCA-based heteroatom polyamides was analyzed by differential scanning calorimetry (DSC). No melting and crystallization peaks were observed. This indicated that the obtained FDCA-based heteroatom polyamides are amorphous materials. As confirmed by the wide-angle X-ray diffraction (WAXD) measurements, no reflection peaks but only broad halo appeared (Figure S8).
The glass-transition temperature (Tg) of the obtained FDCA-based heteroatom polyamides is presented in Table 1 and Figure 4. The Tg was ranging from 41 to 107 °C. PA DETAF showed the highest Tg of 107 °C. This can be explained by two facts. First, the repeating unit of PA DETAF is most rigid because of its shortest chain length. Second, the intermolecular hydrogen bond density in PA DETAF is higher due to the secondary amine groups. Moreover, the Tg of PA EDDAF approaches that of PA DODAF, even though the molecular weight of PA EDDAF is much lower. This can also be explained by the higher intermolecular hydrogen bond density in PA EDDAF. We also noticed that the Tg of FDCA-based heteroatom polyamides from enzymatic polymerization in bulk is lower, despite having higher molecular weight. This could be elucidated by the varied composition of the end groups generated from different synthetic approaches.
Figure 4.
DSC second heating curves of the obtained FDCA-based heteroatom polyamides: (a) PA DODAF, (b) PA DETAF, and (c) EDDAF.
The Tg of the synthesized FDCA-based heteroatom polyamides decreases as the chain length of the heteroatom aliphatic diamine units increases. These results also agreed well with our previous results reported in the literature,33 which indicated that the Tg of semiaromatic polyamides decreased, whereas the chain length of the aliphatic diamine units increased.
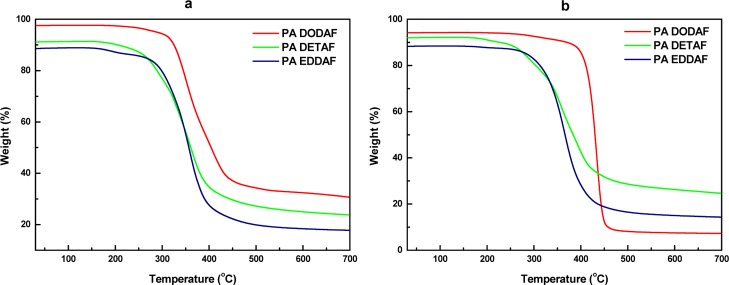
The thermal stability of the tested FDCA-based heteroatom polyamides was determined by thermal gravimetric analysis (TGA). Figure 5 shows the TGA curves of the FDCA-based heteroatom polyamides. The temperature at 5% weight loss (Td-5%) of all FDCA-based heteroatom polyamides was around 186–297 °C. The temperature of maximal rate of decomposition (Td) of all FDCA-based heteroatom polyamides was ranging from 351 to 432 °C. In addition, we also observe 10% weight loss step in PA DETAF at temperature around 288–292 °C. The temperature at the maximum rate of decomposition can mostly associate with the thermal cleavage of the amide bonds in the polymer backbones. However, to obtain additional information for understanding the thermal degradation mechanism steps, further analysis using TGA-GC/MS coupling measurements is needed in the future. Considering their high decomposition temperature, all FDCA-based heteroatom polyamides have a very wide processing window.
Figure 5.
TGA traces of the obtained FDCA-based heteroatom polyamides: (a) enzymatic polymerization in solution and (b) in bulk.
Conclusions
We demonstrate that
enzymatic catalysis is a robust pathway toward
the synthesis of FDCA-based heteroatom polyamides. As confirmed by 1H NMR and ATR–FTIR analysis, a series of FDCA-based
heteroatom polyamides are successfully synthesized, with a 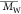 of up
to 16 620 g/mol. We found
that N435 shows the highest catalytic activity toward DODA, a diamine
having ether groups, compared to the other two with secondary amines.
Additionally, we found that enzymatic polymerization in bulk is more
preferred. Furthermore, MALDI-ToF MS results indicated that no polyamide
species can be assigned to the byproducts from undesirable side reaction.
of up
to 16 620 g/mol. We found
that N435 shows the highest catalytic activity toward DODA, a diamine
having ether groups, compared to the other two with secondary amines.
Additionally, we found that enzymatic polymerization in bulk is more
preferred. Furthermore, MALDI-ToF MS results indicated that no polyamide
species can be assigned to the byproducts from undesirable side reaction.
All obtained FDCA-based heteroatom polyamides are amorphous materials with relatively high thermal stability. TGA analysis results show that all obtained FDCA-based heteroatom polyamides possess a Td-5% and Td-max at around 186–297 and 351–432 °C, respectively. Moreover, these heteroatom polyamides possess a glass-transition temperature at around 41–107 °C. Because of the polymer chain rigidity, tested FDCA-based heteroatom polyamides having shorter diamine units generally possess higher glass-transition temperature.
In the future research, it will be of great interest to optimize reaction parameters to facilitate the enzymatic polymerization. Furthermore, we are aiming to thoroughly understand the enzymatic polymerization mechanism; thus, we can design a greener and more efficient pathway toward diverse biobased polymers.
Experimental Section
Materials
Novozym 435 [N435, Candida antartica lipase B (CALB) immobilized on acrylic resin, 5000 + U/g], 4,9-dioxa-1,12-dodecanediamine (DODA, 99%), DETA (reagent plus, 99%), 1,2-bis(3-aminopropylamino) ethane (EDDA, technical grade, 94%), toluene (anhydrous, 99, 8%), formic acid (puriss, 98+%), molecular sieves (4 Å), dimethyl sulfoxide-d6 (DMSO-d6, 99, 5 atom %D), and potassium trifluoroacetate (KTFA, 98%) were purchased from Sigma-Aldrich. Dimethyl 2,5-furandicarboxylate (DMFDCA, 97%) was purchased from Fluorochem UK. 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, 99%) was purchased from TCI Europe. Dithranol (98+%) was purchased from Fluka. Tertrahydrofuran (THF, stabilized with BHT, pro analyze) was purchased from Boom BV. 2,5-Dihydroxybenzoic acid (DHB, 98–100%) was purchased from ThermoFisher scientific.
N435 was predried as reported previously,40 and the molecular sieves were preactivated at 200 °C in vacuo. All of the other chemicals were used without further purification.
Procedure for the N435-Catalyzed Solution Polymerization of DMFDCA with Various Heteroatom Diamines
Predried N435 (20 wt % in relation to the total amount of the monomer) and preactivated molecular sieves (200 wt %) were placed in a 25 mL round-bottle flask under a nitrogen environment. Subsequently, DMFDCA (5.000 mmol), diamines (5.000 mmol), and anhydrous toluene (500 wt %) were added into the flask. The flask was placed in an oil bath, and the reaction mixture was magnetically stirred under atmospheric pressure at 90 °C for 72 h. After that, formic acid (15 mL) was added to dissolve the products and then the solution was filtrated (folded filter type 15 Munktell 240 mm) to remove N435 and molecular sieves. N435, molecular sieves, and filter paper were washed three times using formic acid (10 mL). All of the obtained solutions were then combined and concentrated by a rotary evaporator at 40 °C under reduced pressure of 20–40 mbar. The concentrated solution was poured in an excess amount of THF. The solution with the precipitated products was then stored for several hours at −20 °C. Subsequently, they were isolated via centrifugation (30 min, 4500 rpm, 4 °C in Thermo/Heraeus Labofuge 400 R). The obtained crude products were dissolved by a small amount of formic acid and then added dropwise into THF. The final products were collected via centrifugation following the same procedure mentioned above and dried in vacuo at 40 °C for 3 days. Finally, they were stored in vacuo at room temperature prior to analysis.
Procedure for the N435-Catalyzed Bulk Polymerization of DMFDCA with Various Heteroatom Diamines
DMFDCA (5.000 mmol, 0.9208 g), diamines (5.000 mmol, 1.0216 g), preactivated molecular sieves (200 wt %), and predried N435 (20 wt % in relation to the total amount of the monomer) were added into a 25 mL round-bottle flask. The reaction mixture was magnetically stirred at 90 °C under atmospheric pressure for 2 h, followed by applying 30 mm Hg pressure for 70 h. After that, the obtained products were purified according to the same procedure as described above. Finally, the products were stored in vacuo at room temperature before analysis.
Poly(4,9-Dioxa-1,12-dodecamethylene furanamide) (PA DODAF)
1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.49 (1H, m, −NH–CO–, from DODA), 7.10 (2H, s, =CH–, furan), 3.38–3.30 (4H, 12H, overlap multiplet,–NH–CH2–, −O–CH2–, from DODA), 1.73 (4H, m, −NH–CH2–CH2–CH2–O–, from DODA), 1.50 (4H, s, −O–CH2–CH2–O–, from DODA); 13C NMR (300 MHz, DMSO-d6, δ, ppm): 157.69 (−CO–NH–, from DMFDCA), 148.67 (−NH–CO–C(O)=CH–, from DMFDCA), 114.69 (=CH–, from DMFDCA), 70.36 (−O–CH2–CH2–, from DODA), 68.16 (−O–CH2–CH2–CH2–NH–CO–, from DODA), 36.42 (−CH2–NH–CO–, from DODA), 29.94(−CH2–CH2–NH–CO–,from DODA), 26.43 (−O–CH2–CH2–, from DODA).
Poly(3-Aza-1,5-pentamethylene furanamide) (PA DETAF)
1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.25 (1H, m, NH–CO–, from DETA), 7.12 (2H, s, =CH–, furan), 3.43 (4H, m, −CO–NH–CH2–, from DETA), 2.85 (4H, s, −NH–CH2–, from DETA); 13C NMR (400 MHz, DMSO-d6, δ, ppm): 157.62 (−CO–NH–, from DMFDCA), 148.06 (−NH–CO–C(O)=CH–, from DMFDCA), 114.57 (=CH–, from DMFDCA), 47.29 (−CH2–NH–, from DETA), 36.88 (−NH–CO–CH2–, from DETA), 150.28 (C=O, end groups from DMFDCA), 119.26 (=CH–, end groups from DMFDCA), 52.21 (−OCH3, end groups from DMFDCA).
Poly(4,7-Diaza-1,10-decamethylene furanamide) (PA EDDAF)
1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.35 (1H, m, −NH–CO–, from EDDA), 7.13 (2H, s, =CH–, furan), 3.29–3,25 (4H, 8H, overlap multiplet, −CO–NH–CH2–, −NH–CH2–, from EDDA), 1.76 (4H, m, −NH–CH2–CH2–, from EDDA); 13C NMR (400 MHz, DMSO-d6, δ, ppm): 157.38 (−CO–NH–, from DMFDCA), 148.15 (−NH–CO–C(O)=CH–, from DMFDCA), 114.38 (=CH–, from DMFDCA), 48.63 (−NH–CH2–CH2–NH–, from EDDA), 44.6(−CO–NH–CH2–CH2–CH2–NH–, from EDDA), 36.37 (−CO–NH–CH2–, from EDDA), 27.18 (−CO–NH–CH2–CH2–, from EDDA).
Furanic-aliphatic heteroatom polyamides (ν, cm–1): 3251–3290 (N–H stretching vibrations); 3101–3114 (=C–H stretching vibrations of the furan ring); 2935–2944, 2831–2873 (asymmetric and symmetric C–H stretching vibrations); 1643–1646 (C=O stretching vibrations); 1571–1573 (aromatic C=C bending vibrations); 1500–1533 (N–H bending vibrations); 1429–1500, 1369–1440 (C–H deformation and wagging vibrations); 1342–1365 (C–H rocking vibrations); 1286–1288 (C–N stretching vibrations); 1105 (C–O–C asymmetric stretching vibrations, DODA); 1099–1168, 1012–1016 (=C–O–C= ring vibrations, furan ring); 962–966, 821–825, 744–759 (=C–H out-of-plane deformation vibrations, furan ring).
Instrumental Methods
1H NMR spectra were recorded on a Varian VXR Spectrometer (1H: 400; 13C 300 MHz), using DMSO-d6 as the solvent. Chemical shifts (δ) are reported in parts per million (ppm), whereas the chemical shifts were referenced to the resonances of the residual solvent or tetramethylsilane.
ATR–FTIR spectra were recorded on a Bruker VERTEX 70 spectrometer in the range of 4000–400 cm–1, with 16 scans for each sample at a nominal resolution of 4 cm–1 using a diamond single reflection ATR.
Size exclusion chromatography (SEC) was performed in DMF
(containing
0.01 M LiBr) on Viscotek GPCmax equipped with model 302 TDA detectors,
two columns (Agilent Technologies-PolarGel-L and M, 8 μm 30
cm) at a flow rate of 1.0 mL min–1. The columns
and detectors were held at 50 °C. Data acquisition and calculations
were performed using Viscotek OmniSec software version 5.0. Molecular
weights were determined based on a conventional calibration curve
generated from narrow dispersity polystyrene standards (Agilent and
PSS, 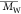 = 550–1 190 000
g/mol).
The samples were filtered over a 0.2 μm PTFE filter prior to
injection.
= 550–1 190 000
g/mol).
The samples were filtered over a 0.2 μm PTFE filter prior to
injection.
Thermal transitions of the obtained polyamides were measured by DSC on TA-Instruments Discovery DSC 2500. The samples were scanned by heating–cooling–heating scans with heating–cooling rates of 10 °C/min. Tzero aluminium pinhole hermetic pans were used for all of the DSC measurements.
Thermal stability of the obtained polyamides was characterized by TGA on TA-Instruments Discovery TGA 5500 on an open pan under a nitrogen environment. The scan rate was 10 °C/min. To remove the remaining water and solvents in the polymer, the tested sample was first heated up to 150 °C and then maintained at this temperature for 30 min before the standard TGA measurement.
MALDI-ToF MS measurements were performed on a Biosystems Voyager-DE PRO spectrometer in the positive ionization and the linear mode using an accelerating voltage of 25 kV. The grid voltage, guide wire voltage, and delay time were optimized for each spectrum to achieve the best signal-to-noise ratio. PA DODAF and PA DETAF samples were prepared using 20 mg/mL matrix solution of dithranol in HFIP. Polymer sample solution in HFIP (1–2 mg/mL), potassium trifluoroacetate in HFIP (KTFA, 5 mg/mL), and dithranol (20 mg/mL) were premixed in a ratio of 5:1:5. After that, the resulting mixture (0,2–0,6 μL) was hand-spotted on a MALDI target plate and left to dry. PA EDDAF samples were prepared using 40 mg/mL matrix solution of DHB in 70/30 acetonitrile/water with 0.1% TFA. Typically, a 1:2 mixture of polymer sample solution in HFIP (1–2 mg/mL) was mixed with the DHB matrix solution. Subsequently, the mixture was hand-spotted on the MALDI target plate and left to dry. Polyamide species having different end groups were determined by the following equation: MP = MEG + (n × MRU) + MK+, where MP is the molecular mass of a polyamide species, MEG is the molecular mass of the end groups, n is the number of the repeating units, MRU is the molecular mass of the repeating units, and MK+ is the molecular mass of the potassium cation.
WAXD spectra were recorded at room temperature using a Bruker D8 Advance diffractometer (Cu Kα radiation, λ = 0.1542 nm) in the angular range of 5–50° (2θ).
Acknowledgments
D.M. thanks the financial support from the Indonesian Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan LPDP)
Glossary
Abbreviations
- FDCA
2,5-furandicarboxylic acid
- N435
Novozyme 435
- DMFDCA
dimethyl 2,5-furandicarboxylate
- DODA
4,9-dioxa-1,12-dodecanediamine
- DETA
diethylenetriamine
- EDDA
3,3-ethylenediiminopropylamine
- TPA
terephthalic acid
- IPA
isophthalic acid
- HMF
5-(hydroxymethyl)furfural
- PA DODAF
poly(4,9-dioxa-1,12-dodecamethylene furanamide)
- PA DETAF
poly(3-aza-1,5-pentamethylene furanamide)
- PA EDDAF
poly(4,7-diaza-1,10-decamethylene furanamide)
- ATR–FTIR
attenuated total reflection–Fourier transform infrared
- 1H NMR
proton nuclear magnetic resonance
- SEC
size exclusion chromatography
- DSC
differential scanning calorimetry
- TGA
thermal gravimetric analysis
- MALDI-ToF MS
matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- WAXD
wide-angle X-ray diffraction
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01106.
MALDI-TOF analysis: additional end groups of the obtained FDCA-based heteroatom polyamides, 13C NMR spectra of FDCA-based heteroatom polyamides produced via enzymatic polymerization in bulk, SEC elution curves of the obtained FDCA-based heteroatom polyamides, and MALDI-ToF MS spectra of the obtained FDCA-based heteroatom polyamides with detailed peak interpretation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Glasscock D.; Atolino W.; Kozielski G.; Martens M.. High Performance Polyamides Fulfill Demanding Requirements for Automotive Thermal Management Components; DuPont Engineering Polymers, 2008. [Google Scholar]
- Marchildon K. Polyamides - Still Strong After Seventy Years. Macromol. React. Eng. 2011, 5, 22–54. 10.1002/mren.201000017. [DOI] [Google Scholar]
- Williams C.; Hillmyer M. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. 10.1080/15583720701834133. [DOI] [Google Scholar]
- van Putten R.-J.; van der Waal J. C.; de Jong E.; Rasrendra C. B.; Heeres H. J.; de Vries J. G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- Rass-Hansen J.; Falsig H.; Jørgensen B.; Christensen C. H. Bioethanol: fuel or feedstock?. J. Chem. Technol. Biotechnol. 2007, 82, 329–333. 10.1002/jctb.1665. [DOI] [Google Scholar]
- Weber J. N.Polyamides. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons, Inc: 2011; Vol. 19, pp 454–518. [Google Scholar]
- Jiang Y.; Maniar D.; Woortman A. J. J.; Alberda van Ekenstein G. O. R.; Loos K. Enzymatic Polymerization of Furan-2,5-Dicarboxylic Acid-Based Furanic-Aliphatic Polyamides as Sustainable Alternatives to Polyphthalamides. Biomacromolecules 2015, 16, 3674–3685. 10.1021/acs.biomac.5b01172. [DOI] [PubMed] [Google Scholar]
- Poojari Y.; Clarson S. J. Lipase-Catalyzed Synthesis and Properties of Silicone Aromatic Polyesters and Silicone Aromatic Polyamides. Macromolecules 2010, 43, 4616–4622. 10.1021/ma100548z. [DOI] [Google Scholar]
- Poulhès F.; Mouysset D.; Gil G.; Bertrand M. P.; Gastaldi S. CAL-B catalyzed synthesis of chiral polyamides. Tetrahedron: Asymmetry 2012, 23, 867–875. 10.1016/j.tetasy.2012.05.021. [DOI] [Google Scholar]
- Stavila E.; Arsyi R. Z.; Petrovic D. M.; Loos K. Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur. Polym. J. 2013, 49, 834–842. 10.1016/j.eurpolymj.2012.12.010. [DOI] [Google Scholar]
- Stavila E.; Alberda van Ekenstein G. O. R.; Loos K. Enzyme-Catalyzed Synthesis of Aliphatic-Aromatic Oligoamides. Biomacromolecules 2013, 14, 1600–1606. 10.1021/bm400243a. [DOI] [PubMed] [Google Scholar]
- Sousa A. F.; Matos M.; Freire C. S. R.; Silvestre A. J. D.; Coelho J. F. J. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. 10.1016/j.polymer.2012.11.081. [DOI] [Google Scholar]
- Gandini A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. 10.1039/c0gc00789g. [DOI] [Google Scholar]
- Gandini A.; Lacerda T. M.; Carvalho A. J. F.; Trovatti E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. 10.1021/acs.chemrev.5b00264. [DOI] [PubMed] [Google Scholar]
- de Jong E.; Dam M. A.; Sipos L.; Gruter G.-J. M.. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters; American Chemical Society, 2012; Vol. 1105, pp 1–13. [Google Scholar]
- Delidovich I.; Hausoul P. J. C.; Deng L.; Pfützenreuter R.; Rose M.; Palkovits R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. 10.1021/acs.chemrev.5b00354. [DOI] [PubMed] [Google Scholar]
- Vilela C.; Sousa A. F.; Fonseca A. C.; Serra A. C.; Coelho J. F. J.; Freire C. S. R.; Silvestre A. J. D. The quest for sustainable polyesters - insights into the future. Polym. Chem. 2014, 5, 3119–3141. 10.1039/c3py01213a. [DOI] [Google Scholar]
- Hülsey M. J.; Yang H.; Yan N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustainable Chem. Eng. 2018, 6, 5694–5707. 10.1021/acssuschemeng.8b00612. [DOI] [Google Scholar]
- Abid S.; El Gharbi R.; Gandini A. Polyamides incorporating furan moieties. 5. Synthesis and characterisation of furan-aromatic homologues. Polymer 2004, 45, 5793–5801. 10.1016/j.polymer.2004.06.046. [DOI] [Google Scholar]
- Gharbi S.; Gandini A. Synthese de nouveaux polyamides entierement furaniques. J. Soc. Chim. Tunis. 2004, 6, 17–26. [Google Scholar]
- Gandini A.; Silvestre A. J. D.; Neto C. P.; Sousa A. F.; Gomes M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. Polym. Chem. 2009, 47, 295–298. 10.1002/pola.23130. [DOI] [Google Scholar]
- Deng J.; Liu X.; Li C.; Jiang Y.; Zhu J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015, 5, 15930–15939. 10.1039/c5ra00242g. [DOI] [Google Scholar]
- Gubbels E.; Jasinska-Walc L.; Koning C. E. Synthesis and Characterization of Novel Renewable Polyesters Based on 2,5-Furandicarboxylic Acid and 2,3-Butanediol. J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 890–898. 10.1002/pola.26446. [DOI] [Google Scholar]
- Moreau C.; Belgacem M. N.; Gandini A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. 10.1023/b:toca.0000013537.13540.0e. [DOI] [Google Scholar]
- Du P.; Wu M.; Liu X.; Zheng Z.; Wang X.; Sun P.; Joncheray T.; Zhang Y. Synthesis of linear polyurethane bearing pendant furan and cross-linked healable polyurethane containing Diels-Alder bonds. New J. Chem. 2014, 38, 770–776. 10.1039/c3nj01245j. [DOI] [Google Scholar]
- Cousin T.; Galy J.; Rousseau A.; Dupuy J. Synthesis and properties of polyamides from 2,5-furandicarboxylic acid. J. Appl. Polym. Sci. 2018, 135, 45901. 10.1002/app.45901. [DOI] [Google Scholar]
- Ma K.; Chen G.; Wang W.; Zhang A.; Zhong Y.; Zhang Y.; Fang X. Partially bio-based aromatic polyimides derived from 2,5-furandicarboxylic acid with high thermal and mechanical properties. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 1058–1066. 10.1002/pola.28982. [DOI] [Google Scholar]
- Gross R. A.; Kumar A.; Kalra B. Polymer synthesis by in vitro enzyme catalysis. Chem. Rev. 2001, 101, 2097–2124. 10.1021/cr0002590. [DOI] [PubMed] [Google Scholar]
- Kobayashi S.; Makino A. Enzymatic polymer synthesis: an opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. 10.1021/cr900165z. [DOI] [PubMed] [Google Scholar]
- Gross R. A.; Ganesh M.; Lu W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. 10.1016/j.tibtech.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Loos K. Enzymatic Synthesis of Biobased Polyesters and Polyamides. Polymers 2016, 8, 243. 10.3390/polym8070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch S.Building Blocks for Polymer Synthesis by Enzymatic Catalysis. Ph.D. Dissertation, KTH Royal Institute of Technology, 2017. [Google Scholar]
- Jiang Y.; Maniar D.; Woortman A. J. J.; Loos K. Enzymatic synthesis of 2,5-furandicarboxylic acid-based semi-aromatic polyamides: enzymatic polymerization kinetics, effect of diamine chain length and thermal properties. RSC Adv. 2016, 6, 67941–67953. 10.1039/c6ra14585j. [DOI] [Google Scholar]
- Jiang Y.; Woortman A.; van Ekenstein G.; Loos K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules 2013, 3, 461–480. 10.3390/biom3030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Woortman A. J. J.; Alberda van Ekenstein G. O. R.; Loos K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. 10.1039/c5py00629e. [DOI] [Google Scholar]
- Jiang Y.; Woortman A. J. J.; Alberda van Ekenstein G. O. R.; Petrović D. M.; Loos K. Enzymatic Synthesis of Biobased Polyesters Using 2,5-Bis(hydroxymethyl)furan as the Building Block. Biomacromolecules 2014, 15, 2482–2493. 10.1021/bm500340w. [DOI] [PubMed] [Google Scholar]
- Schwab L. W.Polyamide Synthesis by Hydrolases. Ph.D. Dissertation, University of Groningen, Groningen, 2010. [Google Scholar]
- Froidevaux V.; Negrell C.; Caillol S.; Pascault J.-P.; Boutevin B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. 10.1021/acs.chemrev.6b00486. [DOI] [PubMed] [Google Scholar]
- Wang S.; Meng X.; Zhou H.; Liu Y.; Secundo F.; Liu Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 6, 32. 10.3390/catal6020032. [DOI] [Google Scholar]
- Jiang Y.; van Ekenstein G. O. R. A.; Woortman A. J. J.; Loos K. Fully Biobased Unsaturated Aliphatic Polyesters from Renewable Resources: Enzymatic Synthesis, Characterization, and Properties. Macromol. Chem. Phys. 2014, 215, 2185–2197. 10.1002/macp.201400164. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.